Abstract
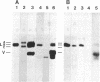
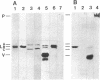
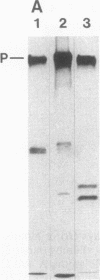
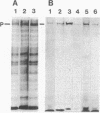
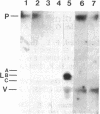
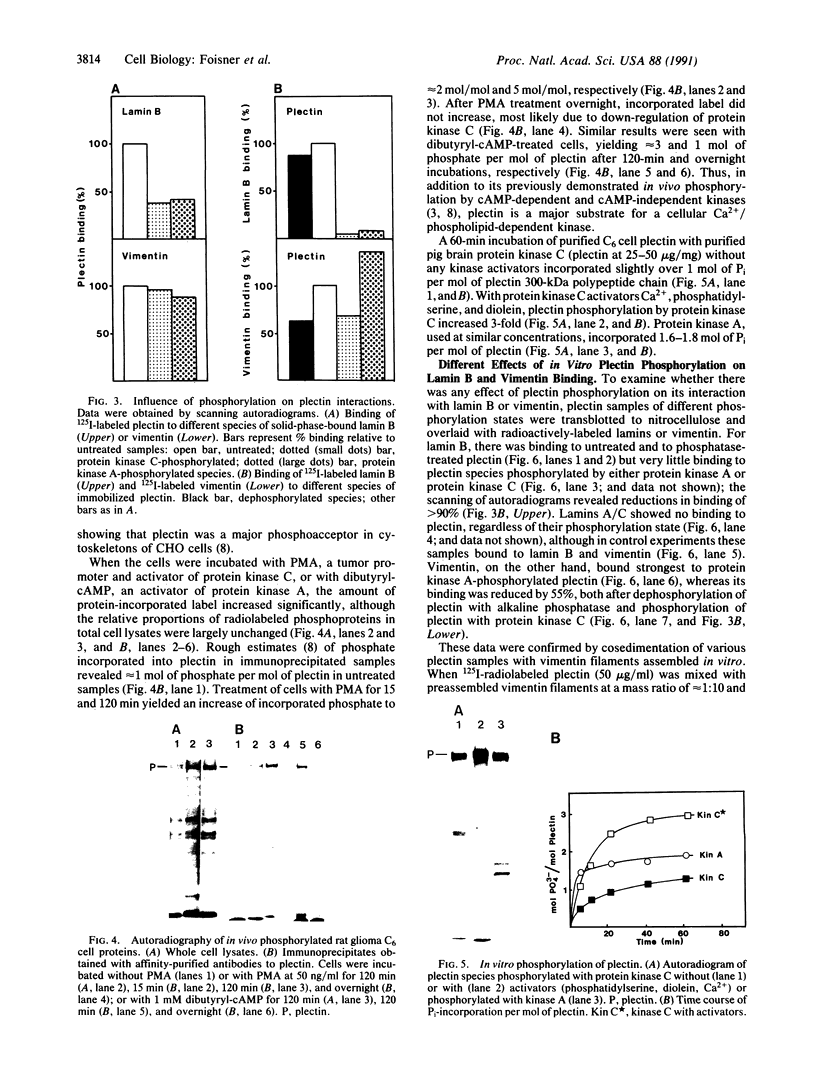
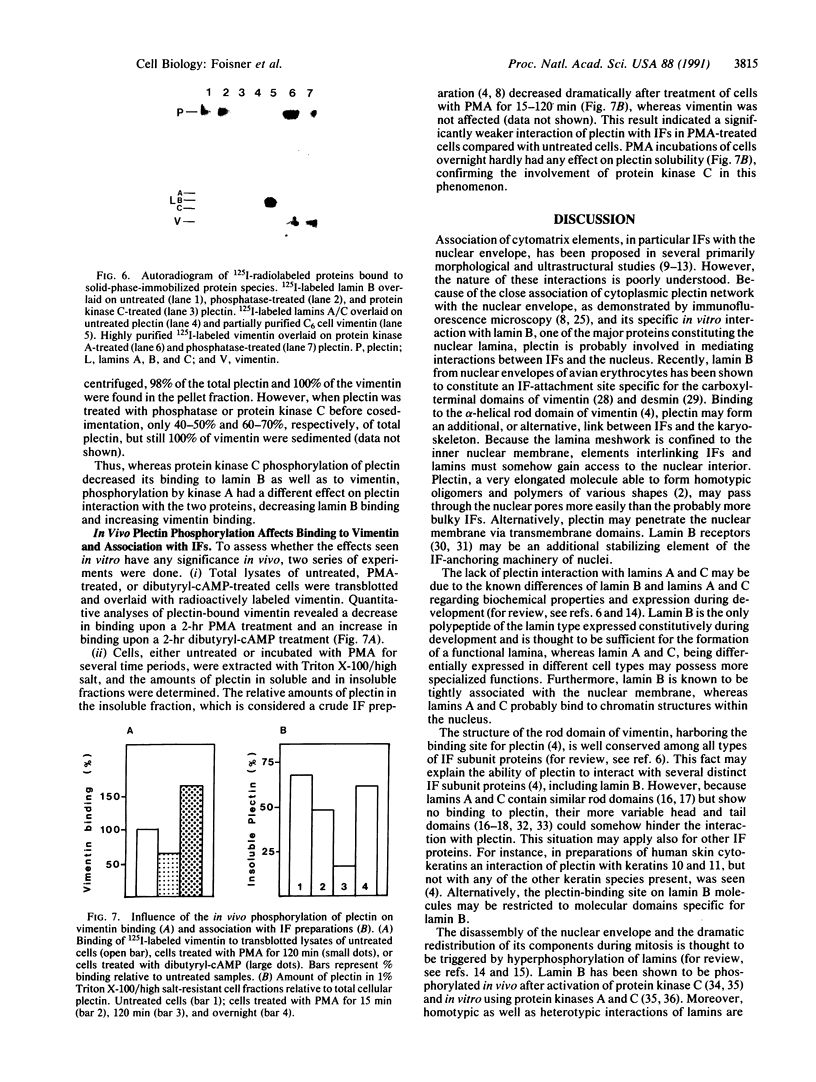
Solid-phase binding assays with protein species purified from cultured rat glioma C6 cells and Ehrlich ascites revealed that plectin bound specifically to lamin B but not to lamins A and C. Lamin B interaction was significantly decreased upon in vitro phosphorylation of either lamin B or plectin with protein kinase A or C. In contrast, phosphorylation of plectin with kinase A increased its binding to vimentin, suggesting a different regulation of plectin interactions by this kinase. 32P-radiolabeling of rat glioma C6 cells revealed plectin as a major in vivo target of protein kinase A and protein kinase C. Plectin, present in lysates of dibutyryladenosine 3',5'-cyclic monophosphate-treated cells, showed a 2.5 times higher binding affinity to vimentin than plectin from phorbol ester-treated cells. Furthermore, the relative amounts of plectin in 1% Triton X-100/high salt-insoluble cell fractions decreased to one-fourth of control values upon treating cells with phorbol esters, whereas vimentin was unaffected. This finding suggested a protein kinase C-dependent weakening of plectin interaction with intermediate filaments in vivo. Taken together, these results point to a role of plectin in interlinking cytoskeletal and nuclear elements and suggest that specific protein kinases are involved in regulating these interactions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Cidadão A. J., David-Ferreira J. F. Filamentous cross-bridges link intermediate filaments to the nuclear pore complexes. Eur J Cell Biol. 1988 Feb;45(2):282–290. [PubMed] [Google Scholar]
- Chou Y. H., Rosevear E., Goldman R. D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. Cyclic AMP-dependent protein kinase-induced vimentin filament disassembly involves modification of the N-terminal domain of intermediate filament subunits. FEBS Lett. 1988 Jul 4;234(1):73–78. doi: 10.1016/0014-5793(88)81306-1. [DOI] [PubMed] [Google Scholar]
