Abstract
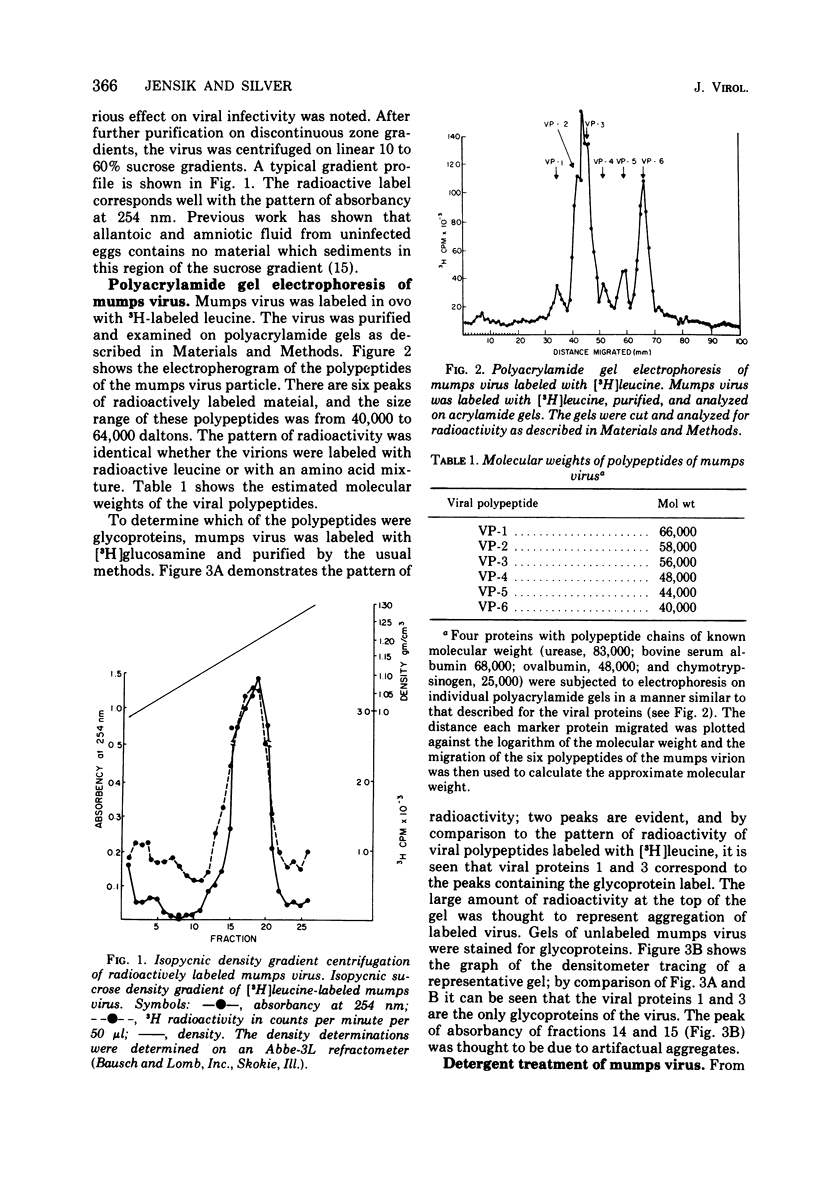
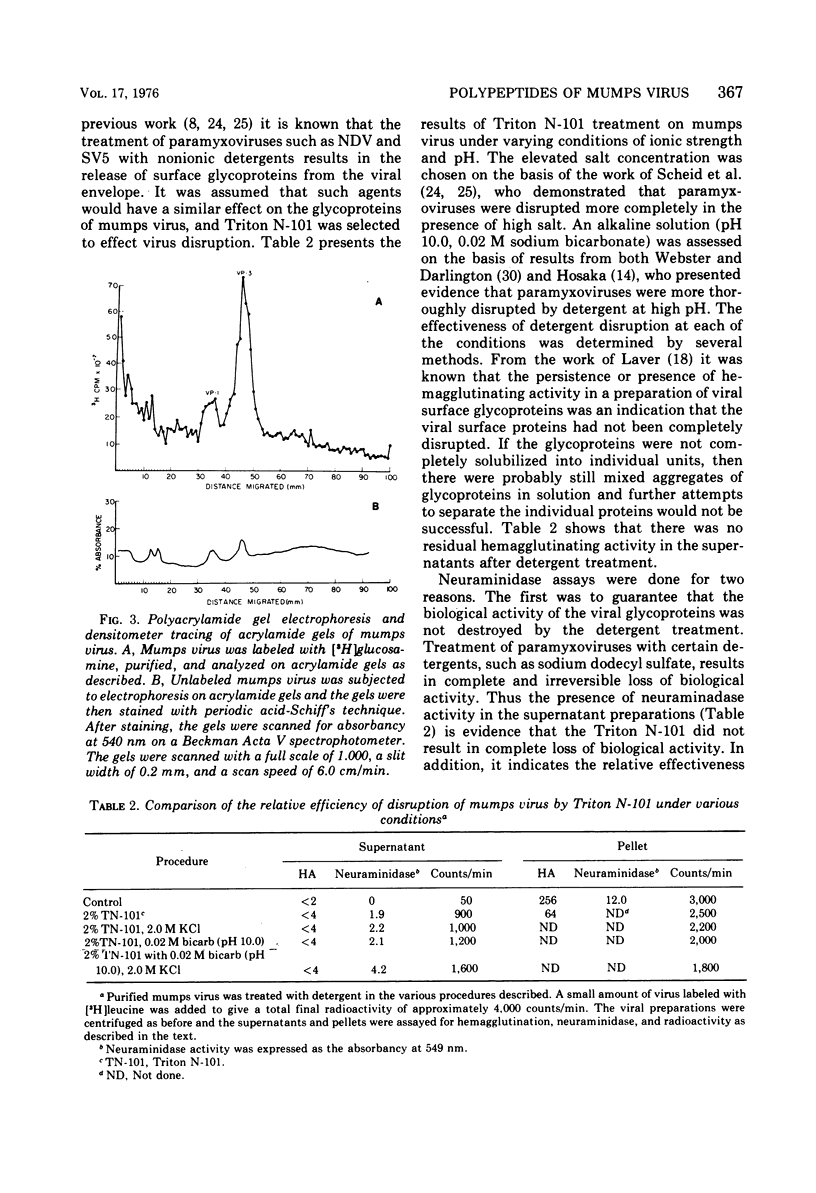
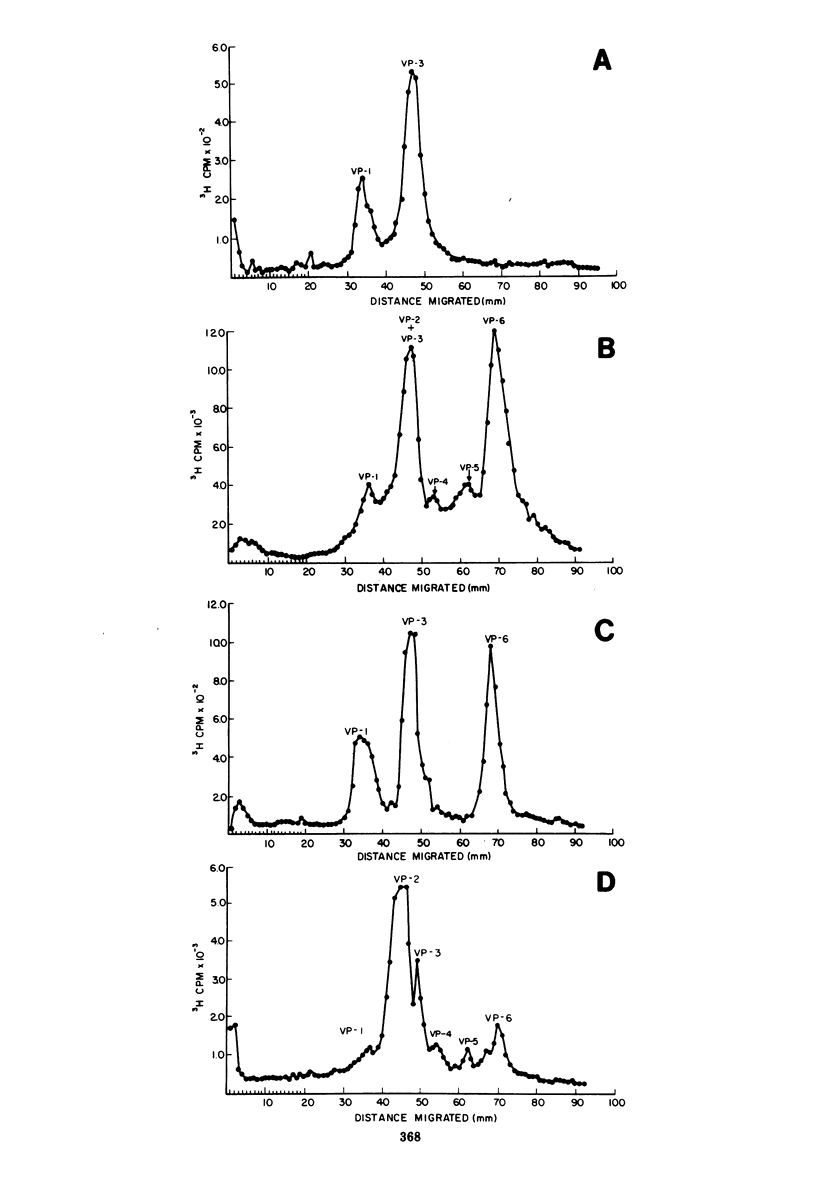
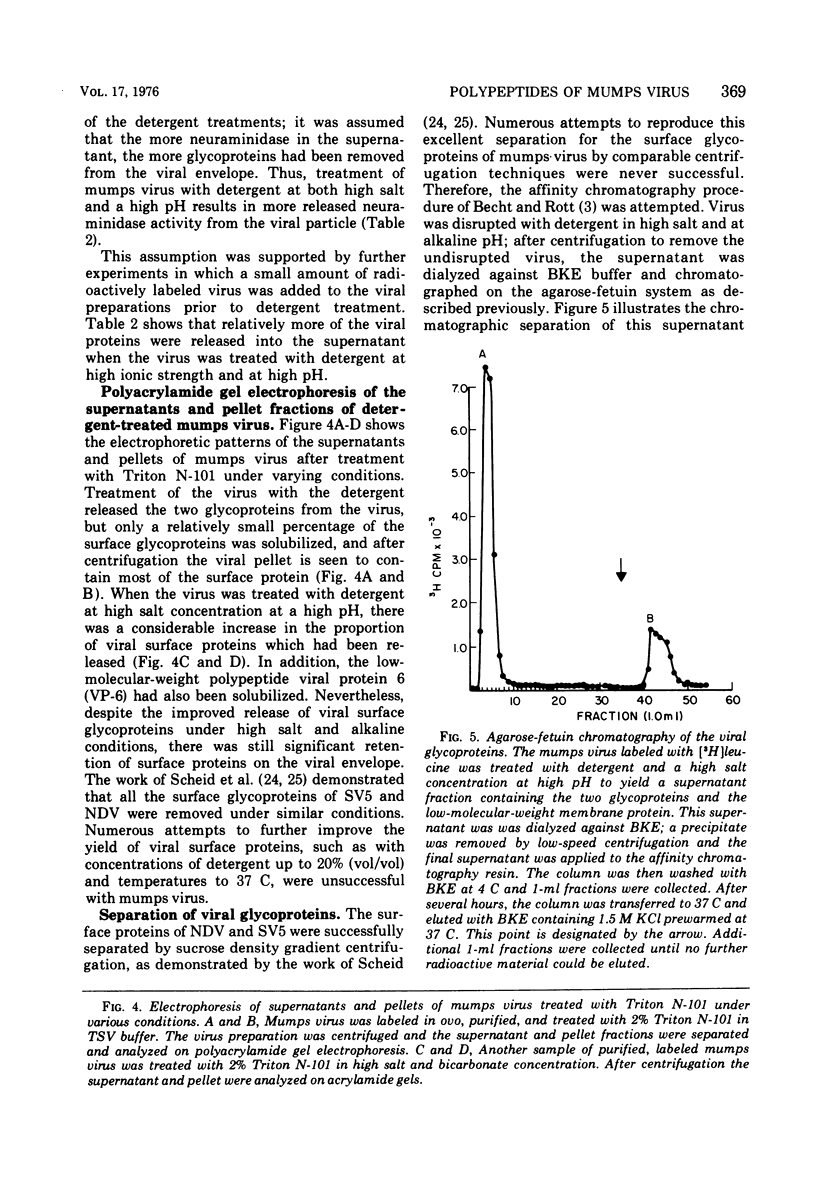
Mumps virus was propagated in the extra-embryonic fluids of embryonated chicken eggs and was labeled by cionjection of radioactively labeled amino acids. The virus was purified by density gradient centrifugation, and its polypeptides were analyzed by polyarylamide gel electrophoresis. The virus was found to be composed of six polypeptides, ranging in size from 40,000 to 64,000 daltons. Viral proteins 1 and 3 were the glycoproteins of the virons. When the virus particle was treated with noniontic detergents, a small fraction of these glycoproteins could be released into the supernatant. After treatment with nonionic detergents in high salt and alkaline conditions, more of the surface glycoproteins were removed. This treatment also released the smallest viral polypeptide from the virion. The glycoproteins were separated using an affinity chromatographic column of agarose-fetuin. The heavier glycoprotein, viral protein 1, was found