Abstract
Objective
To evaluate the frequency of cerebral amyloid in early Parkinsons disease (ePD) and provide a multimodal assessment of the influence of cerebral amyloid on disease phenotype.
Methods
We performed a multicentre cohort study of the Parkinsons Progression Markers Initiative (PPMI), including 369 drug-naïve patients with ePD and 174 healthy controls (HC). Cerebrospinal fluid (CSF) amyloid-β levels were transformed using the linear regression procedure. A cut-off of >198 pg/mL was used to define amyloid-negative (PD−) and amyloid-positive (PD+) subgroups. Grey matter (GM) density and hippocampal volume from the MRI was measured using Advanced Normalisation Tools (ANTs). We compared demographic, genetic, CSF, behavioural, functional and imaging modalities across ePD− and ePD+ groups.
Results
We observed that 16.5% of ePD have CSF evidence of amyloidosis. PD+ was significantly older than PD−, had a higher frequency of APOE e4 alleles and all CSF measures (total-tau, phosphorylated-tau and α-synuclein) were reduced. PD+ had reduced cognitive performance relative to PD− on Symbol–Digit Matching, Verbal Category Fluency and Delayed Recall tests. Imaging analysis in a subset of individuals (PD+ =43; PD− =241) revealed overlapping GM atrophy relative to HC in medial temporal, frontal and brainstem structures. Direct comparisons revealed PD+ GM reductions predominantly located in the frontal cortex while PD− had GM reductions in subcortical structures. These observations remain when controlling for age and APOE e4 allele status.
Conclusions
Cerebral amyloid in ePD yields a unique phenotype across all measured modalities that is consistent with a synergistic interaction between α-synuclein and amyloid pathology. Amyloid status should be considered when screening these individuals for trials involving disease-modifying agents.
INTRODUCTION
Neuropathological studies have identified α-synuclein pathology as the characteristic misfolded protein contributing to Parkinson’s disease (PD)1 and the combination of amyloid-β (Aβ) plaques and tau neurofibrillary tangles (NFTs) as the histopathological hallmark of Alzheimer’s disease (AD).2,3 However, there is now mounting evidence suggestive of a spectrum of pathologies observed in AD and PD with autopsy studies indicating that ~50% of patients with AD and PD have comorbid α-synuclein and Aβ histopathological inclusions.4,5 The co-occurrence of Aβ deposition with α-synuclein pathology has been associated with reduced cognitive performance in patients with PD6–8 and with more severe clinical syndrome in animals.9,10 However, prior evidence of the interaction between α-synuclein and Aβ deposition has largely been based on late-stage neuropathological studies and investigations of the role of amyloid in drug-naïve patients with early PD (ePD) are rare11 with no prior work examining its influence on structural brain changes.
In this study, we report a multimodal evaluation of the influence of amyloid on ePD using data from the Parkinson’s Progression Markers Initiative (PPMI).12 Previous evidence suggests that lower cerebrospinal fluid (CSF) amyloid levels (similar to those seen in AD and presumably related to cortical amyloidosis) predict cognitive decline in PD,7 and specifically that CSF amyloid correlates with cognitive difficulty in non-demented patients with PD13 and in ePD.14 In line with these prior findings, we hypothesised that CSF evidence of cerebral amyloid in ePD would be associated with more significant cognitive dysfunction at baseline and that this reduction in cognitive performance would be related to a higher level of cortical involvement of disease. Since neuroimaging investigations have yet to assess the impact of amyloidosis on the distribution of cortical disease in ePD, we conducted a large-scale imaging analysis of the PPMI cohort. These multimodal evaluations may provide potential insights into the interactions of α-synuclein and Aβ pathology in ePD, as well as important screening tools for clinical trials involving disease-modifying agents.
METHODS
Participants
Data used in the preparation of this article were obtained from the PPMI database (http://www.ppmi-info.org/data). For up-to-date information on the study, visit http://www.ppmi-info.org. All data were treated in compliance with the PPMI Data Use Agreement.
We evaluated 369 drug-naïve patients with ePD and 174 controls from the PPMI database. Inclusion criteria for ePD in PPMI include a 2-year or less diagnosis of PD, at least two Parkinsonism signs/symptoms (resting tremor, bradykinesia and/or rigidity), a baseline Hoehn and Yahr Stage of I or II, and unanticipated need for medication within 6 months of baseline and a minimum age of 30 years. Inclusion criteria for controls include a minimum age of 30 years, a Montreal Cognitive Assessment (MOCA) score of >26, a negative history of neurological impairment and a negative first-degree family history. For the current study, additional inclusion criteria were baseline CSF, APOE ε4 genotyping, baseline neuropsychological testing and functional assessment.
CSF analysis
To analyse the presence of evidence for cerebral amyloidosis, we evaluated CSF Aβ1-42. Using a linear transformation procedure of Aβ1-42 CSF (see online supplementary text and figure S1), we defined a cut-off of 198 pg/mL to subgroup participants as having cerebral amyloid (ePD+) or not (ePD−). This value is relatively consistent with the most commonly reported cut-off of 192 pg/mL,15 and in repeated analysis using a 192 pg/mL cut-off we observed convergent findings: all reported statistical tests remained significant (p<0.05). After subgrouping participants based on low AB and high AB, we then performed group comparisons of total-tau (t-tau), phosphorylated-tau (p-tau), and α-synuclein.
Genetic analysis
APOE ε4 status was available for all ePD and controls. χ2 Analyses tested group differences using a dominant model (presence of at least one APOE ε4 allele) and an additive model (0, 1 or 2 copies of an APOE ε4 allele).
Neuropsychological analysis
We evaluated baseline neuropsychological performance for a range of cognitive domains. These include general cognitive function on the MOCA; verbal delayed and recognition memory was assessed using the Hopkins Verbal Learning Test (HVLT),16 and visuospatial function was assessed using the Benton Judgment of Line Orientation (JOLO);17 semantic language function was evaluated using the Verbal Category Fluency task (we report the mean across categories); executive/attention function was evaluated with Letter-Number Sequencing, which requires participants to repeat a letter-number sequence, that is, read out loud to them (eg, ‘T-9-A-3’) in numerical order and then alphabetical order (eg, ‘3-9-A-T’); and Symbol–Digit Matching, which involves using a key to match as many digits to geometric figures as possible in 90 s.
Functional measures
We evaluated motor function using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale III (UPDRS-III)18 and functional performance using the Modified Schwab and England Activities of Daily Living Scale.
