Abstract
Regulatory T (Treg) cells play an integral role in maintaining immune homeostasis and preventing autoimmune diseases. Foxp3 expression marks the commitment of progenitor cells to the Treg lineage. Although the essential function of the NF-κB family transcription factor c-Rel in the regulation of natural Treg cells (nTregs) has been firmly established, little is known about whether c-Rel is involved in the in vivo generation of peripheral Treg cells (pTregs), which develop from mature CD4+ conventional T cells outside of the thymus. We sought to answer this question through the induction of pTregs in the eye and gut mucosa using ovalbumin-specific T cell receptor transgenic mice that do or do not express c-Rel. Our results showed that Tregs can be induced in the eye in a c-Rel-dependent manner when immune-mediated inflammation occurs. However, c-Rel is dispensable for the induction of pTregs in the gut mucosa after oral antigen administration. Thus, c-Rel may play distinct roles in regulating the development of pTregs in different organs.
Keywords: c-Rel, eye, pTreg, gut mucosa, Foxp3
1. Introduction
CD4+CD25+ Foxp3+T (forkhead box P3+) regulatory cells (Tregs) are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and curb autoimmune disease [1–4]. Tregs are broadly classified into natural Treg (nTreg), peripheral Treg (pTreg) and induced Tregs (iTreg). nTreg cells are generated in the thymus and are among the best-studied Treg cells [3]. Unlike nTreg cells, Treg cells can be generated in peripheral lymphoid organs (pTreg) or in cultures after stimulation with antigens and Treg cell-inducing cytokines such as TGF-β and IL-2 (iTreg) [5–8]. In addition to nTreg, pTreg and iTreg cells, other types of Treg cells have also been described. These include CD8+ Treg cells, Tr1 cells, and Th3 cells [9–10].
Both pTregs and nTregs constitute the Treg pool circulating in the periphery. TCR activation has been shown to be essential for Treg cell differentiation and FoxP3 expression is an indication of progenitor commitment to the Treg lineage. TCR activation induces binding of transcription factors such as c-Rel, p65, NFAT, Sp1, AP1, CREB, and ATF to either the Foxp3 promoter or the intragenic enhancer element in T cells. TGF-β, on the other hand, can induce binding of Smads to intragenic enhancer and TIEG1 protein to extragenic enhancer and oppose methylation mediated silencing of Foxp3 [11–13]. Like nTregs, antigen specific pTregs are able to suppress T cell response. Though nTreg, pTreg and iTreg cells share a similar function, they exhibit some observed variances in execution, specific to cell class, such as differing mRNA transcripts and protein expression, epigenetic modification, and stability [14]. TGF-β has been shown to be essential for iTreg differentiation but it is still controversial as to whether it is needed for nTreg differentiation [15]. nTregs and iTregs also differ in their principal antigen specificities and in the T-cell receptor signal strength and co-stimulatory requirements needed for their generation [16].
The mammalian Rel/NF-κB family consists of five members: c-Rel, RelA (p65), RelB, NF-κB1 (p50/p105), and NF-κB2 (p52/p100). Unlike other members of the Rel/NF-κB family that are constitutively expressed in multiple cell types, c-Rel is expressed primarily in lymphoid and myeloid cells [17–20]. It has been reported that c-Rel plays a crucial role in assembling a Foxp3-specific enhancer complex that is required for initiating Treg cell differentiation [21]. However, since there are no precise or specific surface markers to identify nTregs, pTregs and iTregs [14], little is known whether c-Rel is required for the generation of in vivo induced pTregs.
To address this issue, we generated ovalbumin (OVA)-specific T cell receptor (TcR) transgenic mice that do or do not express c-Rel. pTregs were then induced in the eye under inflammatory conditions, and in the gut mucosa through adoptive T cell transfer and oral antigen administration. Our data clearly demonstrate that transcription factor c-Rel is required for the generation of Tregs in the eye but is dispensable for the induction of pTregs in the gut mucosa.
2. Materials and Methods
Ethics Statement
All mice were housed in the University of Pennsylvania animal care facilities under pathogen-free conditions. The animal use protocol has been reviewed and approved by University of Pennsylvania Animal Care and Use Committee. All efforts were made to minimize suffering.