- Evans R. M. Phosphorylation of vimentin in mitotically selected cells. In vitro cyclic AMP-independent kinase and calcium-stimulated phosphatase activities. J Cell Biol. 1989 Jan;108(1):67–78. doi: 10.1083/jcb.108.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The intermediate-filament proteins vimentin and desmin are phosphorylated in specific domains. Eur J Cell Biol. 1988 Apr;46(1):152–160. [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields A. P., Pettit G. R., May W. S. Phosphorylation of lamin B at the nuclear membrane by activated protein kinase C. J Biol Chem. 1988 Jun 15;263(17):8253–8260. [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Feldman B., Sander L., Wiche G. Monoclonal antibody mapping of structural and functional plectin epitopes. J Cell Biol. 1991 Feb;112(3):397–405. doi: 10.1083/jcb.112.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Leichtfried F. E., Herrmann H., Small J. V., Lawson D., Wiche G. Cytoskeleton-associated plectin: in situ localization, in vitro reconstitution, and binding to immobilized intermediate filament proteins. J Cell Biol. 1988 Mar;106(3):723–733. doi: 10.1083/jcb.106.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Wiche G. Structure and hydrodynamic properties of plectin molecules. J Mol Biol. 1987 Dec 5;198(3):515–531. doi: 10.1016/0022-2836(87)90297-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell. 1987 Jan 16;48(1):3–4. doi: 10.1016/0092-8674(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Furtner R., Wiche G. Binding specificities of purified porcine brain alpha- and beta-tubulin subunits and of microtubule-associated proteins 1 and 2 examined by electron microscopy and solid-phase binding assays. Eur J Cell Biol. 1987 Dec;45(1):1–8. [PubMed] [Google Scholar]
- Geisler N., Weber K. Phosphorylation of desmin in vitro inhibits formation of intermediate filaments; identification of three kinase A sites in the aminoterminal head domain. EMBO J. 1988 Jan;7(1):15–20. doi: 10.1002/j.1460-2075.1988.tb02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Lamin B constitutes an intermediate filament attachment site at the nuclear envelope. J Cell Biol. 1987 Jul;105(1):117–125. doi: 10.1083/jcb.105.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Stournaras C., Blobel G. Heterotypic and homotypic associations between the nuclear lamins: site-specificity and control by phosphorylation. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4325–4329. doi: 10.1073/pnas.85.12.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Weber K., Geisler N., Blobel G. Binding of two desmin derivatives to the plasma membrane and the nuclear envelope of avian erythrocytes: evidence for a conserved site-specificity in intermediate filament-membrane interactions. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6780–6784. doi: 10.1073/pnas.84.19.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H., Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem. 1987 Jan 25;262(3):1320–1325. [PubMed] [Google Scholar]