Statistical analysis
Kolmogorov-Smirnov tests revealed that the majority of measures are not normally distributed, and therefore we performed non-parametric statistical tests. Kruskal-Wallis tests were used to evaluate an overall group difference (ePD+, ePD−, Control+, Control−) for each measure and, if significant, we performed protected post hoc Mann-Whitney tests. Since our analyses revealed an age and APOE difference between ePD+ and ePD− groups, we also repeated analyses for each modality using two types of regression models: one with a single covariate for age and another with covariates for age and APOE ε4 status. Together, we conservatively report non-parametric and parametric analyses of the data and, importantly, observe convergent findings from each analysis.
Image analysis
Research quality T1-weighted MRI scans were available for a subset of 40 ePD+, 226 ePD− and 116 control participants. MRI exclusion criteria included in-plane resolution greater than 1.5 mm, poor-quality MRI at visual inspection (eg, distortion, excessive motion), no MRI available at baseline or processing failure due to image distortion/artefact.
All image preprocessing was performed using Advanced Normalisation Tools (ANTs) which provide a state-of-the-art19,20 pipeline as previously reported.20 Briefly, all MRI scans were resampled to 1 mm isotropic resolution for uniformity and we used N4 bias correction to minimise image inhomogeneity effects.21 We then performed brain extraction using a combination of template-based and segmentation strategies involving registration of a dilated template brain that can then be used to guide brain segmentation from each individual MRI scan. Atropos six-tissue class segmentation (cortex, deep grey, brainstem, cerebellum, white matter and CSF/other) was performed using an optimised combination of prior knowledge from N4 bias correction and template-based priors.22 Voxelwise grey matter (GM) density measures were calculated as the weighted probability of a voxel belonging to a given tissue class. Consistent with recommendations from an independent evaluation,23 we report unmodulated GM density analyses that have been demonstrated to have improved sensitivity and accuracy relative to modulated procedures using similar optimised preprocessing routines. To optimise our analyses, we first use a diffeomorphic and symmetric registration algorithm in ANTs to warp each GM density map to a representative custom template comprising 115 controls and 93 patients with neurodegenerative disease (PD, AD, amyotrophic lateral sclerosis and fronto-temporal degeneration) from the University of Pennsylvania Neurology Clinics who are demographically comparable to the imaging series in this study. We then apply a diffeomorphic and symmetric registration warp to each image, from the custom template to the Montreal Neurological Institute (MNI) stereotactic space, in order to report results using an internationally accepted stereotactic system.
Voxelwise analyses of GM density were performed using the non-parametric randomise tool implemented in the FMRIB Software Library (FSL: http://fsl.fmrib.ox.ac.uk) with 10 000 permutations. We report two sets of Student’s t-tests: (1) each patient group (ePD−, ePD+) relative to controls; (2) a direct comparison between ePD− and ePD+ and (3) an interaction analysis for amyloid status (positive, negative) by group (ePD, Control). Nuisance covariates were included for age and for study/scanner site in all imaging analyses to minimise variance-associated, age-related neurodegeneration and site–site image variation, respectively. We report clusters that survive family-wise error (FWE) correction of p<0.05 with threshold-free cluster enhancement (TFCE) and a minimum of 50 adjacent voxels.
Given the prevalence of hippocampus atrophy associated with cerebral amyloidosis in AD, we additionally performed a focused analysis of hippocampal volumes. For this analysis, we assessed ePD−, ePD+ and Control−, as above, and additionally evaluated 24 Control+ cases. Hippocampal volume was calculated by applying the inverse of the diffeomorphic and symmetric transformations from our whole brain registrations to a publicly available region of interest (ROI) label set24 from the MNI space that includes a hippocampus label and whole brain label. This warping was performed for each individual subject and then we computed the hippocampal and intracranial volume (ICV) in units of mm3. For statistical analyses, we generated the mean hippocampal volume (left hippocampus +right hippocampus/2) and then divided it by ICV to control for individual differences in brain size.
RESULTS
CSF results
Using an Aβ1-42 CSF cut-off, we observed a rate of 16.53% (N=61) patients with ePD+ and 83.47% (N=308) patients with ePD−. We also observed that 16.67% (N=29) of controls were below the Aβ1-42 CSF threshold (Control+).
After subgrouping ePD and Controls based on amyloid evidence, subsequent Kruskal-Wallis analyses revealed group-level differences for all CSF analytes, including Aβ1-42, t-tau, p-tau and α-synuclein (table 1). By definition, mean Aβ1-42 was reduced for Control+ relative to Control− and for ePD+ relative to ePD−. Control+ and Control− did not differ on any other CSF analytes. Direct patient comparisons revealed that ePD+ additionally had reduced t-tau (U=6317; median difference (MD)=13; p<0.0001), p-tau (U=6647; MD=3; p=0.0002) and α-synuclein relative to ePD− (U=5694; MD=528; p<0.0001). Lastly, ePD− had moderately reduced p-tau (U=19 184; MD=1; p<0.0569) and α-synuclein (U=18 825; MD=180; p<0.0072) relative to Control, suggesting that these markers are reduced in PD independent of amyloidosis status. Subsequent regression analyses between ePD+ and ePD−, controlling for age and APOE, were convergent with the group CSF observations above, suggesting that these findings are not confounded by age or genetic status (table 2).
Table 1.
