Abstract
Recent evidence suggests that NK cells require priming to display full effector activity. Here, we demonstrate that IL-18 contributed to this phenomenon. IL-18 signalling-deficient NK cells were found to be unable to secrete IFN-γ in response to ex vivo stimulation with IL-12. This was not due to a co-stimulatory role of IL-18 because blocking IL-18 signalling during the ex vivo stimulation with IL-12 did not alter IFN-γ production by wild-type NK cells. Rather, we demonstrate that IL-18 primes NK cells in vivo to produce IFN-γ upon subsequent stimulation with IL-12. Importantly, IL-12-induced IFN-γ transcription by NK cells was comparable in IL-18 signalling-deficient and -sufficient NK cells. This suggests that priming by IL-18 leads to an improved translation of IFN-γ mRNA. These results reveal a novel type of cooperation between IL-12 and IL-18 that requires the sequential action of these cytokines.
Introduction
Natural Killer cells (NK) are lymphocytes of the innate immune system that play a role in the protection against pathogens and tumours by means of cell cytotoxicity and the secretion of IFN-γ (1, 2). Recent evidence has shown that, similar to other lymphocytes, NK cells need to be primed to acquire their full effector functions. This priming involves an encounter with activated dendritic cells (DC) in lymphoid organs (3). Mechanistically, NK cell priming has been found to be mediated in part by IL-15, trans-presented by IL-15Rα on DC. Accordingly, NK cells isolated from IL-15 deficient mice display low cytotoxicity and reduced IFN-γ secretion (4, 5). In this study, we aimed at identifying other molecules involved in NK cell priming using a candidate-gene approach. We focused our attention on Myeloid Differentiation primary response gene 88 (MyD88), a crucial integrator of innate immune responses, required for the signalling through most Toll-like receptors (TLR) and receptors of the interleukin-1 (IL-1) receptor family.
Methods
Mice
Inbred mice were purchased from Charles River, L’Arbresle, France. All mice were on a C57BL/6 background. Poly(I:C) (Invivogen) was injected i.p. (200 µg/mouse). Bone marrow chimeras were generated as described (6). IL-18 KO mice and IL-18R KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
NK cell culture
NK cells were enriched by negative selection, as described (6). They were cultured with YAC1 cells (1:1 ratio), cytokines, or coated antibodies (29A1.4, anti-NKp46; 4E5, Ly49D) and anti-CD107a / Golgi-stop (BD-Biosciences, San Diego, CA). IL-2 (3,000 U/ml, Peprotech, Rocky Hill), IL-12 (20 ng/ml), IL-18 (R&D Systems, Minneapolis, 5 ng/ml), anti-IL-18 (MBL, 20 µg/ml), anti-IL-18R (R&D Systems 12.5 µg/ml) were used. Following stimulation, IFN-γ production by NK cells was measured, as described (6). For LAK generation, splenocytes were cultured in medium with rhIL-2 (Peprotech, 2000 UI/ml). IFN-γ was also measured by Elisa (R&D Systems). In some experiments, NK cells were cultured 4 h with IL-18 (5 ng/ml), washed three times and then stimulated as described above.
Quantitative RT-PCR
RNA was extracted with the RNeasy micro kit (Qiagen, Hilden, Germany). Reverse transcriptase (Invitrogen, Carlsbad, CA) was used to generate cDNA. PCR was carried out with a SybrGreen-based kit (Qiagen) using the primers: mHprt 5’: GCCCCAAAATGGTTAAGGTT, mHprt 3’: TTGCGCTCATCTTAGGCTTT, mIFN-γ 5’: GAACTGGCAAAAGGATGGTGA, mIFN-γ 3’: TGTGGGTTGTTGACCTCAAAC
Statistics
Non parametric permutation test was used for statistical analysis using StatXxact 8 software (Cytel Studio, Cambridge, MA). 2-slided p-values are shown: * = p value<0.05, ** = p value<0.001, *** = p value<0.0001.