to contain both the neuraminidase and hemagglutinating activity. The two glycoproteins were tested for their ability to react in complement-fixing tests with mumps antisera. Only the heavier glycoprotein reacted with antisera possessing both anti-S and anti-V activity. Neither glycoprotein reacted with antisera specific for the S antigen. Thus, it was concluded that this glycoprotein corresponds to the classical V antigen of mumps virus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDREWES C. H., BANG F. B., BURNET F. M. A short description of the Myxovirus group (influenza and related viruses). Virology. 1955 Jul;1(2):176–184. doi: 10.1016/0042-6822(55)90014-3. [DOI] [PubMed] [Google Scholar]
- Becht H., Rott R. Purification of influenza virus hemagglutinin by affinity chromatography. Med Microbiol Immunol. 1972;158(2):67–70. doi: 10.1007/BF02120470. [DOI] [PubMed] [Google Scholar]
- Bernard J. P., Northrop R. L. RNA polymerase in mumps virion. J Virol. 1974 Jul;14(1):183–186. doi: 10.1128/jvi.14.1.183-186.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. D., Duesberg P. H. Myxovirus ribonucleic acids. Annu Rev Microbiol. 1970;24:539–574. doi: 10.1146/annurev.mi.24.100170.002543. [DOI] [PubMed] [Google Scholar]
