Corticobasal degeneration (CBD) is characterized by 4-repeat misfolded tau (4Rtau) including astrocytic plaques, threads and neuronal tangles[1]. 18F-AV-1451 is a PET radioligand that achieves in vivo binding in Alzheimer's disease (AD)[8] and autoradiographic evidence of binding to paired helical filaments (PHFs) composed of 3-repeat misfolded tau (3Rtau) and 4Rtau characteristic of AD histopathology[4, 5, 7]. However, autoradiographic studies of 18F-AV-1451 on CBD post-mortem tissue failed to demonstrate binding in cortical regions[5, 7] though there was minimal pathology in one study (<1.1% tau-load)[7]. Another study suggests minimal, but present, autoradiographic binding of 18F-AV-1451 for 4Rtau[4]. Given mixed autoradiographic evidence in CBD, there is a need for in vivo evaluations of 18F-AV-1451 in patients with pathological-confirmation.
We report a multimodal evaluation of a 58 year-old male with autopsy-confirmed CBD. He participated in in vivo baseline (15 months pre-death) clinical, 18F-AV-1451 PET, 18F-florbetapir PET, MRI, and DTI and longitudinal (5 months pre-death) 18F-AV-1451 PET research studies (Online Resource 1). When enrolled in research the patient met criteria for progressive supranuclear palsy (Online Resource 2).
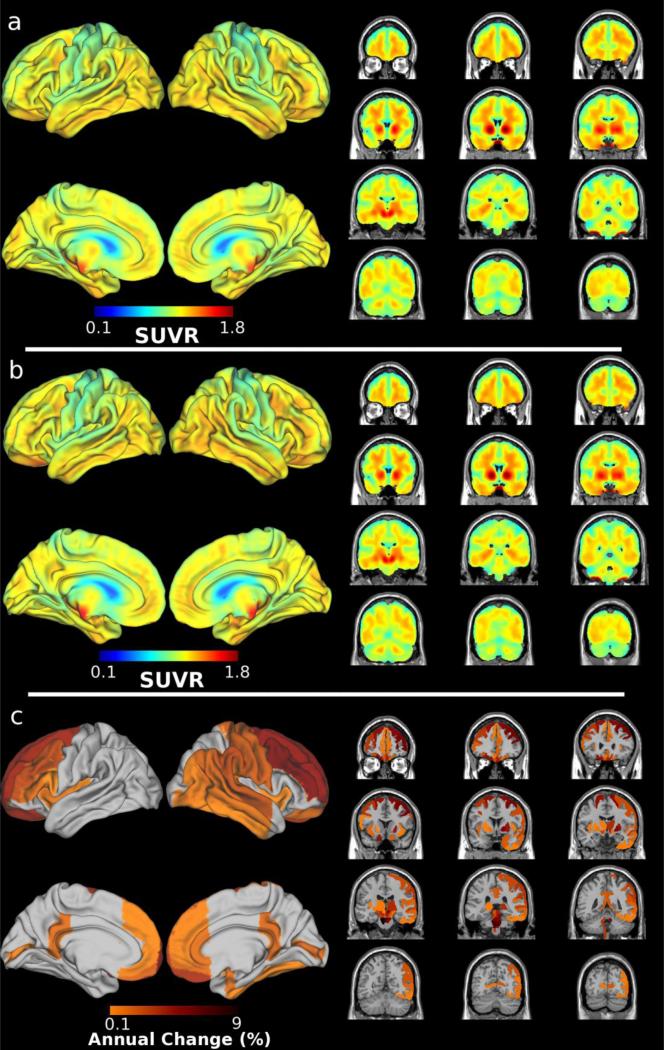
Baseline 18F-AV-1451 (Figure 1.a) revealed the highest retention in deep grey matter areas commonly associated with CBD pathology[1], including bilateral substantia nigra, globus pallidus, and midbrain. Follow-up 18F-AV-1451 revealed more visible retention in bilateral frontal and posterior temporal cortical regions along with midbrain, and pons (Figure 1.b). A direct assessment of annualized change revealed a 1-9% increase in 18F-AV-1451 retention, which was highest in the pons, medulla, and midbrain along with bilateral frontal and right temporo-parietal cortices (Figure 1.c).
Figure 1.
Regional distribution of 18F-AV-1451 at a) baseline (15 months pre-death); b) follow-up (5 months pre-death); and c) annualized change between baseline and follow-up defined as (SUVRfollowup – SUVRbaseline) / SUVRbaseline.