Results and Discussion
MyD88 is required for IFN-γ production by NK cells in response to IL-12
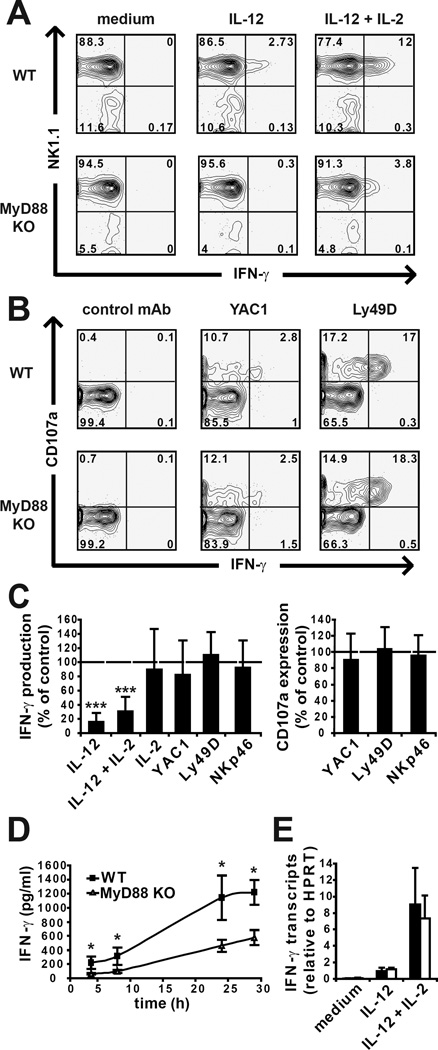
We first compared the function of NK cells isolated from unchallenged wild-type and MyD88 KO mice. For this purpose, we used an ex vivo assay in which freshly purified NK cells were stimulated 4 h with YAC-1 target cells, or cytokines or monoclonal antibody (mAb) specific to the activating NK cell receptors Ly49D and NKp46. IFN-γ secretion and cytotoxic granule release (which is proportional to surface CD107a exposure (7)) were measured. MyD88 KO NK cells produced four times less IFN-γ than wild-type NK cells in response to IL-12 or IL-2 + IL12 stimulation (Fig. 1A–B–C). This was true over a wide range of IL-12 concentrations (data not shown). Similar results were also obtained using ELISA and for longer periods of stimulation (Fig. 1D). By contrast, both cell types produced similar levels of IFN-γ in response to all other stimuli tested, ie IL-2, YAC1 cells, cross-linking of Ly49D, NKp46 (Fig. 1A–B and data not shown). Wild-type and MyD88 KO NK cells were equally potent in their degranulation response to YAC1 cells, Ly49D or NKp46 cross-linking (Fig. 1B–C). Moreover, NK cell development and maturation were found to be normal in MyD88 KO mice (data not shown). Unexpectedly, MyD88 KO NK cells produced similar levels of IFN-γ transcripts as wild-type NK cells in response to stimulation with IL-12 or IL-2 + IL12 (Fig. 1D). Thus, MyD88 regulates NK cell IFN-γ production induced by IL-12 at the posttranscriptional level.
Figure 1. MyD88 is required for IFN-γ production by NK cells in response to IL-12.
NK cells from wild-type and MyD88 KO mice were stimulated in the indicated conditions. (A–C) The expression of NK1.1, CD3, CD107a and IFN-γ were measured. (A) Representative FACS analysis of NK1.1 and IFN-γ expression by gated CD3− cells. Cytokine stimulation did not induce CD107a surface exposure (data not shown). (B) Representative FACS analysis of IFN-γ expression and CD107a surface exposure by gated NK1.1+ CD3− cells. (C) Mean production of IFN-γ (left) and CD107a surface exposure (right) by gated MyD88 NK cells, expressed as the percentage of the wild-type value in each condition. Mean +/− SD of n = 8 mice. (D) IFN-γ level was measured by ELISA in the culture supernatant over time. Mean +/− SD for n = 4 MyD88 KO mice and n = 3 WT mice. (E) Detection of IFN-γ transcripts as assessed by quantitative RT-PCR upon 4 hours of stimulation. The relative quantity of IFN-γ transcripts was determined by normalization to Hprt1 mRNA. Mean +/− SD of n = 3.