Median (IQR) demographic, genetic, cerebrospinal fluid, neuropsychological, and functional measurements for CTRL and patients with ePD with (+) or without (−) cerebrospinal fluid Aβ1-42 evidence of cerebral amyloid
| Measure | CTRL− | CTRL+ | ePD− | ePD+ | Kruskal-Wallis | α-Synuclein
|
Amyloid CTRL− vs CTRL+ |
α-Synuclein+amyloid
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| ePD− vs CTRL− | ePD+ vs CTRL+ | ePD− vs ePD+ | ePD+ vs CTRL− | |||||||
| Demographics | ||||||||||
| Number | 145 | 29 | 309 | 61 | – | |||||
| Gender | 50 F/95 M | 10 F/19 M | 109 F/200 M | 16 F/45 M | χ2=1.86 (p=0.6011) | |||||
| Age | 60 (55–68) | 66 (58–70) | 61 (53–67) | 67 (62–71) | χ2=20.88 (p=0.0001) | *** | *** | |||
| Cerebrospinal fluid | ||||||||||
| Transformed Aβ1-42 | 249 (227–270) | 178 (154–189) | 243 (225–266) | 185 (165–190) | χ2=225.66 (p<0.0001) | *** | *** | *** | ||
| Total-tau | 44 (36–57) | 67 (29–76) | 42 (34–53) | 29 (23–54) | χ2=25.08 (p<0.0001) | * | *** | *** | ||
| Phosphorylated-tau | 14 (11–21) | 15 (10–25) | 13 (10–20) | 10 (8–15) | χ2=24.13 (p<0.0001) | * | * | *** | *** | |
| α-Synuclein | 1982 (1534–2690) | 1970 (1063–2580) | 1802 (1392–2371) | 1274 (958–1921) | χ2=36.30 (p<0.0001) | * | * | *** | *** | |
| Genetics | ||||||||||
| APOE ε4 carrier (±) | 116/29 | 12/17 | 235/74 | 36/25 | χ2=32.21 (p<0.0001) | *** | * | |||
| APOE ε4 alleles (0/1/2) | 116/28/1 | 12/14/3 | 235/69/5 | 36/22/3 | χ2=26.35 (p<0.0001) | *** | * | |||
| Neuropsychological | ||||||||||
| MOCA | 28 (27–29) | 28 (27–29) | 28 (26–29) | 27 (26–29) | χ2=24.17 (p<0.0001) | * | * | *** | ||
| HVLT Trial 1 | 7 (6–8) | 6 (5–7) | 6 (5–8) | 6 (5–7) | χ2=16.29 (p=0.0010) | * | * | *** | ||
| HVLT Delayed Recall | 10 (8–11) | 9 (7–10) | 9 (7–10) | 8 (6–10) | χ2=23.98 (p<0.0001) | * | * | * | *** | |
| HVLT Recognition | 12 (11–12) | 12 (11–12) | 12 (11–12) | 11 (11–12) | χ2=12.49 (p=0.0059) | * | * | *** | ||
| JOLO | 14 (12–15) | 14 (11–15) | 3 (12–15) | 13 (10–14) | χ2=5.51 (p=0.1379) | |||||
| Letter-Number Sequencing | 11 (9–12) | 10 (9–12) | 11 (9–12) | 10 (9–12) | χ2=3.64 (p=0.3029) | |||||
| Verbal Category Fluency | 17 (14–20) | 16 (15.00–20) | 16 (14–19) | 15 (12–18) | χ2=15.67 (p=0.0013) | * | *** | *** | ||
| Symbol–Digit Matching | 47 (40–53) | 43 (37–50) | 43 (36–49) | 38 (31–45) | χ2=41.58 (p<0.0001) | * | * | *** | *** | |
| Functional measures† | ||||||||||
| Modified Schwab and England | – | – | 90 (90–100) | 90 (90–95) | U=7923 (p=0.0360) | * | ||||
| UPDRS III | – | – | 20 (14–26) | 21 (17–29) | U=7928 (p=0.046) | * | ||||
| Imaging | ||||||||||
| Hippocampus % (cc/ICV cc) | 0.19 (0.18–0.20) | 0.19 (0.19–0.21) | 0.19 (0.18–0.20) | 0.19 (0.18–0.20) | χ2=3.727 (p=0.2930) | |||||
p<0.05;
p<0.005.
Group-level comparison only between ePD+ and ePD−.
Aβ, amyloid-β; cc, cubic centimeters; CTRL, controls; ePD, early Parkinson’s disease; F, female; HVLT, Hopkins Verbal Learning Test; ICV, intracranial volume; JOLO, Judgment of Line Orientation; M, male; MOCA, Montreal Cognitive Assessment; UPDRS-III, Unified Parkinson’s Disease Rating Scale III.
Table 2.
Regression results controlling for age and for age+APOE including cerebrospinal fluid, neuropsychological, functional and imaging measurements for patients with early PD with cerebrospinal fluid Aβ1-42 evidence of cerebral amyloid (PD+) or evidence of negative cerebral amyloid (PD−)
| Measure | Median difference (PD+ <PD−) | p Value Adjusted for age |
p Value Adjusted for age and APOE |
|---|---|---|---|
| Cerebrospinal fluid | |||
| Total-tau | 13 | <0.001 | <0.001 |
| Phosphorylated-tau | 3 | 0.009 | 0.010 |
| α-Synuclein | 528 | <0.001 | <0.001 |
| Neuropsychological | |||
| MOCA | 1 | 0.800 | 0.721 |
| HVLT Trial 1 | 0 | 0.210 | 0.372 |
| HVLT Delayed Recall | 1 | 0.173 | 0.224 |
| HVLT Recognition | 1 | 0.896 | 0.722 |
| JOLO | 0 | 0.184 | 0.280 |
| Letter-Number Sequencing | 1 | 0.642 | 0.520 |
| Verbal Category Fluency | 1 | 0.039 | 0.049 |
| Symbol–Digit Matching | 5 | 0.004 | 0.009 |
| Motor | |||
| Modified Schwab and England | 0 | 0.028 | 0.053 |
| UPDRS III | 1 | 0.215 | 0.205 |
| Imaging | |||
| Hippocampus volume/ICV | 0 | 0.901 | 0.766 |
Aβ, amyloid-β; HVLT, Hopkins Verbal Learning Test; ICV, intracranial volume; JOLO, Judgment of Line Orientation; MOCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; UPDRS-III, Unified Parkinson’s Disease Rating Scale III.
Demographic results
We observed significant differences for age between Control+ and Control− (U=1599; MD=6; p<0.0465) and between ePD+ and ePD− (U=6294; MD=6; p<0.0001), suggesting that cerebral amyloidosis is more frequently present in older adults as commonly reported. No other group analyses of age or gender were significant.
Genetic results
We observed significant associations of APOE ε4 status with cerebral amyloidosis in ePD and Controls (table 1). Specifically, ePD+ ε4 carriers (40.9%) are more frequent than ePD− ε4 carriers (23.9%) in a dominant model (χ2=8.29; p=0.0159). We observed a similar effect for APOE ε4 frequency in an additive model (χ2=7.30; p=0.0069). Likewise, ε4 carrier frequency for Control+ (58.6%) is greater than Control− (20.0%) both in a dominant model (χ2=19.25; p=0.0001) and in an additive model (χ2=23.61; p<0.0001). Control+ and ePD+ did not differ in association using dominant (χ2=2.46; p=0.1170) and additive (χ2=2.75; p<0.2532) models, suggesting that there is a relationship between APOE and amyloid status, but that this is independent of a PD diagnosis.
Neuropsychological results
Kruskal-Wallis tests revealed significant group-level differences on several neuropsychological tests, including MOCA, HVLT Trial 1, HVLT Delayed Recall, HVLT Recognition, Verbal Category Fluency and Symbol–Digit Matching. We did not observe differences on JOLO or Letter-Number Sequencing (table 1).