Mice
C57BL/6 (B6) mice that carry a c-Rel gene null mutation (Rel−/−) were generated as described and were backcrossed to B6 mice for 12 generations before being used in this study [22]. C57BL/6-Tg (TcraTcrb) 425Cbn/J ovalbumin mice (hereafter referred to as OVA TcR transgenic mice) and B6.SJL-Ptprca Pepcb/BoyJ (hereafter referred to as B6 CD45.1 mice) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). To generate OVA TcR transgenic mice with c-Rel gene null mutation, Rel−/− B6 mice were crossed with OVA TcR transgenic mice and progenies were genotyped for OVA and Rel by PCR.
Total cell isolation from eye
Intact eyes were removed from mice sacrificed according to approved animal ethics protocols and placed immediately in sterile HBSS. After cleaning the eye with forceps, micro-dissecting scissors were used to cut the eyes into small pieces. Eyes were washed with HBSS twice and then incubated at 37°C in HBSS medium containing 0.2 mg/mL freshly prepared collagenase P (Roche) solutions. After 15 minutes, large pieces were allowed to settle and then digested materials were transferred into growth culture medium (DMEM containing 10% fetal bovine serum, 1% L-glutamine and antibiotics). The remaining tissues were digested as above for two more times and the digested materials were combined, filtered through a cell strainer and used for flow cytometry analysis.
Lymphcyte isolation from mouse lymph nodes
Cervical lymph node and mesenteric lymph node were removed from mice and homogenized through a nylon mash (70 µM) using the bottom side of a 10-cc syringe plunger. The single cell suspension were washed twice with culture medium (DMEM containing 10% fetal bovine serum, 1% L-glutamine and antibiotics) and used for flow cytometry analysis.
Antibodies and Flow Cytometry
Fluorescein isothiocyanate-conjugated anti-Foxp3 was purchased from BD PharMingen. PE-conjugated anti-CD4 and allophycocyanin-conjugated anti-CD25 were purchased from Caltag Laboratories. Flow cytometry analyses were performed on freshly isolated cells from eye or mesenteric lymph node. For Foxp3 intracellular staining, cells were fixed with 4% paraformaldehyde (PFA) and permeabilized with 0.5% saponin (Sigma). Stained cells were analyzed on FACS-Calibur (BD Biosciences). Data were analyzed using FlowJo software.
pTreg induction in the eye
OVA TcR transgenic mice with or without c-Rel deficiency were injected with 50 ug OVA into anterior chamber. 4 days after injection, mice were killed and eye derived cells were isolated by collagenase digestion. The percentage of CD4+CD25+ Foxp3+ cells and the mean fluorescence intensity of Foxp3 were determined by flow cytometry analysis.
RNA isolation and real-time PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription was performed using oligo-dT primers. Quantitative real-time PCR was carried out in the Applied Biosystems 7500 system using Power SYBR Green PCR Master Mix (Applied Biosystems). The relative level of gene expression was determined using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the control. The primers used in this study are: Foxp3-F: 5’-CAGCTGCCTACAGTGCCCCTAG-3’; Foxp3-R: 5’-CATTTGCCAG CAGTGGGTAG-3’; GAPDH-F: 5’-AGTATGACTCCACTCACGGCAA-3’; GAPDH-R: 5’-TCTCGCTCCTGGAAGATGGT-3’.
Adoptive T cell transfer and oral antigen administration
To induce pTregs in the gut mucosa, CD4+CD25−CD45RBHigh cells from OVA TcR transgenic mice (CD45.2) with or without c-Rel-deficiency were flow-sorted and injected into C57BL/6 mice (CD45.1). OVA were added into the drinking water for five days to induce antigen-specific Treg cells.
Statistical Analyses
Student’s t test was used to evaluate the statistical significance of the differences in mRNA levels and the differences in the percentages and number of cells.