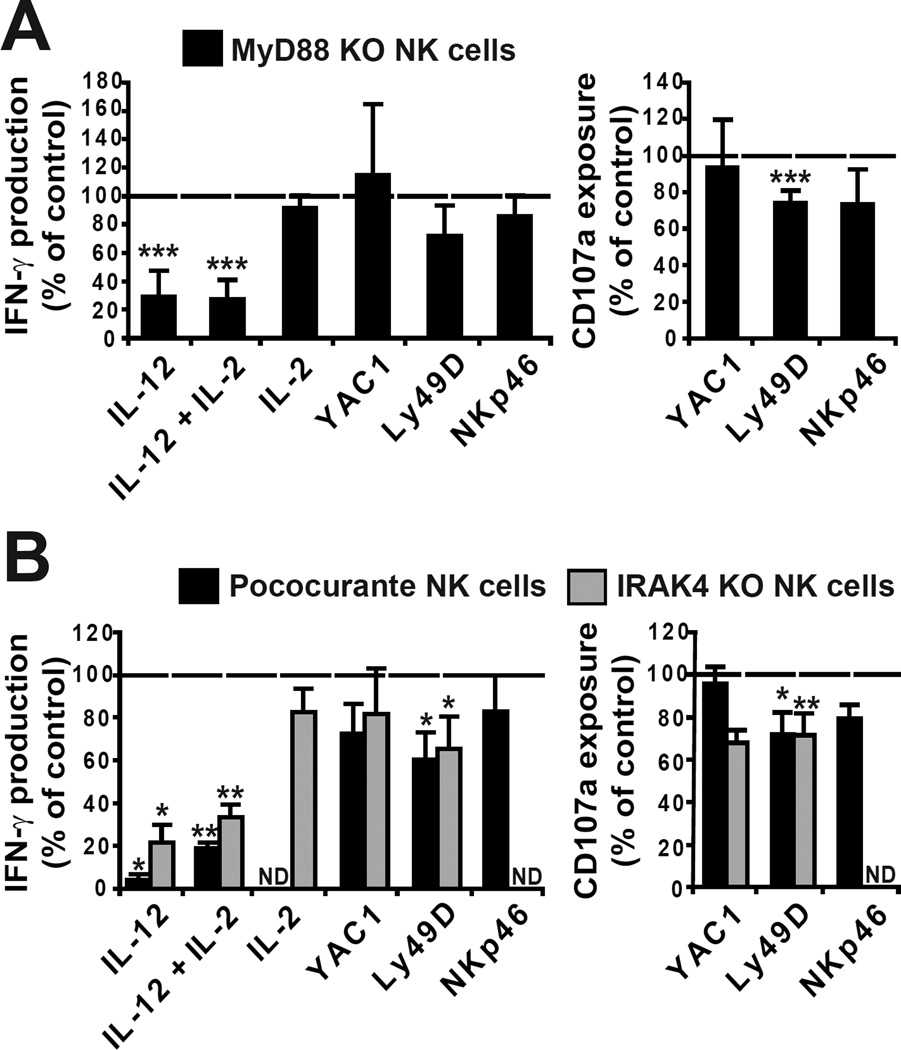
We then tested whether MyD88 functioned in a way intrinsic to NK cells. We reconstituted irradiated recipient mice with a 1:1 mixture of wild-type CD45.1+ and MyD88-deficient CD45.1− bone marrow cells. The NK cell response was assessed 8 weeks after transplantation. MyD88 KO NK cells displayed a reduced IFN-γ production in response to IL-12 or IL-2 + IL12 stimulation in comparison with CD45.1+ wild-type NK cells (Fig. 2A). Their response was otherwise similar to that of wild-type NK cells (Fig. 2A). These results indicate that MyD88 has a cell autonomous function in IL-12-induced production of IFN-γ by NK cells. Next, we analyzed the response of NK cells in other mutants of the IL-1/TLR/MyD88 pathway. MyD88 associates with receptors of the TLR and IL-1R families through a homotypic interaction of their respective TLR/IL-1 receptor (TIR) domains (8). The Pococurante point mutation in the mouse MyD88 TIR domain abrogates this interaction (9). Signalling downstream MyD88 is also dependent on IL-1 Receptor Associated Kinase 4 (IRAK4) (10). We found that both Pococurante and IRAK4 KO mice phenocopied MyD88 KO mice for the NK cell response (Fig. 2B). These results show that the NK cell response to IL-12 requires the association of MyD88 with a TIR-domain containing receptor signalling through the canonical MyD88/IRAK4 signalling pathway.
