Abstract
Objective
Evidence suggests that Alzheimer’s disease (AD) biomarkers become abnormal many years prior to the emergence of clinical symptoms of AD, raising the possibility that biomarker levels measured in cognitively normal individuals would be associated with cognitive performance many years later. This study examined whether performance on computerized cognitive tests is associated with levels of cerebrospinal fluid (CSF) biomarkers of amyloid, tau, and phosphorylated tau (p-tau) obtained approximately 10 years earlier, when individuals were cognitively normal and primarily middle-aged.
Method
Individuals from the BIOCARD cohort (mean age at testing = 69 years) were tested on two computerized tasks hypothesized to rely on brain regions affected by the early accumulation of AD pathology: (1) a Paired Associates Learning (PAL) task (n = 67) and (2) a visual search task (n = 86).
Results
In regression analyses, poorer performance on the PAL task was associated with higher levels of CSF p-tau obtained years earlier, whereas worse performance in the visual search task was associated with lower levels of CSF Aβ1-42.
Conclusions
These findings suggest that AD biomarker levels may be differentially predictive of specific cognitive functions many years later. In line with the pattern of early accumulation of AD pathology, the PAL task, hypothesized to rely on medial temporal lobe function, was associated with CSF p-tau, whereas the visual search task, hypothesized to rely on frontoparietal function, was associated with CSF amyloid. Studies using amyloid and tau PET imaging will be useful in examining these hypothesized relationships further.
Keywords: cerebrospinal fluid, Alzheimer’s disease, preclinical, amyloid, phosphorylated tau, memory, visual perception, cognition
It is now recognized that the symptomatic phase of Alzheimer’s disease (AD) is preceded by an asymptomatic phase during which AD pathology (i.e., amyloid plaques and tau neurofibrillary tangles) is accumulating in the brain while individuals are still cognitively normal (Sperling et al., 2011). This phase, also known as the preclinical phase of AD, is thought to last for years to decades and may represent an important time period for therapeutic interventions given that the disease has not yet progressed to a point where clinical impairment is evident. Therefore, the ability to identify individuals who are in the preclinical phase of AD is highly relevant for clinical trials.
Studies of biomarkers that reflect underlying AD pathology in cognitively normal individuals are among the primary data in support of the existence of a preclinical phase of AD. For example, among middle-aged and older cognitively normal adults, lower levels of beta amyloid 1–42 (Aβ1-42) and higher levels of tau and phosphorylated tau (p-tau), as measured in cerebrospinal fluid (CSF), are associated with an increased likelihood of developing clinical symptoms of mild cognitive impairment (MCI) due to AD several years later (e.g., Fagan et al., 2007; Moghekar et al., 2013; Roe et al., 2011; Soldan et al., 2013). Cognitively normal individuals with more abnormal levels of both CSF Aβ1-42 and tau/p-tau at baseline also show the greatest global cognitive decline over the subsequent 5 – 10 years (Soldan et al., in press; Vos et al., 2013).
In addition to biomarker abnormalities, the preclinical phase of AD is also characterized by subtle cognitive decline. For example, lower baseline performance and greater longitudinal decline on neuropsychological tests of memory, executive function, and language are associated with an increased likelihood of developing clinical symptoms of MCI several years later (e.g., Albert et al., 2014; Blacker et al., 2007; Howieson et al., 2008; Wilson, Leurgans, Boyle, & Bennett, 2011).
While prior investigations of the preclinical phase of AD have primarily utilized standard neuropsychological tests to assess cognition, little is known about performance on computerized cognitive measures and their relationship with measures of AD biomarkers, such as CSF. The current study examined whether performance on computerized cognitive tests is associated with levels of CSF amyloid, tau, and p-tau obtained approximately 10 years earlier among cognitively normal individuals.
We investigated two computer-based cognitive tests that are thought to rely on brain regions affected by the early accumulation of AD pathology. The visual-spatial Paired Associates Learning (PAL) test from the Cambridge Neuropsychological Test Automated Battery (CANTAB) was used, since episodic memory performance depends heavily on medial-temporal lobe function, the earliest site of AD-related tau (Braak, Alafuzoff, Arzberger, Kretzschmar, & Del Tredici, 2006; Braak & Braak, 1991). Previous studies have indicated that impaired PAL performance is evident in individuals with prodromal AD or with mild AD dementia (de Jager, Milwain, & Budge, 2002; Egerhazi, Berecz, Bartok, & Degrell, 2007; Junkkila, Oja, Laine, & Karrasch, 2012). Performance on this test has also been associated with hippocampal function and structure in both individuals with normal cognition and MCI patients (de Rover et al., 2011). Because neurofibrillary tangles initially accumulate in the hippocampus and entorhinal cortex during the early phase of AD (Braak & Braak, 1991), we hypothesized that performance on this test would be associated with CSF levels of tau and p-tau, the primary constituent of the tangles, obtained 10 years prior to test administration.
A visual search test was used, since visual search performance is heavily dependent on frontal-parietal networks (e.g., Anderson et al., 2007; Corbetta & Shulman, 1998; Donner et al., 2002; Muller-Oehring, Schulte, Rohlfing, Pfefferbaum, & Sullivan, 2013; Viskontas et al., 2011) and fibrillar amyloid accumulation is initially seen in cortical regions (Thal, Rub, Orantes, & Braak, 2002), including the precuneus, posterior cingulate, and prefrontal cortex (Bilgel, Jedynak, Wong, Resnick, & Prince, 2015; Yotter et al., 2013). Visual search performance is impaired in individuals with amnestic MCI (McLaughlin, Borrie, & Murtha, 2010; Tales et al., 2011; Tales, Haworth, Nelson, Snowden, & Wilcock, 2005) relative to older controls. We hypothesized that visual search performance would correlate with CSF Aβ1-42 levels measured 10 years prior to task administration among cognitively normal middle age and older adults.
METHODS
Study design and participant selection
Data used in the present analyses were collected from participants in the BIOCARD study, which was designed to identify variables among cognitively normal, primarily middle-aged individuals that could predict the subsequent development of mild to moderate symptoms of AD. Recruitment procedures, baseline evaluations, and annual clinical and cognitive assessments have been described in detail elsewhere (Albert et al., 2014). Briefly, the BIOCARD study was initiated at the National Institutes of Health (NIH) in 1995, with recruitment conducted by the staff of the Geriatric Psychiatry Branch (GPB) of the intramural program of the NIMH from 1995 to 2005. During that time, participants were administered a comprehensive neuropsychological battery and clinical examination annually, and MRI scans, cerebrospinal fluid (CSF), and blood specimens were obtained approximately every two years. By design, approximately 75% of the participants had a first degree relative with dementia of the Alzheimer type. In 2005, the study was stopped for administrative reasons.
