Abstract
Hemostasis requires the tightly regulated interaction of the coagulation system, platelets, other blood cells and components of the vessel wall at a site of vascular injury. The dysregulation of this response may result in excessive bleeding if the response is impaired, and pathologic thrombosis with vessel occlusion and tissue ischemia if the response is overly robust. Extensive studies over several decades have elucidated the major molecular signaling pathways responsible for platelet activation and aggregation, and anti-thrombotic agents targeting several of these pathways are in widespread clinical use. This review will summarize more recent research examining mechanisms by which these multiple platelet signaling pathways are integrated in time and space at a site of vascular injury in vivo to produce an optimal hemostatic response.
Introduction
The hemostatic response to vascular injury is a complex process requiring regulated activation of coagulation proteins, platelets and components of the vascular wall to form a localized hemostatic plug that prevents bleeding. Many aspects of this process have been well characterized at the molecular and cellular level in vitro, and the major biochemical pathways responsible for coagulation and platelet activation have been reviewed extensively elsewhere1–5. This review will focus primarily on how the multiple components of the hemostatic system are integrated in time and space to generate an optimal response, including how fluid dynamics and the physical architecture of platelet plugs contribute to the formation of complex biochemical gradients at a site of vascular injury. While presented in the context of hemostasis, all of the players and processes discussed have an important role in pathologic thrombosis as well, as indicated by the clinical utility of multiple therapeutics directed against platelet and coagulation targets as anti-thrombotics. As we continue to gain a better understanding of how coagulation and various cellular signaling pathways are coordinated in time and space during hemostasis, we are more likely to uncover differences that may exist between hemostasis and thrombosis that could be targeted for safer anti-thrombotic treatments.
The hemostatic response to vascular injury
In a fairly simplified view, the hemostatic response can be considered as a sequence of cellular and molecular events delineated into the overlapping phases of initiation, extension and stabilization. Each of these phases involves pro-hemostatic molecular processes that result in the rapid plugging of a hole in the vessel wall to stem bleeding, balanced with anti-hemostatic processes that limit the response to the site of injury and prevent unwarranted vascular occlusion. The molecular players involved include adhesion molecules and their ligands, platelet surface receptors that initiate intracellular signaling pathways, and the coagulation cascade to generate thrombin and fibrin among others.
Initiation
Hemostasis is triggered by the exposure of blood to a breach in the vessel wall. During the initiation phase, circulating platelets are recruited to the injury site via adhesive interactions between von Willebrand factor (vWf) bound to collagen fibers in the vessel wall and the platelet GPIb-IX-V receptor complex. VWf is normally found circulating in the plasma in an inactive form, is secreted constitutively from endothelial cells as part of the extracellular matrix, and is also secreted from Weibel-Palade bodies of activated endothelial cells6,7. Following a breach in the vessel wall, circulating vWf is deposited on collagen fibers exposed at the injury site8. Unfolding of the protein as a result of shear forces exposes binding sites for platelet surface GPIb to rapidly recruit platelets from the circulation6,7,9. As the vWf-GPIb complex interaction is relatively weak, additional adhesive interactions mediated by integrin family adhesion molecules on the platelet surface are also required for firm platelet attachment at the site of injury. These include α2β1 integrin binding to collagen and αIIbβ3 binding to vWf and other ligands. In order for platelet integrins to bind their ligands, they must undergo a conformational change from a resting to active state that requires platelet activation. Platelet activation during the initiation phase is likely mediated via multiple platelet signaling pathways, including activation of the GPVI collagen receptor, activation of platelet ATP and ADP receptors via release of these molecules from damaged cells and by signaling downstream of the GPIb-IX-V complex. In addition, escaping blood at the site of injury encounters tissue factor expressed by cells in the vessel wall and extravascular tissue initiating the generation of thrombin, which is a potent platelet activator.
Extension
Following initial platelet adhesion and activation, additional platelets are recruited from the circulation to form a platelet aggregate via platelet-platelet cohesion during the extension phase. This cohesion is mediated primarily by binding of the plasma protein fibrinogen to αIIbβ3 integrin (aka GPIIbIIIa). Each fibrinogen molecule has two αIIbβ3 binding sites and can therefore mediate platelet-platelet interactions by binding to receptors on two adjacent platelets. Platelet recruitment and αIIbβ3 mediated cohesion require platelet activation by ADP released from platelet dense granules and thromboxane A2 (TxA2) generated by platelets already adherent at the site of injury. Thrombin activity also continues to contribute to platelet activation. The importance of αIIbβ3 in mediating platelet aggregation is demonstrated by the lack of aggregation of platelets from Glanzmann’s thrombasthenia patients, which results in a bleeding diathesis. Inhibition of platelet aggregation using αIIbβ3 antagonists is effective to prevent thrombosis in the setting of percutaneous coronary intervention10,11.
Stabilization
Once formed, the nascent hemostatic plug must condense and become firmly anchored at the injury site to resist the force of flowing blood and prevent rebleeding. In addition to activating platelets, thrombin converts fibrinogen to fibrin forming a network of fibrin fibers that helps stabilize the platelet plug. Stabilization is also facilitated by consolidation of the platelet mass via actin-myosin mediated platelet retraction. Platelet activation is reinforced by positive feedback from soluble agonists (thrombin, ADP, TxA2), as well as contact-dependent signaling pathways that are initiated once platelets come in close proximity to one another such that receptor/ligand pairs on adjacent platelets become engaged. Again, αIIbβ3 integrin has an important role in this stage, now acting as a signaling molecule regulating platelet retractile processes12.
