Abstract
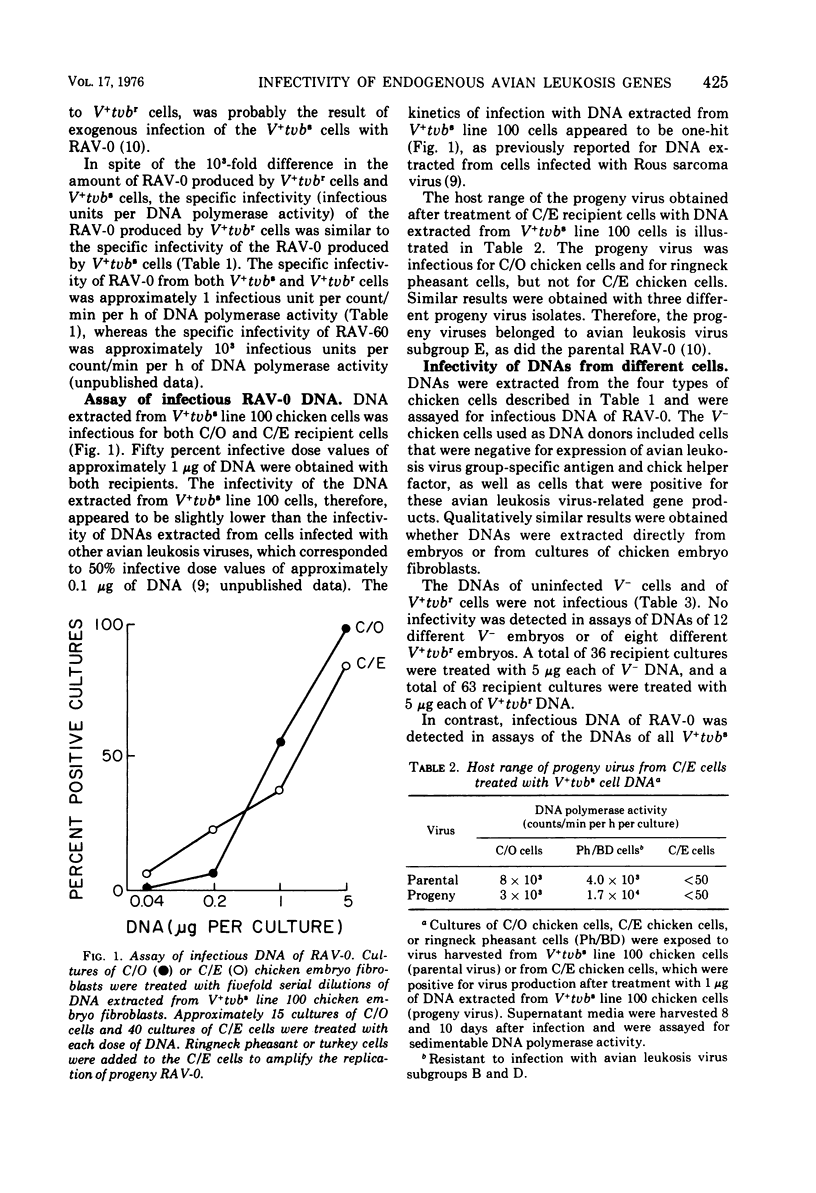
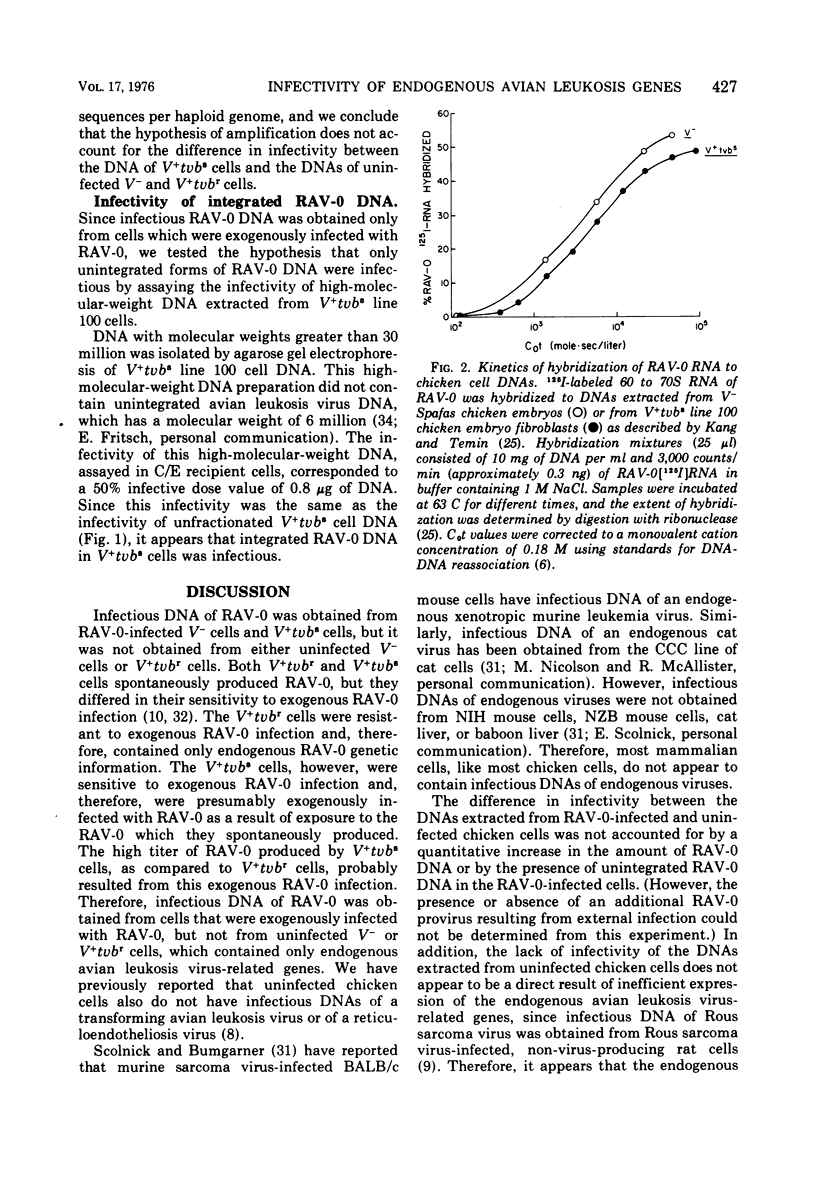
The infectivity of the avian leukosis virus-related genes in the DNA of four genetically distinct types of chicken cells was determined. Infectious DNA of Rous-associated virus-O(RAV-O) was obtained from V- chicken cells which were experimentally infected with RAV-O and from V+tvbs chicken cells, which spontaneously produced RAV-O and were sensitive to exogenous RAV-O infection. However, infectious DNA of RAV-O was not obtained from uninfected V- chicken cells or from V+tvbr chicken cells, which spontaneously produced a low titer of RAV-O but were resistant to exogenous RAV-O infection. No detectable amplification of the RAV-O related DNA sequences in the V+tvbs cells was found by hybridization of RAV-O 125I-labeled RNA to the DNAs of V+tvbs and uninfected V- cells. These results indicate that the endogenous avian leukosis virus-related genes in uninfected V- and V+tvbr cells differ from the RAV-O proviruses in RAV-O-infected V- and V+tvbs cells. The lack of infectivity of the DNA of V+tvbr cells is consistent with the hypothesis that the endogenous RAV-O genome in V+tvbr cells is linked to a cis-acting control element, which results in its inefficient expression.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, MacDonald D. W. A gene cluster in Aspergillus nidulans with an internally located cis-acting regulatory region. Nature. 1975 Mar 6;254(5495):26–31. doi: 10.1038/254026a0. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, Scazzocchio C. Initiator constitutive mutation with an 'up-promoter' effect in Aspergillus nidulans. Nature. 1975 Mar 6;254(5495):31–34. doi: 10.1038/254031a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P., Daams J. H. Oncornaviruses and their proviruses. Rev Eur Etud Clin Biol. 1972 Mar;17(3):245–248. [PubMed] [Google Scholar]