In 2009, a research team at the Johns Hopkins School of Medicine was funded to re-establish the cohort, continue the annual clinical and cognitive assessments and evaluate the previously acquired MRI scans, CSF, and blood specimens. The neuropsychological test battery covers a broad range of cognitive domains (see Albert et al., 2014). Annual examinations include a physical and neurological examination, record of medication use, behavioral and mood assessments (Cummings et al., 1994; Yesavage et al., 1982), family history of dementia, history of symptom onset, and a Clinical Dementia Rating (CDR). All participants who agreed to continued follow-up signed consent forms approved by the Johns Hopkins Institutional Review Board.
Each participant undergoes annual consensus review by the staff of the BIOCARD Clinical Core at Johns Hopkins using procedures comparable to those used in the National Institute on Aging Alzheimer’s Disease Centers program: (1) clinical data pertaining to the medical, neurologic, and psychiatric status of the participant are examined; (2) reports of changes in cognition by the participant and collateral sources are reviewed; and (3) decline in cognitive performance, based on review of longitudinal testing from multiple domains, is established. These three sources of data are used to determine whether a participant is impaired; if impaired, the likely etiology of the impairment is identified. Then, the age at which the clinical symptoms began is estimated, based primarily on the reports of the participant and collateral source provided during the CDR interview. This same diagnostic process was retrospectively applied to participants who had become cognitively impaired while the study was at the NIH.
Subjects in the analyses
The present analyses included 132 participants who completed at least one of the computer-based cognitive tests discussed above (22 completed both tests) and had CSF measures obtained when they were cognitively normal (i.e., approximately 10 years prior, while the study was at the NIH). For participants with more than one CSF measure, the most recent CSF measure was selected (M = 10.2 years between CSF draw and computerized cognitive testing, SD = 1.3, range = 8.2 – 17.5). Although all participants were cognitively normal when their CSF was obtained, 19 have since developed mild to moderate clinical symptoms of AD, resulting in a diagnosis of either Mild Cognitive Impairment (MCI, N=14) or dementia (N=5) due to AD at the time of their computer-based cognitive testing. For these individuals, the mean time between the CSF draw included in the analysis and clinical symptom onset of MCI was 4.8 years, (SD = 3.3, range = 1 - 12 years).
Computer-based cognitive tests
The computer-based tests mentioned above were administered as part of a participant’s annual visit. The tests were added to the standard neuropsychological battery, between 2012 and 2015.
Paired Associate Learning (PAL) task
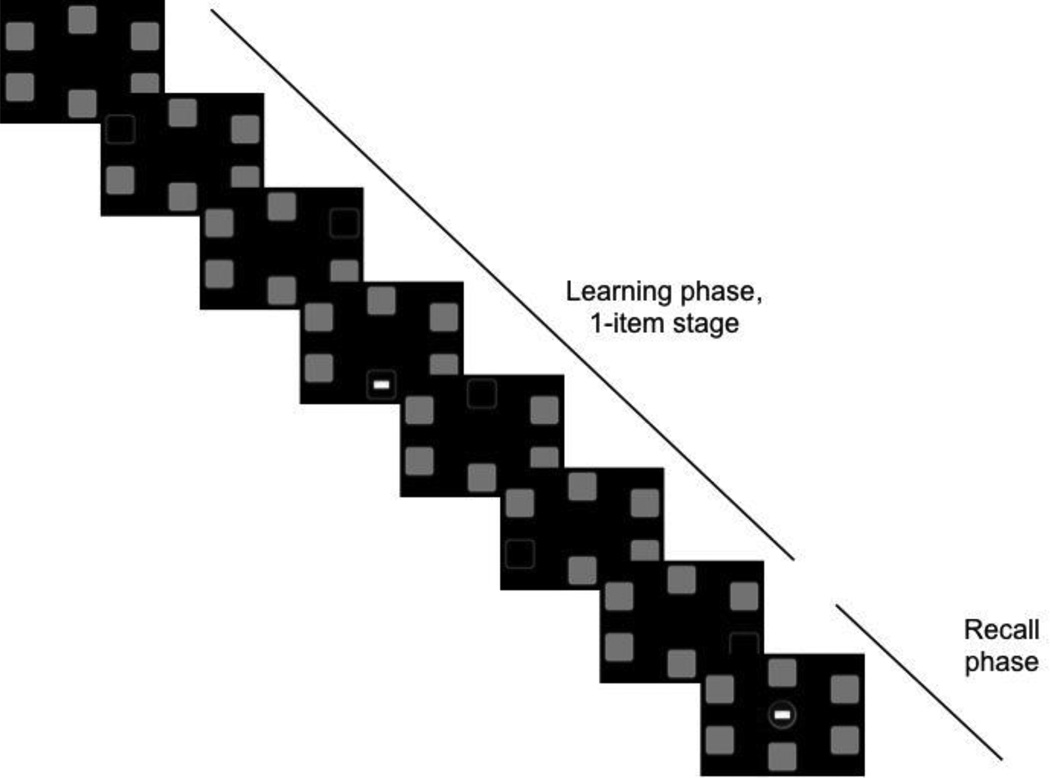
Sixty-seven participants completed the visuospatial PAL task from the CANTAB mobile battery (www.camcog.com). Participants were instructed to remember the location of colorful abstract patterns presented within six possible locations (e.g., Sahakian et al., 1988) on an iPad screen. Over the course of the task, participants completed four successive stages that involved learning 1, 2, 3, or 6 pattern-location pairings. Each trial consisted of a learning phase and a recall phase. During the learning phase, subjects saw 6 white boxes configured in a circle on a black background, indicating possible target locations. All boxes were then sequentially ‘opened’, for 3 seconds each, and either revealed an empty box (i.e., a black box with a white border) or a pattern to be remembered (see Figure 1, learning phase). After the sixth block was ‘opened’, the recall phase began. During this phase, previously presented patterns were presented sequentially in the middle of the screen and participants responded by tapping the location in which the pattern had appeared (Figure 1, recall phase). During the 1-item stage, only one box contained a pattern, while the remaining boxes were empty. During the 2, 3, and 6 item stages, the corresponding number of boxes contained a pattern to be remembered. If all patterns were correctly recalled, the task advanced to the next stage. If any of the patterns were incorrectly recalled, the trial was repeated: patterns were redisplayed in their initial location and recall was attempted again. Participants were given up to 6 attempts per stage, and the task terminated if all 6 attempts at a given stage were failed. The patterns and their locations were randomized across trials and participants. The task took approximately 5 minutes to administer. The dependent variable was the total number of errors across all stages. Using confirmatory factor analysis, this task has been shown to load on the same factor as other neuropsychological tests of episodic memory, including the Rey auditory verbal learning test, Logical Memory delayed story recall, and Rey Figure reproduction (Lenehan, Summers, Saunders, Summers, & Vickers, 2015).