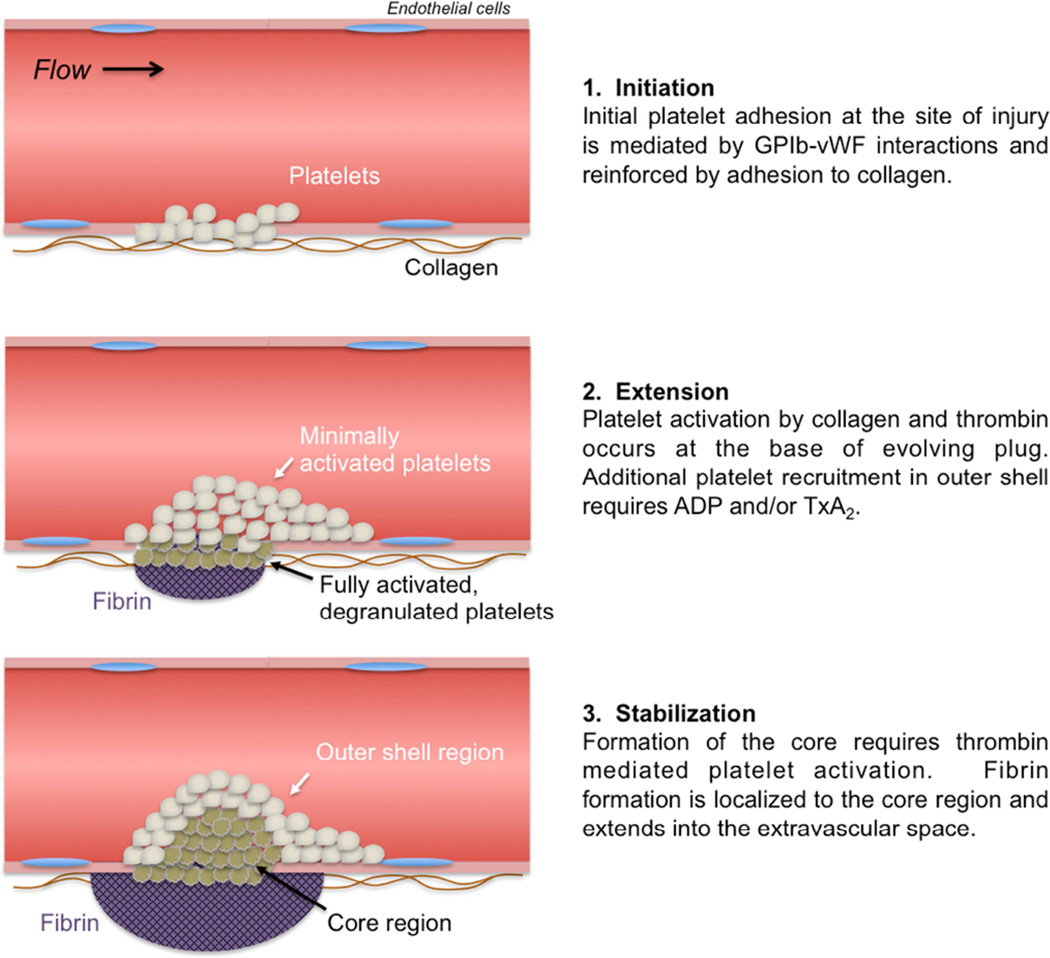
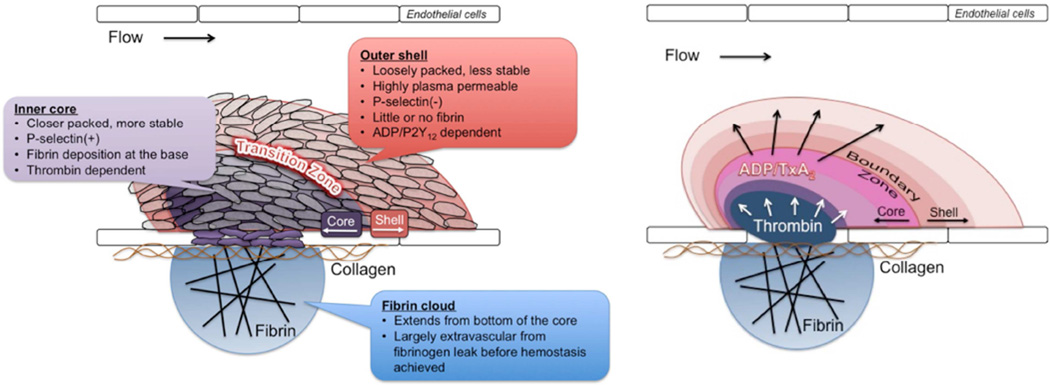
An updated model of hierarchical hemostatic plug architecture
The above description of the hemostatic response provides a general sequence of events. It is consistent with, and in large part derived from, clinical experience regarding the importance of the various molecular players involved as defined by bleeding diatheses that result from either genetic or acquired deficiencies of specific molecular components. However, recent studies examining hemostasis and thrombosis in vivo show that this model is overly simplistic. Rather than a mass of uniformly activated platelets contained in a fibrin meshwork, hemostatic plugs formed in vivo develop a regional architecture where not all platelets are activated in the same way, and fibrin is distinctly localized (Figure 1). Platelets in different regions are morphologically as well as molecularly distinct, reflecting differences in activation state. The architecture of a hemostatic plug has been described as consisting of a core of highly activated, densely packed, degranulated platelets overlaid by a shell of less activated, loosely associated platelets (Figure 2A). This description is derived primarily from intravital imaging studies in the microcirculation13, but evidence suggests a similar hierarchical organization of platelet activation in large vessels as well. The mechanisms responsible for heterogeneity of platelet activation include a complex interplay of platelet agonists and the physical microenvironment present within a platelet mass resulting in the development of gradients of soluble platelet agonists such as thrombin, ADP and TxA2 (Figure 2B). Critical concentrations of these agonists are reached in different regions within the hemostatic plug, which means that individual platelets are exposed to different combinations of agonists that can vary over time as well as space. Importantly, as a direct consequence of the variable contribution of platelet agonists in time and space, therapeutic approaches targeting specific platelet signaling pathways, such as P2Y12 ADP receptor antagonists, aspirin and thrombin inhibitors have distinct effects on hemostatic plug architecture.