Figure 2. MyD88 and IRAK4 have an intrinsic role in NK cell response to IL-12.
NK cells from mixed bone marrow chimera mice (wild-type CD45.1 with MyD88 KO or Pococurante or IRAK4 KO) were stimulated as indicated. The expression of NK1.1, CD45.1, CD3, CD107a and IFN-γ were measured. (A) Mean production of IFN-γ (left) and CD107a surface exposure (right) by gated MyD88 KO NK cells, expressed as the percentage of the wild-type value in each condition. Mean +/− SD of n = 8 mice. (B) Mean production of IFN-γ (left) and CD107a surface exposure (right) by gated Pococurante or IRAK4 KO NK cells, expressed as the percentage of the wild-type value in each condition. Mean +/− SD of n = 4 mice. ND: not determined.
IL-18 signalling is required for optimal IL-12-induced IFN-γ production by NK cells
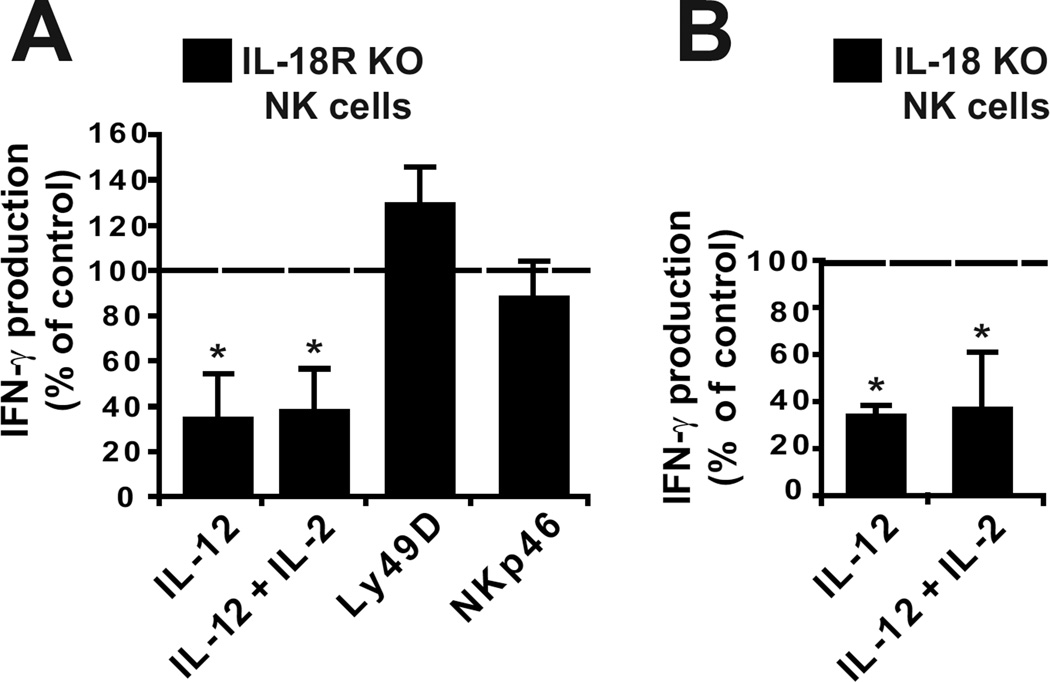
We sought to determine which MyD88-coupled receptor(s) were involved in the NK cell response to IL-12. NK cells isolated from TLR1, TLR2, TLR 3, TLR4, TLR 5, TLR7 or TLR9 knockout mice responded normally to stimulation with IL-12 (data not shown). NK cells from 3d mutant mice, bearing a mutation in Unc93b impairing TLR3, TLR7 and TLR9 (11) also responded normally to stimulation with IL-12 (data not shown). By contrast, and similarly to MyD88 KO NK cells, IL-18R KO NK cells responded poorly to IL-12 and IL-2 + IL12 but normally to Ly49D and NKp46 cross-linking (Fig. 3A). Thus, the IL-18R/MyD88/IRAK4 pathway is required for the ex vivo IFN-γ production by NK cells in response to IL-12. In support of this conclusion, NK cells isolated from IL-18 KO mice produced four times less IFN-γ than wild-type NK cells in response to IL-12 or IL-2 + IL12 stimulation (Fig. 3B).
