Abstract
Vitamin D can exert anticancer effect beyond bone and calcium metabolism. We aimed to investigate whether postoperative vitamin D supplementation affects quality of life (QOL) and survival in esophageal cancer (EC) patients. We utilized the widely used EORTC QLQ-C30 and QLQ-OES18 to assess QOL at EC diagnosis and 24 months after surgery. Generalized estimating equations (GEEs) were used to analysis the association of vitamin D supplement use with QOL. Kaplan-Meier method and Cox regression model were used to evaluate the prognostic value of vitamin D supplementation. The notably improved QOL were found among vitamin D supplementation users compared with non-users (p < 0.05). Kaplan-Meier analysis revealed that vitamin D supplement use was significantly associated with improved disease-free survival (DFS) (p = 0.030), but not related to overall survival (OS) (p = 0.303). The multivariable analysis further demonstrated vitamin D supplement use as an independent prognostic factor for DFS (p = 0.040; HR 0.610; 95% CI 0.381–0.978). In conclusion, these results showed that vitamin D supplement use could serve as a promising intervention to enhancing QOL and prolonging DFS in EC.
EC is the sixth cause of cancer-related death and the eighth most common carcinoma in the world1. There are estimated 455,800 new EC cases and 400,200 EC-caused deaths in 2012 worldwide2. Surgery, as the best curative option for nonmetastatic patients, is the major treatment for it. Even though patients undergo esophagectomy, the 5-year OS rate is only 15–20%3. As previous studies reported, high incidence and mortality, as well as poor QOL and prognosis are well-recognized features of EC. Undoubtedly, it has already become a major public health concern in the world. As a result, there is an urgent need to improve QOL and survival in EC patients following surgery.
As is known to all, cancer patients’ QOL and survival are not only determined by tumor pathology but also by host factors, such as healthy diet4. An interest in dietary supplementation has been particularly popular among cancer survivors5, although limited existing data supported that it could contribute to positive clinical outcomes after cancer diagnosis. One widely used dietary supplementation is vitamin D. No vitamins have received more attention in recent years for their potential relationships with cancer causation and outcomes than vitamin D6. At present, the role of vitamin D is not only limited to regulate bone metabolism and maintain calcium homeostasis7. A large number of preclinical studies have demonstrated several effects of vitamin D on the hallmarks of cancer, including anti-proliferative, anti-metastasis, anti-angiogenesis, pro-apoptosis and pro-differentiation activities8,9,10,11,12,13,14. Observational studies have also revealed significant relationships of vitamin D with breast cancer, colorectal cancer, prostate cancer and pancreatic cancer6,10,15,16,17. Additionally, vitamin D deficiency could increase the risk of developing cancer and lead to a lower QOL18,19. Therefore, more and more evidence supported that vitamin D supplement use might enhance QOL and decrease risk of recurrence or mortality among cancer patients. However, no work has been done to address the role of vitamin D supplementation that plays in clinical outcomes in EC patients following surgery.
To investigate the chemopreventive potential and anticancer action of vitamin D, we conducted a longitudinal, observational study of supplementation with vitamin D for improving poor clinical outcomes in EC patients. We primarily hypothesized that vitamin D supplement use could improve QOL during EC treatment and recovery phases. Our secondary hypotheses addressed the relationships between vitamin D supplementation and the risk of recurrence or mortality in cancer patients who underwent esophagectomy.
Results
Patients’ characteristics
A total of 303 patients were eventually recruited in our study. 49 (16.2%) patients regularly used vitamin D supplementation after surgery, and they usually took it 200–400 IU daily over one year via self-report. However, 254 (83.8%) patients were vitamin D supplementation non-users. Additionally, there were only 181 EC survivors at 2 years of follow-up. 32 cases took regularly vitamin D supplementation among these survivors, whereas 149 did not. Patients’ baseline characteristics are listed in Table 1. There were significant differences in age (p = 0.006), education (p = 0.008), income (p = 0.003), fat intake (p = 0.020), physical activity (p = 0.013), smoking (p = 0.003) and history of diabetes (p = 0.009) between vitamin D supplementation users group and non-users group. Besides, no significant differences were found in other characteristics (all p > 0.05).
Table 1. Patients’ characteristics.