Figure 1. An updated model of the hemostatic response to vascular injury.
This updated model takes into account the gradient of platelet activation observed emanating from the site of injury.
Figure 2. Regional architecture of a hemostatic plug.
A) The properties of the core and shell regions of a hemostatic plug are described. B) The core and shell architecture develops as a result of local platelet agonist gradients that are shaped by physical forces within the platelet mass microenvironment.
Regulation of platelet activation in vivo
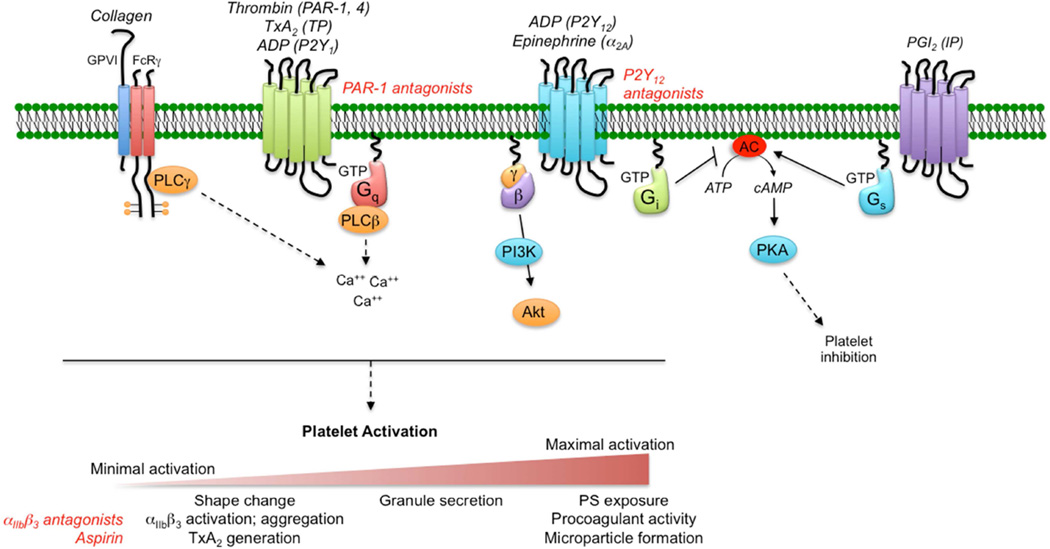
Once platelets are captured at the damaged vessel wall, the primary drivers for platelet activation are collagen, thrombin, ADP, TxA2 and, to a more limited extent, epinephrine (Figure 3). With the exception of collagen, each of these agonists signal through one or more members of the G protein coupled receptor (GPCR) superfamily. In general terms, activation of receptors results in an increase in the cytosolic Ca2+ concentration, followed by a series of downstream cell signaling events leading to multiple cellular responses that in aggregate are referred to as platelet activation (Figure 3). Although often depicted as such, platelet activation is not a binary process, but rather a graded sequence of events, some of which are reversible and some that are not. Early, reversible components of platelet activation include shape change from a discoid morphology to a variety of other shapes, αIIbβ3 integrin activation leading to platelet aggregation, and thromboxane A2 formation. Later, irreversible activation events include dense and alpha granule secretion, phosphtidylserine (PS) exposure supporting coagulation factor complex assembly, and finally membrane blebbing and microparticle formation. The degree to which platelets are activated at a site of injury depends on the integration of the activating inputs each platelet experiences.
Figure 3. A simplified illustration of the platelet signaling network.
Platelets respond to chemical inputs in their local microenvironment via cell surface receptors that initiate multiple intracellular signaling cascades, ultimately leading to a graded series of platelet activation events, including shape change, integrin activation, aggregation, granule secretion and procoagulant activity. The degree of activation achieved by individual platelets within a hemostatic plug or thrombus is the net result of the integration of multiple inputs. For the GPCRs shown, the text in parentheses indicates the receptor(s) name for each agonist indicated. Red text indicates anti-platelet therapeutic targets.
As described above and illustrated in Figure 2, platelet activation within an evolving hemostatic plug or thrombus is heterogeneous, resulting in the development of a platelet mass with a gradient of platelet activation extending from the injury site13. The recognition that platelet activation is not uniform during the hemostatic response leads to the question of how such a platelet activation gradient develops and whether there are implications for both the use of existing anti-platelet therapies and the development of new ones. One possible explanation is that platelets with different degrees of activation represent subpopulations of circulating platelets with distinct properties. While intriguing, there is at present little experimental evidence to support this hypothesis. Instead, the available evidence suggests that heterogeneity of platelet activation reflects non-uniformity in agonist distribution (Figure 2B). Here, we will discuss what is known about each of the major platelet agonist signaling pathways with regard to their contribution to the spatio-temporal regulation of platelet activation.
