Abstract
The glutamatergic system directs central nervous system (CNS) neuronal activity and may underlie various neuropsychiatric disorders. Glutamate transmits its effects through multiple receptor classes. Class II metabotropic glutamate receptors, mGlu2 and mGlu3, play an important role in regulating synaptic release of different neurotransmitter systems and consequently modulate signaling across several neuronal subtypes. Drugs targeting mGlu2 and mGlu3 are seen as potential therapeutics for various psychiatric and neurological disorders, and defining their expression through development can aid in understanding their distinct function.
Here, non-radioactive in situ hybridization was used to detect mGlu2 and mGlu3 mRNA in the CNS of 129SvEv mice at PN1, PN8, PN25, PN40, and PN100. At PN1, mGlu2 and mGlu3 are strongly expressed cortically, most notably in layer III and V. Subcortically, mGlu2 is detected in thalamic nuclei; mGlu3 is highly expressed in the striatum. By PN8, the most notable changes are in hippocampus and cortex, with mGlu2 densely expressed in the dentate gyrus, and showing increased cortical levels especially in medial cortex. At PN8, mGlu3 is observed in cortex and striatum, with highest levels detected in reticular thalamic nucleus. At PN25 patterns of expression approximated those observed across adulthood (PN40 & PN100): mGlu2 expression was high in cortex and dentate gyrus while mGlu3 showed expression in the reticular thalamic nucleus, cortex, and striatum. These studies provide a foundation for future research seeking to parse out the roles of mGlu2 from mGlu3, paving the way for better understanding of how these receptors regulate activity in the brain.
Keywords: Metabotropic glutamate receptor, mGlu2, mGlu3, Mouse, In situ hybridization
1. Introduction
Glutamate is the primary excitatory neurotransmitter in the brain, acting at ionotropic ligand-gated channels (Glu) and metabotropic G protein-coupled receptors (mGlu) to finely tune neuronal activity (Neki et al., 1996b,; Ohishi et al., 1998) reviewed in (Ferraguti and Shigemoto, 2006). Glutamate receptors have broad and overlapping expression patterns within the brain, and the metabotropic class comprises 8 distinct receptors that are further classified into 3 subgroups based upon their structure and function (Conn and Pin, 1997). Class I couple to Gq and activate signaling via phospholipase C. Class II couple to Gi/o, negatively regulating adenyl cyclase, as do Class III receptors (for review see Ferraguti and Shigemoto, 2006). Clinical and preclinical studies of class II mGlu receptors have examined their potential role in ranging from psychiatric (schizophrenia, drug abuse, depression) to neurological (epilepsy, stroke) (reviewed in Imre, 2007).
Class II mGlu receptors (e.g., mGlu2 and mGlu3) are found on different types of neurons where they act either presynaptically to inhibit adenyl cyclase, negatively regulating glutamate release into the synapse or postsynaptically where they negatively modulate excitability via intracellular mechanisms such as inhibition of adenylate cyclase, mTOR signaling, modulation of ion channels, and induction of long term depression (Anwyl, 1999). Receptors of the mGlu2/3 class can heterodimerize with each other and with other receptors (Gonzalez-Maeso et al., 2008; Doumazane et al., 2011), controlling release of GABA, monoamines and other neuropeptides (Cartmell et al., 2001; Coplan et al., 2001; Schoepp, 2001). In this way, mGlu2/3 play important roles at both sides of the synapse in modulating neuronal activity and brain function.
There is very little work attempting to distinguish between mGlu2 and mGlu3 pharmacology and function. This is largely due to the fact that mGlu2 and mGlu3 are highly conserved, and subtype-specific ligands have only recently been identified (Sheffler et al., 2010; Cid et al., 2012). Attempts to dissociate mGlu2 from mGlu3 suggest distinct roles and interactions for each subtype (Spooren et al., 2000; Linden et al., 2005; Hetzenauer et al., 2008). The temporal expression of mGlu2 and mGlu3 and the role of these receptors in brain development is also not well understood, and a systematic analysis of mGlu2 and mGlu3 expression across development has not previously been conducted in species used as model systems for CNS disorders. To address this lack of information, in this study we assess mGlu2 and mGlu3 mRNA expression in the mouse brain across development.
2. Methods
2.1. Animals
Mice were bred and housed under standard husbandry conditions, approved by the Institutional Animal Care and Use Committee, and monitored daily for birth dates. Mice were euthanized, whole brains dissected and fresh frozen in O.C.T.; PN1 mice were dissected in ice-cold PBS, while older brains were removed in ambient air (N = 3 per timepoint). All brains were stored at −80 °C prior to sectioning. 18 μm sections were cut directly onto Super-Frost Plus Slides on a Leica CM 3050 S cryostat, onto series of 8 slides, and ISH was performed on a set of 5 slides (with 15–21 sections per slide depending on the age and bregma level totally 75–105 sections per animal) covering the distance from olfactory bulb to hindbrain (Bregma −4.16), then air dried for 20 min and stored at −80 °C.
2.2. Design and generation of probes for in situ hybridization
Anti-sense mGlu2 and mGlu3 in situ probes were generated by in vitro transcription with DIG-labeled NTPs from linearized cDNA templates, using DIG RNA Labeling SP6/T7 Kit (Roche, 11175025910). A full-length cDNA clone BC115866 (Thermo Fisher, Open Biosystems) was used as a template for mGlu2. A partial cDNA template for mGluR3 was cloned from 129SvEv whole brain cDNA by PCR using primers: 5′-AAAGGCACTGGAACTGAAGA-3′ and 5′-GGTTGAGGCTCTGGAAGTAG-3′, and subcloned into a pCR-II-TOPO vector (Life Technologies).