To evaluate the influence of amyloidosis in PD, we performed direct post hoc comparisons and observed reduced performance for ePD+ relative to ePD− on HVLT Trial 1 (U=7817; MD=0; p=0.0296), HVLT Delayed Recall (U=7845; MD=1; p=0.0340), Verbal Category Fluency (U=7143; MD=1; p=0.0025) and Symbol–Digit Matching (U=6607; MD=5; p=0.0002). Direct comparisons between Control+ and Control−, however, did not reveal any significant neuropsychological differences between groups. We additionally observed that ePD− had reduced performance on MOCA (U=17 164; MD=0; p=0.0001), HVLT Trial 1 (U=18 812; MD=1; p=0.0062), HVLT Delayed Recall (U=17 333; MD=1; p=0.0001) and Symbol–Digit Matching (U=16 335; MD=4; p<0.0001) relative to Control−, suggesting that difficulty on these tasks is in part related to PD independent of amyloid status. Likewise, ePD+ also differed from Control− on MOCA (U=2944; MD=1; p=0.0001), HVLT Trial 1 (U=2897; MD=1; p=0.0001), HVLT Delayed Recall (U=2770; MD=2; p<0.0001) and Symbol–Digit Matching (U=2174; MD=9; p<0.0001). ePD+ additionally displayed reduced performance on Verbal Category Fluency relative to Control− (U=2946; MD=2; p=0.0002).
Analyses including age and age with APOE ε4 status largely revealed converging results (see table 2). The only exception is that the observation of reduced HVLT Trial 1 and HVLT Delayed Recall for ePD+ relative to ePD− is no longer significant when controlling for age.
Functional measure results
Functional measures were only available for patients with ePD and are summarised in table 1. We observed that patients with ePD+ have reduced performance in motor (U=7928, MD=1; p=0.0360) and activity of daily living (U=7923; MD=0; p=0.0460) measurements relative to ePD−, as measured with the UPDRS-III and Modified Schwab and England Assessment. Our analyses that include covariates for age reveal similar findings for Modified Schwab and England Assessment where ePD+ exhibit more difficulty relative to ePD−; however, UPDRS-III differences between ePD groups are diminished when controlling for age. This could be associated with age-related parkinsonism and motor decline that have previously been reported in a large-scale population-based Religious Orders Study of healthy ageing.25 Notably, both ePD groups were still within the ‘completely independent’ range that may include some slowness and early awareness of difficulties.
Imaging results
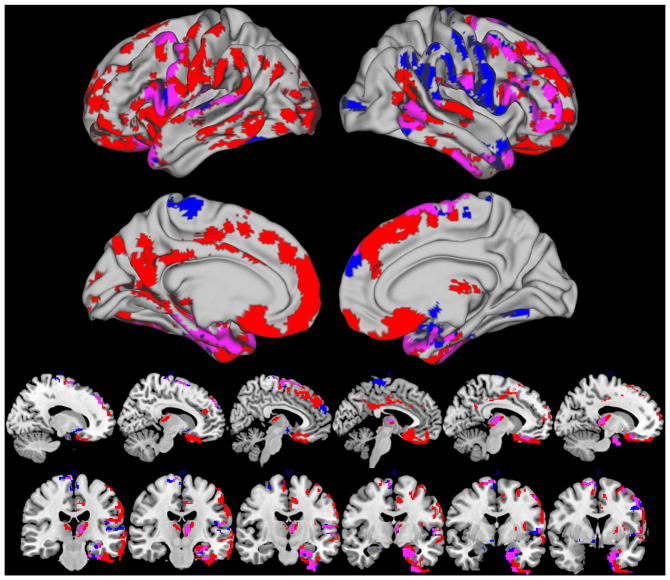
Whole brain analysis of GM density revealed extensive bilateral atrophy in ePD+ relative to Control− including the medial and lateral frontal, temporal and parietal cortex. We also observed GM reductions in deeper subcortical structures that included the thalamus and putamen (table 3 and figure 1/red). A comparison of ePD− relative to Control− also revealed medial and lateral temporal cortex atrophy along with the thalamus and putamen with moderate right lateral frontal cortex disease and relative sparing of the medial frontal and parietal regions (table 3 and figure 1/blue). Overlapping regions of atrophy for ePD+ and ePD− relative Control− were observed in the bilateral precentral gyrus, superior temporal gyrus and medial temporal lobes including the perirhinal cortex (table 3 and figure 1/magenta). Whole brain analysis for Control− relative to ePD+ and relative to ePD− revealed no significantly reduced GM regions.
Table 3.
Peak loci of reduced grey matter density for patients with ePD who are amyloid negative (ePD−) and amyloid positive (ePD+) relative to demographically comparable healthy controls who are amyloid negative (Control−) and direct comparisons relative to each other
| Contrast | Cluster size | p Value of cluster maxima (FWE) | MNI coordinates
|
L/R/M | Neuroanatomical region (Brodmann area) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| ePD− <Control− | 2135 | 0.003 | 52 | 6 | 32 | R | Precentral gyrus (6) |
| 1686 | 0.001 | 26 | −2 | −50 | R | Perirhinal cortex/temporal pole (38) | |
| 1405 | 0.003 | −18 | −6 | −24 | L | Amygdala | |
| 612 | 0.006 | −6 | −38 | −52 | M | Brainstem | |
| 605 | 0.005 | −46 | −20 | 14 | L | Angular gyrus (40/41) | |
| 409 | 0.002 | 62 | −56 | −10 | R | Fusiform gyrus (37) | |
| 333 | 0.006 | 12 | 0 | 68 | R | Precentral gyrus (6) | |
| 292 | 0.005 | 24 | 14 | −2 | R | Putamen | |
| 287 | 0.005 | −58 | −28 | 8 | L | Heschl’s gyrus (42) | |
| 202 | 0.011 | −6 | −20 | 8 | L | Thalamus | |
| 168 | 0.012 | 18 | 48 | 42 | R | Superior frontal gyrus (8) | |
| 134 | 0.007 | −14 | 22 | −16 | L | Putamen | |
| 103 | 0.004 | −38 | 10 | 60 | L | Precentral gyrus (6) | |
| 75 | 0.028 | −46 | −52 | −24 | L | Fusiform gyrus (36) | |
| 66 | 0.017 | 30 | −54 | −10 | R | Fusiform gyrus (37/19) | |