Thrombin
Thrombin is a key regulator of robust platelet activation in response to vascular injury. It activates platelets via two G protein coupled receptors on human platelets, PAR-1 and PAR-4. These receptors are coupled to Gq signaling pathways that lead to a rise in platelet cytosolic Ca2+ concentration, among other effects. Downstream signaling pathways culminate in activation of αIIbβ3 integrin and platelet aggregation, platelet granule secretion, generation of thromboxane A2 and platelet retraction (Figure 3).
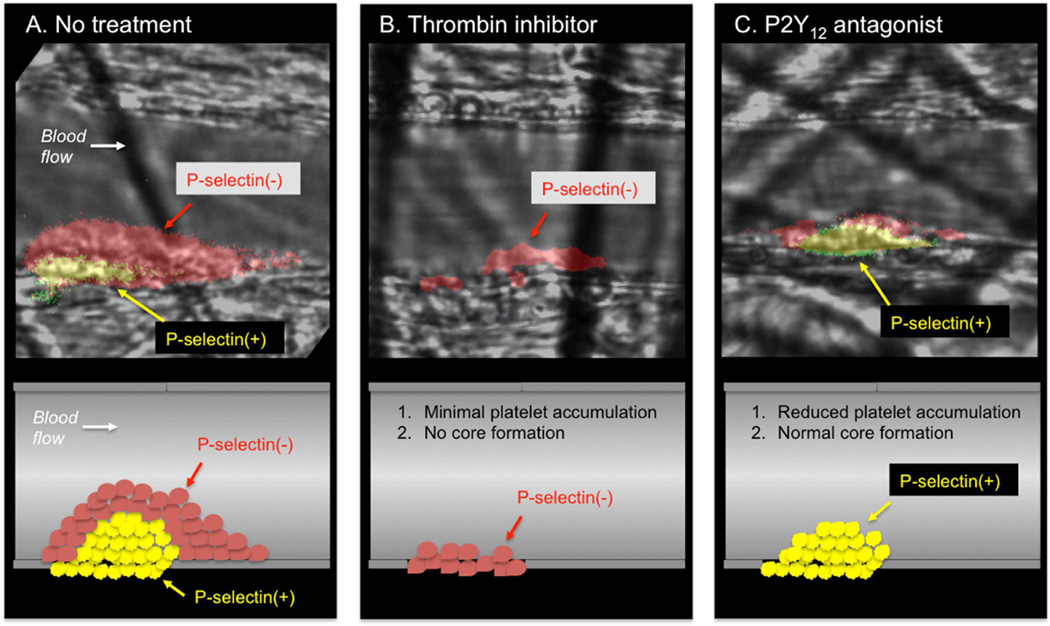
In mouse models, inhibition of thrombin generation/activity or genetic deletion of the platelet thrombin receptor (PAR-4 on mouse platelets) results in significantly impaired platelet accumulation and a near complete lack of intracellular calcium mobilization14,15 and P-selectin expression13,16 as markers of platelet activation (Figure 4A–B). Thus, thrombin activity is critical for development of a stable core composed of fully activated platelets. However, the localization of thrombin activity in time and space is limited. The prothrombinase complex (factors Va and Xa) that generates thrombin is localized to procoagulant membranes of platelets and endothelial cells at or immediately adjacent to the site if injury, thus limiting the distribution of thrombin within a platelet mass17–19. Once generated, thrombin distribution is limited by its ability to diffuse away from the site of generation (discussed in more detail below) and by plasma-borne inhibitors that either directly inhibit its activity (e.g. antithrombin) or generation (e.g. tissue factor pathway inhibitor and activated protein C). This combination of factors limits thrombin activity to the core region of the hemostatic plug as demonstrated by studies using a fluorogenic thrombin sensor bound to the surface of platelets20 and studies showing the localization of fibrin formation restricted to the core and extravascular space13,16,21.
Figure 4. The effect of anti-thrombotic agents on hemostatic plug architecture in an experimental model.
A) Hemostatic plug formation in the mouse cremaster microcirculation is characterized by formation of a core of P-selectin positive platelets overlaid by a shell of P-selectin negative platelets. B) In the presence of the thrombin inhibitor hirudin, platelet accumulation is significantly attenuated and none of the adherent platelets become P-selectin positive. C) In contrast, a P2Y12 antagonist results in a decrease in platelet accumulation in the outer shell region with no effect on full platelet activation in the core region. Data from Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885.
ADP
ADP is released from damaged cells at the site of injury as well as from dense granules of activated platelets. It acts on two platelet receptors, P2Y1 and P2Y12, to reinforce platelet activation in a paracrine or autocrine fashion (Figure 2)22–27. P2Y1 is coupled to a Gq signaling pathway, although activation of Gq signaling by ADP is relatively weak compared to Gq signaling downstream of thrombin receptors, for example. P2Y12 is coupled to a Gi signaling pathway28,29. Gi signaling results in inhibition of adenylyl cyclase and a decrease in cAMP levels in platelets. cAMP is an important inhibitor of platelet activation, normally acting to keep platelets in a quiescent state in the circulation via stimulation of adenylyl cyclase as a result of prostacyclin receptor activation. One effect of P2Y12 signaling, therefore, is to turn off this inhibitory pathway to facilitate platelet activation. P2Y12 signaling also contributes to αIIbβ3 activation and granule secretion. P2Y12/Gi signaling is required for platelet activation in response to weak agonist stimulation, such as ADP activation of P2Y1, and it potentiates platelet activation in response to submaximal concentrations of thrombin or thromboxane A2. It is also required for maximal platelet activation downstream of the collagen receptor GPVI.