2.3. DIG-labeled in situ hybridization
All solutions and stocks were made up prior to the experiment with RNase free reagents. On the day of experiment sections were air dried for 15 min, then placed in a slide basket. Slides were immersed in cold 4% paraformaldehyde (PFA) for 15 min, washed 3 × 3 min in PBS, then treated with Proteinase K for 5 min (Proteinase K solution: 1 μg/ml Proteinase K, 50 mM Tris pH7.5, 5 mM EDTA pH8.0). Slides were immersed in 4% PFA for a second time, for 5 min, washed 3 × 3 min in PBS, then placed in acetylation solution for 10 min (84 mM acetic anhydride, 24 mM triethanolamine, 20 mM HCl). Slides were washed 3 × 5 min in fresh PBS prior to being removed from the metal slide rack and placed in a tray. Sections were then covered with 500 μl hybridization solutions per slide and incubated ~2 h in a humidified slide tray.
During this incubation, hybridization probes were prepared. Probes were diluted in hybridization solution (0.1 μM/μL), heated at 80 °C for 5 min then returned immediately to ice prior to being placed on sections. 100 μL probe solution was placed on each slide, which were then cover-slipped and placed in boxes humidified with 5 × SSC, %50 formamide, and incubated overnight at 72 °C.
The following day slides were immersed in 5xSSC preheated to 72 °C, and incubated for 5 min. Coverslips were removed and slides were transferred to 0.2xSSC at 72 °C, and incubated for 1 h. Slides were transferred to 0.2xSSC at room temperature for 5 min prior to being transferred to buffer B1 (0.1 M Tris-HCl pH7.5, 0.15 M NaCl). Slides were then returned to the humidified tray and covered with 0.5 ml Buffer B2 (B1 +10% heat inactivated normal goat serum), and incubated for 1 h. Slides were rinsed with buffer B1 then incubated overnight at 4 °C with anti-DIG antibody (Buffer B1 + %1 HINGS + 1:5000 Sheep anti-DIG-AP FAb fragments (Roche, 11093274910)), in a humidified tray.
On day 3, slides were washed 3 × 5 min in Buffer B1, and equilibrated in Buffer B3 (0.1 M Tris-HCl pH9.5, 0.1 M NaCl, 50 mM MgCl2) for 5 min. 0.5 ml of BCIP/NBT substrate solution (Vector Laboratories, SK-5400) was placed on each slide, slides were cover-slipped and the DIG colorimetric reaction was allowed to proceed in the dark, at room temperature until visualization of the DIG labeled probes occurred. A ddH2O wash ended the color reaction, and slides were dried and mounted with Glycergel medium (Dako USA, C0563) for imaging.
To test probe specificity, sense probes were hybridized in tandem for both receptors, and for mGlu2 in situ hybridization was performed on mGlu2 knockout mouse tissue. No hybridization was detected in this mouse, allowing us to confirm that the probe did not cross-react with mGlu3, or any other protein in the mouse brain (data not shown).
2.4. Imaging
Sections were imaged using a Nikon DS-Fi1 microscope, and captured using NIS Elements software. Captured images were compiled in Photoshop 6.0.
3. Results
3.1. mGlu2 expression across a developmental time-course
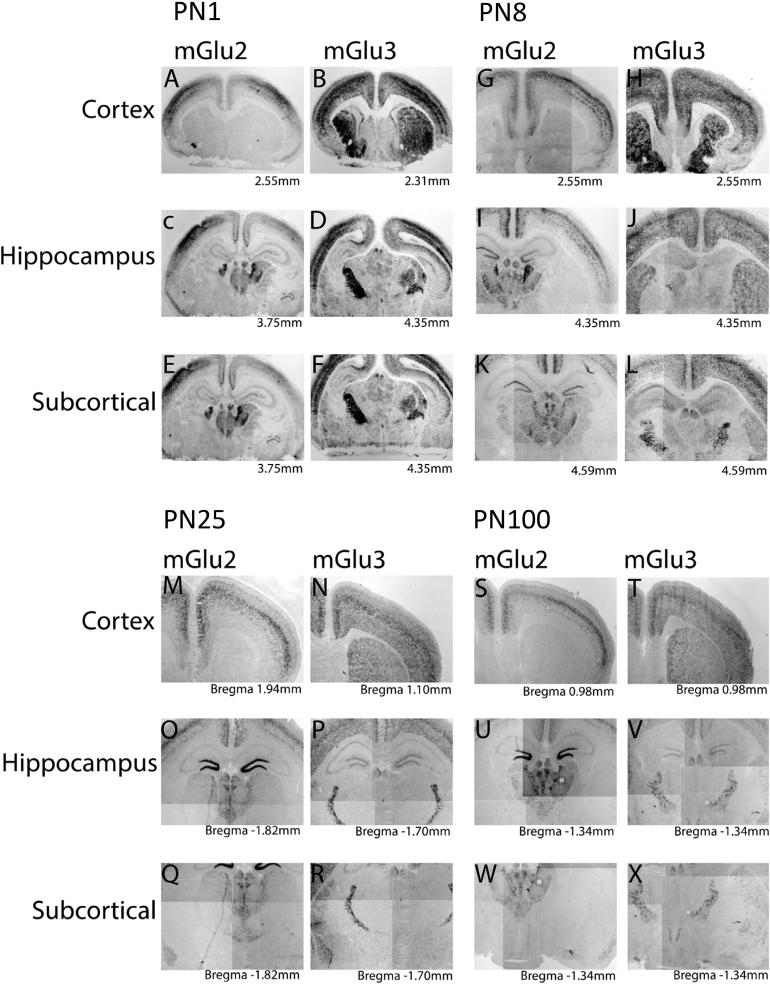
At postnatal day 1 (PN1), mGlu2 expression is observed in cortical layers IV and V (Fig. 1A). Strongest cortical expression is in somatosensory regions, with lower levels detected in the cingulate, insular, and parietal cortices, and the pyramidal layer of the piri-form cortex. Diffuse punctate expression is observed in the dentate gyrus of the hippocampus (Fig. 1C). Subcortically, numerous thalamic nuclei express mGlu2 at this time point (detailed in Table 1). Strong expression is observed in the basolateral amygdala and the medial habenula with no expression detected in the lateral habenula (Fig. 1E).