| Characteristics | Postoperative vitamin D supplementation |
p value | |
|---|---|---|---|
| Users (n = 49) | Non-users (n = 254) | ||
| Age (years) | 61.694 ± 6.884 | 64.886 ± 7.552 | 0.006* |
| Gender (M:F) | 40:9 | 212:42 | 0.754 |
| Education | 0.008* | ||
| High school and below | 35 (71.429%) | 220 (86.614%) | |
| Some college and above | 14 (28.571%) | 34 (13.386%) | |
| Income (RMB/month) | 0.003* | ||
| <1000 | 12 (24.490%) | 105 (41.339%) | |
| 1000~2000 | 10 (20.408%) | 72 (28.346%) | |
| >2000 | 27 (55.102%) | 77 (30.315%) | |
| BMI (kg/m2) | 21.153 ± 3.599 | 21.576 ± 2.971 | 0.329 |
| Physical activity (h/week) | 16.764 ± 5.698 | 14.500 ± 5.807 | 0.013* |
| Sun exposure (days/week)a | 0.658 | ||
| <7 | 12 (24.490%) | 70 (27.559%) | |
| =7 | 37 (75.510%) | 184 (72.441%) | |
| Smoking (pack-year) | 0.003* | ||
| <20 | 38 (77.551%) | 138 (54.331%) | |
| ≥20 | 11 (22.449%) | 116 (45.669%) | |
| Alcohol (kg/day) | 0.142 | ||
| ≤0.025 | 38 (77.551%) | 170 (66.929%) | |
| >0.025 | 11 (22.449%) | 84 (33.071%) | |
| Fruit and vegetable intakeb | 0.519 | ||
| ≥300 g/day & ≥200 g/day | 26 (53.061%) | 122 (48.031%) | |
| Fat intake | 0.020* | ||
| High | 10 (20.408%) | 54 (21.260%) | |
| Medium | 15 (30.612%) | 125 (49.213%) | |
| Low | 24 (48.980%) | 75 (29.527%) | |
| Past medical history | |||
| HBP | 8 (16.327%) | 51 (20.079%) | 0.544 |
| CAD | 5 (10.204%) | 15 (5.906%) | 0.426 |
| Diabetes | 6 (12.245%) | 7 (2.756%) | 0.009* |
| Tumor information | |||
| Pathology | 0.897 | ||
| SCC | 46 (93.878%) | 234 (92.126%) | |
| AC | 3 (6.122%) | 20 (7.874%) | |
| Histological grade | 0.252 | ||
| Well | 4 (8.163%) | 43 (16.929%) | |
| Moderately | 19 (38.776%) | 99 (38.976%) | |
| Poorly/undifferentiated | 26 (53.061%) | 112 (44.095%) | |
| Location | 0.143 | ||
| Cervical/upper/middle | 25 (51.020%) | 158 (62.205%) | |
| Low | 24 (48.980%) | 96 (37.795%) | |
| Length <3 cm | 22 (44.900%) | 105 (41.339%) | 0.644 |
| T category | 0.696 | ||
| 0 | 2 (4.082%) | 6 (2.362%) | |
| 1 | 7 (14.286%) | 36 (14.173%) | |
| 2 | 15 (30.612%) | 77 (30.315%) | |
| 3 | 25 (51.020%) | 126 (49.606%) | |
| 4 | 0 (0%) | 9 (3.544%) | |
| TNM stage | 0.884 | ||
| 0/I/II | 32 (65.306%) | 158 (62.205%) | |
| III/IV | 17 (34.694%) | 96 (37.795%) | |
| Lymph node metastasis | 23 (46.939%) | 113 (44.488%) | 0.752 |
| No. of metastatic lymph nodes | 1.102 ± 1.862 | 1.591 ± 3.012 | 0.918 |
| Ratio of lymph node <0.2 | 41 (83.673%) | 202 (79.528%) | 0.505 |
| Type of surgery | 0.966 | ||
| L-thoracic esophagectomy | 40 (81.633%) | 208 (81.890%) | |
| R-thoracic esophagectomy | 9 (18.367%) | 46 (18.110%) | |
| Treatment regimen | 0.179 | ||
| S | 21 (42.857%) | 153 (60.236%) | |
| S plus postoperative R | 2 (4.082%) | 23 (9.055%) | |
| S plus postoperative C | 12 (24.490%) | 41 (16.142%) | |
| S plus postoperative CRT | 14 (28.571%) | 37 (14.567%) | |
M, male; F, female; BMI, body mass index; HBP, high blood pressure; CAD, coronary artery disease; SCC, squamous cell carcinoma; AC, adenocarcinoma; L, left; R, right; S, surgery; R, radiotherapy; C, chemotherapy; CRT, chemoradiotherapy. aSun exposure was defined as the number of days per week on which at least half an hour was spent outside. bThe cutoff values of fruit and vegetable are ≥300 g/day and ≥200 g/day, respectively. *p < 0.05
Association between vitamin D supplement use and QOL
Table 2 presents the association between postoperative vitamin D supplement use and QOL over 24 months of follow-up. After adjustment for potential confounders, which are age at diagnosis, gender, education, income, BMI, dietary habits, physical activity, sun exposure, smoking, alcohol, past medical history, tumor characteristics, type of surgery, treatment regimen and QOL at diagnosis, the significant associations were observed between vitamin D supplement use and certain aspects of QOL, including global health (β = −2.985, 95% CI −5.880–−0.089, p = 0.043), physical functioning (β = −3.640, 95% CI −6.085–−1.196, p = 0.004), social functioning (β = −6.347, 95% CI −11.178–−1.516, p = 0.010), fatigue (β = 6.110, 95% CI 0.531–11.689, p = 0.032) and appetite loss (β = 10.435, 95% CI 1.107–19.763, p = 0.028) measured by QLQ-C30 as well as eating (β = 5.365, 95% CI 0.876–9.853, p = 0.019) and trouble with taste (β = 8.491, 95% CI 0.882–16.100, p = 0.029) measured by QLQ-OES18.