Results and Discussion
1. OVA TcR transgenic mice with c-Rel deficiency produced less eye-derived Tregs
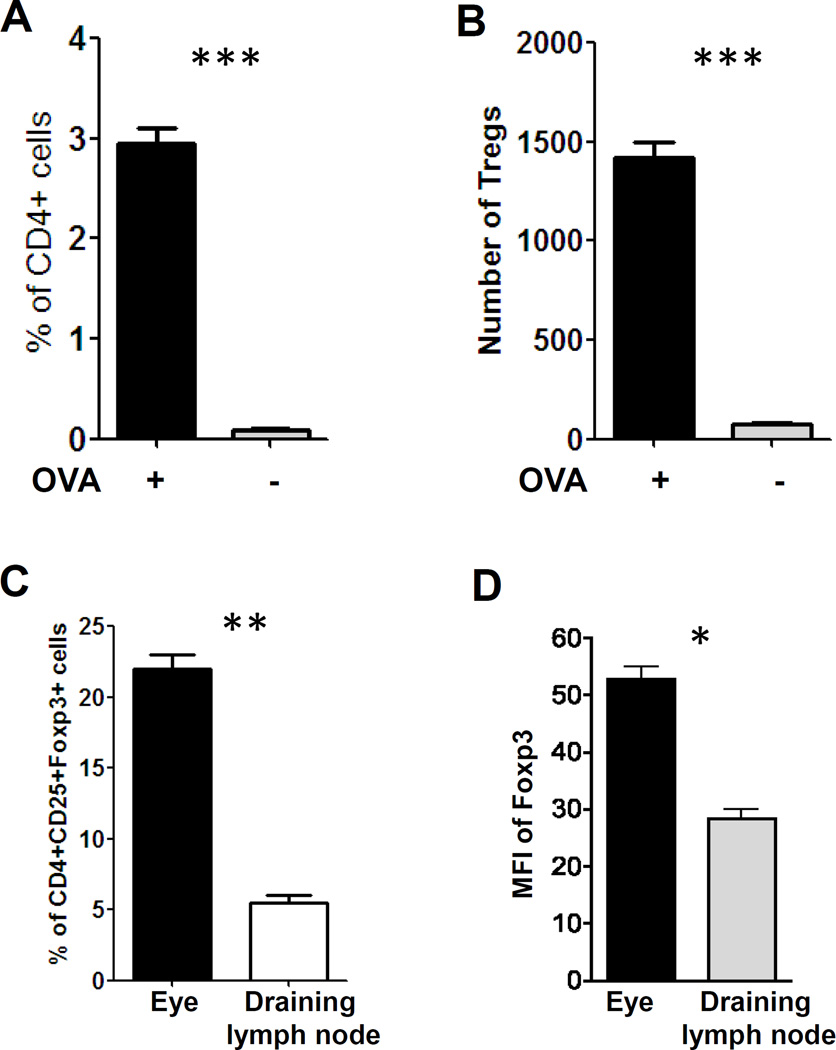
The eye is known to be endowed with immune privilege and inflammation is self-regulated to preserve organ function. However, despite its immune privilege, the eye is susceptible to autoinflammatory conditions such as autoimmune uveoretinitis. Treg cells are detected in experimental autoimmune uveoretinitis (EAU) and play an important role in the regression of EAU [23]. Tregs within the eye could come from thymic-derived natural Tregs or Tregs induced within the eye draining lymph node. Conventional T cells could also be converted into Tregs within the eye under inflammatory conditions. The anterior chamber contains biologically relevant concentrations of various immunomodulatory neuropeptides, growth factors and cytokines, such as transforming growth factor (TGF-β), α-melanocyte-stimulating hormone (α-MSH), vasoactive intestinal peptide (VIP), and calcitonin gene-related peptide (CGRP) as well as others. These factors are capable of suppressing innate and adaptive immunity [24–26]. Recently two groups have reported that Foxp3-expressing Tregs can be induced locally within the retina [27–28]. To induce Tregs in the eye, we injected OVA into the ocular anterior chamber of OVA TcR transgenic mice. 4 days after microinjection, the frequency of CD4+ T cells (Fig. 1A) and the number of CD4+Foxp3+ T cells (Fig. 1B) in the eye were increased ~40-fold and ~18-fold, respectively. More importantly, the percentage of Treg cells (Fig. 1C) and the mean fluorescence intensity (MFI) of Foxp3 expression (Fig. 1D) from OVA-treated eyes were significantly higher than Treg cells from the draining lymph node. It has been reported that levels of Foxp3 in regulatory T cells are far more relevant to their functional status in transplantation rejection than the frequency of Tregs [29]. Based on these results, we speculate that it is unlikely that the increase of CD4+CD25+Foxp3+ cells in the eye is due to the migration of Tregs from eye draining lymph node. More likely, these Tregs are generated locally in the eye. To further support this, there are a few reports concluded that Tregs that mediated ACAID-mediated immune tolerance are originating from conversion of conventional T cells into pTregs upon exposure to high concentrations of TGF-β and IL-10 in the anterior chamber [26–28]..