Fig. 1.
mGlu2 and mGlu3 mRNA expression throughout development. In situ hybridization with DIG-labeled antisense RNA probes for mGlu2 and mGlu3 of representative coronal sections along the rostrocaudal axis at postnatal day 1 (PN1), PN8, PN25 and PN100 (adult) mouse brain. Bregma levels are indicated for each section.
Table 1. mGlu2 expression across the developmental period.
Summary of in situ hybridization data for mGlu2 mRNA expression levels across five developmental time points – postnatal day 1 (PN1), PN8, PN25, PN40 and PN100 (adult). A qualitative expression level scale was defined to correspond to the strength of observed DIG-BCIP/NBT colorimetric reaction as follows: ‘−’ no detectable expression, ‘+’ weak, ‘++’ moderate, ‘+++’ strong, ‘++++’ very strong, ‘+++++’ extremely strong.
| Brain region | PN1 | PN8 | PN25 | PN40 | PN100 |
|---|---|---|---|---|---|
| Olfactory Bulb | |||||
| AOB | ++ | +++++ | +++ | +++ | ++ |
| AOM | +++ | ++ | ++ | + | |
| Cortex | |||||
| Layer 1 | − | − | − | − | − |
| Layer 2/3 | ++ | + | + | + | + |
| Layer 4 | ++++ | +++ | +++ | +++ | ++ |
| Layer 5 | +++ | +++ | ++ | + | + |
| Layer 6 | − | − | − | − | − |
| Hippocampus | |||||
| CA Stratum oriens | − | − | − | − | − |
| CA Stratum pyramidale | − | + | − or + | − | − |
| CA Stratum radiens | − | − | − | − | − |
| CA Stratum lacunosum moleculare | − | − | − | − | − |
| DG molecular layer | − | − | − | − | − |
| DG granular layer | + | +++++ | +++++ | +++++ | +++++ |
| DG hilus | − | − | + | − | − |
| Amygdala | |||||
| Basloateral | − | ++ | ++ | ++ | +++ |
| Central | − | − | − | − | − |
| Anterior amygdaloid area | − | + | ++ | − | − |
| Medial nucleus | − | − | − | − | − |
| Cortical nucleus | − | + | + | − | − |
| Lateral olfactory tract nucleus | − | − | − | − | − |
| Intercalated nuclei | − | − | − | − | − |
| Basal Ganglia | |||||
| Striatum | − | − | − | − | − |
| Nucleus Accumbens Core | − | − | − | − | − |
| Nucleus Accumbens Shell | − | − | − | − | − |
| Thalamus | |||||
| Habenula – medial | ++++(caudal) | +++ | ++ | ++ | ++ |
| Habenula – lateral | +(caudal) | + | + | + | − |
| Zona Incerta | +++ | + | − | ||
| Dorsomedial laterodorsal | ++++ | ++ | + | − | +++ |
| Ventrolateral laterodorsal | +++ | ++ | + | − | ++ |
| Ventrolateral | +++ | ++ | + | + | + |
| Anteroventral | + | ++++ | − | ++ | |
| Anteromedial | + | ++ | ++ | ++ | |
| Anterodorsal | ++ | ++ | +++ | ++ | +++ |
| Intermediodorsal | + | ++++ | |||
| Mediodorsal – central | +++ | +++ | ++ | ++ | ++ |
| Mediodorsal – lateral | +++ | ++++ | + | + | + |
| Xiphoid | − | + | +++ | ++ | + |
| Reunions | +++ | ++ | + | + | + |
| Rhomboid | +++ | + | +++ | + | + |
| Central medial | +++ | +++ | − | ++ | ++ |
| Posterior | ++ | +++ | + | ||
| Ventral posteromedial | ++ | − | ++ | ||
| Reticular | − | − | − | − | − |
| Dorsal Tenia tecta | − | ++ | + | − | − |
| Medial Pretectal | − | ||||
| Lithoid | ++++ | ++++ | ++++ | ||
| Paraventricular nucleus | +++ | +++ | ++ |
By PN8, the most notable changes are in the hippocampus and cortex (Table 1, Fig. 1G and I). Dense staining is seen in the granular layer of the dentate gyrus. Rostral cortex expression is observed along a dorsoventral gradient with strongest levels in the somatosensory areas. More caudally, the cingulate cortex shows intense expression, acquiring a layered pattern with dense expression observed in layers IV and V (Table 1). In the olfactory bulb, mGlu2 is expressed in the glomerular layer, the inter-peduncular nucleus, lateral subnucleus and the mitral cell layer. Strong expression is also seen in the external anterior olfactory nucleus and the dorsal tenia tecta, along the rostrocaudal axis (Table 1). The lateral septal nucleus exhibits diffuse but strong expression. The medial habenula and the basolateral amygdala also have demonstrable mGlu2 expression, and staining in several thalamic nuclei is evident (Table 1, Fig. 1K). At PN25 strong cortical expression is evident in layer IV (Fig. 1M), with diffuse expression in layer II/III, and V, and the pyramidal layer of the piriform cortex with no positive cells observed in Layer I or VI. Highest levels of mGlu2 are observed in the granular cell layer of the dentate gyrus (Fig. 1O). This pattern of expression persists into adulthood as observed at PN40 and PN100 time points (Table 1, Fig. 1S, U, W and Fig. 2A–D). At PN100 mGlu2 is also observed at low levels in the pyramidal layer of the cornu ammonis of the hippocampus (Figs. 1U and 2C).