Table 2. Multivariable-adjusted association between postoperative vitamin D supplement use and QOL assessed by EORTC QLQ-C30 and QLQ-OES18 over 24-month follow up.
| β coefficient | 95% CI | p value | |
|---|---|---|---|
| QLQ-C30 | |||
| Global Health | −2.985 | −5.880, −0.089 | 0.043* |
| Functional Scales | |||
| Physical Functioning | −3.640 | −6.085, −1.196 | 0.004* |
| Role Functioning | −0.449 | −4.070, 3.173 | 0.808 |
| Emotional Functioning | −2.621 | −7.336, 2.095 | 0.276 |
| Cognitive Functioning | −2.017 | −9.191, 5.158 | 0.582 |
| Social Functioning | −6.347 | −11.178, −1.516 | 0.010* |
| Symptom Scales | |||
| Fatigue | 6.110 | 0.531, 11.689 | 0.032* |
| Nausea/vomiting | −0.717 | −4.476, 3.042 | 0.709 |
| Pain | 0.894 | −4.579, 6.368 | 0.749 |
| Dyspnoea | −1.099 | −4.777, 2.580 | 0.558 |
| Insomnia | 3.456 | −0.827, 7.739 | 0.114 |
| Appetite loss | 10.435 | 1.107, 19.763 | 0.028* |
| Constipation | 3.131 | −7.324, 13.585 | 0.557 |
| Diarrhea | −0.380 | −4.160, 3.401 | 0.844 |
| Financial difficulties | 1.972 | −3.551, 7.495 | 0.484 |
| QLQ-OES18 | |||
| Dysphagia Scale | −0.614 | −5.509, 4.281 | 0.806 |
| Eating Scale | 5.365 | 0.876, 9.853 | 0.019* |
| Reflux Scale | 5.341 | −3.428, 14.109 | 0.233 |
| Pain Scale | −4.160 | −8.924, 0.604 | 0.087 |
| Trouble swallowing saliva | 3.151 | −1.916, 8.218 | 0.223 |
| Choking when swallowing | −4.470 | −9.737, 0.796 | 0.096 |
| Dry mouth | 2.206 | −1.907, 6.318 | 0.293 |
| Trouble with taste | 8.491 | 0.882, 16.100 | 0.029* |
| Trouble with coughing | 2.470 | −1.410, 6.349 | 0.212 |
| Trouble with talking | 0.425 | −3.681, 4.530 | 0.839 |
CI, confidence interval. *p < 0.05.
The prognostic value of vitamin D supplement use
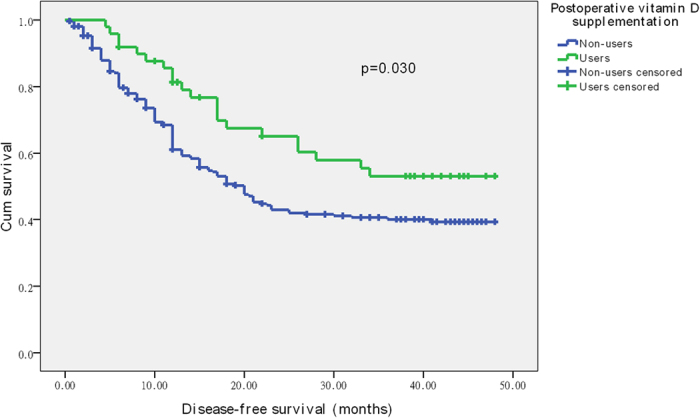
The 3-year OS and 3-year DFS associated with vitamin D supplement use, calculated by Kaplan-Meier method, are shown in Figs 1 and 2. The 3-year OS rates were 49.5% in the non-users group and 55.1% in the users group. The 3-year DFS rates were 40.1% and 53.1% in the non-users group and users group, respectively. Patients who received vitamin D supplementation were more likely to have improved DFS (p = 0.030). However, no positive association of vitamin D supplement use with OS was found in our study (p = 0.303).
Figure 1. Overall survival related to postoperative vitamin D supplementation.
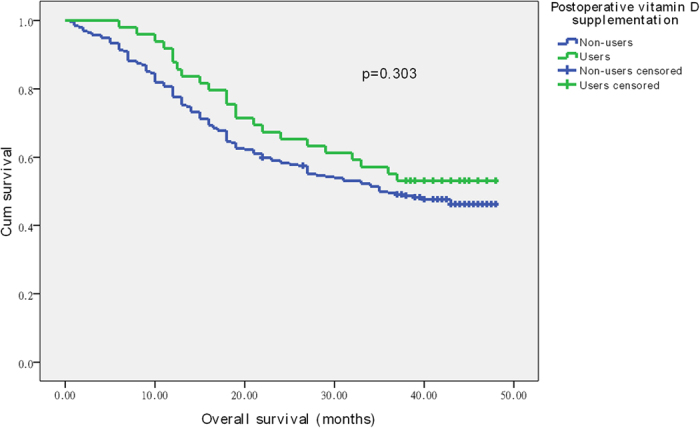
Figure 2. Disease-free survival related to postoperative vitamin D supplementation.
Univariate and multivariate analysis
The factors related to OS and DFS on univariate analysis are shown in Table 3. In univariate analysis, physical activity, pathological type, tumor length, T stage, TNM stage, lymph node metastasis, the number of lymph node metastases, positive lymph node ratio and treatment regimen (all p < 0.05) were associated with both OS and DFS. However, vitamin D supplementation was only associated with DFS (p = 0.035; HR 0.611; 95% CI 0.386–0.966) but not related to OS (p = 0.308; HR 0.795; 95% CI 0.511–1.237). The results of multivariate Cox regression analysis of the factors related to OS and DFS are shown in Table 4. It further suggested that vitamin D supplement use was an independent prognostic factor for DFS (p = 0.040; HR 0.610; 95% CI 0.381–0.978). Interestingly, we found physical activity was an independent prognostic factor for both OS (p = 0.004; HR 0.957; 95% CI 0.928–0.986) and DFS (p < 0.001; HR 0.949; 95% CI 0.922–0.978).