Figure 1.
Example of one trial of the Paired Associates Learning (PAL) task, featuring the learning and recall phases of the 1-item stage.
Visual search task
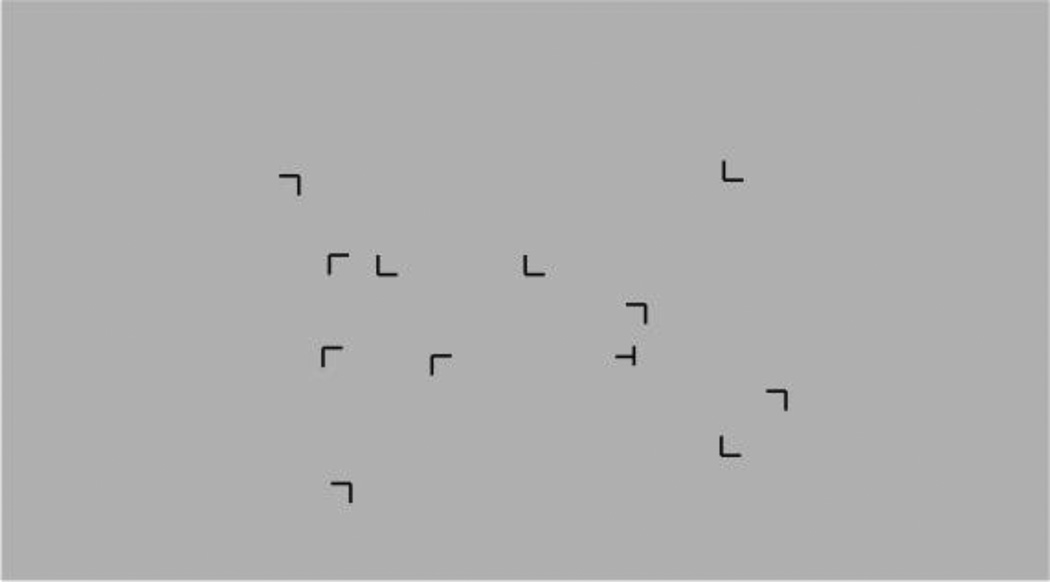
Eighty-seven participants completed a visual search task that involved searching for a target (the letter ‘T’ rotated 90°) among a field of distractors (the letter ‘L’ rotated 0°, 90°, 180°, or 270°) (see Figure 2). All stimuli were presented in black on a gray background. Each trial started with a fixation dot presented in the center of the screen for 500 milliseconds (ms), followed by the onset of a stimulus array containing 1 target and 11 distractors. Participants were instructed to find the target as quickly as possible and then to press the ‘a’ key if the target was rotated clockwise (i.e., if the leg of the T pointed to the left) or the ‘l’ key if rotated counterclockwise (i.e., if the leg of the T pointed to the right). Each response was followed by auditory feedback consisting of a high-pitch tone for correct responses or a low-pitch tone for incorrect responses, with subsequent trials beginning immediately after auditory feedback. Participants first completed a practice block consisting of 12 trials, followed by 10 experimental blocks of 12 trials each. Each block ended with a gray screen indicating that participants should press any key to continue. Stimulus arrays were generated randomly at each trial and presented within the cells of an invisible 8 row x 12 column grid, with stimuli balanced across quadrants. The task was run on a Dell laptop running MatLab’s PsychToolbox3 (version 3.0.11) and took approximately 10 minutes to administer. This task is a modified version of a previously published visual search task that included a contextual cuing component (Bennett, Barnes, Howard, & Howard, 2009; Chun & Jiang, 1998). Our version of the task did not include the contextual cuing component and was therefore significantly shorter. Using factor analysis, visual search performance has been shown to load on the same factor as standard neuropsychological tests of executive function and attention (Trails A, Trails B – A, and Digit Symbol Matching (Anstey, Wood, Kerr, Caldwell, & Lord, 2009), though other studies have failed to find associations between visual search performance and standard neuropsychological tests (Rosler et al., 2000).
Figure 2.
Example of one trial of the visual search task, in which participants search for the target (rotated T) in a field of distractors.
To remove reaction time (RT) outliers from the analysis, all RTs more than 2.5 standard deviations from the mean RT for that subject were excluded from analyses, as were all RTs faster than 300 ms (Whelan, 2008). This led to the removal of 2.8% of the trials and no more than 5 trials per subject. Two dependent variables were examined: (1) mean visual search RT across all correct trials and (2) change in search speed over the 10 blocks, which was measured by first finding the mean RT (for correct trials) separately for each of the 10 blocks and then computing the slope of the mean RT over the 10 blocks.
Cerebrospinal fluid assessments
CSF specimens were analyzed using the same protocol employed in the Alzheimer’s Disease Neuroimaging Initiative. As reported in Moghekar et al. (2013), this protocol used the xMAP-based AlzBio3 kit (Innogenetics, Ghent, Belgium) run on the Bioplex 200 system. The kit contains monoclonal antibodies specific for Aβ1-42 (4D7A3), total tau (t-tau) (AT120), and phosphorylated tau181p (p-tau) (AT270), each chemically bonded to unique sets of color-coded beads, and analyte-specific detector antibodies (HT7 and 3D6). Calibration curves were produced for each biomarker using aqueous buffered solutions that contained the combination of 3 biomarkers at concentrations ranging from 25 to 1555 pg/mL for recombinant tau, 54 to 1,799 pg/mL for synthetic Aβ1-42 peptide, and 15 to 258 pg/mL for a tau synthetic peptide phosphorylated at the threonine 181 position (i.e., the p-tau181p standard). Each participant had all samples (run in triplicate) analyzed on the same plate. (See Moghekar, Goh, Li, Albert, & O’Brien, 2012 for additional details regarding these procedures.)