Figure 3. IL-18 signalling is required for IL-12-induced IFN-γ production by NK cells.
NK cells from wild-type, IL-18R KO or IL-18 KO mice were stimulated as indicated. The expression of NK1.1, CD3, and IFN-γ were measured. Results show the mean production of IFN-γ by gated NK cells from IL-18R KO mice (A) or from IL-18 KO mice (B). Results are expressed as the percentage of the wild-type value in each condition. Mean +/− SD of n = 10 mice for each genotype.
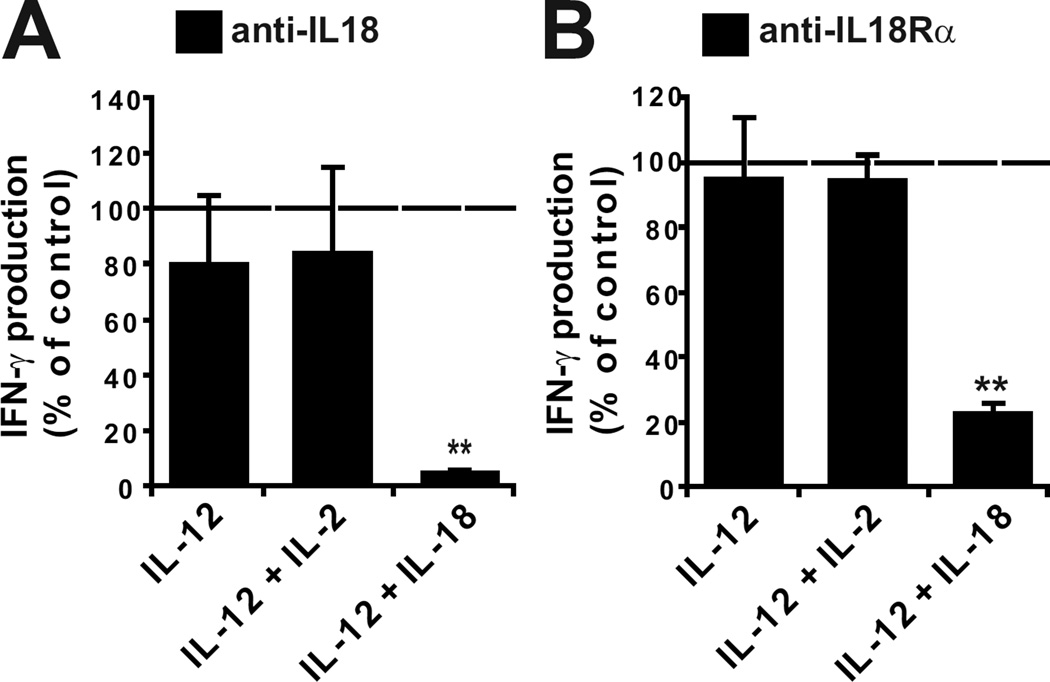
IL-12 and IL-18 are well-known to synergize in inducing IFN-γ production by NK cells when co-administered in vitro (12, 13). It has also been previously reported that during a 24h culture of splenocytes with exogenous IL-12, endogenous production of IL-18 can occur and subsequently costimulate IL-12 induced IFN-γ production (14). This phenomenon could potentially explain why, in our experiments, NK cells deficient for the IL-18 pathway were impaired for IFN-γ production in response to stimulation by exogenous IL-12. However, addition of blocking mAbs to IL-18 and IL-18 receptor or NFκB inhibitors did not block the production of IFN-γ by wild-type NK cells induced by IL-12 or IL-2 + IL12 stimulation (Fig. 4A–B and data not shown). As a control, these reagents efficiently blocked the production of IFN-γ by wild-type NK cells induced by exogenously added IL-18 (Fig. 4A–B and data not shown). Thus, IL-18 signalling is not required for NK cells to respond to IL-12 during the 4 hour in vitro assay, but it is mandatory in vivo to prime NK cells to respond to a subsequent stimulation with IL-12.