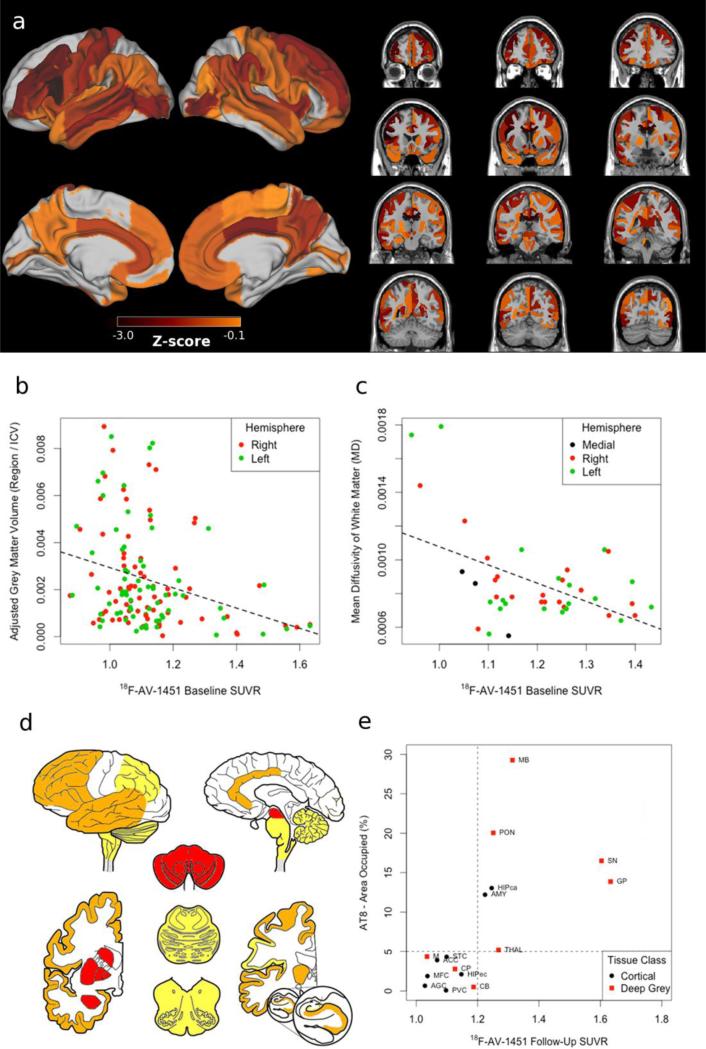
Baseline MRI (Figure 2.a) revealed predominantly reduced cortical grey matter in bilateral frontal cortex and right angular gyrus along with deep grey structures including the midbrain, putamen, right globus pallidus, right caudate, and left hippocampus. White matter revealed increased mean diffusivity (a measure of reduced white matter integrity) in corpus callosum, bilateral tapetum, pontine crossing fibers, and right lateralized corticospinal tract and posterior corona radiata. These observations are consistent with previously reported distributions of disease in CBD[6]. Spearman correlations revealed inverse associations between baseline 18F-AV-1451 and grey matter volume (rs=−0.209;p=0.016;Figure 2.b) and mean diffusivity (rs=−0.329;p=0.032;Figure 2.c). Furthermore, we observed that increased 18F-AV-1451 retention was related to reduced grey matter volume (rs=0.208;p=0.015) and lower mean diffusivity (rs=0.506;p<0.001) (Online Resource 3). Future longitudinal MRI/DTI studies are necessary to evaluate if these regions are becoming more atrophic over time due to tau accumulation.
Figure 2. MRI/DTI neuroimaging, Histopathology and 18F-AV-1451.
a) Z-scores of grey matter MRI and white matter DTI relative to 100 demographically-comparable healthy controls; correlations demonstrating increased baseline (15 months pre-death) 18F-AV-1451 retention associated with reduced b) grey matter volume and c) white matter integrity; d) heatmap reflecting ordinal ratings of PHF-1 tau burden rated as severe (3+; red) in midbrain (MB), pons (PON), substantia nigra (SN), globus pallidus (GP), putamen (CP), and amygdala (AMY); moderate (2+; orange) in thalamus (THAL), medulla (M), and CA1/subiculum (HIPca) along with anterior cingulate (ACC), middle frontal (MFC), entorhinal (HIPec), angular gyrus (AGC), superior temporal (STC) cortices; mild scattered tangles (1+; yellow) in cerebellum (CB); and no pathology in primary visual cortex (PVC); e) Correlation between AT-8 percent area occupied and follow-up (5 months pre-death) 18F-AV-1451 retention.