Figure 1. Foxp3+Tregs can be induced in the eye under inflammatory condition.
A & B. OVA or PBS was injected into the ocular anterior chamber (AC) of OVA TcR transgenic mice. 4 days after microinjection, single cell suspensions were prepared from the eye and percentage of CD4+ cells (A) and the number of Tregs (B) were determined by flow cytometry. C & D. Mice were treated as in (A) and single cell suspensions were prepared from the eye and draining lymph node. The percentages of CD4+CD25+Foxp3+ cells (C) and mean fluorescence intensity (MFI) of Foxp3 (D) were determined by flow cytometry. The percentages of Tregs are the ration to CD4+ cells. Results are representative of two independent experiments. *: P < 0.05; **: P < 0.01; ***: P < 0.001.
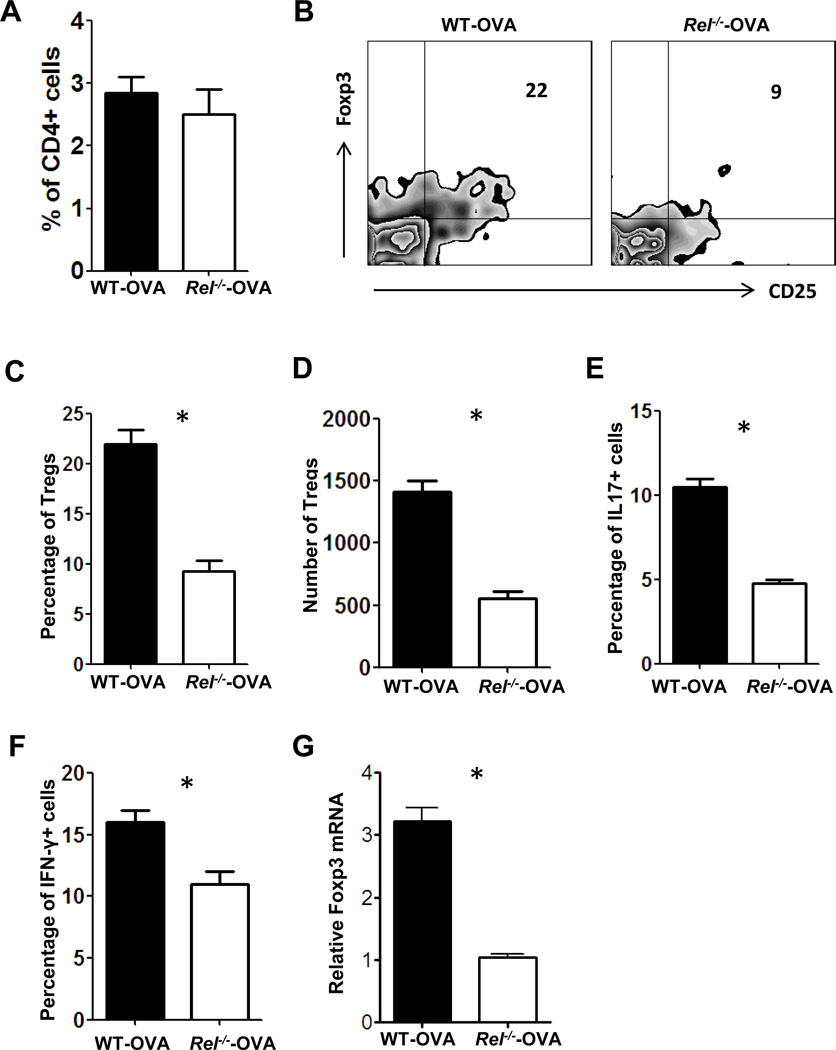
It has been reported that deficiency in c-Rel causes up to ten-fold reductions in the number of nTreg cells in mice [21]. To examine whether c-Rel is also involved in the generation of pTregs within the eye, we injected OVA into the ocular anterior chamber of OVA TcR transgenic mice with or without c-Rel deficiency. 4 days after microinjection, we found a marginal difference in the frequency of total CD4+ T cells (Fig. 2A). However, the frequency (Fig. 2B & 2C) and number (Fig. 2D) of CD4+CD25+Foxp3+ cells, as well as the frequency of CD4+IL-17+ (Fig. 2E) and CD4+IFN-γ+ (Fig. 2F) cells, were significantly decreased in c-Rel deficient mice. Consistent with the flow cytometry data, Foxp3 mRNAs were also decreased in c-Rel deficient mice (Fig. 2G). These results indicate that c-Rel is required for the generation of intra-ocular pTregs under inflammatory condition. However, many c-Rel deficient T cells were still converted to CD4+Foxp3+ cells in vivo, suggesting that other NF-κB family members, like p65, are compensating for the loss of c-Rel [21].