- Chen J. H., Hayward W. S., Hanafusa H. Avian tumor virus proteins and RNA in uninfected chicken embryo cells. J Virol. 1974 Dec;14(6):1419–1429. doi: 10.1128/jvi.14.6.1419-1429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious DNA from cells infected with Rous sarcoma virus, reticuloendotheliosis virus or Rous-associated virus-O. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1027–1032. doi: 10.1101/sqb.1974.039.01.118. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L. B., Smith E. J., Weiss R. A., Sarma P. S. Host gene control of endogenous avian leukosis virus production. Virology. 1974 Jan;57(1):128–138. doi: 10.1016/0042-6822(74)90114-7. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Lack of relationship between infection with avian leukosis virus and the presence of COFAL antigen in chick embryos. Virology. 1966 Aug;29(4):586–595. doi: 10.1016/0042-6822(66)90282-0. [DOI] [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne D. C. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966 Sep;54(3):911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita D. J., Chen Y. C., Friis R. R., Vogt P. K. RNA tumor viruses of pheasants: characterization of avian leukosis subgroups F and G. Virology. 1974 Aug;60(2):558–571. doi: 10.1016/0042-6822(74)90350-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Friis R. R., Mason W. S. Expression of the Major Viral Glycoprotein of Avian Tumor Virus in Cells of chf(+) Chicken Embryos. J Virol. 1975 May;15(5):1131–1140. doi: 10.1128/jvi.15.5.1131-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Aoki T., Kawai S., Miyamoto T., Wilsnack R. E. Presence of antigen common to avian tumor viral envelope antigen in normal chick embryo cells. Virology. 1973 Nov;56(1):22–32. doi: 10.1016/0042-6822(73)90284-5. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hayward W. S., Chen J. H., Hanafusa T. Control expression of tumor virus genes in uninfected chicken cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1139–1144. doi: 10.1101/sqb.1974.039.01.130. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H. Isolation of leukosis-type virus from pheasant embryo cells: possible presence of viral genes in cells. Virology. 1973 Jan;51(1):247–251. doi: 10.1016/0042-6822(73)90388-7. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T., Fleissner E. Existence and expression of tumor virus genes in chick embryo cells. Virology. 