Statistical analyses
The association between CSF biomarker levels and performance on computerized cognitive tests administered 10 years later was examined with linear regression. Model fit and the assumptions of linear regression were tested using standard techniques. For each cognitive test score (the dependent variable), we ran separate regressions with CSF Aβ1-42 (for the visual search task) or CSF tau/ p-tau (for the PAL task) as predictors. Each model also included age at testing and diagnosis at testing (coded as 0 for cognitively normal or 1 for MCI or dementia) as covariates. The models were not adjusted for years of education and gender because preliminary analyses indicated that neither education nor gender were associated with task performance (all ps > 0.32 for both of the computerized tests). Additionally, in regressions with a step-wise selection procedure using a p=0.2 threshold for model inclusion (data not shown), neither education nor gender were selected for inclusion. Individuals with a diagnosis of “Impaired not MCI” at the time of the computerized cognitive testing were included in the group of normal participants; however, when we excluded these participants from analysis, the same pattern of results was obtained.
RESULTS
Demographic characteristics and descriptive statistics for participants in the analyses are shown in Table 1. The subjects were primarily middle-aged (mean = 59.2 years, SD = 8.3) at their CSF assessment, approximately 10 years earlier.
Table 1.
Demographic characteristics and descriptive statistics for participants in the analyses. Unless otherwise indicated, values reflect means with standard deviations in parentheses.
| Participants with visual search data |
Participants with PAL data |
|
|---|---|---|
| Demographics at cognitive testing | ||
| N* | 87 | 67 |
| Age (years) | 67.9 (8.5) | 70.9 (7.9) |
| Education (years) | 17.1 (2.3) | 17.4 (2.2) |
| Gender (% female) | 63% | 64% |
| MMSE † | 29.2 (1.1) | 28.8 (1.6) |
| N (%) with MCI or dementia due to AD | 3 (3%) | 16 (24%) |
| Performance on cognitive testing | ||
| Visual search reaction time (mean) | 1717 (503) | - |
| Visual search learning (slope) | −23.3 (27.6) | - |
| Paired associate learning (total errors) | - | 6.4 (6.0) |
| CSF biomarkers (pg/ml) | ||
| Years between CSF and cognitive testing |
10.2 (1.0) | 10.2 (1.6) |
| Aβ1-42 | 402.2 (87.4) | 390.4 (81.1) |
| t-tau | 64.9 (23.3) | 62.5 (23.3) |
| p-tau | 38.7 (12.2) | 35.9 (12.4) |
22 participants were tested on both computerized tasks. Therefore, the total number of subjects (N = 132) is less than the sum the number of subjects reported for both taska.
MMSE, Mini-Mental State Exam (Folstein, Folstein & McHugh, 1975)
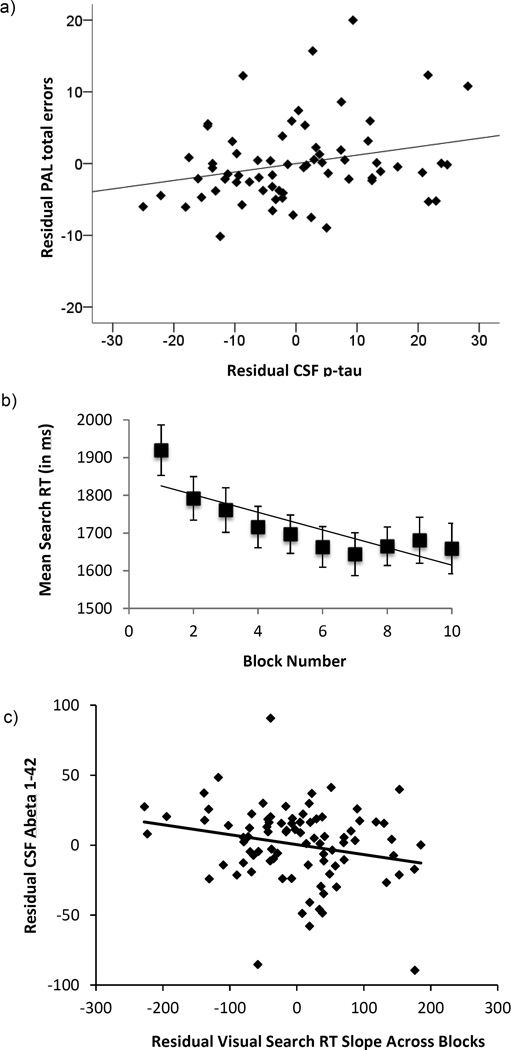
Table 1 shows the average number of errors participants made in the PAL task (across all stages), the mean RT in the visual search task, and the mean visual search slope across blocks (i.e., the visual search learning effect). The results of the regression analyses for both tasks are shown in Table 2. For the PAL task, there was a significant positive association between the total number of errors (across all stages) and levels of p-tau obtained approximately 10 years earlier (β = 0.24, p = 0.04). See Figure 3.a for a scatterplot of this association. The association between the number of errors on the PAL task and levels of total tau was not significant (β = 0.11, p = 0.39). To examine if the association between PAL performance and p-tau was independent of CSF Aβ1-42 levels obtained at the same time as the p-tau measurement, we added CSF Aβ1-42 to the regression model. This analysis showed that p-tau remained significantly associated with the number of errors on the PAL, while CSF Aβ1–42, was not significant in this model (p = 0.37).
Table 2.