Figure 2. OVA TcR transgenic mice with c-Rel deficiency produced less eye-derived Tregs.
OVA TcR transgenic mice with (Rel−/−-OVA) or without c-Rel deficiency (WT-OVA) were injected with 50 ug OVA into the anterior chamber. 4 days after injection, mice were killed and eye-derived cells were isolated by collagenase digestion. Cells were pooled from 3 mice for each group. A. The percentage of CD4+ cells was determined by flow cytometry analysis. B, C & D. The percentage (B& C) and number (D) of CD4+CD25+ Foxp3+ was determined by intracellular staining and flow cytometry analysis. E. mRNA of Foxp3 was determined by real-time PCR. F & G. The percentages of IL-17+ (F) and IFN-γ+ (G) cells were determined by intracellular staining and flow cytometry analysis. The percentages of Tregs, IL-17+ and IFN-γ+ cells are the ration to CD4+ cells. The experiment was repeated twice more, yielding similar results. *: P < 0.02.
2. c-Rel is dispensable for the induction of Tregs in the gut mucosa
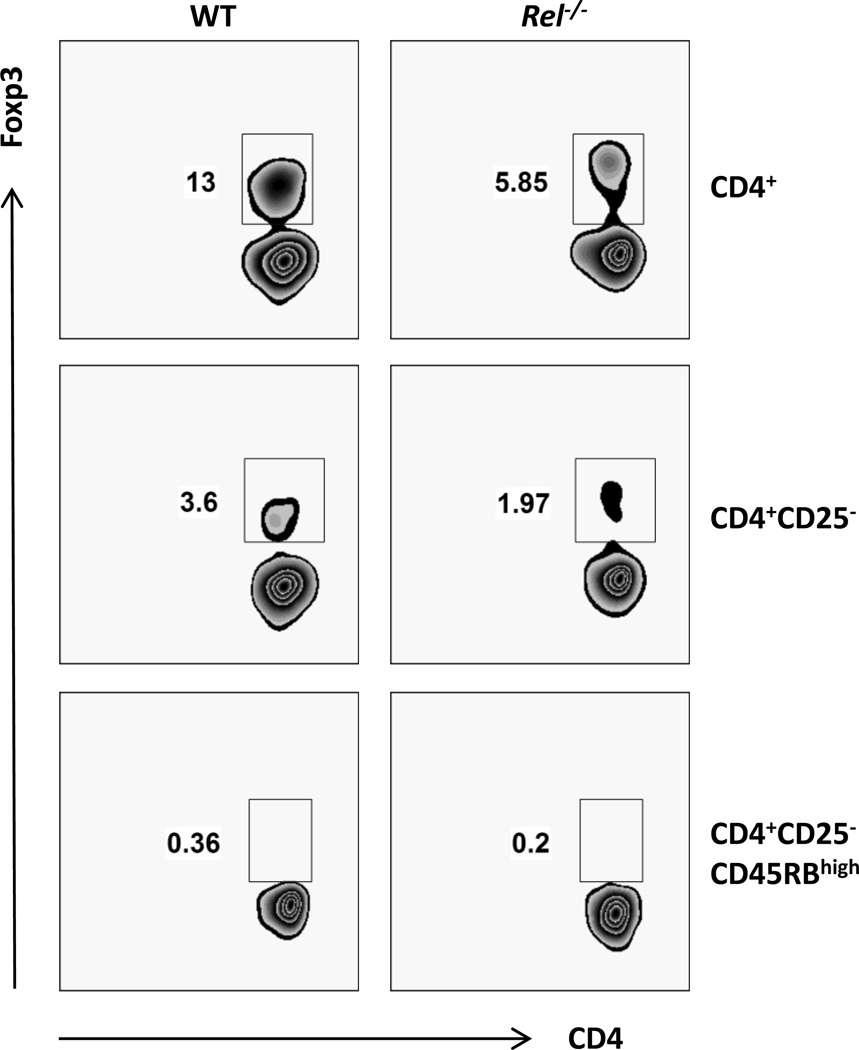
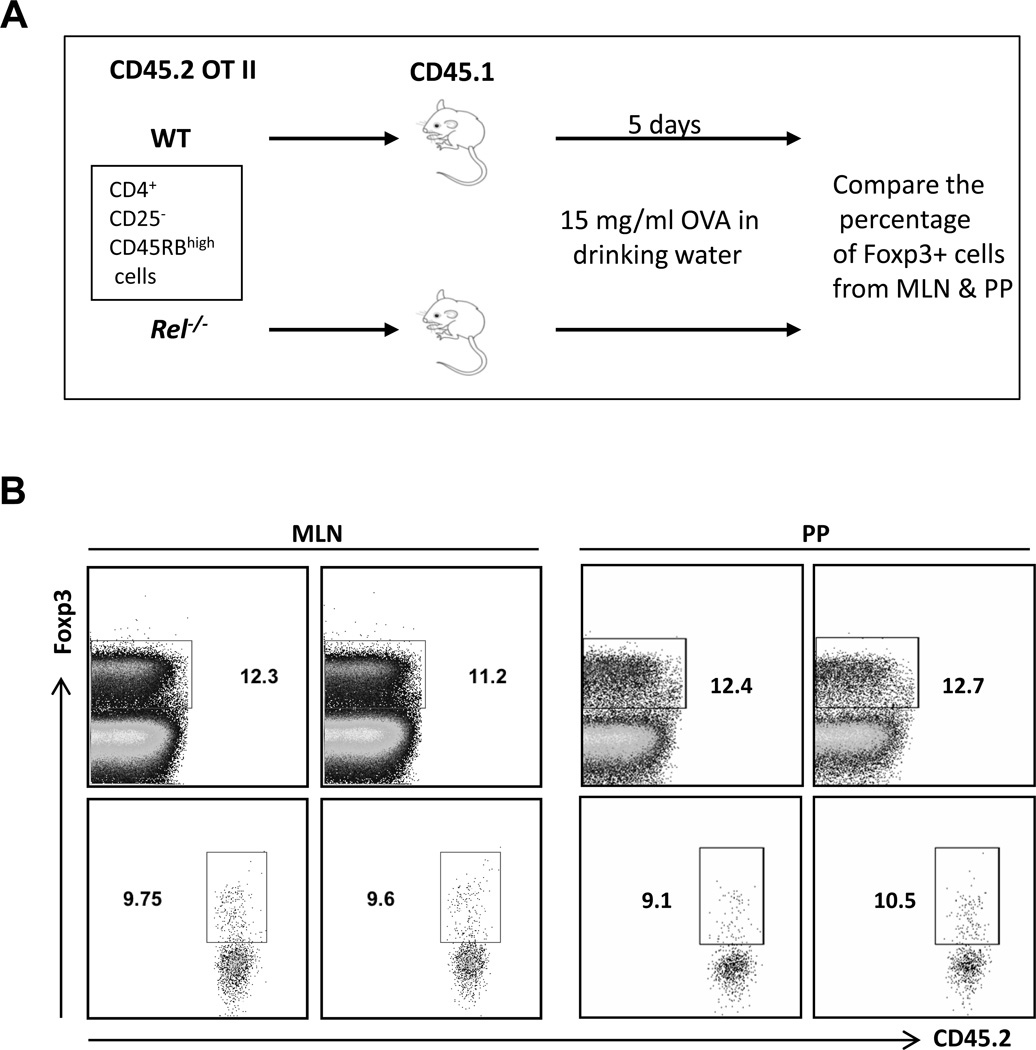
The gut mucosal immune system is the largest lymphoid organ in the body and experiences continuous antigenic challenges from food, bacterial flora, and pathogens. Despite this constant antigenic stimulation, inflammatory responses are tightly controlled. CD4+ Foxp3+ cells are prominent immune regulatory T cells (Tregs). The highest concentrations of these cells are found in the intestine [30]. Intestinal Treg cells consist of thymically and extrathymically developed cells. It has been shown that oral antigen administration may induce oral tolerance and promote the generation of regulatory T lymphocytes [31]. To examine whether c-Rel is involved in the generation of Tregs within the gut mucosa, Foxp3-negative antigen-specific T cells (CD45.2) were adoptively transferred into B6 CD45.1 mice and the generation of pTreg cells was monitored. In order to isolate cells that do not express Foxp3, CD4+CD25-CD45RBHigh cells were