| 62 | 0.015 | −4 | −34 | 74 | L | Postcentral gyrus (4) | |
| 57 | 0.019 | 26 | −90 | 6 | R | Cuneus (17) | |
| ePD+ <Control− | 10 851 | 0.001 | −28 | −10 | −46 | L | Perirhinal cortex/temporal pole (38) |
| 2103 | 0.001 | 48 | 46 | −8 | R | Middle frontal gyrus (44/46) | |
| 1796 | 0.001 | 30 | 16 | −46 | R | Perirhinal cortex/temporal pole (38) | |
| 805 | 0.001 | 68 | −46 | 4 | R | Superior temporal gyrus (22) | |
| 657 | 0.002 | −34 | −74 | 20 | L | Angular gyrus (39/19) | |
| 331 | 0.001 | −6 | −18 | 2 | L | Thalamus | |
| 296 | 0.003 | −4 | −12 | 34 | L | Cingulate gyrus (24/23) | |
| 190 | 0.013 | −24 | 22 | 62 | L | Precentral gyrus (6) | |
| 101 | 0.011 | −56 | −46 | 38 | L | Angular gyrus (40) | |
| 99 | 0.006 | −18 | 8 | −6 | L | Putamen | |
| 93 | 0.024 | 10 | −22 | 14 | R | Thalamus | |
| 92 | 0.012 | 36 | 8 | 48 | R | Precentral gyrus (6) | |
| 85 | 0.025 | 54 | −46 | −20 | R | Fusiform gyrus (37) | |
| 79 | 0.023 | 50 | −64 | 14 | R | Angular gyrus (39/19) | |
| 75 | 0.019 | 44 | −32 | 22 | R | Intraparietal sulcus (40) | |
| 60 | 0.026 | 20 | −54 | 4 | R | Lingual gyrus (19) | |
| 52 | 0.016 | 64 | −12 | 26 | R | Postcentral gyrus (4) | |
| 50 | 0.021 | −48 | −26 | 62 | L | Postcentral gyrus (1/2) | |
| 50 | 0.011 | −16 | −76 | 38 | L | Cuneus (19) | |
| ePD+ <ePD− | 3518 | 0.001 | 4 | 30 | −30 | R | Rectus gyrus (11) |
| 547 | 0.016 | −38 | −12 | −30 | L | Middle temporal gyrus (21) | |
| 331 | 0.003 | 20 | 58 | 16 | R | Anterior cingulate cortex (24) | |
| 325 | 0.007 | 46 | −50 | 14 | R | Middle temporal gyrus (39) | |
| 317 | 0.004 | −36 | 12 | 38 | L | Dorsolateral prefrontal cortex (9) | |
| 285 | 0.003 | −8 | −60 | 16 | L | Posterior cingulate gyrus (23) | |
| 265 | 0.008 | −46 | −60 | 10 | L | Middle temporal gyrus (21) | |
| 212 | 0.01 | −6 | −20 | 6 | L | Thalamus | |
| 187 | 0.011 | −26 | −8 | −36 | L | Parahippocampal gyrus (36/28) | |
| 139 | 0.012 | −24 | −10 | 52 | L | Precentral gyrus (4) | |
| 135 | 0.013 | 8 | −24 | 10 | R | Thalamus | |
| 132 | 0.001 | −14 | −28 | 38 | L | Cingulate gyrus (31) | |
| 102 | 0.018 | 16 | 60 | −4 | R | Superior frontal gyrus (10) | |
| 63 | 0.021 | 38 | 22 | 26 | R | Dorsolateral prefrontal cortex (9) | |
| 61 | 0.014 | 6 | 38 | 42 | R | Superior frontal gyrus (8) | |
| 60 | 0.025 | −56 | −30 | 30 | L | Angular gyrus (40) | |
| 60 | 0.023 | 64 | −24 | −26 | R | Fusiform gyrus (20/36) | |
| 60 | 0.032 | 28 | 0 | −46 | R | Perirhinal cortex/temporal pole (38) | |
| ePD− <ePD+ | 1120 | 0.001 | 14 | −14 | −14 | R | Substantia nigra |
| 620 | 0.001 | −6 | −42 | −68 | M | Brainstem | |
| 433 | 0.002 | −8 | −14 | −6 | L | Substantia nigra | |
| 65 | 0.015 | −4 | −58 | 64 | L | Precuneus (7) | |
All loci are reported in the MNI stereotactic space using FWE correction (p<0.05) and TFCE.
ePD, early Parkinson’s disease; FWE, family-wise error; L, left; M, medial; MNI, Montreal Neurological Institute; R, right; TFCE, threshold-free cluster enhancement.
Figure 1.
Regions of reduced grey matter for amyloid-negative early Parkinsons disease (ePD−; blue) and amyloid-positive ePD (ePD+; red) relative to amyloid-negative healthy controls (p<0.05 threshold-free cluster enhancement family-wise error-corrected). Magenta indicates regions of overlapping ePD− and ePD+ reduced GM relative to healthy controls.
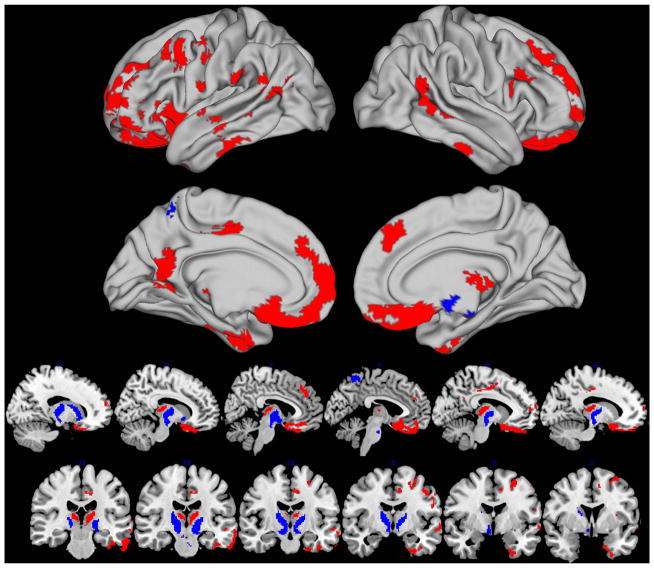
Whole brain direct comparisons of ePD+ relative to ePD− revealed much more extensive atrophy that included the medial and lateral frontal regions, thalamus and middle temporal cortex (table 3 and figure 2/red). In contrast, ePD− only had reduced GM in the bilateral substantia nigra, brainstem and superior medial parietal cortex relative to ePD+ (table 3 and figure 2/blue). Importantly, since these imaging analyses included a covariate for age, it is more likely that the observed GM regions are related to cerebral amyloid in ePD+ rather than age-related neurodegeneration.
Figure 2.
Regions of reduced grey matter for amyloid-negative early Parkinsons disease (ePD−; blue) and amyloid-positive ePD (ePD+; red) relative to each other in direct comparisons (p<0.05 threshold-free cluster enhancement family-wise error-corrected).
To evaluate whether regions associated with cerebral amyloid in ePD+ relative to ePD− survive correction for α-synuclein pathology, we performed an additional exploratory analysis. In this analysis, we evaluated ePD+ relative to ePD−, as above, while additionally covarying for CSF α-synuclein and haemoglobin levels, and observed a similar pattern of reduced GM in ventomedial prefrontal, dorsomedial prefrontal, and dorsolateral prefrontal cortex and regions in temporoparietal cortex (see online supplementary figure S2).