- Herrmann H., Wiche G. Specific in situ phosphorylation of plectin in detergent-resistant cytoskeletons from cultured Chinese hamster ovary cells. J Biol Chem. 1983 Dec 10;258(23):14610–14618. [PubMed] [Google Scholar]
- Hornbeck P., Huang K. P., Paul W. E. Lamin B is rapidly phosphorylated in lymphocytes after activation of protein kinase C. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2279–2283. doi: 10.1073/pnas.85.7.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höger T. H., Krohne G., Franke W. W. Amino acid sequence and molecular characterization of murine lamin B as deduced from cDNA clones. Eur J Cell Biol. 1988 Dec;47(2):283–290. [PubMed] [Google Scholar]
- Inagaki M., Nishi Y., Nishizawa K., Matsuyama M., Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987 Aug 13;328(6131):649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Goldman A. E., Yang H. Y., Goldman R. D. The organizational fate of intermediate filament networks in two epithelial cell types during mitosis. J Cell Biol. 1985 Jan;100(1):93–102. doi: 10.1083/jcb.100.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma Y., Swierenga S. H., Marceau N., French S. W. Connections of intermediate filaments with the nuclear lamina and the cell periphery. Biol Cell. 1987;59(3):193–203. doi: 10.1111/j.1768-322x.1987.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Krohne G., Wolin S. L., McKeon F. D., Franke W. W., Kirschner M. W. Nuclear lamin LI of Xenopus laevis: cDNA cloning, amino acid sequence and binding specificity of a member of the lamin B subfamily. EMBO J. 1987 Dec 1;6(12):3801–3808. doi: 10.1002/j.1460-2075.1987.tb02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Raymond Y., Gagnon G. Lamin B shares a number of distinct epitopes with lamins A and C and with intermediate filament proteins. Biochemistry. 1988 Apr 5;27(7):2590–2597. doi: 10.1021/bi00407a048. [DOI] [PubMed] [Google Scholar]