The importance of P2Y12 in platelet activation in vivo is highlighted by the efficacy of P2Y12 antagonists in inhibiting platelet activation and protecting against thrombotic events in humans30. This role for P2Y12 signaling is recapitulated in animal models, where deletion of P2Y1223,31 and the introduction of P2Y12 receptor antagonists have been shown to attenuate thrombus formation32. In general, these studies have ascribed a role for P2Y12 in regulating thrombus stability31,33,34. Viewed from the perspective of spatio-temporal regulation of platelet activation, the effect of P2Y12 signaling on thrombus stability is due to the importance of this signaling pathway in platelet recruitment and retention in the outer layers of a developing platelet plug, a region where thrombin activity rapidly declines. Inhibition of P2Y12 activation greatly reduces platelet accumulation in the outer platelet shell (Figure 4C), while a gain of function mutation in Gi2α, the principal G protein coupled to P2Y12 receptors, leads to an expansion of the shell13. In contrast, a P2Y12 antagonist had no effect on robust platelet activation in the thrombus core, where thrombin activity is high (Figure 4C)13. This latter finding may help to explain the relative safety of P2Y12 antagonists used clinically.
Thromboxane A2
Like ADP, thromboxane A2 (TxA2) generated and released by activated platelets acts to reinforce platelet activation in an autocrine and paracrine fashion. TxA2 is generated via the aspirin sensitive cyclooxygenase-1 (COX-1) pathway in platelets. Upon release, it binds its receptors (TPα and β) on the platelet surface to activate a Gq signaling pathway (Figure 3). Like other Gq coupled receptors, downstream signaling includes a rise of cytosolic calcium concentration, activation of αIIbβ3 and granule release29.
The importance TxA2 in platelet activation in vivo is shown by a number of large clinical studies demonstrating the efficacy of aspirin treatment in the prevention of platelet-mediated cardiovascular events (i.e. myocardial infarction and stroke)35. Thrombus formation is also attenuated in TP-deficient mice36. The spatio-temporal distribution of TxA2 within a growing hemostatic plug is not yet well defined. As a highly diffusible molecule, its localization will primarily be determined by its source (activated platelets) and its rapid metabolism in plasma to inactive metabolites. Initial studies indicate that like ADP, TxA2 contributes primarily to platelet recruitment and retention in the outer shell region of the hemostatic plug (Stalker et al, unpublished observation).
Collagen/vWf
Collagen is a potent platelet agonist. Unlike most soluble platelet agonists, the collagen receptor GPVI is not a GPCR, but instead belongs to the family of immune-type signaling receptors. GPVI is coupled to the Fc receptor gamma chain, which acts as the signaling component of the collagen receptor complex. Collagen binding to GPVI initiates a signaling cascade similar to Gq signaling in that it results in an increase in cytosolic CA2+ concentration and subsequent downstream platelet activation events (Figure 3). In vitro, collagen mediated platelet activation is highly dependent on secondary signaling by ADP and thromboxane A2.
In contrast to soluble platelet agonists, collagen is an insoluble component of the vessel wall and extravascular tissue. As such, its direct contribution to platelet signaling via activation of the collagen receptor GPVI is restricted to platelets in contact with the damaged vessel wall and those that escape into the extravascular compartment. The contribution of GPVI signaling in experimental models is therefore highly dependent on the mechanism and extent of injury, as well as the amount of thrombin generated37. This likely explains varying reports of GPVI being a critical regulator of platelet accumulation and activation in some settings, yet completely dispensable in others37–40. Thus, while collagen is clearly a potent activator of platelets in vitro, its contribution to hemostasis and thrombosis in vivo is likely much more context dependent than other platelet agonists such as thrombin, ADP and TxA2.
The contribution of signaling from the GPIb-V-IX complex (the platelet vWf receptor) to platelet activation in vivo remains unclear. In vitro studies have suggested that GPIb-V-IX may act as a mechanosensor, including the demonstration of platelet calcium transients following GPIb complex engagement under flow conditions in vitro41,42. However, these results have not been directly recapitulated in an in vivo system. In fact, vWf mediated signaling appears to be dispensable for platelet activation as measured by cytoplasmic calcium concentration in the mouse cremaster laser-injury model14. In contrast, a mouse line in which the cytoplasmic tail of GPIb-alpha was truncated showed defective thrombus formation in a FeCl3 injury model43.
Influence of local hemodynamics
The importance of the local hemodynamic conditions on platelet accumulation has been documented using in vivo and in vitro methods. The presence of geometrical features that disrupt the local flow pattern, such as stenosis or aneurysms, can exacerbate pre-existing conditions for platelet recruitment and activation. For instance, stenotic regions or developing platelet masses restrict the volume of the vessel available to blood flow, and in so doing generate zones of fluid acceleration and deceleration44–47. In the deceleration zones platelet aggregation occurs via vWF/GPIb and αIIbβ3 integrin dependent interactions44,45,48. Anti-platelet agents blocking the TxA2 and ADP signaling pathways prevent the shear-dependent aggregation occurring in the deceleration zones, indicating that platelet activation is a necessary component of the deposition process44,47,48.
Aneurysms also present flow disturbances and are frequently associated with intramural thrombi49. A combination of experimental and computational studies shows that the presence of these thrombi further deteriorate the local conditions of the aneurysm via distinct mechanisms including local release of degenerative enzymes50, flow-induced hypoxia and changes in wall shear stress distribution51,52. All these mechanisms contribute to vessel wall weakening and may explain why the presence of intramural thrombi are associated with aneurysm growth53, rupture54, and mortality55.