flow sorted from OVA TcR transgenic mice with or without c-Rel deficiency. Consistent with published data [32], only 0.36% of CD4+CD25−CD45RBHigh cell expressed Foxp3, comparing to 13% in CD4+ cells and 3.6% in CD4+CD25− cells (Fig. 3). Because it is observed that c-Rel plays a crucial role during the initiation of Treg cell differentiation [21], it is not surprising to find that c-Rel deficient CD4+ T cells expressed significant less Foxp3 (Fig. 3). Figure 4A illustrates the protocol used for pTreg generation in the gut mucosa. As expected, the percentage of recipient-derived CD4+Foxp3+ (CD45.2-negative) cells were comparable in mice transferred with naïve T cells with or without c-Rel deficiency (Fig. 4B & 4C). Surprisingly, we also found that the same amount of WT and c-Rel deficient naïve T cells converted into Foxp3+ (CD45.2-positive) cells in both mesenteric lymph node (MLN) and Peyer’s patch (PP) (Fig. 4B). It has been reported that the capacity of c-rel-deficient Treg cells to undergo homeostatic expansion is reduced [33]. However, since we only waited for 5 days after OVA feeding to analyze the conversion of Tregs, the reduced homeostatic expansion by c-Rel deficiency Tregs may not be apparent at this early time-point. Taken together, these data indicate that c-Rel is not required for the induction of pTregs in the gut muscoa.
Figure 3. Percentage of Foxp3+ cells on gated CD4+, CD4+CD25− and CD4+CD25−CD45RBhigh cells.
Splenocytes from C57BL/6 mice with (Rel−/−) or without c-Rel deficiency (WT) were stained with antibodies against murine CD4, CD25, CD45RB and Foxp3, and analyzed by flow cytometry. Cells were pooled from 3 mice for each group. Results are representative of three independent experiments.
Figure 4. c-Rel is dispensable for the induction of Tregs in the gut mucosa.
(A). Protocol used for pTreg generation in the gut mucosa. B. Mice were treated as in (A). Total cells from mesenteric lymph node (MLN) and Peyer’s patch (PP) were stained with antibodies to murine CD4, CD45.2 and Foxp3, and analyzed by flow cytometry. Results are representative of two independent experiments.