Table 3. Univariate analysis of factors associated with OS and DFS.
| OS |
DFS |
|||||
|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | |
| Age | 0.160 | 1.015 | 0.994-1.036 | 0.075 | 1.019 | 0.998–1.039 |
| Male | 0.023* | 0.566 | 0.346–0.925 | 0.619 | 0.900 | 0.595–1.361 |
| Education | 0.777 | 1.063 | 0.698–1.619 | 0.250 | 1.262 | 0.849–1.877 |
| High school and below | ||||||
| Income | ||||||
| <1000 RMB/month | 0.336 | Ref. | 0.571 | Ref. | ||
| 1000~2000 | 0.152 | 1.330 | 0.900–1.964 | 0.957 | 0.989 | 0.664–1.474 |
| >2000 | 0.320 | 1.208 | 0.833–1.753 | 0.356 | 1.183 | 0.828–1.690 |
| BMI | 0.558 | 0.985 | 0.937–1.036 | 0.906 | 1.003 | 0.954–1.054 |
| Physical activity | <0.001* | 0.942 | 0.916–0.968 | <0.001* | 0.946 | 0.920–0.972 |
| Sun exposure | 0.691 | 1.074 | 0.754–1.530 | 0.907 | 0.980 | 0.696–1.379 |
| <7 days/week | ||||||
| Smoking | 0.991 | 1.002 | 0.730–1.375 | 0.626 | 1.081 | 0.791–1.476 |
| <20 pack-year | ||||||
| Alcohol | 0.299 | 0.831 | 0.587–1.178 | 0.310 | 0.837 | 0.593–1.180 |
| ≤0.025 kg/d | ||||||
| Fruit and vegetable | 0.318 | 0.852 | 0.622–1.167 | 0.364 | 0.866 | 0.635–1.181 |
| ≥300 g/d &≥200 g/d | ||||||
| Fat intake | ||||||
| High | 0.526 | Ref. | 0.397 | Ref. | ||
| Medium | 0.354 | 1.218 | 0.803–1.848 | 0.700 | 0.926 | 0.626–1.370 |
| Low | 0.903 | 1.028 | 0.656–1.613 | 0.205 | 0.760 | 0.497–1.161 |
| Past medical history | ||||||
| HBP | 0.672 | 1.089 | 0.735–1.613 | 0.409 | 0.840 | 0.556–1.271 |
| CAD | 0.687 | 0.877 | 0.462–1.664 | 0.855 | 0.944 | 0.512–1.742 |
| Diabetes | 0.981 | 0.991 | 0.464–2.114 | 0.991 | 0.996 | 0.467–2.124 |
| Tumor information | ||||||
| Pathology | 0.026* | 1.798 | 1.071–3.017 | 0.003* | 2.172 | 1.311–3.598 |
| SCC | ||||||
| Histological grade | ||||||
| Well | 0.118 | Ref. | 0.452 | Ref. | ||
| Moderately | 0.299 | 1.316 | 0.784–2.209 | 0.762 | 0.930 | 0.582–1.487 |
| Poorly/undifferentiated | 0.054 | 1.636 | 0.992–2.699 | 0.536 | 1.153 | 0.735–1.809 |
| Location | 0.792 | 0.958 | 0.696–1.319 | 0.548 | 0.908 | 0.662–1.245 |
| Cervical/upper/middle | ||||||
| Length<3 cm | <0.001* | 0.430 | 0.305–0.607 | <0.001* | 0.540 | 0.391–0.747 |
| T category | ||||||
| 0 | 0.016* | 0.076 | 0.009–0.615 | 0.056 | 0.259 | 0.065–1.038 |
| 1 | <0.001* | 0.115 | 0.041–0.317 | 0.001* | 0.207 | 0.078–0.546 |
| 2 | 0.013* | 0.363 | 0.163–0.806 | 0.103 | 0.494 | 0.211–1.153 |
| 3 | 0.118 | 0.542 | 0.251–1.169 | 0.238 | 0.607 | 0.265–1.391 |
| 4 | <0.001* | Ref. | 0.002* | Ref. | ||
| TNM stage | <0.001* | 2.905 | 2.116–3.990 | <0.001* | 2.452 | 1.793–3.355 |
| 0/I/II | ||||||
| Lymph node metastasis | <0.001* | 2.771 | 2.002–3.836 | <0.001* | 2.093 | 1.531–2.860 |
| No. of metastatic lymph nodes | <0.001* | 1.131 | 1.089–1.174 | <0.001* | 1.164 | 1.113–1.218 |
| Ratio of lymph node<0.2 | <0.001* | 0.393 | 0.279–0.553 | <0.001* | 0.418 | 0.293–0.597 |
| Type of surgery | ||||||
| Left-thoracic esophagectomy | 0.305 | 1.226 | 0.831–1.807 | 0.263 | 1.251 | 0.845–1.852 |
| Treatment regimen | ||||||
| S | 0.013* | Ref. | 0.004* | Ref. | ||
| S plus postoperative R | 0.003* | 2.129 | 1.287–3.522 | 0.042* | 1.774 | 1.020–3.085 |
| S plus postoperative C | 0.155 | 1.358 | 0.891–2.070 | 0.098 | 1.419 | 0.937–2.149 |
| S plus postoperative CRT | 0.040* | 1.555 | 1.020–2.370 | 0.001* | 1.976 | 1.327–2.942 |
| Vitamin D supplementation | 0.308 | 0.795 | 0.511–1.237 | 0.035* | 0.611 | 0.386–0.966 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; HBP, high blood pressure; CAD, coronary artery disease; SCC, squamous cell carcinoma; S, surgery; R, radiotherapy; C, chemotherapy; CRT, chemoradiotherapy. *p < 0.05.