Taken together these studies indicate that flow disturbances affect platelet deposition, and that platelet deposition in turn causes flow disturbances. This positive feedback can lead to disastrous consequences when platelet accumulation occurs at sites of atherosclerotic plaque formation. Platelet accumulation, however, in all cases discussed is always activation-dependent45. Some in vitro evidence suggests that under conditions of non-physiologic shear rates (>20,000 s−1) vWF fibers become tissue plasminogen activator and ADAMTS13 resistant56, and can support activation-independent platelet aggregation57. This mechanism of platelet aggregation may be particularly problematic when blood moves through left ventricular assist and other artificial devices.
The intrathrombus microenvironment shapes agonist distribution
Even as a growing platelet mass disturbs the bulk flow around it causing accelerations and decelerations, the flow conditions inside of the platelet mass are exceedingly calm. Within this calm domain many chemical reactions occur that result in complex gradients of soluble platelet agonists. Location, mode of release, stability, and ease of movement are all factors that contribute to an agonist specific gradient. The movement of a soluble agonist within the platelet mass can become restricted due to both agonist-dependent and platelet mass-dependent effects. Agonist-dependent effects include its size, charge, and binding interactions. The platelet mass-dependent effects are determined by the pore space formed between platelets, the size of the pores, and the connectivity and plasma velocity between the pores.
While each of these factors has a physical meaning the net effect on a solute movement is estimated by measuring two multifactorial parameters, permeability and diffusivity. Permeability measures the ability of a porous material to allow the passage of fluids. Diffusivity measures how quickly a solute spreads as a function of temperature and concentration. Studies have used experimental and computational approaches to estimate the permeability and diffusivity values of a platelet mass. Clot permeability, as measured in vitro, is small, has a value close to that of the endothelium (2 × 10−18 m2), and is determined by platelet retraction and fibrin58. The small and heterogeneous permeability of a clot translates to a model of hindered or absent flow within the platelet mass microenvironment59–62.
When the contribution of flow becomes negligible diffusion becomes the controlling factor for molecular movement58,61,62. In the platelet mass microenvironment, a solute’s diffusivity is determined by the ratio of the solute size to the size of the pores. Solutes much smaller than the size of the pores diffuse faster while solutes close to the size of the pores become excluded. Due to differences in the extent of platelet packing density, diffusivity within the platelet mass is higher in the shell and lower in the core61–63, where it may even be absent due to size exclusion13. Thus, the net effect of permeability and diffusivity is reduced and heterogeneous solute transport properties in a platelet mass, with lower transport in the core61,64,65.
Understanding how solute transport is regulated is critical to understand the delivery, removal, and depletion of pro- and anti-coagulant factors within the platelet mass66–68. Studies have shown that individual reactions within the coagulation cascade can switch from being reaction-to transport-limited depending on the local conditions69. Limited transport increases the residence time of solutes in the platelet mass, which translates into reduced generation of new thrombin, and at the same time increased biological activity of locally trapped thrombin. Evidence from experimental and theoretical studies show that as the platelet mass develops platelets physically cover tissue factor sites, which also leads to reduced thrombin generation68,70–72.
Taken together, these observations describe a progression of coordinated events that depend on the tight interaction between the hemodynamic environment and the biological response of platelets: 1) platelets accumulate at an injury site, 2) as the platelet mass grows the transport of soluble agonists is restricted and shifts from being convection to being diffusion dominated, 3) diffusion increases the residence time of larger agonists (e.g. thrombin) more so than smaller agonists (e.g. ADP), 4) the platelet mass consolidates in response to longer exposure to agonists creating zones that are closed to solute exchange, 5) hemostasis is achieved61,64,65. When this balance is perturbed using mice deficient in platelet retraction, solute transport is increased resulting in fewer platelets becoming maximally activated64.
Summary
In summary, extensive research by many investigators over the past several decades has provided a thorough understanding of the major molecular mechanisms responsible for thrombin generation and platelet activation. We are now beginning to understand how the multiple cell signaling and biochemical events involved in the hemostatic response are integrated in time and space to achieve an optimal response to injury, and how perturbations in the regulation of these events cause pathologic thrombosis. Recent findings highlight the importance of the local microenvironment, including hemodynamic and other physical factors, in shaping the biochemical gradients present at a site of injury to elicit specific cellular responses. Finally, the spatio-temporal distribution of thrombin, ADP and other platelet agonists helps explain effects of therapeutic approaches that target these specific pathways. By gaining a better understanding of how coagulation and platelet activation are regulated in time and space we may be able to better delineate differences between hemostasis and pathologic thrombosis, and identify novel targets for safer, effective anti-thrombotic treatment.
Key Points.
The platelet response to vascular injury involves multiple cell signaling pathways that are coordinated in both time and space.
Local conditions within the evolving platelet plug microenvironment result in the development of platelet agonist gradients.
Anti-platelet therapeutics targeting specific platelet activation pathways have disparate effects on platelet mass architecture depending on the spatio-temporal regulation of the target pathway.
Acknowledgments
Disclosures:
The authors acknowledge research funding from the National Heart, Lung and Blood Institute (P01-HL40387 and P01-HL120846) and The Medicines Company (MDCO 9256).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maurizio Tomaiuolo, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 821 BRB II/III, 421 Curie Blvd., Philadelphia, PA 19104, mtomai@mail.med.upenn.edu
Lawrence F. Brass, Perelman School of Medicine, University of Pennsylvania, 815 BRB II/III, 421 Curie Blvd., Philadelphia, PA 19104, brass@mail.med.upenn.edu.
Timothy J. Stalker, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 809 BRB II/III, 421 Curie Blvd., Philadelphia, PA 19104, tstalker@mail.med.upenn.edu.