1972 Feb;47(2):475–482. doi: 10.1016/0042-6822(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Hillova J. RNA and DNA forms of the genetic material of C-type viruses and the integrated state of the DNA form in the cellular chromosome. Biochim Biophys Acta. 1974 Apr 29;355(1):7–48. doi: 10.1016/0304-419x(74)90006-7. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. A cis-dominant regulatory mutation affecting enzyme induction in the eukaryote Aspergillus nidulans. Nature. 1975 Jan 17;253(5488):210–212. doi: 10.1038/253210a0. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Kang C. Y., Temin H. M. Endogenous RNA-directed DNA polymerase activity in virions of RNA tumor viruses and in a fraction from normal chicken cells. Methods Enzymol. 1974;29:119–124. doi: 10.1016/0076-6879(74)29014-1. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Purchase H. G., Okazaki W. Chicken leukosis virus genome sequences in DNA from normal chick cells and virus-induced bursal lymphomas. Cell. 1975 Apr;4(4):311–319. doi: 10.1016/0092-8674(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Bumgarner S. J. Isolation of infectious xenotropic mouse type C virus by transfection of a heterologous cell with DNA from a transformed mouse cell. J Virol. 1975 May;15(5):1293–1296. doi: 10.1128/jvi.15.5.1293-1296.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. J., Crittenden L. B., Brinsfield T. H., Jr Status of the endogenous avian leukosis virus in resistant cells from a producing line. Virology. 1974 Oct;61(2):594–596. doi: 10.1016/0042-6822(74)90293-1. [DOI] [PubMed] [Google Scholar]
- Thuriaux P., Ramos F., Piérard A., Grenson M., Wiame J. M. Regulation of the carbamoylphosphate synthetase belonging to the arginine biosynthetic pathway of Saccharomyces cerevisiae. J Mol Biol. 1972 Jun 20;67(2):277–287. doi: 10.1016/0022-2836(72)90241-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Deng C. T., Bishop J. M. Synthesis, structure and function of avian sarcoma virus-specific DNA in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):987–996. doi: 10.1101/sqb.1974.039.01.113. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]