Table 4. Multivariate analysis of factors associated with OS and DFS.
| OS |
DFS |
|||||
|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | |
| Male | 0.132 | 0.675 | 0.404–1.126 | — | — | — |
| Physical activity | 0.004* | 0.957 | 0.928–0.986 | <0.001* | 0.949 | 0.922–0.978 |
| Pathological type | 0.589 | 1.169 | 0.663–2.061 | 0.059 | 1.682 | 0.981–2.883 |
| SCC | ||||||
| Length<3 cm | 0.096 | 0.729 | 0.503–1.058 | 0.095 | 0.733 | 0.509–1.056 |
| T category | ||||||
| 0 | 0.186 | 0.230 | 0.026–2.034 | 0.935 | 1.066 | 0.227–5.011 |
| 1 | 0.022* | 0.253 | 0.078–0.817 | 0.530 | 0.686 | 0.212–2.220 |
| 2 | 0.304 | 0.606 | 0.233–1.576 | 0.630 | 1.325 | 0.459–3.822 |
| 3 | 0.423 | 0.703 | 0.297–1.665 | 0.764 | 1.160 | 0.441–3.049 |
| 4 | 0.094 | Ref. | 0.376 | Ref. | ||
| TNM stage | 0.968 | 1.012 | 0.558–1.836 | 0.158 | 1.574 | 0.839–2.955 |
| 0/I/II | ||||||
| Lymph node metastasis | 0.097 | 1.619 | 0.917–2.856 | 0.786 | 0.922 | 0.514–1.655 |
| No. of metastatic lymph nodes | 0.246 | 1.040 | 0.973–1.111 | 0.059 | 1.076 | 0.997–1.161 |
| Ratio of lymph node<0.2 | 0.406 | 0.812 | 0.496–1.327 | 0.993 | 1.002 | 0.593–1.695 |
| Treatment regimen | ||||||
| S | 0.480 | Ref. | 0.158 | Ref | ||
| S plus postoperative R | 0.142 | 1.475 | 0.878–2.480 | 0.323 | 1.336 | 0.752–2.375 |
| S plus postoperative C | 0.831 | 0.952 | 0.608–1.493 | 0.339 | 1.236 | 0.800–1.910 |
| S plus postoperative CRT | 0.964 | 1.010 | 0.650–1.571 | 0.026* | 1.628 | 1.060–2.500 |
| Vitamin D supplementation | — | — | — | 0.040* | 0.610 | 0.381–0.978 |
HR, hazard ratio; CI, confidence interval; SCC, squamous cell carcinoma; S, surgery; R, radiotherapy; C, chemotherapy; CRT, chemoradiotherapy.
*p < 0.05
Discussion
To the best of our knowledge, this is the first report on relationship between vitamin D supplement use and clinical outcomes of EC. We found a significant improvement in QOL and DFS of EC patients undergoing esophagectomy who received vitamin D supplementation during cancer treatment and recovery phases. Considering imperfect medicare system, relatively low socioeconomic status and weak health conscious in our developing countries, it was not surprising to find that the majority of patients were vitamin D non-users and only 49 patients (16.2%) self-reported to regularly receive vitamin D supplement. The dosage of it was 200–400 IU daily, which was suggested by clinician and also recommended by Chinese Nutrition Society and World Health Organization. In summary, compared to non-users, vitamin D users tended to be younger, attended some college, had a higher income, participated in more physical activity, had lower fat intake, used less tobacco and were more likely to suffer from diabetes in our study.
In view of the limited data supporting positive effect of vitamin D supplement use on QOL for cancer, our results undoubtedly confirmed and extended recently published articles. Although we followed up the patients for 3 years, the sample size of survivors who received vitamin D was too small to sufficient statistical analysis at that time. As a result, the associations between vitamin D supplement use and QOL assessed at 2 years of follow-up were observed. In our study, EC patients who were supplemented with vitamin D had higher scores of physical functioning, social functioning and global health as well as lower scores of fatigue and appetite loss via a 24-month follow-up compared with non-users. It suggested that vitamin D users were more likely to maintain physical function, have more energy and better appetite, feel less faintness as well as enjoy life. These findings were consistent with previous study20, which reported that stage II colorectal cancer patients who supplemented vitamin D tended to have better QOL. Another cross-sectional study indicated that vitamin D deficiency was quite common as well as associated with fatigue and poor physical and functional well-being in advanced cancer patients19. Therefore, it pointed to vitamin D supplementation as a potential therapy to improve cancer patients’ QOL. Although vitamin D status is unknown in the current study, vitamin D supplement use did show a significant relationship with QOL.