Results from regression analyses for both cognitive tasks.
| Variable | df | Unstandardized Coefficient (SE) |
Standardized Coefficient |
t-value | p-value |
|---|---|---|---|---|---|
| Dependent Variable: Number of errors on the Paired Associates Learning Task | |||||
| Model 1 | |||||
| Age | 1 | 0.13 (0.09) | 0.17 | 1.33 | 0.19 |
| Diagnosis | 1 | 2.84 (1.73) | 0.20 | 1.64 | 0.11 |
| CSF p-tau | 1 | 0.18 (0.06) | 0.24 | 2.10 | 0.04 |
| Model 2 | |||||
| Age | 1 | 0.12 (0.10) | 0.16 | 1.21 | 0.23 |
| Diagnosis | 1 | 2.91 (1.78) | 0.21 | 1.64 | 0.11 |
| CSF tau | 1 | 0.02 (0.03) | 0.11 | 0.89 | 0.37 |
| Dependent Variable: Visual search learning (RT slope) | |||||
| Age | 1 | −0.24 (0.35) | −0.07 | −0.68 | 0.50 |
| Diagnosis | 1 | −8.10 (16.0) | −0.05 | −0.50 | 0.61 |
| CSF Aβ1-42 | 1 | −0.07 (0.03) | −0.23 | −2.09 | 0.04 |
| Dependent Variable: Mean visual search speed (mean RT) | |||||
| Age | 1 | 21.0 (6.16) | 0.35 | 3.42 | 0.001 |
| Diagnosis | 1 | −95.7 (281.7) | −0.03 | −0.34 | 0.73 |
| CSF Aβ1-42 | 1 | 0.25 (0.60) | 0.04 | 0.43 | 0.67 |
Figure 3.
Top panel (a): Scatterplot showing the partial correlation between CSF p-tau levels approximately 10 years prior to testing and the total number of errors on the Paired Associates Learning (PAL) task (adjusted for age and diagnosis at test). Middle panel (b): Mean visual search RT (and standard error) across the 10 blocks of the task. The line represents the mean slope across blocks. Bottom panel (c): Scatterplot showing partial correlation between CSF Aβ1-42 approximately 10 years prior to testing and visual search learning (i.e., slope across block, adjusted for age and diagnosis at test).
To further examine if participants’ cognitive performance at the time the CSF was drawn could account for the observed relationship between CSF p-tau levels and subsequent PAL performance, we ran an additional regression with number of errors on the PAL as the outcome and included the Mini-Mental State Examination (MMSE) score (Folstein, Folstein, & McHugh, 1975), and Rey Recall score (Rey, 1941) obtained at the same visit as the CSF collection, in addition to diagnosis at computerized testing, age, and CSF p-tau as predictors (the MMSE was used as a measure of general cognitive status and the Rey Recall evaluates visual spatial episodic memory. In this analysis, the association between accuracy on the PAL task and CSF p-tau levels 10 years prior remained significant (β = 0.22, p = 0.05). We also reran this analysis, excluding the 16 subjects with cognitive impairment at time of computerized testing; the association between CSF p-tau levels and number of errors on the PAL task was not significant when the 16 subjects with cognitive impairment were excluded from analysis (β = 0.07, p = 0.63), possibly reflecting the significant reduction in sample size.
For the visual search task, accuracy was at ceiling for all participants across all blocks (mean accuracy = 98.2%, SD = 2.2); we were therefore able to use RT as the primary dependent variable without modeling speed-accuracy tradeoffs (Heitz, 2014). The slope in visual search RT across blocks was significantly less than zero (i.e., mean slope = −23.3, t(86) = −7.89, p < 0.0001). This indicates that subjects’ search speed reliably decreased across blocks and provides evidence for a visual search learning effect (see Figure 3.b).
The association between visual search learning and CSF Aβ1-42 levels was significant (β = −0.23, p = 0.037): participants with higher (i.e., more normal) levels of CSF Aβ1-42 approximately 10 years prior to testing showed a greater improvement in visual search speed across blocks (i.e., a more negative slope). See Figure 3.c for a scatterplot. This association remained significant when CSF tau or CSF p-tau were added to the regression model, but neither tau nor p-tau were significant in these models (both ps > 0.29). This suggests that the association between CSF Aβ1-42 and visual search learning is independent of CSF tau and p-tau levels. In contrast, there was no association between mean search RT and CSF Aβ1-42 levels (β = 0.04, p = 0.67).
To further examine if cognitive performance at the time the CSF was collected could account for the association between CSF Aβ1-42 and visual-search learning, we ran a regression with visual search RT slope as the dependent variable and included the MMSE score and Trails B score (Reitan, 1958), obtained at the same visit as the CSF collection, in addition to diagnosis at computerized testing, age and CSF Aβ1-42 as predictors (the Trails B is a paper and pencil based measure of visual search speed and executive function). In this analysis, the association between visual search RT slope and CSF Aβ1-42 remained significant (β = −0.23, p = 0.04). The pattern of results also remained the same when the 3 individuals with cognitive impairment at the time of computerized testing were excluded from analysis (β = −0.22, p =0.05).
Additional post-hoc analyses were conducted to examine if the differential association between CSF Aβ1-42 levels and visual search learning on the one hand, and CSF-p-tau levels and PAL performance on the other hand would be observed in the subset of participants who completed both tasks (n = 22). In these analyses, the associations between the CSF biomarkers and the cognitive outcome measures did not reach significance (all ps > 0.12), likely reflecting the significant reduction in power due to the much smaller sample size. Of note, the correlation between PAL performance and visual search learning slope (partialing out age and diagnosis at computerized testing) was not significant, r(20) = 0.12, p = 0.61, consistent with the view that they tap different cognitive processes.
DISCUSSION
The current study examined if performance on two computerized cognitive tests is associated with levels of CSF AD biomarkers obtained approximately 10 years earlier when individuals were primarily middle aged and cognitively normal. We found that higher levels of CSF p-tau 10 years earlier were associated with increased errors on the episodic memory PAL test, independent of CSF Aβ1-42 levels. By comparison, lower (i.e., more abnormal) levels of CSF Aβ1-42 10 years earlier were associated with smaller practice-related improvements on a visual search test; this relationship was independent of CSF tau and p-tau levels 10 years earlier. Taken together, these results suggest that AD biomarker levels may be predictive of performance on specific cognitive tests many years later.
The observed association between lower accuracy on the PAL task and higher CSF p-tau levels from approximately 10 years prior is consistent with studies reporting that elevated levels of p-tau among cognitively normal individuals are associated with subsequent cognitive decline, particularly on tests of episodic memory (Glodzik et al., 2011; Insel et al., 2015; Steenland, Zhao, Goldstein, Cellar, & Lah, 2014), as well as an increased risk of developing clinical symptoms of MCI (Moghekar et al., 2013). The finding that CSF p-tau but not total tau levels were associated with performance on the PAL task may reflect the fact that p-tau is a more direct measure of AD neuropathology, being the primary constituent of the neurofibrillary tangles that characterize AD. Total CSF tau, by comparison, is a more general measure of neurodegeneration and neuronal injury and, as such, may be a less sensitive marker of early tangle pathology. Consistent with this interpretation, we have previously observed that CSF p-tau, but not total tau, measured in middle-aged, cognitively normal individuals is associated with the time to onset of clinical symptoms of MCI. This may indicate that CSF p-tau is a more sensitive marker of tangle pathology than total tau during the earliest phase of the disease. In later stages of the disease, both total tau and p-tau may be associated with performance on the PAL test.