References
- 1.Brass LF, Stalker TJ, Zhu L, Woulfe DS. Signal transduction during initiation, extension and perpetuation of platelet plug formation. In: Michelson AD, editor. Platelets. 2nd. Academic Press; 2006. [Google Scholar]
- 2.Stalker TJ, Newman DK, Ma P, Wannemacher KM, Brass LF. Platelet signaling. Handbook of experimental pharmacology. 2012;(210):59–85. doi: 10.1007/978-3-642-29423-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 4.Mintz KP, Mann KG. Detection of Procollagen Biosynthesis Using Peptide-Specific Antibodies. Matrix. 1990;10(3):186–199. doi: 10.1016/s0934-8832(11)80168-x. [DOI] [PubMed] [Google Scholar]
- 5.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120(Suppl 1):S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100(12):1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 8.Rand JH, Glanville RW, Wu XX, et al. The significance of subendothelial von Willebrand factor. Thromb Haemost. 1997;78(1):445–450. [PubMed] [Google Scholar]
- 9.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 10.Investigators E. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. The New England journal of medicine. 1994;330(14):956–961. doi: 10.1056/NEJM199404073301402. [DOI] [PubMed] [Google Scholar]
- 11.Investigators E. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The New England journal of medicine. 1997;336(24):1689–1696. doi: 10.1056/NEJM199706123362401. [DOI] [PubMed] [Google Scholar]
- 12.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401(6755):808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 13.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117(4):953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gestel MA, Reitsma S, Slaaf DW, et al. Both ADP and thrombin regulate arteriolar thrombus stabilization and embolization, but are not involved in initial hemostasis as induced by micropuncture. Microcirculation. 2007;14(3):193–205. doi: 10.1080/10739680601139294. [DOI] [PubMed] [Google Scholar]
- 16.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci U S A. 2007;104(1):288–292. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi T, Mogami H, Murakami Y, et al. Real-time analysis of platelet aggregation and procoagulant activity during thrombus formation in vivo. Pflugers Archiv : European journal of physiology. 2008;456(6):1239–1251. doi: 10.1007/s00424-008-0466-9. [DOI] [PubMed] [Google Scholar]
- 18.Munnix IC, Kuijpers MJ, Auger J, et al. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation. Arterioscler Thromb Vasc Biol. 2007;27(11):2484–2490. doi: 10.1161/ATVBAHA.107.151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanciu L, Krishnaswamy S, Camire RM. New insights into the spatiotemporal localization of prothrombinase in vivo. Blood. 2014;124(11):1705–1714. doi: 10.1182/blood-2014-03-565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh JD, Colace TV, Muthard RW, Stalker TJ, Brass LF, Diamond SL. Platelet-targeting sensor reveals thrombin gradients within blood clots forming in microfluidic assays and in mouse. J Thromb Haemost. 2012;10(11):2344–2353. doi: 10.1111/j.1538-7836.2012.04928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8(10):1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 22.Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998;273(4):2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- 23.Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107(12):1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 25.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273(4):2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 26.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95(14):8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang FL, Luo L, Gustafson E, et al. ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem. 2001;276(11):8608–8615. doi: 10.1074/jbc.M009718200. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Wu J, Jiang H, et al. Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J Biol Chem. 2002;277(48):46035–46042. doi: 10.1074/jbc.M208519200. [DOI] [PubMed] [Google Scholar]
- 29.Brass LF, Newman DK, Wannemacher KM, Zhu L, Stalker TJ. Signal transduction during platelet plug formation. In: Michelson AD, editor. Platelets. 3rd. Boston, MA: Academic Press; 2013. pp. 367–398. [Google Scholar]
- 30.Cattaneo M. Platelet P2 receptors: old and new targets for antithrombotic drugs. Expert review of cardiovascular therapy. 2007;5(1):45–55. doi: 10.1586/14779072.5.1.45. [DOI] [PubMed] [Google Scholar]
- 31.Andre P, Delaney SM, LaRocca T, et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112(3):398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacology & therapeutics. 2005;108(2):180–192. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Nergiz-Unal R, Cosemans JM, Feijge MA, et al. Stabilizing role of platelet P2Y(12) receptors in shear-dependent thrombus formation on ruptured plaques. PLoS One. 2010;5(4):e10130. doi: 10.1371/journal.pone.0010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolla M, Stefanini L, Roden RC, et al. The kinetics of alphaIIbbeta3 activation determines the size and stability of thrombi in mice: implications for antiplatelet therapy. Blood. 2011;117(3):1005–1013. doi: 10.1182/blood-2010-07-297713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauer MS. Clinical practice. Aspirin for primary prevention of coronary events. The New England journal of medicine. 2002;346(19):1468–1474. doi: 10.1056/NEJMcp012672. [DOI] [PubMed] [Google Scholar]
- 36.Thomas DW, Mannon RB, Mannon PJ, et al. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102(11):1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangin P, Yap CL, Nonne C, et al. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006;107(11):4346–4353. doi: 10.1182/blood-2005-10-4244. [DOI] [PubMed] [Google Scholar]
- 38.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107(10):3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massberg S, Gawaz M, Gruner S, et al. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med. 2003;197(1):41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl(3) -induced thrombosis. J Thromb Haemost. 2011;9(7):1423–1426. doi: 10.1111/j.1538-7836.2011.04328.x. [DOI] [PubMed] [Google Scholar]