- Senior A., Gerace L. Integral membrane proteins specific to the inner nuclear membrane and associated with the nuclear lamina. J Cell Biol. 1988 Dec;107(6 Pt 1):2029–2036. doi: 10.1083/jcb.107.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stewart M. Intermediate filaments: structure, assembly and molecular interactions. Curr Opin Cell Biol. 1990 Feb;2(1):91–100. doi: 10.1016/s0955-0674(05)80037-7. [DOI] [PubMed] [Google Scholar]
- Traub P., Scherbarth A., Willingale-Theune J., Paulin-Levasseur M., Shoeman R. Differential sensitivity of vimentin and nuclear lamins from Ehrlich ascites tumor cells toward Ca2+ -activated neutral thiol proteinase. Eur J Cell Biol. 1988 Aug;46(3):478–490. [PubMed] [Google Scholar]
- Traub P., Scherbarth A., Willingale-Theune J., Traub U. Large scale co-isolation of vimentin and nuclear lamins from ehrlich ascites tumor cells cultured in vitro. Prep Biochem. 1988;18(4):381–404. doi: 10.1080/00327488808062539. [DOI] [PubMed] [Google Scholar]
- Vikstrom K. L., Borisy G. G., Goldman R. D. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):549–553. doi: 10.1073/pnas.86.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G. M., Bertics P. J., Hudson L. G., Vedvick T. S., Gill G. N. A three-step purification procedure for protein kinase C: characterization of the purified enzyme. Anal Biochem. 1987 Mar;161(2):425–437. doi: 10.1016/0003-2697(87)90471-4. [DOI] [PubMed] [Google Scholar]
- Weitzer G., Wiche G. Plectin from bovine lenses. Chemical properties, structural analysis and initial identification of interaction partners. Eur J Biochem. 1987 Nov 16;169(1):41–52. doi: 10.1111/j.1432-1033.1987.tb13578.x. [DOI] [PubMed] [Google Scholar]
- Wiche G., Baker M. A. Cytoplasmic network arrays demonstrated by immunolocalization using antibodies to a high molecular weight protein present in cytoskeletal preparations from cultured cells. Exp Cell Res. 1982 Mar;138(1):15–29. doi: 10.1016/0014-4827(82)90086-6. [DOI] [PubMed] [Google Scholar]
- Wiche G. Plectin: general overview and appraisal of its potential role as a subunit protein of the cytomatrix. Crit Rev Biochem Mol Biol. 1989;24(1):41–67. doi: 10.3109/10409238909082551. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Krohne G., Kirschner M. W. A new lamin in Xenopus somatic tissues displays strong homology to human lamin A. EMBO J. 1987 Dec 1;6(12):3809–3818. doi: 10.1002/j.1460-2075.1987.tb02717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H. J., Yuan J., Blobel G., Georgatos S. D. A lamin B receptor in the nuclear envelope. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]