Neurofibrillary tangles initially accumulate in the transentorhinal and entorhinal cortex; they then spread to the hippocampus, and adjacent medial-temporal lobe structures before moving into other cortical regions (Braak et al., 2006; Braak & Braak, 1991). Higher CSF p-tau levels have been linked to longitudinal atrophy of the hippocampus and temporal cortex (Mattsson, Insel, Nosheny, Trojanowski, et al., 2014; Stricker et al., 2012), as well as smaller temporal lobe volumes cross-sectionally (Glodzik et al., 2012). Similarly to CSF abeta1-42, levels of p-tau increase with age in middle-aged individuals (Sutphen et al., 2015). Thus, the current results are consistent with the view that cognitively normal individuals with higher levels of p-tau may accumulate additional p-tau at a more rapid rate over time, which may be associated with atrophy of medial-temporal lobe structures and subsequently poorer performance on sensitive tests of episodic memory, such as the PAL. It is also possible that those individuals with higher levels of p-tau at baseline already had functional and/or structural changes in medial temporal lobe regions that underlie their poorer performance on the PAL test a decade later. Consistent with this interpretation, Pettigrew et al. (2015) reported a cross-sectional association between a visual-spatial episodic memory composite score and CSF p-tau levels among cognitively normal, middle-aged individuals.
The relationship between low CSF abeta and reduced learning in the visual search task a decade later is consistent with the possibility that brain regions showing early amyloid deposition (i.e., frontal-parietal regions) show downstream changes that are detectable using sensitive cognitive tasks. Decreasing levels of CSF Aβ1-42 are hypothesized to be one of the earliest biomarkers of amyloid deposition and AD pathological processes (Jack et al., 2013). Lower levels of CSF Aβ1-42 among cognitively normal individuals have been associated with longitudinal cognitive decline (Li et al., 2014), higher rates of progression from normal cognition to MCI or AD-dementia (Moghekar et al., 2013), and lower cross-sectional performance on tests of attentional control (Aschenbrenner, Balota, Fagan, et al., 2015; Aschenbrenner, Balota, Tse, et al., 2015). Low or declining levels of CSF Aβ1-42 among cognitively normal subjects have also been linked to greater atrophy rates, particularly in frontal-parietal regions (Mattsson, Insel, Nosheny, Tosun, et al., 2014) and reduced cortical thickness (Tosun, Schuff, Shaw, Trojanowski, & Weiner, 2011).
Interestingly, there was no association between CSF Aβ1-42 and mean visual search speed across all trials of the task. This likely reflects the fact that visual search speed is not only a measure of attention and executive function, but also of motor and sensory speed that may be affected by processes unrelated to AD. Moreover, early AD pathology tends to spare motor and early visual regions (Braak et al., 2006; Braak & Braak, 1991). As such, a measure of overall visual search speed may be less likely to capture disruptions in frontal-parietal networks that are subject to early amyloid pathology. The visual search learning effect, by comparison, may be less related to motor and early perceptual processes, and instead reflect an increase in the efficiency of task performance due to the learning of task-related parameters, such as developing a better search strategy, knowing what buttons to press, and having more efficient stimulus-response mappings. Visual search learning, as measured in the current study, has been specifically linked to activation changes in frontal-parietal regions, including the posterior cingulate and precuneus (Manelis & Reder, 2012). It may therefore represent a sensitive measure of the functional integrity of frontal-parietal networks. Consistent with this interpretation, post-hoc analyses showed that mean visual search speed was associated with performance on the Trail Making Tests A and B and the Digit Symbol Substitution test, two measures of processing speed that were administered at the same visit as the visual search task (all p <=0.007, data not shown). Partialling out performance on those three tests did not alter the association between visual search learning and CSF Aβ1-42 (β=-0.076, p=0.03), supporting the view that this association is independent of general processing speed differences.
Another possibility is that amyloid does not directly impact the structure or function of frontal-parietal networks, but rather that early amyloid accumulation is associated with a downstream cascade of neuopathological processes (such as tau-related neurodegeneration or neuroinflammation), which ultimately impacted performance on the visual search task. Longitudinal studies comparing performance on computerized tasks and CSF measures in the same individuals will be able to address the timing of changes in CSF Abeta and p-tau in relation to cognitive performance.
The PAL and visual search tests used in the current study were specifically chosen because they are (1) easy to administer, (2) short in duration, (3) non-verbal, which means they can be administered to individuals from a variety of language backgrounds, and (4) they show no or only weak associations with education, suggesting they are useful for individuals from a broad range of educational and socio-economic backgrounds. Tests with such characteristics may be practical to employ in clinical settings. The present finding of associations between current performance on these two computerized cognitive tasks and AD biomarker levels obtained a decade earlier provide initial data that these tests may be useful for detecting individuals in the preclinical phase of AD. Of note, performance on traditional neuropsychological tests obtained concurrent with the computerized tasks (i.e., Digit Symbol Substitution and Trails A and B), was not associated with CSF abeta1-42 levels 10 years prior (all p >0.35, data not shown) and performance on neuropsychological tests of episodic memory (Rey Recall and Verbal Paired Associates), was unrelated to CSF p-tau levels from 10 years prior (all p>0.4, data not shown). This suggests that the computerized cognitive measures may be more sensitive to the effects of preclinical AD pathology than traditional neuropsychological tests. Future studies are necessary for determining whether performance on these two computerized tasks is sensitive to concurrent levels of AD biomarkers. Amyloid and tau PET imaging will be important for validating the hypothesized relationship between localized amyloid and tau brain pathology and performance on the computerized cognitive measures.