It has been reported before that c-Rel-deficient naïve CD4+ T cells can convert to Treg cells in vivo and the percentage of c-Rel deficient Treg cells was reduced when compared with the WT population [33]. However, our data clearly indicate that c-Rel deficient naïve T cells can convert to Foxp3+ Treg cells with the same efficiency as WT naïve T cells. The use of different naïve cells and an alternate protocol for Treg induction may account for this discrepancy; CD4+CD25− cells were used for adoptive transfer according to published data. As described in Figure 3, ~3.6% of CD4+CD25− cells expresses Foxp3. However, previously published data showed that only ~1% of the CD4+CD25− cells converted to Foxp3-producing Treg cells. This raises the question of whether Treg cells were indeed induced in vivo. Contrastingly, we flow sorted CD4+CD25−CD45RBHigh cells for adoptive transfer and found only 0.36% of the cells expressed Foxp3. Additionally, antigen-specific Treg cells were induced in our system, facilitating an almost 10% conversion of the transferred naïve T cells to Tregs.
In conclusion, we have shown that transcription factor c-Rel is required for the induction of Foxp3+ regulatory T cells in the eye but not in the gut mucosa. This suggests that Treg inducing conditions in the eye and in the gut mucosa may be different. The anterior chamber contains biologically relevant concentrations of various immunomodulatory neuropeptides, growth factors and cytokines [24–26]. However, Tregs induced in the gut mucosa relied mainly on oral tolerance [32]. Indeed it has been shown before that, depending on the induction conditions used, c-Rel may or may not be required for the induction of Tregs in vitro [21, 33]. We propose that, under certain conditions, other NF-κB family members, like p65, which also regulate Foxp3 expression compensate for the loss of c-Rel.