To date, the association of vitamin D with cancer has been reported by several reviews,6,9,10,21,22,23,24,25 which proposed it might have potential role as anticancer drug. In addition to the influence of vitamin D on cancer risk26,27,28, numerous reports29,30,31,32 showed that vitamin D sufficiency could improve survival in lung cancer, breast cancer, prostate cancer and follicular lymphoma. However, the studies concerning associations between vitamin D supplementation and DFS or OS were limited and contradictory. In this study, we found that vitamin D supplement use could prolong DFS but not OS of EC patients. Our results were consistent with the findings of Zeichner et al.33. They also reported that vitamin D supplementation in patients with nonmetastatic HER2+ breast cancer was associated with improved DFS but not related to OS. While, Lewis et al.20 studied the correlation of vitamin D supplementation with risk of recurrence or mortality in stage II colorectal cancer patients, no association was observed with DFS or OS. The statistical power for survival analysis may be insufficient or confounding variables could not be completely adjusted to influence the final results or measurement bias, which may in a way explain their null results. In another recent study in women in the UK, Jeffreys et al.34 found no evidence that pre-diagnostic vitamin D supplementation was associated with survival among women with breast cancer, colorectal cancer, lung cancer and gynaecologic cancer. By contrast, we studied the correlation between post-operative vitamin D supplementation after cancer diagnosis and survival in EC patients, which did show that vitamin D supplement use after surgery could reduce the risk of recurrence to improve DFS. According to numerous existing evidences, the different results might be partially explained that pre-diagnostic vitamin D supplementation was more likely to play a role in reducing cancer risk but little associated with long-term clinical outcomes, while post-diagnostic vitamin D supplement use during cancer treatment and recovery phases might have a crucial effect on long-term survival.
Additionally, we interestingly observed physical activity was an independent prognostic factor for both DFS and OS in our study. A number of articles, which have reported the inverse relation of lower physical activity with increased risk of mortality and poor survival, were consistent with our results35,36,37. We strongly supported a protective effect of physical activity on prognosis of EC.
In brief, vitamin D can be obtained from foods, sun exposure and supplementation. However, the quantities of vitamin D from dietary are quite small in our country. What’s more, cancer patients are more likely to spend relatively large amounts of time indoors because of physical weakness and severe symptoms. It leads to less sun exposure contrasted with healthy people. Therefore, compared to other sources, the crucial role of vitamin D supplementation in cancer prevention cannot be ignored. Furthermore, it is worth mentioning that in addition to the well-known classic metabolic pathway, recently published articles reported that novel pathway of vitamin D metabolism in vivo can be initiated by CYP11A1 and modified by CYP27B1 to generate previously unrecognized vitamin D-hydroxyderivatives, which are different from 25-hydroxyvitamin D [25(OH)D] and 1,25(OH)2D38,39,40. An amount of studies have revealed that they can not only regulate bone mineralization and calcium homeostasis41, but also play an important role in antiproliferative, prodifferentiation and anticancer11,12,42,43. Taken together, the in-depth studies about vitamin D as a promising anticancer drug need to be urgently carried out.
At present, the mechanisms, account for the beneficial impacts of vitamin D supplement use on clinical outcomes of cancer, can be summarized as follows. One is that vitamin D are involved in various signaling pathways by its role of anticancer active to affect many important molecular events. As mentioned above, they include inhibition of proliferative, angiogenesis, invasion and metastasis, induction of apoptosis and stimulation of differentiation for malignant cells6,10,22. Another recognized mechanism is that vitamin D is also related to immune regulation and inflammation6,22, which play an important role in cancer pathogenesis and progression. In addition, the other novel mechanisms will be further studied in future.
There are a few limitations in this study. Firstly, because of a retrospective and single-center study, the collected data is somewhat limited. Secondly, vitamin D concentration in blood is not routinely measured among cancer patients in our clinical practice. As a result, the associations of the level of vitamin D with clinical outcomes could not be observed. Thirdly, pre-operative vitamin D supplement use, which may be related to both post-operative vitamin D supplement use and EC outcomes, is unavailable in our study. Therefore, further studies will be needed to verify that our results are not bias or occasional.
In summary, we initially reported that vitamin D supplement use could enhance QOL and prolong DFS of EC. Our study supported that clinician should recommend the use of vitamin D supplementation as an intervention during cancer treatment and recovery phases to improve poor clinical outcomes. Although our results was strongly supported by previous studies, further prospective, well-designed randomized controlled trials with larger samples are necessary to further verify the impacts of vitamin D supplementation on cancer’s clinical outcomes.
Materials and Methods
Patient recruitment
Between January 2012 and December 2012, newly diagnosed and pathologically proven EC patients were recruited from the Department of Thoracic Surgery, Qilu Hospital of Shandong University. All patients enrolled in our study had detailed and authentic clinicopathological data. Patients were excluded from the study: (1) if they were treated with chemotherapy and/or radiotherapy before surgery to reduce the size of the tumor; (2) if they could not clarify whether vitamin D supplementation was used or not after esophagectomy; (3) if they received other supplementations post operative in addition to vitamin D supplement; (4) if they were lost to follow-up. This study was approved by Ethics Committee of Qilu Hospital of Shandong University. Informed consent was obtained from all the patients. All data has been anonymized and de-identified. Moreover, the patient data collection methods were carried out in accordance with the Declaration of Helsinki.
Data collection
Clinicopathological data were obtained from patients’ medical records. Metastases to lymph nodes resected at surgery were counted and pathologically examined. Major factors known to influence QOL, recurrence and mortality of EC were extracted from medical records and follow-up, including age at diagnosis, gender, education, income, body mass index (BMI), physical activity, sun exposure, smoking, alcohol, dietary habits, past medical history, tumor characteristics, type of surgery and treatment regimen. All tumors were staged on the basis of the American Joint Committee on Cancer staging manual44. The cutoff points of alcohol, as well as fruit and vegetable intake were based on the 2011 Chinese Inhabitant Dietary Guideline. The cutoff values of tumor length and the lymph node ratio were in view of previous articles45.