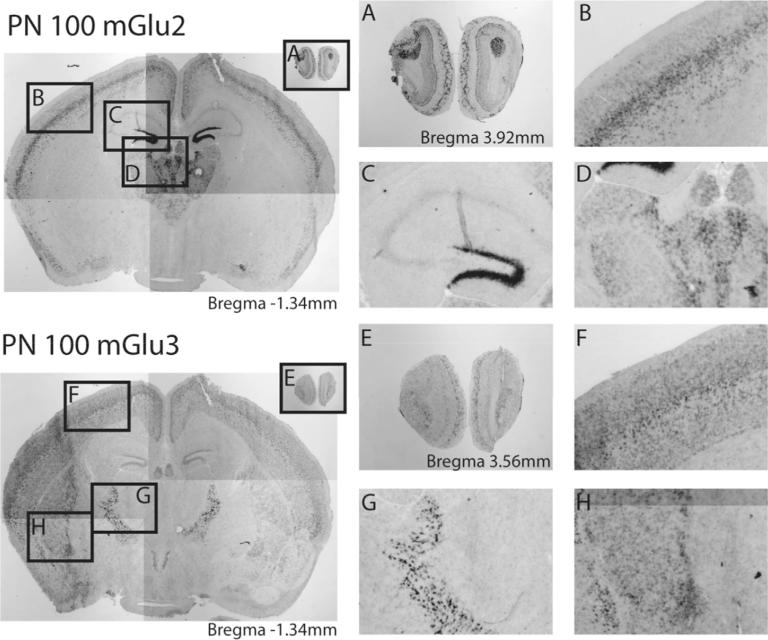
Fig. 2.
Distinct patterns of mGlu2 and mGlu3 in the adult mouse brain. Representative images of different brain subregions expressing mGlu2 and mGlu3 mRNA at postnatal day 100 (PN100). High magnification panels correspond to outlined borders on low magnification map as indicated for mGlu2 (A–D) and mGlu3 (E–H). Distinct expression of mGlu2 and mGlu3 is seen in the olfactory bulb (A and E), cortical layers (B and F), and subcortical nuclei (D, H). mGlu2 is enriched in the dentate gyrus of the hippocampus (C), whereas high levels of mGlu3 are seen in the reticular thalamic nucleus (G).
3.2. mGlu3 expression across a developmental time-course
At PN1, robust mGlu3 expression is already detectable in many regions: the glomerular and mitral layers of the olfactory bulb, and cortex, with especially strong levels in layer IV and layer V, the nucleus accumbens core and shell, and in the striatum (Fig. 1B). Weaker expression is seen in the zona incerta at this age. The region most strongly expressing mGlu3 is the reticular thalamic nucleus (Fig. 1D, F). Interestingly, at this timepoint expression is also observed along the medial wall of the lateral ventricle, an area coincident with the corridor where neuronal progenitors are still migrating at this early stage (Fig. 1B). Scattered punctate expression is observed throughout the brain, suggestive of glial expression. By PN8 mGlu3 expression is clearly evident in the anterior olfactory nucleus, dorsal tenia tecta, and granular insular cortex (Table 2). A notable difference between mGlu2 and mGlu3 is the high level of mGlu3 expression seen in the striatum and the nucleus accumbens core and shell (Fig. 1H, J). Lower levels of mGlu3 are observed in the subgeniculate nucleus, dorsal lateral geniculate nucleus, and the medial habenular nucleus (Table 2, Fig. 1 L). At PN25 cells expressing mGlu3 continue to be observed throughout the cortex with densest expression now restricted to layer IV (Fig. 1N). Strong punctate expression is also seen in the basolateral amygdala, and dense, uniform staining is seen across the entire extent of the reticular thalamic nucleus (Fig. 1P, R). mGlu3 expression at PN40 and PN100 is similar to that observed at PN25; strongest expression is in the reticular thalamic nucleus (Fig. 1V, X). The cortex expresses mGlu3, across the rostrocaudal and dorsoventral axes, and mGlu3 is abundant in the nucleus accumbens and the striatal matrix (Table 2 and Fig. 1T). Expression is also seen in the pyramidal layer of the hippocampus, the granular layer of the dentate gyrus, superficial gray superior colliculus, and the claustrum (Fig. 2E–H).
Table 2. mGlu3 expression across the developmental period.