Figure 4. Endogenous IL-18 does not costimulate NK cells stimulated with IL-12.
(A–B) NK cells isolated from wild-type mice were stimulated 4 hours in vitro as indicated in the presence or absence of anti-IL-18 antibody (A) or anti-IL-18Rα mAbs (B). Results show the mean production of IFN-γ by gated NK cells, expressed as the percentage of the control (no antibody) value. Mean ± SD of (A) n = 7; (B) n = 8.
In vitro or in vivo NK cell pre-activation restores IL-12-induced IFN-γ production by MyD88 KO NK cells
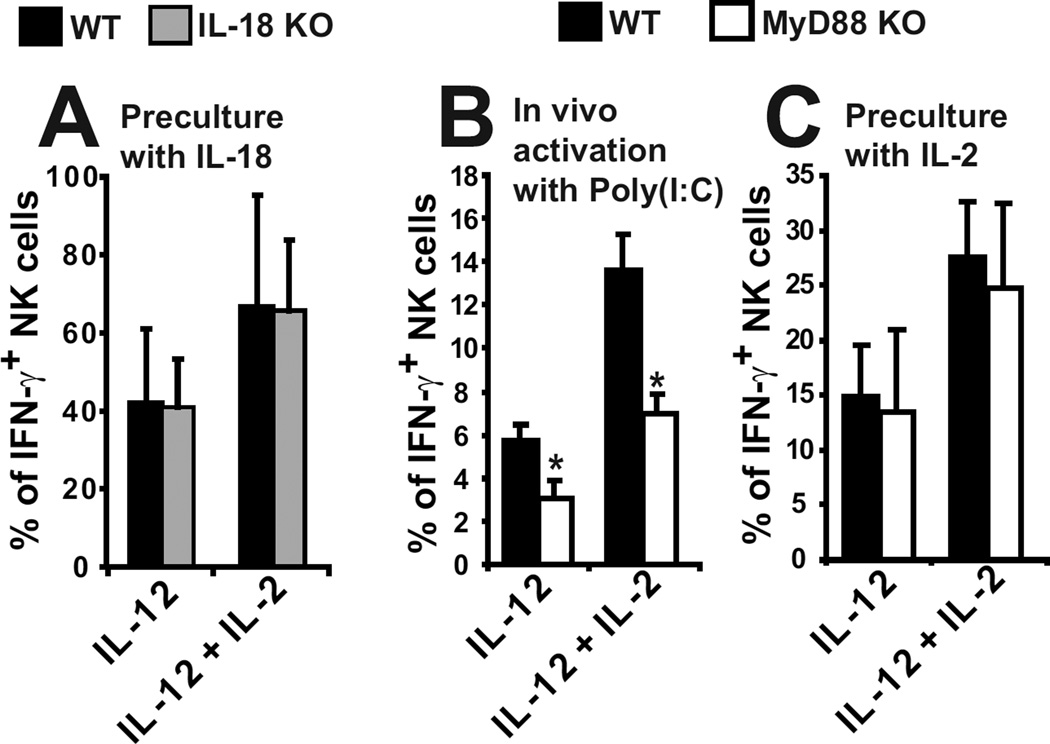
Next, we asked whether the defective NK cell response to IL-12 observed in IL-18 signalling-deficient mice could be overcome by prior in vivo or in vitro NK cell activation. We first cultured WT and IL-18 KO NK cells with IL-18 prior to the stimulation with either IL-12 or IL-2 + IL12. In these conditions IL-18 KO NK cells produced IFN-γ as efficiently as WT NK cells in response to both stimuli (Fig. 5A). Second, MyD88 KO and WT mice were injected with Poly(I:C) to activate NK cells in vivo. IFN-γ production by in vivo activated MyD88 KO NK cells in response to IL-12 or IL2/12 was lower than that of WT NK cells (Fig. 5B), but the difference was lesser than between resting WT and MyD88 KO NK cells (Fig. 1A). Third, NK cells from MyD88 KO and WT mice were cultured for 6 days in vitro with IL-2 to generate lymphokine-activated killers (LAK). The response of MyD88 KO LAK in response to IL-12 or IL2/12 was comparable to the response of WT LAK (Fig. 5C). Thus, the responsiveness of MyD88 KO NK cells to IL-12 can be restored by in vitro activation with IL-2 and partially recovered by in vivo activation with Poly(I:C). Altogether, these results show that the defective production of IFN-γ by NK cells in IL-18 signalling deficient mice is not due to a developmental deficiency but rather to a defect of priming.