Vitamin D supplementation assessment
The data of vitamin D supplement use were ascertained through self-report. Each patient or their kin was asked as follows: (1) Did patient take regularly vitamin D supplementation after esophagectomy during EC treatment and recovery phases? (2) If did, what’s the frequency and dose of vitamin D supplementation? (3) What’s the duration of taking it? (4) What’s the brand of vitamin D supplementation? On the basis of these collected data, we finally identified two groups of patients for comparison—those who received vitamin D supplementation after surgery during EC treatment and recovery phases (vitamin D supplementation users group) and those who did not use it (vitamin D supplementation non-users group).
QOL assessment
EC survivors’ QOL were ascertained by using the cancer-specific European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) and oesophagus-specific module EORTC QLQ-OES18 at diagnosis and 24 months after surgery. Both the two questionnaires are well validated and their utilities among EC patients have been previously described46,47. The EORTC QLQ-C30 comprises one global health scale, five function scales (physical, role, emotional, cognitive and social), three symptom scales (fatigue, pain and nausea/vomiting), and six single items (dyspnoea, insomnia, appetite loss, constipation, diarrhea and financial difficulties). The EORTC QLQ-OES18 contains four symptom scales (dysphagia, eating difficulties, reflux and esophageal pain) and six single items (trouble swallowing saliva, choking when swallowing, dry mouth, trouble with taste, trouble with cough and trouble with talking). Each item in both questionnaires, except for the global health scale which has seven responses ranging from “very poor” to “excellent”, have four response categories: “not at all”, “a little”, “quite a bit” and “very much”. Patients’ responses were converted to a score of 0–100 in accordance with the EORTC scoring manual46, with higher scores reflecting better QOL in function scales and global health scale, whereas higher scores in symptom scales and items representing more serious symptoms.
Follow-up and survival assessment
Patients were informed routinely examined in our outpatient clinics every 3 months for the first 2 years post operative and every 6 months interval or until death thereafter. Physical examination, laboratory tests, barium meal fluoroscopy, esophagoscopy, computed tomography scans and other examinations as it fits were included in the follow-up assessments. In brief, the condition of patients’ recurrence or death can be timely obtained through a combination of follow-up and medical record review. The follow-up end point was death or January 2016.
Statistical analysis
Baseline characteristics of patients were presented as mean ± standard deviation or proportion by postoperative vitamin D supplementation status (users versus non-users). The Student’s t test or Mann-Whitney U test where appropriate was used to evaluate continuous variables. Categorical variables were assessed by using the Pearson’s chi square test or Fisher’s exact test. The primary end point of this study was the QOL of EC survivors at 24 months post operative. GEEs were utilized to analysis the association of QOL with vitamin D supplement use. The secondary end points were 3-year DFS and 3-year OS. DFS was calculated from the date of surgery to the first date of tumor recurrence. OS was defined as the time from the date of surgery to the date of death or to last follow-up. Kaplan-Meier curves and log-rank tests were used for survival analyses. The Cox regression model was used in the univariate and multivariate analyses. The variables having statistically significant in the univariate analysis were selected into the multivariable analysis. All p values were two-sided and p < 0.05 indicated statistically significant.
All data were performed with the Statistical Package for Social Science program (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL).
Additional Information
How to cite this article: Wang, L. et al. Longitudinal, observational study on associations between postoperative nutritional vitamin D supplementation and clinical outcomes in esophageal cancer patients undergoing esophagectomy. Sci. Rep. 6, 38962; doi: 10.1038/srep38962 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors wish to thank patients and their kin for supporting our work.
Footnotes
Author Contributions All authors contributed significantly to this work. L.W., C.W. and Y.C. designed the study; L.W., C.W., J.W. and X.H. performed the study and collected the data; L.W. and Y.C. analyzed the data; L.W. wrote the first draft of the manuscript; L.W. and C.W. revised the manuscript. All authors reviewed, edited and approved the manuscript.
