Abstract
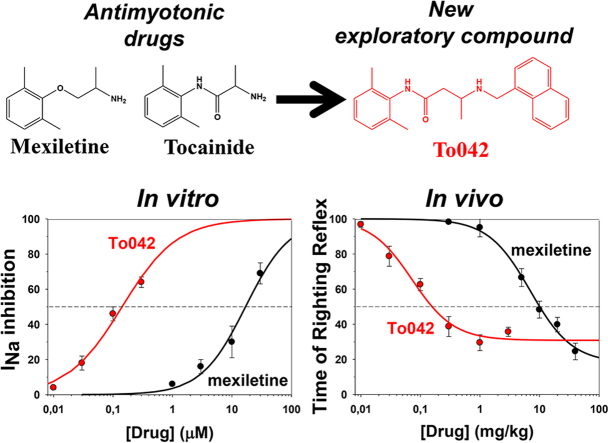
Although the sodium channel blocker, mexiletine, is the first choice drug in myotonia, some myotonic patients remain unsatisfied due to contraindications, lack of tolerability, or incomplete response. More therapeutic options are thus needed for myotonic patients, which require clinical trials based on solid preclinical data. In previous structure-activity relationship studies, we identified two newly-synthesized derivatives of tocainide, To040 and To042, with greatly enhanced potency and use-dependent behavior in inhibiting sodium currents in frog skeletal muscle fibers. The current study was performed to verify their potential as antimyotonic agents. Patch-clamp experiments show that both compounds, especially To042, are greatly more potent and use-dependent blockers of human skeletal muscle hNav1.4 channels compared to tocainide and mexiletine. Reduced effects on F1586C hNav1.4 mutant suggest that the compounds bind to the local anesthetic receptor, but that the increased hindrance and lipophilia of the N-substituent may further strengthen drug-receptor interaction and use-dependence. Compared to mexiletine, To042 was 120 times more potent to block hNav1.4 channels in a myotonia-like cellular condition and 100 times more potent to improve muscle stiffness in vivo in a previously-validated rat model of myotonia. To explore toxicological profile, To042 was tested on hERG potassium currents, motor coordination using rotarod, and C2C12 cell line for cytotoxicity. All these experiments suggest a satisfactory therapeutic index for To042. This study shows that, owing to a huge use-dependent block of sodium channels, To042 is a promising candidate drug for myotonia and possibly other membrane excitability disorders, warranting further preclinical and human studies.
Keywords: Sodium channel, Use-dependence, Myotonia, Membrane hyperexcitability, Tocainide derivative, Mexiletine
Abbreviations: TRR, time of righting reflex; 9-AC, anthracene-9-carboxylic acid; SAR, structure-activity relationship
Graphical abstract
Highlights
-
•
To040 and To042 are potent use-dependent hNav1.4 sodium channel blockers.
-
•
The compounds strengthen the molecular interaction at the local anesthetic receptor.
-
•
To042 is 120-fold more potent than mexiletine in vitro in myotonia-like conditions.
-
•
To042 is 100-fold more potent than mexiletine in vivo in a rat model of myotonia.
-
•
To042 is a promising antimyotonic drug deserving further investigation.
1. Introduction
Blockers of voltage-gated sodium channels are clinically used in a number of disorders of plasma membrane excitability, including cardiac arrhythmias, epileptic seizures, pain, and myotonia (Imbrici et al., 2016). Many sodium channel blockers bind to the local anesthetics receptor located within the pore of the channel (Ragsdale et al., 1994, Ragsdale et al., 1996). This receptor is highly conserved among sodium channel subtypes, thereby allowing most blockers to exert similar block of sodium channels expressed in central and peripheral neurons, cardiomyocytes and skeletal muscle fibers (England and de Groot, 2009). In most cases, the safety of these drugs relies on their ability to block sodium channels in a frequency-dependent manner, allowing a selective inhibition of over-excited cells while sparing the healthy organs. Recently, more selective blockers have been identified, especially for the peripheral nerve Nav1.7 and Nav1.8 subtypes, and some of them are being evaluated in clinical studies for the treatment of chronic pain (De Lera Ruiz and Kraus, 2015, Theile and Cummins, 2011).