Neuropathological examination including immunohistochemistry (IHC) for phosphorylated tau and tau-isoform specific antibodies confirmed a diagnosis of CBD and Western blot analyses confirmed 4Rtau pathology (Figure 2.d; Online Resource 4). A Spearman correlation revealed that percent area occupied (%AO) of tau was related to follow-up 18F-AV-1451 SUVR (rs=0.768;p=0.001; Figure 2.e). Moreover, a 18F-AV-1451 SUVR≥1.2 (high retention) captured all 7 regions of tau pathology with high tau pathology (%AO≥5.0) and did not capture any regions with low tau pathology (%AO<5.0). Using this pathologically-defined cutoff (SUVR≥1.2) we also observed more deep grey matter and white matter atrophy in regions above the cutoff relative to those below the cutoff (Online Resource 5). While we did not observe a correlation between annualized 18F-AV-1451 change and %AO of tau (rs=−0.308;p=0.246), 6/7 regions with %AO≥5.0 of tau exhibited an increase in annualized18F-AV-1451 retention (0.7-7.2%) (Online Resource 3). Prior studies have suggested that 8F-AV-1451 may have “off-target” binding to pigmented cells[5]. In this patient, the vast majority of melanin-containing cells in the substantia nigra were tau-positive and therefore it was not possible to discriminate between “off-target” or “pathological” 18F-AV-1451 retention.
Potential differences in 18F-AV-1451 retention between AD and CBD may reflect different structures of the tau filaments, which are tubular or straight filaments in CBD and not PHFs as in AD[1]. An autoradiographic study of 18F-AV-1451 in AD observed more binding to neurofibrillary tangles than neuritic tau pathology[4]. We evaluated differential affinity for the tangle and thread pathological features of CBD[1, 2], and observed that 18F-AV-1451 uptake was more highly related to thread (rs=0.712;p=0.004), than tangle (rs=0.630;p=0.022) pathology. Future studies must assess the mechanisms for possible differential affinity of 18F-AV-1451 for various forms of tau pathology.
This multimodal assessment provides suggestive evidence that in vivo PET imaging with 18F-AV-1451 correlates with 4Rtau, including threads and plaques that are presumably glial in origin[1]. Additionally, 18F-AV-1451 retention is related to MRI and DTI at baseline and appears to increase over disease course in brain regions containing with 4Rtau pathological burden. These findings converge with other post-mortem evidence of 18F-AV-1451 retention in MAPT mutation carriers [9] and a patient with CBD[3]. However, single case data should be interpreted cautiously and future cohort studies, additional autoradiography studies, and direct comparisons between CBD and AD are necessary to validate 18F-AV-1451 imaging for 4Rtau in CBD.
Supplementary Material
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH; AG043503; AG017586; AG10124) and the Dana Foundation. PET imaging data acquisition was supported by AVID Radiopharmaceuticals (Philadelphia, PA), a wholly owned subsidiary of Eli Lilly and Company.
References
- 1.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs KA, Whitwell JL, Tacik P, Duffy JR, Senjem ML, Tosakulwong N, et al. [18F]AV- 1451 tau- PET uptake does correlate with quantitatively measured 4R- tau burden in autopsy- confirmed corticobasal degeneration. Acta Neuropathol. :1–3. doi: 10.1007/s00401-016-1618-1. in press. doi: 10.1007/s00401-016-1618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016:1–19. doi: 10.1186/s40478-016-0315-6. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV- 1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan CT, Boyd C, Gross RG, Weinstein J, Firn K, Toledo JB, et al. Multimodal imaging evidence of pathology-mediated disease distribution in corticobasal syndrome. Neurology. 2016;87:1227–1234. doi: 10.1212/WNL.0000000000003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sander K, Lashley T, Gami P, Gendron T, Lythgoe MF, Rohrer JD, et al. Characterization of tau positron emission tomography tracer [(18)F]AV-1451 binding to postmortem tissue in Alzheimer's disease, primary tauopathies, and other dementias. Alzheimers Dement. doi: 10.1016/j.jalz.2016.01.003. in press. doi: 10.1016/j.jalz.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Sepulcre J, Schultz AP, Sabuncu M, Gomez-Isla T, Chhatwal J, Becker A, et al. In vivo tau, amyloid, and gray matter profiles in the aging brain. J Neurosci. 2016;36:7364–7374. doi: 10.1523/JNEUROSCI.0639-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith R, Puschmann A, Schöll M, Ohlsson T, van Swieten J, Honer M, et al. 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain. 2016;139:2372–2379. doi: 10.1093/brain/aww163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.