Summary of in situ hybridization data for mGlu3 mRNA expression levels across five developmental time points – postnatal day 1 (PN1), PN8, PN25, PN40 and PN100 (adult). A qualitative expression level scale was defined to correspond to the strength of observed DIG-BCIP/NBT colorimetric reaction as follows: ‘−’ no detectable expression, ‘+’ weak, ‘++’ moderate, ‘+++’ strong, ‘++++’ very strong, ‘+++++’ extremely strong.
| Brain region | PN1 | PN8 | PN25 | PN40 | PN100 |
|---|---|---|---|---|---|
| Olfactory Bulb | |||||
| AOB | +++++ | ++++ | + | + | + |
| AOM | +++++ | +++++ | ++ | ++ | ++ |
| Cortex | |||||
| Layer 1 | + | + | + | + | + |
| Layer 2/3 | ++++ | +++ | ++ | ++ | +++ |
| Layer 4 | +++++ | ++++ | ++++ | ++++ | ++++ |
| Layer 5 | +++++ | ++++ | +++ | +++ | +++ |
| Layer 6 | +++ | +++ | +++ | +++ | +++ |
| Hippocampus | |||||
| CA Stratum oriens | + | + | + | + | + |
| CA Stratum pyramidale | + | + | + | + | + |
| CA Stratum radiens | + | + | + | + | + |
| CA Stratum lacunosum moleculare | + | + | + | + | + |
| DG molecular layer | + | + | + | + | + |
| DG granular layer | + | + | + | + | + |
| DG hilus | + | + | + | + | + |
| Amygdala | |||||
| Basloateral | ++ | + | +++ | ++ | + |
| Central | + | + | + | + | + |
| Anterior amygdaloid area | + | + | + | + | + |
| Medial nucleus | + | + | + | + | + |
| Cortical nucleus | + | + | + | + | + |
| Lateral olfactory tract nucleus | + | + | + | + | + |
| Intercalated nuclei | + | + | + | + | + |
| Basal Ganglia | |||||
| Striatum | +++++ | ++++ | +++ | +++ | +++ |
| Nucleus Accumbens Core | +++++ | ++++ | ++++ | ++++ | +++ |
| Nucleus Accumbens Shell | +++++ | ++++ | ++++ | ++++ | +++ |
| Thalamus | |||||
| Habenula – medial | ++++ | ++++ | + | +++ | ++ |
| Habenula – lateral | +++ | +++ | + | + | + |
| Zona Incerta | ++ | + | + | + | + |
| Dorsomedial laterodorsal | ++ | + | + | + | + |
| Ventrolateral laterodorsal | ++ | + | + | + | + |
| Ventrolateral | ++ | + | + | + | + |
| Anteroventral | ++ | + | + | + | + |
| Anterodorsal | ++ | + | + | + | + |
| Intermediodorsal | ++ | + | + | + | + |
| Mediodorsal – central | ++ | + | + | + | + |
| Mediodorsal – lateral | ++ | + | + | + | + |
| Xiphoid | ++ | + | + | + | + |
| Reunions | ++ | + | + | + | + |
| Rhomboid | ++ | + | + | + | + |
| Central medial | ++ | + | + | + | + |
| Paracentral | ++ | + | + | + | + |
| Posterior | ++ | + | + | + | + |
| Ventral posteromedial | ++ | + | + | + | + |
| Reticular | +++++ | +++++ | +++++ | +++++ | +++++ |
| Dorsal Tenia tecta | +++ | ++ | +++ | ++ | ++ |
| Medial Pretectal | ++++ | + | + | + | |
| Lithoid | ++ | + | + | + | + |
Expression of both mGlu2 and mGlu3 appear to decrease with age. While this cannot be quantified using DIG-labeled ISH, a relationship between mGlu2/3 and age has been shown previously, using [3H]LY341495 radioligand binding (Frank et al., 2011; Matosin et al., 2014; McOmish et al., 2016).
4. Discussion
In this study we mapped expression of mGlu2 and mGlu3 mRNA throughout development and in adulthood, in the mouse CNS. We show that while both mGlu2 and mGlu3 are each expressed across many brain regions, they display distinct regional localization. Expression of mGlu2 was most notable in cortical and hippocampal subregions with high enrichment in the dentate gyrus; cortical mGlu2 appeared to decline across the time periods assessed, while concentration in the dentate gyrus increased from PN1 to PN8, remaining consistently high throughout later developmental stages and adulthood. mGlu3 was comparatively more strongly expressed than mGlu2; however, levels of mGlu3 appeared to decrease more significantly with age. The reticular thalamic nucleus was an exception to this trend, retaining very strong mGlu3 expression throughout all time points investigated. In addition, a tendency for mGlu3 expression to predominate in the basal ganglia was noted, while subcortically mGlu2 was more widely expressed in central and medial thalamic nuclei. Both receptors were expressed in amygdalar regions. In sum, these studies demonstrate robust expression of mGlu2 and mGlu3 in development and in adulthood, in distinct and complementary patterns.