Figure 5. In vitro or in vivo NK cell pre-activation restores IL-12 induced IFN-γ production by MyD88 KO NK cells.
(A) NK cells from WT and IL-18 KO mice were cultured 4 hours in vitro with IL-18, extensively washed and then stimulated 4 hours as indicated. The percentage of IFN-γ+ NK cells was measured. n = 4–5. (B) NK cells from Poly(I:C) injected wild-type and MyD88 KO mice were stimulated 4 hours in vitro as indicated. The percentage of IFN-γ+ NK cells was measured. n = 4. (C) LAK cells from wild-type and MyD88 KO NK cells were stimulated 4 hours in vitro as indicated. The percentage of IFN-γ+ LAK cells was measured. n = 4.
Concluding remarks
Our results reveal a novel type of cooperation between IL-12 and IL-18 that requires a sequential activity of these cytokines. What is the molecular basis of this phenomenon? IL-12 and IL-18 have been previously reported to act synergistically to induce IFN-γ secretion by T cells and NK cells (12, 13). Synergy between IL-12 and IL-18 signalling arises from the distinct transcription factors induced and by the cooperation between these factors. For instance, IL-12-induced STAT4 up regulates the binding activity of IL-18-induced AP1 on the IFN-γ promoter (15). However, we found that the IFN-γ gene was transcribed at a similar level in WT and MyD88 KO NK cells in response to IL-12 stimulation. Accordingly, we found that i) IL-12R chains were expressed normally in MyD88 KO NK cells, both at mRNA and protein levels and that ii) STAT4 was phosphorylated at a normal level in MyD88 KO NK cells upon stimulation with IL-12 (data not shown). These results suggest an unexpected role of the IL-18 pathway in the post-transcriptional regulation of IFN-γ induced by IL-12. Post-transcriptional regulation of IFN-γ has been shown to occur at different stages, from the localization of IFN-γ mRNA (16), to the stability (17) and even the translation of this mRNA (18, 19). Our results do not favour a role for IL-18 in IFN-γ mRNA stabilization, as the level of IFN-γ mRNA is similar in wild-type and MyD88 KO NK cells stimulated with IL-12. They are, however, compatible with the two other possibilities. Interestingly, Hodge et al found that IL-12 induces IFN-γ mRNA accumulation in the nucleus of cells of the NK92 line. They also found that stimulation with IL-2 improves the translocation of IFN-γ mRNA to the cytoplasm, which could be linked to an improved translation of this mRNA (16). Thus, it is possible that stimulation of NK cells with IL-12 induces a sub-optimal production of IFN-γ by NK cells because translation is limiting. In this model, a prior stimulation by IL-18 could result in a better nuclear export and translation of IFN-γ mRNA. Of note, other NK cell effector functions such as cytotoxicity have been found to be primed at the level of translation. In particular, perforin and granzyme B mRNA are translated upon IL-15 mediated priming (20). Thus, the control of mRNA translation could be central in NK cell priming. Interestingly, although we found no effect of IL-18 signalling on NK cell capacity to degranulate, others previously reported that NK cells isolated from IL-18 KO mice had defective cytotoxicity against YAC1 cells (21). One possible explanation to this discrepancy could be that the level of granule proteins are also limiting in IL-18 KO NK cells.