- CANTELL K. Mumps virus. Adv Virus Res. 1961;8:123–164. doi: 10.1016/s0065-3527(08)60684-3. [DOI] [PubMed] [Google Scholar]
- Chen C., Compans R. W., Choppin P. W. Parainfluenza virus surface projections: glycoproteins with haemagglutinin and neuraminidase activities. J Gen Virol. 1971 Apr;11(1):53–58. doi: 10.1099/0022-1317-11-1-53. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East J. L., Kingsbury D. W. Mumps virus replication in chick embryo lung cells: properties of ribonucleic acids in virions and infected cells. J Virol. 1971 Aug;8(2):161–173. doi: 10.1128/jvi.8.2.161-173.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. The biochemical and biological characteristics of the surface components of measles virus. J Gen Virol. 1974 Mar;22(3):363–374. doi: 10.1099/0022-1317-22-3-363. [DOI] [PubMed] [Google Scholar]
- Hosaka Y. Isolation and structure of the nucleocapsid of HVJ. Virology. 1968 Jul;35(3):445–457. doi: 10.1016/0042-6822(68)90223-7. [DOI] [PubMed] [Google Scholar]
- Jensik S. C., Northrop R. L. Incorporation of radioactive seleno-(75Se)-methionine into mumps virus. Appl Microbiol. 1971 Mar;21(3):451–455. doi: 10.1128/am.21.3.451-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Choppin P. W. Proteins and glycoproteins of paramyxoviruses: a comparison of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1971 Jan;7(1):47–52. doi: 10.1128/jvi.7.1.47-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shramek G., Deinhardt F. Development of an attenuated mumps virus vaccine. II. Immune response of animals to vaccination with inactivated and live attenuated mumps viruses. J Immunol. 1969 Apr;102(4):1093–1098. [PubMed] [Google Scholar]
- WATERSON A. P. Two kinds of myxovirus. Nature. 1962 Mar 24;193:1163–1164. doi: 10.1038/1931163a0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Darlington R. W. Disruption of myxoviruses with Tween 20 and isolation of biologically active hemagglutinin and neuraminidase subunits. J Virol. 1969 Aug;4(2):182–187. doi: 10.1128/jvi.4.2.182-187.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