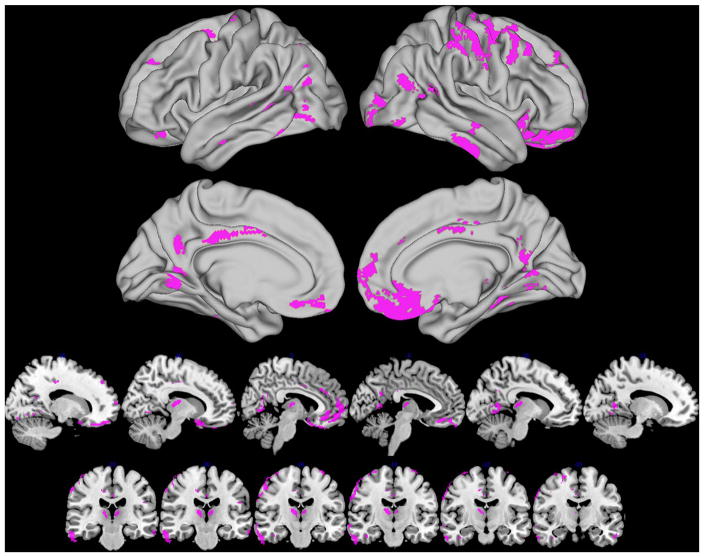
To further evaluate whether the regions of reduced GM observed in the ePD+ group are associated with a synergistic interaction between cerebral amyloidosis and α-synuclein pathology, we additionally performed an amyloid status (positive, negative) by group (ePD, healthy controls) interaction test. This analysis identified many GM regions that are similar to the direct ePD+ relative to ePD− comparisons, including the ventromedial and orbitofrontal cortex, dorsolateral prefrontal cortex, inferior temporal cortex, thalamus and angular gyrus (table 4 and figure 3).
Table 4.
Peak loci of reduced grey matter density for an interaction effect of amyloid status (positive, negative) by group (early Parkinson’s disease, healthy controls)
| Contrast | Cluster size | p Value of cluster maxima (FWE) | MNI coordinates
|
L/R/M | Neuroanatomical region (Brodmann area) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Amyloid×group interaction | 1899 | 0.003 | 61 | −21 | −26 | R | Fusiform gyrus (20) |
| 1487 | 0.005 | 57 | −22 | 48 | R | Postcentral gyrus (1/2) | |
| 1106 | 0.007 | −10 | −59 | 4 | L | Lingual gyrus (18) | |
| 787 | 0.012 | 47 | −62 | 16 | R | Angular gyrus (39) | |
| 776 | 0.018 | −45 | −67 | −7 | L | Fusiform gyrus (37/19) | |
| 713 | 0.005 | −26 | 43 | 37 | L | Dorsolateral prefrontal cortex (9) | |
| 675 | 0.019 | 10 | −18 | 10 | R | Thalamus | |
| 630 | 0.025 | 6 | −59 | 10 | R | Posterior cingulate gyrus (23) | |
| 448 | 0.021 | 31 | −89 | 3 | R | Lingual gyrus (18) | |
| 400 | 0.024 | 53 | −44 | 15 | R | Superior temporal gyrus (22) | |
| 393 | 0.026 | −8 | −22 | 9 | L | Thalamus | |
| 364 | 0.038 | 32 | −38 | −15 | R | Fusiform gyrus (20/36) | |
| 346 | 0.019 | −56 | −40 | 3 | L | Middle temporal gyrus (22) | |
| 264 | 0.029 | −45 | −71 | 45 | L | Intraparietal sulcus (7/40) | |
| 235 | 0.036 | −4 | 36 | −15 | L | Rectus gyrus (11) | |
| 212 | 0.025 | −2 | −15 | 36 | M | Anterior cingulate cortex (24) | |
| 209 | 0.032 | 20 | −55 | 9 | R | Posterior cingulate gyrus (23) | |
| 209 | 0.023 | −6 | −57 | 27 | L | Posterior cingulate gyrus (31) | |
| 182 | 0.040 | −64 | −23 | −20 | L | Fusiform gyrus (20) | |
| 182 | 0.027 | −46 | −59 | 12 | L | Angular gyrus (39/19) | |
| 177 | 0.027 | −50 | −30 | −22 | L | Fusiform gyrus (20/36) | |
| 177 | 0.027 | 46 | −73 | −10 | R | Fusiform gyrus (37/19) | |
| 163 | 0.034 | −3 | 50 | −21 | L | Rectus gyrus (11) | |
| 162 | 0.007 | −22 | 1 | 56 | L | Supplementary motor cortex (6) | |
| 160 | 0.030 | −20 | 37 | −17 | L | Rectus gyrus (11) | |
| 127 | 0.038 | −54 | −54 | −18 | L | Fusiform gyrus (37) | |
| 123 | 0.034 | 29 | 55 | 1 | R | Superior frontal gyrus (10) | |
| 115 | 0.035 | −45 | −74 | 22 | L | Angular gyrus (39) | |
| 109 | 0.007 | 19 | −83 | −12 | R | Lingual gyrus (18) | |
| 107 | 0.039 | −57 | −10 | −26 | L | Fusiform gyrus (20) | |
| 93 | 0.040 | 6 | 36 | 27 | R | Dorsomedial prefrontal cortex (9) | |
| 87 | 0.034 | 40 | −13 | −28 | R | Fusiform gyrus (20) | |
| 67 | 0.036 | −50 | −22 | 25 | L | Inferior parietal (40) | |
| 65 | 0.029 | 19 | −47 | −3 | R | Hippocampal gyrus (30) | |
| 60 | 0.034 | 4 | −5 | 37 | M | Anterior cingulate cortex (24) | |
| 59 | 0.036 | 9 | −13 | 44 | R | Precentral gyrus (4/6) | |
| 56 | 0.022 | 14 | −23 | 42 | R | Precuneus (7) | |
| 55 | 0.031 | −20 | −76 | −10 | L | Lingual gyrus (18) | |
| 55 | 0.029 | 17 | −70 | −7 | R | Lingual gyrus (18) | |
| 55 | 0.040 | 52 | −64 | −5 | R | Fusiform gyrus (37/19) | |
| 50 | 0.041 | −44 | −72 | 4 | L | Middle occipital gyrus (19) | |
All loci are reported in the MNI stereotactic space using FWE correction (p<0.05) and TFCE.
FWE, family-wise error; L, left; M, medial; MNI, Montreal Neurological Institute; R, right; TFCE, threshold-free cluster enhancement.