References
- Ferlay J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 127, 2893–2917 (2010). [DOI] [PubMed] [Google Scholar]
- Torre L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin. 65, 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin. 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W. et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 65, 167–189 (2015). [DOI] [PubMed] [Google Scholar]
- Rock C. L. et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 62, 243–274 (2012). [DOI] [PubMed] [Google Scholar]
- Feldman D., Krishnan A. V., Swami S., Giovannucci E. & Feldman B. J. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 14, 342–357 (2014). [DOI] [PubMed] [Google Scholar]
- Bhan I. Vitamin d binding protein and bone health. Int J Endocrinol. 2014, 561214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K. C. et al. MART-10, the new brand of 1α,25(OH)2D3 analog, is a potent anti-angiogenic agent in vivo and in vitro. J Steroid Biochem Mol Biol. 155, 26–34 (2016). [DOI] [PubMed] [Google Scholar]
- Chiang K. C., Yeh C. N., Chen M. F. & Chen T. C. Hepatocellular carcinoma and vitamin D: a review. J Gastroenterol Hepatol. 26, 1597–1603 (2011). [DOI] [PubMed] [Google Scholar]
- Deeb K. K., Trump D. L. & Johnson C. S. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 7, 684–700 (2007). [DOI] [PubMed] [Google Scholar]
- Pan L. et al. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A /sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. FEBS J. 277, 989–999 (2010). [DOI] [PubMed] [Google Scholar]
- Pilon C. et al. 1α,25-Dihydroxyvitamin D3 inhibits the human H295R cell proliferation by cell cycle arrest: a model for a protective role of vitamin D receptor against adrenocortical cancer. J Steroid Biochem Mol Biol. 140, 26–33 (2014). [DOI] [PubMed] [Google Scholar]
- Piotrowska A. et al. Antiproliferative activity of double point modified analogs of 1,25-Dihydroxyvitamin D2 against human malignant melanoma cell lines. Int J Mol Sci. 17, pii: E76 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Wang X. & Studzinski G. P. 1,25-Dihydroxyvitamin D3 induces monocytic differentiation of human myeloid leukemia cells by regulating C/EBPbeta expression through MEF2C. J Steroid Biochem Mol Biol. 148, 132–137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri B. et al. Vitamin D and pancreas: the role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr. e-pub ahead of print 31 March 2016, doi: 10.1080/10408398.2015.1136922 (2016). [DOI] [PubMed] [Google Scholar]
- Meeker S., Seamons A., Maggio-Price L. & Paik J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J Gastroenterol. 22, 933–948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E. T., Kohler L. N., Kunihiro A. G. & Jurutka P. W. Vitamin D and colorectal, breast, and prostate cancers: a review of the epidemiological evidence. J Cancer. 7, 232–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky A. et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 35, 308–316 (2011). [DOI] [PubMed] [Google Scholar]
- Martinez-Alonso M., Dusso A., Ariza G. & Nabal M. Vitamin D deficiency and its association with fatigue and quality of life in advanced cancer patients under palliative care: a cross-sectional study. Palliat Med. 30, 89–96 (2016). [DOI] [PubMed] [Google Scholar]
- Lewis C., Xun P. & He K. Vitamin D supplementation and quality of life following diagnosis in stage II colorectal cancer patients: a 24-month prospective study. Support Care Cancer. 24, 1655–1661 (2016). [DOI] [PubMed] [Google Scholar]
- Fleet J. C., DeSmet M., Johnson R. & Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 441, 61–76 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. V. & Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 51, 311–336 (2011). [DOI] [PubMed] [Google Scholar]
- Leyssens C., Verlinden L. & Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 20, R31–47 (2013). [DOI] [PubMed] [Google Scholar]
- Mehta R. G., Peng X., Alimirah F., Murillo G. & Mehta R. Vitamin D and breast cancer: emerging concepts. Cancer Lett. 334, 95–100 (2013). [DOI] [PubMed] [Google Scholar]
- Trump D. L., Deeb K. K. & Johnson C. S. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 16, 1–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschasaux M. et al. A prospective study of plasma 25-hydroxyvitamin D concentration and prostate cancer risk. Br J Nutr. 115, 305–314 (2016). [DOI] [PubMed] [Google Scholar]
- Gandini S. et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 128, 1414–1424 (2011). [DOI] [PubMed] [Google Scholar]
- Yin L. et al. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 46, 2196–2205 (2010). [DOI] [PubMed] [Google Scholar]
- Mohr S. B., Gorham E. D., Kim J., Hofflich H. & Garland C. F. Meta-analysis of vitamin D sufficiency for improving survival of patients with breast cancer. Anticancer Res. 34, 1163–1166 (2014). [PubMed] [Google Scholar]
- Mondul A. M., Weinstein S. J., Moy K. A., Mannisto S. & Albanes D. Circulating 25-hydroxyvitamin D and prostate cancer survival. Cancer Epidemiol Biomarkers Prev. 25, 665–669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W. et al. Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 14, 2303–2309 (2005). [DOI] [PubMed] [Google Scholar]
- Kelly J. L. et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA Studies. J Clin Oncol. 33, 1482–1490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichner S. B. et al. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin Breast Cancer. 15, e1–11 (2015). [DOI] [PubMed] [Google Scholar]
- Jeffreys M., Redaniel M. T. & Martin R. M. The effect of pre-diagnostic vitamin D supplementation on cancer survival in women: a cohort study within the UK Clinical Practice Research Datalink. BMC Cancer. 15, 670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper J. G. et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 23, 1939–1948 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechuta S. et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int J Cancer. 138, 2088–2097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. H. et al. Impact of very low physical activity, BMI, and comorbidities on mortality among breast cancer survivors. Breast Cancer Res Treat. 155, 551–557 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 26, 3901–3915 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 383, 181–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 5, 14875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. J., Dusso A. & Slatopolsky E. Vitamin D. Am J Physiol. 277, F157–175 (1999). [DOI] [PubMed] [Google Scholar]
- Slominski A. T. et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 5, e9907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 300, C526–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice T. W., Blackstone E. H. & Rusch V. W. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 17, 1721–1724 (2010). [DOI] [PubMed] [Google Scholar]
- Bollschweiler E., Baldus S. E., Schroder W., Schneider P. M. & Holscher A. H. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 94, 355–363 (2006). [DOI] [PubMed] [Google Scholar]
- Aaronson N. K. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 85, 365–376 (1993). [DOI] [PubMed] [Google Scholar]
- Blazeby J. M. et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 39, 1384–1394 (2003). [DOI] [PubMed] [Google Scholar]