Another open question is the source of IL-18 in vivo. IL-18 has been shown to be produced at basal level (14) by different cell types including macrophages (22) and DC (23). Interactions between macrophages or DC and NK cells have been documented these past years. In particular, DC have been shown to be essential for IL-15-mediated NK cell priming (3). Therefore, they could also potentially prime NK cells through IL-18 production. This could occur during NK cell development as bone marrow NK cells were found to produce IFN-γ in response to stimulation with IL-12 (24). The absence of one cytokine could be partly compensated by the others during inflammation. Indeed, we found that in vitro stimulation with IL-2 or in vivo stimulation with Poly(I:C) restored at least partially the ability of MyD88 KO NK cells to produce IFN-γ in response to stimulation with IL-12. Similarly, IL-18 deficiency has been shown to be compensated by other pathways in the production of IFN-γ in the liver upon infection with mouse cytomegalovirus (25). Redundancy in different cytokine pathways might thus contribute to the robustness of NK cell responses.
Acknowledgments
EV lab is supported by European Union (« Allostem »), Ligue Nationale contre le Cancer ('Equipe labellisée'), Agence Nationale de la Recherche, INSERM, CNRS, Ministère de l'Enseignement Supérieur et de la Recherche and Institut Universitaire de France. This work was also supported by National Institutes of Health Grant AI058181 (to L.B.).
References
- 1.Yokoyama WM. Natural killer cell immune responses. Immunol Res. 2005;32:317–326. doi: 10.1385/IR:32:1-3:317. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakazato K, Yamada H, Yajima T, Kagimoto Y, Kuwano H, Yoshikai Y. Enforced expression of Bcl-2 partially restores cell numbers but not functions of TCRgammadelta intestinal intraepithelial T lymphocytes in IL-15-deficient mice. J Immunol. 2007;178:757–764. doi: 10.4049/jimmunol.178.2.757. [DOI] [PubMed] [Google Scholar]
- 5.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 6.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 7.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL- 18-mediated function [In Process Citation] Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Z, Georgel P, Li C, Choe J, Crozat K, Rutschmann S, Du X, Bigby T, Mudd S, Sovath S, Wilson IA, Olson A, Beutler B. Details of Toll-like receptor:adapter interaction revealed by germ-line mutagenesis. Proc Natl Acad Sci U S A. 2006;103:10961–10966. doi: 10.1073/pnas.0603804103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki N, Chen NJ, Millar DG, Suzuki S, Horacek T, Hara H, Bouchard D, Nakanishi K, Penninger JM, Ohashi PS, Yeh WC. IL-1 receptor-associated kinase 4 is essential for IL-18-mediated NK and Th1 cell responses. J Immunol. 2003;170:4031–4035. doi: 10.4049/jimmunol.170.8.4031. [DOI] [PubMed] [Google Scholar]
- 11.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 12.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 13.Shibuya K, Robinson D, Zonin F, Hartley SB, Macatonia SE, Somoza C, Hunter CA, Murphy KM, O'Garra A. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708–1716. [PubMed] [Google Scholar]
- 14.Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakahira M, Ahn HJ, Park WR, Gao P, Tomura M, Park CS, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol. 2002;168:1146–1153. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 16.Hodge DL, Martinez A, Julias JG, Taylor LS, Young HA. Regulation of nuclear gamma interferon gene expression by interleukin 12 (IL-12) and IL-2 represents a novel form of posttranscriptional control. Mol Cell Biol. 2002;22:1742–1753. doi: 10.1128/MCB.22.6.1742-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye J, Ortaldo JR, Conlon K, Winkler-Pickett R, Young HA. Cellular and molecular mechanisms of IFN-g production induced by IL-2 and IL-12 in a human NK cell line. J. Leukoc. Biol. 1995;58:225–233. doi: 10.1002/jlb.58.2.225. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Asouli Y, Banai Y, Pel-Or Y, Shir A, Kaempfer R. Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell. 2002;108:221–232. doi: 10.1016/s0092-8674(02)00616-5. [DOI] [PubMed] [Google Scholar]
- 19.Khabar KS, Young HA. Post-transcriptional control of the interferon system. Biochimie. 2007;89:761–769. doi: 10.1016/j.biochi.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of Murine NK Cell Cytotoxicity Requires the Translation of a Pre-existing Pool of Granzyme B and Perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Interleukin-18. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 23.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 25.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]