Figure 3.
Regions of reduced grey matter associated with an interaction between amyloid status (positive and negative) by group (early Parkinsons disease and healthy controls) (p<0.05 threshold-free cluster enhancement family-wise error-corrected).
Since the hippocampus is such a characteristic region of atrophy in AD, we additionally performed a targeted ROI analysis in the hippocampus that we hypothesised may be reduced in ePD+ relative to ePD−. This initial analysis revealed no group differences in hippocampus volume (table 1), and despite covarying for age and age with APOE ε4 status, we still did observe a significantly reduced hippocampus volume for ePD+ relative to ePD− (see table 2).
DISCUSSION
In this study, we performed a multimodal assessment of the influence of amyloid on drug-naïve patient with ePD and were able to identify several phenotypic features that were PD specific or modulated by amyloid. This study additionally provides the largest neuroimaging assessment of the publicly available PPMI cohort until now.
Importantly, we found a similar proportion of individuals with evidence of cerebral amyloidosis in the control and ePD cohorts, with age and APOE status appearing to be the primary factors associated with a higher risk of amyloidosis. These findings are consistent with prior studies of cognitively normal adults26 and suggest that ePD has a similar risk for amyloidosis, as detected by CSF, as healthy controls. Further, there was no difference in the APOE carrier status between ePD− and Control−, consistent with findings from genome-wide association studies that have not found evidence supporting APOE as being a PD risk factor.27,28 Interestingly, some work has suggested that APOE ε4 carriers are at an increased risk of developed dementia in pure synucleinopathies,29 and it will be interesting to see if ePD− individuals who are carriers of an APOE ε4 allele have a more rapid cognitive decline relative to ePD− non-allele carriers.
While the current findings do not find a higher frequency of amyloidosis in ePD than controls, those with CSF evidence of cerebral amyloid had evidence for more severe disease in four (ie, biofluid, cognitive, motor and neuroimaging) modalities. This may suggest that amyloid pathology may be synergistic with the severity and distribution of α-synuclein pathology, as previously suggested in human autopsy and transgenic animal studies.4,10
First, we observed that both ePD groups had reduced α-synuclein CSF concentrations relative to the control group, which is consistent with lower CSF α-synuclein levels being sensitive to PD.11,30 Importantly, the ePD+ group had an average CSF α-synuclein level that was reduced by ~25% (1435 vs 1942 pg/mL; refer to table 1) relative to the ePD− group, and this pattern is suggestive of more severe α-synuclein pathology for those with cerebral amyloid. We also observed that ePD+ have lower CSF t-tau and p-tau levels than ePD−, which is less consistent with the typically reported elevation of these analytes in AD.15 However, given that CSF α-synuclein is highly correlated with CSF p-tau in PD11 and preliminary evidence suggests that CSF α-synuclein may increase over the natural history of PD,31 future investigation and longitudinal follow-up in the PPMI cohort is critical to further elucidate the CSF t-tau and p-tau biomarker profiles in ePD.
Second, low CSF amyloid was associated with more widespread cortical changes in ePD+ relative to ePD− that included disproportionate frontal cortex disease rather than a more typical AD pattern and these regions were present even after controlling for α-synuclein CSF levels. In particular, orbitofrontal and anterior cingulate regions displayed significantly reduced GM and are also early cortical regions associated with Lewy Body pathology based on Braak staging.32 Additionally, our amyloid status by group interaction analysis revealed convergent evidence for reduced GM in ventromedial, orbitofrontal and dorsolateral prefrontal cortical regions along with some inferior temporal cortices associated with an interactive, synergistic effect of amyloidosis and α-synuclein. A previous pathological series also found the presence of cerebral amyloid to be associated with greater limbic and cortical α-synuclein deposition.6,33 In contrast to cortical involvement in ePD+, we observed that ePD− had additional subcortical GM disease relative to ePD+, including reduced GM in the substantia nigra and brainstem. Additional studies are required to interpret this observation. One might argue that the maintenance of these differences when covaried for α-synuclein CSF levels would suggest that α-synuclein pathology is not the primary driver of the differential neuroanatomic effects associated with cerebral amyloid. Alternatively, one could speculate that ePD+ and ePD− differ with respect to the localisation of α-synuclein pathological burden in which ePD+ may have a more extensive distribution of disease compared with ePD− with a more focal but denser accumulation of α-synuclein pathology, though detailed neuropathological studies are required to empirically evaluate this speculation.
While the driver of the neurodegenerative changes observed on structural MRI is unclear, it is possible that the cerebral amyloid itself is driving this neurodegeneration and the anterior cingulate is also an area of significant amyloid plaque burden. That said, in AD, NFTs are most associated with neuronal and synaptic loss and the reduced CSF total and p-tau levels argue against a more AD-like neurodegenerative process in the ePD+ cases. Studies of transgenic mice suggest that there is cell-to-cell propagation of α-synuclein from the striatum to the frontal cortex,34 and from this perspective we can speculate that amyloidosis may accelerate this trajectory of pathological spread.
Third, the ePD+ group exhibited significantly reduced cognitive performance that was not observed in the ePD− or Control+ groups, suggesting that the combined presence of synucleinopathy and amyloid contributes to a more severe form of disease. This is consistent with neuropathological assessments of PD that suggest that concomitant cortical amyloid and α-synuclein deposition is correlated with dementia severity.8 One of the observed cognitive deficits included delayed recall, though our analyses controlling for age suggest that deficits in the episodic memory domain may in part be related to ageing, and deficits in memory are perhaps not surprising given that this is a hallmark cognitive deficit of age-related diseases such as AD. Notably, domains outside of episodic memory were also modulated by evidence of amyloidosis and were equally apparent when controlling for age and APOE status. In particular, measures of processing speed (ie, Symbol–Digit Matching) and Verbal Category Fluency were the most saliently affected cognitive domains. This more broad profile of poorer cognitive performance is consistent with the significantly greater cortical GM atrophy, particularly in the dorsolateral and medial frontal cortex. It is notable that structural differences in the medial temporal lobe were minimal between the ePD groups with no evidence of amyloid modulating hippocampal integrity, suggesting that cortical differences or perhaps direct effects of the amyloid on synaptic function resulted in the poorer delayed recall of the ePD+ group. Finally, while a fairly weak difference, there was also evidence of a somewhat reduced motor performance in ePD+ relative to ePD−.