The present study has several limitations. Participants were highly educated, primarily Caucasian, majority female, and many had a family history of dementia, which limits the generalizability of our findings to the population at large. We note, however, that the visual-spatial nature of the tasks renders them less sensitive to the influence of education and socio-economic background than more commonly used verbal tasks. In addition, we did not observe differences in performance by gender, suggesting that the results would be similarly applicable to both males and females. Investigations of the effect of family history and other risk factors on the observed associations will require follow-up studies in larger cohorts. Although participants in the current study did not have any problems performing the tasks on the computer and iPad, it is possible that older individuals with low levels of education and little or no prior exposure to computers and iPads may find it difficult to interact with these technologies. To help overcome this possibility, elaborate instructions and practice trials were included at the beginning of each task. Because the sample size is modest, it is important to replicate our findings in larger cohorts, as well as in those of differing ages.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (U19-AG03365, P50-AG005146, and T32-AG027668). The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Barbara Rodzon); (2) the Clinical Core (Ola Selnes, Marilyn Albert, Anja Soldan, Rebecca Gottesman, Ned Sacktor, Guy McKhann, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Daniel D’Agostino, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Thomas Reigel, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Richard O’Brien, Abby Spangler); (5) the Informatics Core (Roberta Scherer, David Shade, Ann Ervin, Jennifer Jones, Matt Toepfner, Lauren Parlett, April Patterson, Aisha Mohammed); (6) the Biostatistics Core (Mei-Cheng Wang, Qing Cai, Daisy Lu); and (7) the Neuropathology Core (Juan Troncoso, Barbara Crain, Olga Pletnikova, Gay Rudow, and Karen Fisher). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs John Cernansky, David Holtzman, David Knopman, Walter Kukull, and John McArdle, and Drs Neil Buckholtz, John Hsiao, Laurie Ryan, and Jovier Evans, who provide oversight on behalf of the National Institute on Aging and the National Institute of Mental Health (NIMH), respectively. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principle investigator: Dr. Trey Sunderland). The authors are particularly indebted to Dr. Karen Putnam, who has provided ongoing documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Footnotes
Disclosure Statement
Dr. Soldan reports no disclosures.
Dr. Pettigrew reports no disclosures.
Dr. Moghekar reports no disclosures.
Dr. Albert Dr. Albert is an advisor to Eli Lilly.
References
- Albert M, Soldan A, Gottesman R, McKhann G, Sacktor N, Farrington L, Selnes O. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Current Alzheimer Research. 2014;11(8):773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Mannan SK, Husain M, Rees G, Sumner P, Mort DJ, Kennard C. Involvement of prefrontal cortex in visual search. Experimental Brain Research. 2007;180(2):289–302. doi: 10.1007/s00221-007-0860-0. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR. Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology. 2009;23(4):500–508. doi: 10.1037/a0015389. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner AJ, Balota DA, Fagan AM, Duchek JM, Benzinger TL, Morris JC. Alzheimer Disease Cerebrospinal Fluid Biomarkers Moderate Baseline Differences and Predict Longitudinal Change in Attentional Control and Episodic Memory Composites in the Adult Children Study. Journal of the International Neuropsychological Society. 2015;21(8):573–583. doi: 10.1017/S1355617715000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner AJ, Balota DA, Tse CS, Fagan AM, Holtzman DM, Benzinger TL, Morris JC. Alzheimer disease biomarkers, attentional control, and semantic memory retrieval: Synergistic and mediational effects of biomarkers on a sensitive cognitive measure in non-demented older adults. Neuropsychology. 2015;29(3):368–381. doi: 10.1037/neu0000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Barnes KA, Howard JH, Jr, Howard DV. An abbreviated implicit spatial context learning task that yields greater learning. Behavioral Research Methods. 2009;41(2):391–395. doi: 10.3758/BRM.41.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgel M, Jedynak B, Wong DF, Resnick SM, Prince JL. Temporal Trajectory and Progression Score Estimation from Voxelwise Longitudinal Imaging Measures: Application to Amyloid Imaging. Information Processing in Medical Imaging. 2015;24:424–436. doi: 10.1007/978-3-319-19992-4_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Archives of Neurology. 2007;64(6):862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cueing: implicit learning and memory of visual context guides spatial attention. Cognitive Psychology. 1998;36(1):28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philosophical Transactions of the Royal Society B: Biological Sciences. 1998;353(1373):1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- de Jager CA, Milwain E, Budge M. Early detection of isolated memory deficits in the elderly: the need for more sensitive neuropsychological tests. Psychological Medicine. 2002;32(3):483–491. doi: 10.1017/s003329170200524x. [DOI] [PubMed] [Google Scholar]
- de Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, Sahakian BJ. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011;49(7):2060–2070. doi: 10.1016/j.neuropsychologia.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage. 2002;15(1):16–25. doi: 10.1006/nimg.2001.0951. [DOI] [PubMed] [Google Scholar]
- Egerhazi A, Berecz R, Bartok E, Degrell I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Progress in Neuropsychopharmacology & Biological Psychiatry. 2007;31(3):746–751. doi: 10.1016/j.pnpbp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Annals of Neurology. 2009;65(2):176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Archives of Neurology. 2007;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glodzik L, de Santi S, Tsui WH, Mosconi L, Zinkowski R, Pirraglia E, de Leon MJ. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiology of Aging. 2011;32(12):2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodzik L, Mosconi L, Tsui W, de Santi S, Zinkowski R, Pirraglia E, de Leon MJ. Alzheimer’s disease markers, hypertension, and gray matter damage in normal elderly. Neurobiology of Aging. 2012;33(7):1215–1227. doi: 10.1016/j.neurobiolaging.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80(14):1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz RP. The speed-accuracy tradeoff: history, physiology, methodology, and behavior. Front Neurosci. 2014;8:150. doi: 10.3389/fnins.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howieson DB, Carlson NE, Moore MM, Wasserman D, Abendroth CD, Payne-Murphy J, Kaye JA. Trajectory of mild cognitive impairment onset. Journal of the International Neuropsychological Society. 2008;14(2):192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Insel PS, Mattsson N, Mackin RS, Kornak J, Nosheny R, Tosun-Turgut D, Weiner MW. Biomarkers and cognitive endpoints to optimize trials in Alzheimer’s disease. Annals of Clinical and Translational Neurology. 2015;2(5):534–547. doi: 10.1002/acn3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junkkila J, Oja S, Laine M, Karrasch M. Applicability of the CANTAB-PAL computerized memory test in identifying amnestic mild cognitive impairment and Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2012;34(2):83–89. doi: 10.1159/000342116. [DOI] [PubMed] [Google Scholar]
- Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Does the Cambridge Automated Neuropsychological Test Battery (CANTAB) Distinguish Between Cognitive Domains in Healthy Older Adults? Assessment. 2015 doi: 10.1177/1073191115581474. [DOI] [PubMed] [Google Scholar]
- Li G, Millard SP, Peskind ER, Zhang J, Yu CE, Leverenz JB, Montine TJ. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurology. 2014;71(6):742–751. doi: 10.1001/jamaneurol.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Reder LM. Procedural learning and associative memory mechanisms contribute to contextual cueing: Evidence from fMRI and eye-tracking. Learning & Memory. 2012;19(11):527–534. doi: 10.1101/lm.025973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel P, Nosheny R, Trojanowski JQ, Shaw LM, Jack CR, Jr, Weiner M. Effects of cerebrospinal fluid proteins on brain atrophy rates in cognitively healthy older adults. Neurobiology of Aging. 2014;35(3):614–622. doi: 10.1016/j.neurobiolaging.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Nosheny R, Tosun D, Trojanowski JQ, Shaw LM, Weiner MW. Emerging beta-amyloid pathology and accelerated cortical atrophy. JAMA Neurology. 2014;71(6):725–734. doi: 10.1001/jamaneurol.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PM, Borrie MJ, Murtha SJ. Shifting efficacy, distribution of attention and controlled processing in two subtypes of mild cognitive impairment: response time performance and intraindividual variability on a visual search task. Neurocase. 2010;16(5):408–417. doi: 10.1080/13554791003620306. [DOI] [PubMed] [Google Scholar]
- Moghekar A, Goh J, Li M, Albert M, O’Brien RJ. Cerebrospinal fluid Abeta and tau level fluctuation in an older clinical cohort. Archives of Neurology. 2012;69(2):246–250. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A, Li S, Lu Y, Li M, Wang MC, Albert M, O’Brien R. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology. 2013;81(20):1753–1758. doi: 10.1212/01.wnl.0000435558.98447.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Rohlfing T, Pfefferbaum A, Sullivan EV. Visual search and the aging brain: discerning the effects of age-related brain volume shrinkage on alertness, feature binding, and attentional control. Neuropsychology. 2013;27(1):48–59. doi: 10.1037/a0030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, Moghekar A, Wang MC, Gross AL, O’Brien R, Albert M. Relationship between cerebrospinal fluid biomarkers of Alzheimer’s disease and cognition in cognitively normal older adults. Neuropsychologia. 2015;78:63–72. doi: 10.1016/j.neuropsychologia.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Resnick SM, Bilgel M, Moghekar A, An Y, Cai Q, Wang MC, Albert MS. Changes in Abeta biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiology of Aging. 2015;36(8):2333–2339. doi: 10.1016/j.neurobiolaging.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Roe CM, Fagan AM, Grant EA, Marcus DS, Benzinger TL, Mintun MA, Morris JC. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Archives of Neurology. 2011;68(9):1145–1151. doi: 10.1001/archneurol.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler A, Mapstone ME, Hays AK, Mesulam MM, Rademaker A, Gitelman DR, Weintraub S. Alterations of visual search strategy in Alzheimer’s disease and aging. Neuropsychology. 2000;14(3):398–408. doi: 10.1037//0894-4105.14.3.398. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111(Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Li S, Wang MC, Moghekar A, Selnes OA, O’Brien R. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiology of Aging. 2013;34(12):2827–2834. doi: 10.1016/j.neurobiolaging.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Wang MC, Moghekar A, O’Brien R, Selnes O, Albert M. Hypothetical preclinical Alzheimer’s disease groups and longitudinal cognitive change. JAMA Neurology. doi: 10.1001/jamaneurol.2016.0194. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Zhao L, Goldstein F, Cellar J, Lah J. Biomarkers for predicting cognitive decline in those with normal cognition. Journal of Alzheimer’s Disease. 2014;40(3):587–594. doi: 10.3233/JAD-2014-131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NH, Dodge HH, Dowling NM, Han SD, Erosheva EA, Jagust WJ. CSF biomarker associations with change in hippocampal volume and precuneus thickness: implications for the Alzheimer’s pathological cascade. Brain Imaging Behav. 2012;6(4):599–609. doi: 10.1007/s11682-012-9171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphen CL, Jasielec MS, Shah AR, Macy EM, Xiong C, Vlassenko AG, Fagan AM. Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA Neurology. 2015;72(9):1029–1042. doi: 10.1001/jamaneurol.2015.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tales A, Bayer AJ, Haworth J, Snowden RJ, Philips M, Wilcock G. Visual search in mild cognitive impairment: a longitudinal study. Journal of Alzheimer’s Disease. 2011;24(1):151–160. doi: 10.3233/JAD-2010-101818. [DOI] [PubMed] [Google Scholar]
- Tales A, Haworth J, Nelson S, Snowden RJ, Wilcock G. Abnormal visual search in mild cognitive impairment and Alzheimer’s disease. Neurocase. 2005;11(1):80–84. doi: 10.1080/13554790490896974. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Tosun D, Schuff N, Shaw LM, Trojanowski JQ, Weiner MW. Relationship between CSF biomarkers of Alzheimer’s disease and rates of regional cortical thinning in ADNI data. Journal of Alzheimer’s Disease. 2011;26(Supplement 3):77–90. doi: 10.3233/JAD-2011-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Masters CL. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. The Lancet Neurology. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, Boxer AL, Fesenko J, Matlin A, Heuer HW, Mirsky J, Miller BL. Visual search patterns in semantic dementia show paradoxical facilitation of binding processes. Neuropsychologia. 2011;49(3):468–478. doi: 10.1016/j.neuropsychologia.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Fagan AM. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. The Lancet Neurology. 2013;12(10):957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R. Effective analysis of reaction time data. The Psychological Record. 2008;58:475–482. [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Archives of Neurology. 2011;68(3):351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yotter RA, Doshi J, Clark V, Sojkova J, Zhou Y, Wong DF, Davatzikos C. Memory decline shows stronger associations with estimated spatial patterns of amyloid deposition progression than total amyloid burden. Neurobiol Aging. 2013;34(12):2835–2842. doi: 10.1016/j.neurobiolaging.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]