Acknowledgments
This study was supported by the Shenzhen Kongque antibody drug program grant; Shenzhen Basic Research Program (JCYJ20140610151856705); National Institutes of Health, USA (AI-077533); National Natural Science Foundation of China (81471554, 81470611, 81530027); National Basic Research Program of China (2013CB967004); Taishan Scholar Program (ts 20150215); and Shandong Province Key Research and development program, China (grant no.: 2015GGH318010)
Abbreviations
- pTreg
peripheral T regulatory cell
- MFI
mean fluorescence intensity
- OVA
Ovalbumin
- nTreg
natural T regulatory cell
- Foxp3
forkhead box P3
- EAU
experimental autoimmune uveoretinitis
- TGF-β
transforming growth factor β
- α-MSH
α-melanocyte-stimulating hormone
- VIP
vasoactive intestinal peptide
- CGRP
calcitonin gene-related peptide
- TcR
T cell receptor
References
- 1.Bommireddy R, Doetschman T. TGFbeta1 and Treg cells: alliance for tolerance. Trends Mol. Med. 2007;13:492–501. doi: 10.1016/j.molmed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol. Rev. 2006;212:149–162. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. Journal of Experimental Medicine. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bommireddy R, Doetschman T. TGFbeta1 and Treg cells: alliance for tolerance. Trends in Molecular Medicine. 2007;13:492–501. doi: 10.1016/j.molmed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. Journal of Experimental Medicine. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14(4):307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat. Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 11.Adalid-Peralta L, Fragoso G, Fleury A, Sciutto E. Mechanisms underlying the induction of regulatory T cells and its relevance in the adaptive immune response in parasitic infections. Int J Biol Sci. 2011;7(9):1412–1426. doi: 10.7150/ijbs.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 13.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9(2):83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol. 2013;6(2):116–123. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Konkel JE. TGF-beta and 'adaptive' Foxp3(+) regulatory T cells. J Mol Cell Biol. 2010;2(1):30–36. doi: 10.1093/jmcb/mjp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+)regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29(9):429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 18.Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52:348–355. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 19.Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huguet C, Bouali F, Enrietto PJ, Stehelin D, Vandenbunder B, Abbadie C. The avian transcription factor c-Rel is expressed in lymphocyte precursor cells and antigen-presenting cells during thymus development. Developmental Immunology. 1998;5:247–261. doi: 10.1155/1998/58608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, Chen YH. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. Journal of Clinical Investigation. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keino H, Takeuchi M, Usui Y, Hattori T, Yamakawa N, Kezuka T, Sakai JI, Usui M. Supplementation of CD4+CD25+ regulatory T cells suppresses experimental autoimmune uveoretinitis. British journal of ophthalmology. 2007;91:105–110. doi: 10.1136/bjo.2006.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AW, Streilein JW, Cousins SW. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. Journal of Immunology. 1994;153:1080–1086. [PubMed] [Google Scholar]
- 25.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. Journal of Leukocyte Biology. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 26.Denniston AK, Kottoor SH, Khan I, Oswal K, Williams GP, Abbott J, Wallace GR, Salmon M, Rauz S, Murray PI, Curnow SJ. Endogenous cortisol and TGF-beta in human aqueous humor contribute to ocular immune privilege by regulating dendritic cell function. Journal of Immunology. 2011;186:305–311. doi: 10.4049/jimmunol.1001450. [DOI] [PubMed] [Google Scholar]
- 27.Zhou R, Horai R, Silver PB, Mattapallil MJ, Zárate-Bladés CR, Chong WP, Chen J, Rigden RC, Villasmil R, Caspi RR. The living eye "disarms" uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. Journal of Immunology. 2012;188:1742–1750. doi: 10.4049/jimmunol.1102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McPherson SW, Heuss ND, Gregerson DS. Local "on-demand" generation and function of antigen-specific Foxp3+ regulatory T cells. Journal of Immunology. 2013;190:4971–4981. doi: 10.4049/jimmunol.1202625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan SK1, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizrahi M, Ilan Y. The gut mucosa as a site for induction of regulatory T-cells. Curr Pharm Des. 2009;15(11):1191–1202. doi: 10.2174/138161209787846784. [DOI] [PubMed] [Google Scholar]
- 31.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5(3):232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelenay S1, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25− CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci U S A. 2005;102(11):4091–4096. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, McNally A, Steptoe RJ, Thomas R, Shannon MF, Gerondakis S. J Exp Med. 2009 Dec 21;206(13):3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]