While our CSF, imaging, cognitive and motor evidence is suggestive that cerebral amyloid in combination with a synucleinopathy yields a more severe ePD phenotype, there are several caveats to consider in our study. The finding of reduced performance on several measures for the ePD− cases supports the notion that amyloidosis alone cannot account for neuropsychological difficulties in ePD, and therefore a primary synucleinopathy also has cognitive consequences even in very early disease stages and in the absence of amyloid pathology. It is possible that some patients in the PPMI cohort clinically diagnosed with ePD may have an alternative aetiology other than α-synuclein pathology such as vascular or other neurodegenerative diseases,35 and long-term follow-up of these patients will be necessary to confirm whether pathological α-synuclein inclusions are contributing to their clinical PD syndrome. It is also possible that early cognitive difficulties in ePD may be related to dopaminergic depletion independent of observable prefrontal GM atrophy and that cognition may improve after these drug-naïve patients with ePD receive dopaminergic treatment.36 Another potential limitation to consider is that our categorisation of patients into ePD+ and ePD− was based on transformed, rather than raw, Aβ1-42 CSF values. We also used a cut-off value that was slightly higher than that in previous reports, 18837–192 pg/mL,15 in an effort to maximise sensitivity. This may have yielded some misclassifications; however, despite this noise, we observed patterns consistent with prior reports such as APOE ε4 frequency and age-related amyloid-positive status. While CSF is widely considered a signature biomarker of amyloid pathology,15 future studies using positron emission tomography (PET)-amyloid imaging may provide additional information about the regional deposition of amyloid pathology in the context of PD. One PET-amyloid study of Parkinson’s disease with mild cognitive impairment (PD-MCI) revealed a very similar detection rate of amyloid positivity (15%) compared with our detection rate (16.5%), suggesting that neither approach has an increased detection advantage over the other,38 and other work suggests high correspondence between PET-amyloid and CSF Aβ1-42 in PD.39 However, until now, the majority of PET-amyloid studies in PD have typically focused on later dementia stages of the disease.40 Also, while this study focused on associations between CSF Aβ1-42-defined cohorts to investigate the influence of amyloid in ePD, future studies that parallel the preliminary PPMI CSF investigation11 should also evaluate other potential CSF markers, such as t-tau and α-synuclein, that may also have associations with clinical characteristics of ePD. Another caveat to consider is that our current evidence is based on a cross-sectional evaluation and future longitudinal studies will be necessary to directly assess whether amyloid contributes to a more rapid disease course in PD. Finally, our interaction analysis of amyloidosis by group should be interpreted cautiously since there were relatively small numbers of Control+ cases in the PPMI cohort and structural imaging changes between Control+ adults relative to those without evidence of amyloidosis (Control−) tend to be quite subtle.
With these caveats in mind, this study provides novel evidence that cerebral amyloid contributes to a distinct phenotype of ePD in the largest published neuroimaging study of PPMI until now. We suggest that amyloid may synergistically interact with α-synuclein in the earliest stages of PD and the copresence of amyloid and α-synuclein should be considered in the development of clinical trials involving disease-modifying agents. We also demonstrate the feasibility of merging neuroimaging data from multicentre clinical scans in the PPMI cohort which hold promise for future investigations using large data sets of clinical MRI scans such as those obtained in clinical trials.
Supplementary Material
Acknowledgments
Funding This work was supported by BAND-14-338181, a joint funding effort through the Alzheimer’s Association, Michael J. Fox Foundation and Weston Brain Institute. Support was also provided by the National Institutes of Health (AG043503). Data acquisition was provided by PPMI—a public-private partnership—that is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squib, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier and UCB.
Footnotes
Competing interests None declared.
Ethics approval Parkinson’s Progression Markers Initiative (PPMI).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data used in the preparation of this article were obtained by CTM and DAW from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
References
- 1.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–7. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 2.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin DJ, Lee VMY, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14:626–36. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger KA. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm Gen Sect. 2004;111:1219–35. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- 6.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–98. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–61. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compta Y, Parkkinen L, O’Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinton LK, Blurton-Jones M, Myczek K, et al. Synergistic Interactions between Aβ, Tau, and α-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–9. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masliah E, Rockenstein E, Veinbergs I, et al. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:12245–50. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang J-H, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013;70:1277–87. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–35. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compta Y, Marti MJ, Ibarretxe-Bilbao N, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord. 2009;24:2203–10. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 14.Alves G, Bronnick K, Aarsland D, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatr. 2010;81:1080–6. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 15.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro AM, Benedict RH, Schretlen D, et al. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13:348–58. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 17.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol. 1978;35:364–7. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 18.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–7. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 19.Klein A, Ghosh SS, Avants B, et al. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage. 2010;51:214–20. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tustison NJ, Cook PA, Klein A, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–79. doi: 10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 21.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–20. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avants BB, Tustison NJ, Wu J, et al. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radua J, Canales-Rodríguez EJ, Pomarol-Clotet E, et al. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage. 2014;86:81–90. doi: 10.1016/j.neuroimage.2013.07.084. [DOI] [PubMed] [Google Scholar]
- 24.Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci. 2012;6:171. doi: 10.3389/fnins.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson RS, Schneider JA, Bienias JL, et al. Parkinsonian like signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003;60:539–44. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simón-Sánchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–12. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalls MA, Plagnol V, Hernandez DG, et al. International Parkinson Disease Genomics Consortium. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–9. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuang D, Leverenz JB, Lopez OL, et al. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70:223–8. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mollenhauer B, Trautmann E, Taylor P, et al. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett. 2013;532:44–8. doi: 10.1016/j.neulet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Stewart T, Sossi V, Aasly JO, et al. Phosphorylated α-synuclein in Parkinson’s disease: correlation depends on disease severity. Acta Neuropathol Commun. 2015;3:7. doi: 10.1186/s40478-015-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 33.Pletnikova O, West N, Lee MK, et al. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005;26:1183–92. doi: 10.1016/j.neurobiolaging.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Luk KC, Kehm VM, Zhang B, et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–12. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulisevsky J. Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson’s disease. Drugs Aging. 2000;16:365–79. doi: 10.2165/00002512-200016050-00006. [DOI] [PubMed] [Google Scholar]
- 37.De Meyer G, Shapiro F, Vanderstichele H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–56. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrou M, Bohnen NI, Müller MLTM, et al. Aβ-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology. 2012;79:1161–7. doi: 10.1212/WNL.0b013e3182698d4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buddhala C, Campbell MC, Perlmutter JS, et al. Correlation between decreased CSF α-synuclein and Aβ1–42 in Parkinson disease. Neurobiol Aging. 2015;36:476–84. doi: 10.1016/j.neurobiolaging.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrou M, Dwamena BA, Foerster BR, et al. Amyloid deposition in Parkinson’s disease and cognitive impairment: a systematic review. Mov Disord. 2015;30:928–35. doi: 10.1002/mds.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.