- 41.Mazzucato M, Pradella P, Cozzi MR, De Marco L, Ruggeri ZM. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibalpha mechanoreceptor. Blood. 2002;100(8):2793–2800. doi: 10.1182/blood-2002-02-0514. [DOI] [PubMed] [Google Scholar]
- 42.Nesbitt WS, Kulkarni S, Giuliano S, et al. Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem. 2002;277(4):2965–2972. doi: 10.1074/jbc.M110070200. [DOI] [PubMed] [Google Scholar]
- 43.Jain S, Zuka M, Liu J, et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2007;104(21):9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109(2):566–576. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- 45.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15(6):665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 46.Westein E, van der Meer AD, Kuijpers MJ, Frimat JP, van den Berg A, Heemskerk JW. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci U S A. 2013;110(4):1357–1362. doi: 10.1073/pnas.1209905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain A, Graveline A, Waterhouse A, Vernet A, Flaumenhaft R, Ingber DE. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat Commun. 2016;7:10176. doi: 10.1038/ncomms10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westein E, van der Meer AD, Kuijpers MJ, Frimat JP, van den Berg A, Heemskerk JW. Proc Natl Acad Sci U S A. Vol. 110. United States: 2013. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner; pp. 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harter LP, Gross BH, Callen PW, Barth RA. Ultrasonic evaluation of abdominal aortic thrombus. J Ultrasound Med. 1982;1(8):315–318. doi: 10.7863/jum.1982.1.8.315. [DOI] [PubMed] [Google Scholar]
- 50.Fontaine V, Jacob MP, Houard X, et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol. 2002;161(5):1701–1710. doi: 10.1016/S0002-9440(10)64447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vorp DA, Lee PC, Wang DH, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34(2):291–299. doi: 10.1067/mva.2001.114813. [DOI] [PubMed] [Google Scholar]
- 52.Wang DH, Makaroun MS, Webster MW, Vorp DA. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J Vasc Surg. 2002;36(3):598–604. doi: 10.1067/mva.2002.126087. [DOI] [PubMed] [Google Scholar]
- 53.Wolf YG, Thomas WS, Brennan FJ, Goff WG, Sise MJ, Bernstein EF. Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. J Vasc Surg. 1994;20(4):529–535. doi: 10.1016/0741-5214(94)90277-1. discussion 535-528. [DOI] [PubMed] [Google Scholar]
- 54.Stenbaek J, Kalin B, Swedenborg J. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;20(5):466–469. doi: 10.1053/ejvs.2000.1217. [DOI] [PubMed] [Google Scholar]
- 55.Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357(4):349–359. doi: 10.1056/NEJMoa063232. [DOI] [PubMed] [Google Scholar]
- 56.Herbig BA, Diamond SL. Pathological von Willebrand factor fibers resist tissue plasminogen activator and ADAMTS13 while promoting the contact pathway and shear-induced platelet activation. J Thromb Haemost. 2015;13(9):1699–1708. doi: 10.1111/jth.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108(6):1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muthard RW, Diamond SL. Blood clots are rapidly assembled hemodynamic sensors: flow arrest triggers intraluminal thrombus contraction. Arterioscler Thromb Vasc Biol. 2012;32(12):2938–2945. doi: 10.1161/ATVBAHA.112.300312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim OV, Xu Z, Rosen ED, Alber MS. Fibrin networks regulate protein transport during thrombus development. PLoS computational biology. 2013;9(6):e1003095. doi: 10.1371/journal.pcbi.1003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leiderman K, Fogelson AL. Grow with the flow: a spatial-temporal model of platelet deposition and blood coagulation under flow. Mathematical medicine and biology : a journal of the IMA. 2011;28(1):47–84. doi: 10.1093/imammb/dqq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomaiuolo M, Stalker TJ, Welsh JD, Diamond SL, Sinno T, Brass LF. A systems approach to hemostasis: 2. Computational analysis of molecular transport in the thrombus microenvironment. Blood. 2014;124(11):1816–1823. doi: 10.1182/blood-2014-01-550343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voronov RS, Stalker TJ, Brass LF, Diamond SL. Simulation of intrathrombus fluid and solute transport using in vivo clot structures with single platelet resolution. Annals of biomedical engineering. 2013;41(6):1297–1307. doi: 10.1007/s10439-013-0764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leiderman K, Fogelson AL. The Influence of Hindered Transport on the Development of Platelet Thrombi Under Flow. B Math Biol. 2013;75(8):1255–1283. doi: 10.1007/s11538-012-9784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stalker TJ, Welsh JD, Tomaiuolo M, et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood. 2014;124(11):1824–1831. doi: 10.1182/blood-2014-01-550319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welsh JD, Stalker TJ, Voronov R, et al. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood. 2014;124(11):1808–1815. doi: 10.1182/blood-2014-01-550335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fogelson AL, Tania N. Coagulation under flow: the influence of flow-mediated transport on the initiation and inhibition of coagulation. Pathophysiol Haemost Thromb. 2005;34(2–3):91–108. doi: 10.1159/000089930. [DOI] [PubMed] [Google Scholar]
- 67.Hathcock JJ, Nemerson Y. Platelet deposition inhibits tissue factor activity: in vitro clots are impermeable to factor Xa. Blood. 2004;104(1):123–127. doi: 10.1182/blood-2003-12-4352. [DOI] [PubMed] [Google Scholar]
- 68.Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98(7):1344–1352. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rana K, Neeves KB. Blood flow and mass transfer regulation of coagulation. Blood Rev. 2016 doi: 10.1016/j.blre.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colace TV, Muthard RW, Diamond SL. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow: role of thrombin with and without fibrin. Arterioscler Thromb Vasc Biol. 2012;32(6):1466–1476. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111(7):3507–3513. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuharsky AL, Fogelson AL. Surface-mediated control of blood coagulation: the role of binding site densities and platelet deposition. Biophys J. 2001;80(3):1050–1074. doi: 10.1016/S0006-3495(01)76085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]