At PN40 and PN100, the expression patterns described herein align with previous research describing mGlu2 and mGlu3 in adulthood, in rats (Ohishi et al., 1993b; a; Testa et al., 1994; Petralia et al., 1996; Ohishi et al., 1998; Tamaru et al., 2001; Crook et al., 2002) showing overlapping yet distinct patterns for the two receptor subtypes. mGlu3 has been reported to be widely expressed in rat CNS ((Ohishi et al., 1993b; a; Testa et al., 1994; Petralia et al., 1996; Ohishi et al., 1998; Tamaru et al., 2001) with high expression seen across most cortical regions, striatum, nucleus accumbens, lateral amygdala, and in the thalamic reticular nucleus. These studies also show that mGlu2 is strongly expressed in the golgi cells of the cerebellar cortex and the mitral cells of the olfactory bulb, with weaker expression in the neocortex, and amygdala ((Ohishi et al., 1993a, 1998) ((Sahara et al., 2001). In contrast with the present investigation, these studies suggested that mGlu3 expression predominates in the dentate gyrus of the hippocampus, with lower levels of mGlu2 being observable. Here we show striking mGlu2 expression in the mouse dentate gyrus pyramidal layer, with minimal mGlu3 mRNA detected in this region, in agreement with another recent study in adult rat CNS (Gu et al., 2008). Subtle differences in response to mGlu2/3 antagonists have been demonstrated across mouse strains (Linden et al., 2005; Hetzenauer et al., 2008), and thus strain-, or indeed species-specific differences may account for the inconsistency between studies.
Extending our knowledge of mGlu2 and mGlu3 expression, this study is the first to systemically investigate mGlu2 and mGlu3 mRNA in the CNS across a developmental time course spanning early postnatal stages and adulthood. One published study has investigated expression of mGlu2 and mGlu3 in rats across a postnatal developmental period up to PN30 (Catania et al., 1994). While many similarities are observed, in contrast to our findings in mice, expression of mGlu2 in rats appears to be relatively low at PN1, increasing to a peak at PN14, and then decreasing again by PN30.
More recently, an immunohistochemical study assessed mGlu2 protein in the juvenile mouse brain (PN 10-13) (Venkatadri and Lee, 2014). The pattern of mGlu2 protein described in this study is closely mimicked by our findings at the mRNA level, with some notable differences including rostrocaudal and dorsoventral gradients in cortical expression intensity, and labelling in the striatum and thalamic reticular nucleus – not observed in the present study. In the absence of further information, it is difficult to identify the reasons for these discrepancies, however mouse strain, protein versus RNA distribution, lack of antibody specificity between mGlu2 and mGlu3, or methodological attributes are all potential sources of variability.
In many of the regions assessed, most notably the cortex, both mGlu2 and mGlu3 expression were discernable, with greatest overlap in layer IV and V. In regions of co-expression, mGlu2 and mGlu3 have been suggested to be able to substitute for each other (Ceolin et al., 2011) allowing for redundancy and functional flexibility. Contrastingly, several regions of the CNS either express a single mGlu subtype, or preferentially express one subtype to such an extent that one can begin to assign more precise roles to each receptor. Consistent with this, global knockouts of these receptors in mice demonstrate specific functions; mGlu2 appears to be critical for the antipsychotic-like effects of mGlu2/3 ligands (Spooren et al., 2000), while both subtypes appear to be involved in the anxiolytic actions of mGlu2/3 antagonists (Linden et al., 2005). Meanwhile mGlu3, but not mGlu2, appear to underlie the cellular response to mGlu2/3 antagonists in the centromedial amygdala and ventral lateral septal nucleus (Hetzenauer et al., 2008). Thus the results of this study provide further evidence for understanding how distinct and overlapping mGlu2 and mGlu3 circuits contribute to the behavioral outcomes of genetic or pharmacological targeting of class II mGlu.
The most notable differences in expression were observed in the thalamic reticular nucleus (strong mGlu3 expression with negligible mGlu2) and the dentate gyrus (strong mGlu2 expression with negligible mGlu3). The thalamic reticular nucleus (TRN) is a highly complex, subcortical structure that forms a capsule around the thalamic nuclei. While it receives input from cortex and other thalamic locales, it only sends efferent projections back to the thalamus. It is a predominantly GABAergic structure, nonetheless containing heterogeneous cell populations (Pinault, 2004), and has been reported to be broadly involved in functional modalities, with at least seven sectors of this region having been defined electrophysiologically, including five sensory regions, one motor region and one limbic region (Shosaku and Sumitomo, 1983; Shosaku et al., 1984). The TRN has been suggested to be involved in cognitive and sensory modalities that are disrupted in schizophrenia (Pratt and Morris, 2015), and the extremely high levels of mGlu3 seen throughout the region underscore the importance of mGlu3 in mediating these important functional consequences. Interestingly, mGlu3 has been implicated in the pathophysiology of schizophrenia by genetic studies (Schizophrenia Working Group of the Psychiatric Genomics C, 2014), supporting the possible involvement of this region in the schizophrenia syndrome.
In contrast, mGlu2 expression was especially strong in the granular layer of the dentate gyrus. The dentate gyrus (DG) is unique in many respects. It is widely known to undergo neurogenesis in adulthood, while maintaining a constant cell count suggestive of balanced cell turnover. It also appears to be unidirectional in its projections, with few reciprocal connections described. Granule cells of the DG are innervated by the entorhinal cortex – the major input to the DG – and give rise to unmyelinated ‘mossy fiber’ axons that project into the CA3 and the polymorphic layer of the hippocampus. The granule cell layer of the DG is composed of densely packed granule cells which are excitatory and, consistent with high levels of mGlu2 expression, use glutamate as their primary neurotransmitter. They are most widely noted for their role in memory formation, and accumulating evidence implicates this region in schizophrenia. Reduced neurogenesis has been observed in the DG of postmortem tissue from schizophrenia patients (Allen et al., 2016). Additionally, hippocampal glutamate levels have been implicated as a key driver of psychosis (Schobel et al., 2013), and changes in glutamate receptor expression in the DG have been suggested to underlie altered glutamate concentrations across the hippocampus more broadly (Stan et al., 2015). Together, these findings support an important role for class II mGlu2 in the development of schizophrenia.
The observation that both mGlu2 and mGlu3 begin to be expressed during early (PN1- PN25) stages, with the strongest DIG labelling observed at the earlier time points, suggests an important role for these receptors in brain development. While little is known regarding an active role of these receptors during embryonic or early postnatal periods, they can be modulated during development by environmental stressors (Melendez et al., 2004; Nasca et al., 2013), placing class II mGlu in a key position to be regulators of gene × environment interactions.
While the results described herein provide novel insight into glutamate receptor expression, further experimentation would significantly add to these findings. Firstly, the study measured mRNA in brain sections in a qualitative manner. While expression can be judged as being weak, moderate or strong, in situ hybridization does not provide quantitative information, and we are thus unable to comment on the absolute level of expression seen across time points and probes. Moreover, protein levels were not measured, in large part due to the dearth of specific antibodies, and thus we cannot comment on the distribution or subcellular localization of functional receptors, during these time points. While a high correlation between RNA and protein expression for these receptors has previously been shown (Neki et al., 1996a; b; Ohishi et al., 1998), this would certainly require future investigation.
Given the known importance of this class of receptors in modulating glutamatergic tone, these findings will facilitate further investigation into the regulation of glutamatergic signaling. A better understanding of the spatio-temporal expression dynamics of these receptors will allow us to form more cogent hypotheses on how mGlu2 and/or mGlu3 work together, or independently, in brain development and adult function. To do so requires increasing our understanding of mGlu2 and mGlu3's role in maintaining healthy brain function. Here we show that both mGlu2 and mGlu3 are present at high levels during important developmental periods, and in regions of the CNS known to be involved in critical functions that are disrupted in disorders such as schizophrenia, anxiety and depression. Our results support a model in which these two receptors likely play precise and distinct roles in controlling neural activity, while potentially possessing some functional redundancy, allowing us to refine our view of how each of these receptors contributes to brain function in health and in disease.
Acknowledgements
The authors would like to thank Joshko Ivica and Anil Jonathan for their assistance with animal husbandry, and Indigo Pratt Kelly for her contributions to the microscopy. Over the course of these experiments, the investigators were supported by several grants. CEM was supported by an NHMRC overseas biomedical research fellowship (628906), and a Brain and Behavior Research Foundation Young Investigator award (19543). JAG was supported by NIMH (2R01MH080116-06A1), EYD was supported by NIMH (1R21MH099458-01A1).
The funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Abbreviations
- AOB
accessory olfactory bulb
- AOM
anterior olfactory nucleus, medial part
- CA
Cornu Ammonis
- CNS
central nervous system
- DIG
digoxigenin
- DG
dentate gyrus
- Glu
glutamate
- ISH
in situ hybridization
- mGlu
metabotropic glutamate receptor
- PN
postnatal day
- TRN
thalamic reticular nucleus
Footnotes
Author contribution statement
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CEM, EYD, JAG. Acquisition of data: CEM, EYD. Analysis and interpretation of data: CEM, EYD. Drafting of the manuscript: CEM. Critical revision of the manuscript for important intellectual content: EYD, JAG. All authors have approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Allen KM, Fung SJ, Shannon Weickert C. Cell proliferation is reduced in the hippocampus in schizophrenia. Aust. N. Z. J. Psychiatry. 2016;50(5):473–480. doi: 10.1177/0004867415589793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev. 1999;29(1):83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Perry KW, Salhoff CR, Monn JA, Schoepp DD. Acute increases in monoamine release in the rat prefrontal cortex by the mGlu2/3 agonist LY379268 are similar in profile to risperidone, not locally mediated, and can be elicited in the presence of uptake blockade. Neuropharmacology. 2001;40(7):847–855. doi: 10.1016/s0028-3908(01)00034-x. [DOI] [PubMed] [Google Scholar]
- Catania MV, Landwehrmeyer GB, Testa CM, Standaert DG, Penney JB, Jr., Young AB. Metabotropic glutamate receptors are differentially regulated during development. Neuroscience. 1994;61(3):481–495. doi: 10.1016/0306-4522(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Ceolin L, Kantamneni S, Barker GR, Hanna L, Murray L, Warburton EC, Robinson ES, Monn JA, Fitzjohn SM, Collingridge GL, Bortolotto ZA, Lodge D. Study of novel selective mGlu2 agonist in the temporoammonic input to CA1 neurons reveals reduced mGlu2 receptor expression in a Wistar substrain with an anxiety-like phenotype. J. Neurosci. Off. J. Soc. Neurosci. 2011;31(18):6721–6731. doi: 10.1523/JNEUROSCI.0418-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid JM, Duvey G, Tresadern G, Nhem V, Furnari R, Cluzeau P, Vega JA, de Lucas AI, Matesanz E, Alonso JM, Linares ML, Andres JI, Poli SM, Lutjens R, Himogai H, Rocher JP, Macdonald GJ, Oehlrich D, Lavreysen H, Ahnaou A, Drinkenburg W, Mackie C, Trabanco AA. Discovery of 1,4-disubstituted 3-cyano-2-pyridones: a new class of positive allosteric modulators of the metabotropic glutamate 2 receptor. J. Med. Chem. 2012;55(5):2388–2405. doi: 10.1021/jm2016864. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Mathew SJ, Smith EL, Trost RC, Scharf BA, Martinez J, Gorman JM, Monn JA, Schoepp DD, Rosenblum LA. Effects of LY354740, a novel glutamatergic metabotropic agonist, on nonhuman primate hypothalamic-pituitary-adrenal axis and noradrenergic function. CNS Spectr. 2001;6(7):607–612. 617. doi: 10.1017/s1092852900002157. [DOI] [PubMed] [Google Scholar]
- Crook JM, Akil M, Law BC, Hyde TM, Kleinman JE. Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann's area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol. Psychiatry. 2002;7(2):157–164. doi: 10.1038/sj.mp.4000966. [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25(1):66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326(2):483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Frank E, Newell KA, Huang XF. Density of metabotropic glutamate receptors 2 and 3 (mGluR2/3) in the dorsolateral prefrontal cortex does not differ with schizophrenia diagnosis but decreases with age. Schizophr. Res. 2011;128(1–3):56–60. doi: 10.1016/j.schres.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452(7183):93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Lorrain DS, Wei H, Cole RL, Zhang X, Daggett LP, Schaffhauser HJ, Bristow LJ, Lechner SM. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: implication in emotional responses and central disinhibition. Brain Res. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- Hetzenauer A, Corti C, Herdy S, Corsi M, Ferraguti F, Singewald N. Individual contribution of metabotropic glutamate receptor (mGlu) 2 and 3 to c-Fos expression pattern evoked by mGlu2/3 antagonism. Psychopharmacology. 2008;201(1):1–13. doi: 10.1007/s00213-008-1236-2. [DOI] [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13(4):444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Shannon H, Baez M, Yu JL, Koester A, Schoepp DD. Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology. 2005;179(1):284–291. doi: 10.1007/s00213-004-2098-x. [DOI] [PubMed] [Google Scholar]
- Matosin N, Fernandez-Enright F, Frank E, Deng C, Wong J, Huang XF, Newell KA. Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: implications for novel mGluR-based therapeutics. J. Psychiatry Neurosci. JPN. 2014;39(6):407–416. doi: 10.1503/jpn.130242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmish CE, Pavey G, Gibbons A, Hopper S, Udawela M, Scarr E, Dean B. Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J. Affect. Disord. 2016;190:241–248. doi: 10.1016/j.jad.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004;29(11):1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, Battaglia G, Mathe AA, Pittaluga A, Lionetto L, Simmaco M, Nicoletti F. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc. Natl. Acad. Sci. U. S. A. 2013;110(12):4804–4809. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Metabotropic glutamate receptors mGluR2 and mGluR5 are expressed in two non-overlapping populations of Golgi cells in the rat cerebellum. Neuroscience. 1996a;75(3):815–826. doi: 10.1016/0306-4522(96)00316-8. [DOI] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Preand postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci. Lett. 1996b;202(3):197–200. doi: 10.1016/0304-3940(95)12248-6. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci. Res. 1998;30(1):65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993a;53(4):1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1993b;335(2):252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, pre-synaptic and glial localizations. Neuroscience. 1996;71(4):949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res. Brain Res. Rev. 2004;46(1):1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. J. Psychopharmacol. 2015;29(2):127–137. doi: 10.1177/0269881114565805. [DOI] [PubMed] [Google Scholar]
- Sahara Y, Kubota T, Ichikawa M. Cellular localization of metabotropic glutamate receptors mGluR1, 2/3, 5 and 7 in the main and accessory olfactory bulb of the rat. Neurosci. Lett. 2001;312(2):59–62. doi: 10.1016/s0304-3940(01)02184-x. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299(1):12–20. [PubMed] [Google Scholar]
- Sheffler DJ, Wenthur CJ, Brunner JA, Daniels JS, Morrison RD, Blobaum AL, Dawson ES, Engers JL, Niswender CM, Conn PJ, Lindsley CW. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD: 2010. Development of the First Selective mGlu3 NAM from an mGlu5 PAM Hit. [PubMed] [Google Scholar]
- Shosaku A, Kayama Y, Sumitomo I. Somatotopic organization in the rat thalamic reticular nucleus. Brain Res. 1984;311(1):57–63. doi: 10.1016/0006-8993(84)91398-2. [DOI] [PubMed] [Google Scholar]
- Shosaku A, Sumitomo I. Auditory neurons in the rat thalamic reticular nucleus. Exp. Brain Res. 1983;49(3):432–442. doi: 10.1007/BF00238784. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R. Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic gluta-mate receptor 2 knockout mice. Eur. J. Pharmacol. 2000;397(1):R1eR2. doi: 10.1016/s0014-2999(00)00269-7. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M, Morris SU, Bartko JJ, Choi C, Tamminga CA. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Mol. Psychiatry. 2015;20(4):433–439. doi: 10.1038/mp.2014.54. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabo-tropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106(3):481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr. Metabotropic gluta-mate receptor mRNA expression in the basal ganglia of the rat. J. Neurosci. Off. J. Soc. Neurosci. 1994;14(5 Pt 2):3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatadri PS, Lee CC. Differential expression of mGluR2 in the developing cerebral cortex of the mouse. J. Biomed. Sci. Eng. 2014;7(13):1030–1037. doi: 10.4236/jbise.2014.713100. [DOI] [PMC free article] [PubMed] [Google Scholar]