Myotonic syndromes are characterized by skeletal muscle stiffness due to sarcolemma hyper-excitability, which can be painful and badly interfere with daily motor activities and quality of life (Statland et al., 2014, Suetterlin et al., 2014). A randomized controlled clinical trial recently demonstrated the efficacy of the antiarrhythmic drug mexiletine in myotonia (Hoffman and Kaminski, 2012, Statland et al., 2012). Consequently, mexiletine received orphan drug designation for treatment of myotonic disorders by F.D.A. and E.M.A. (U.SFood and Drug Administration, 2010, European Medicines Agency, 2013a, European Medicines Agency, 2013b, European Medicines Agency, 2014). By blocking skeletal muscle Nav1.4 sodium channels in a use-dependent manner, the drug inhibits the myotonic discharges of action potentials and favors muscle relaxation (De Luca et al., 1997, Desaphy et al., 1999). The drug also alleviates the transitory muscle weakness associated with recessive myotonia congenita due to chloride channel ClC-1 mutations (Lo Monaco et al., 2015). Nevertheless, a number of myotonic patients obtain little benefits from mexiletine due to contraindications, side effects, lack of tolerability, or lack of response (Matthews and Hanna, 2014, Suetterlin et al., 2015). In addition, mexiletine distribution has been interrupted by the manufacturer in a number of countries, making very difficult access to the drug. In sodium channel-related myotonias, unsatisfactory response to mexiletine may stem from a reduced affinity of the mutated channel to the drug, and other sodium channel blockers, like flecainide, may be successful in such cases (Desaphy et al., 2004, Desaphy et al., 2013c, Desaphy et al., 2016). The other orally-available lidocaine analogue, tocainide, has been also used in myotonia (Streib, 1986, Streib, 1987), but was associated with an elevated incidence of serious adverse reactions and discontinued in many countries. There is thus a general opinion that more therapeutic options are needed for myotonic patients, and that clinical trials based on solid preclinical data are urgently needed (Matthews and Hanna, 2014).
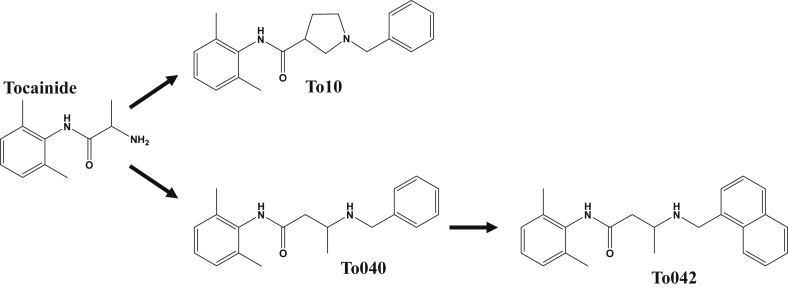
Searching for other useful antimyotonic drugs, we recently developed a pharmacologically-induced model of myotonia in the rat and investigated a number of marketed sodium channel blockers (Desaphy et al., 2013b, Desaphy et al., 2014). We found that the antimyotonic activity of drugs in vivo was closely parallel to the in vitro inhibition of sodium currents elicited by high-frequency voltage-clamp protocols in mammalian cells transfected with hNav1.4 cDNA. Beside the study of marketed drugs, we also explored the possibility to increase potency and use-dependent behavior through chemical optimization of mexiletine and tocainide in a series of SAR studies (De Bellis et al., 2013, De Luca et al., 2000, De Luca et al., 2003a, De Luca et al., 2003b, De Luca et al., 2012). We have identified beta-proline derivatives of tocainide with a 10-fold increase in potency and use dependence for blockade of sodium channels in frog muscle fibers (Catalano et al., 2008). One of these compounds, namely NeP1 or To10 (Fig. 1), was 10 times more potent than tocainide in blocking human sodium channels and showed considerable analgesic activity in animal models of neuropathic pain (Ghelardini et al., 2010). Lately, on the basis of a 3D-QSAR study, new chemical maneuvers allowed to obtain two promising compounds 100 times more potent than tocainide, namely To040 and To042 (Carrieri et al., 2009, Muraglia et al., 2014) (Fig. 1).
Fig. 1.
Chemical structures of tocainide and explorative compounds. The compound To10 is a benzyl-N-substituted β-proline derivative of tocainide (known also as NeP1, Ghelardini et al., 2010). In To040, the β-proline cycle was eliminated. In To042, the benzyl moiety was substituted for by a naphthalene group (Muraglia et al., 2014).
The aim of the present study was to verify the potency of these two new compounds on the human Nav1.4 isoform expressed in mammalian cell line, obtain information about the molecular binding site, and test their antimyotonic activity in vivo in the rat model of myotonia (Desaphy et al., 2013b, Desaphy et al., 2014). Beside efficacy, we also performed experiments to explore the toxicological profile, including effects on hERG potassium channels and cytotoxicity. The results indicate To042 as a promising candidate drug for myotonia and, possibly, other plasma membrane excitability disorders, warranting further preclinical studies and hopefully clinical trials.
2. Materials and methods
2.1. Patch-clamp recording in HEK293 or tSA201 cell lines
Sodium or potassium currents were recorded using the whole-cell patch-clamp configuration in Human Embryonic Kidney 293 (HEK293) cells or tSA201 cells (a derivative of HEK293 cells) expressing the human skeletal muscle voltage-gated (hNav1.4) sodium channel or the human Ether-a-go-go Related Gene (hERG) potassium channel. Permanent transfection of HEK293 cells with wild-type hNav1.4 channel or its F1586C mutant has been previously described (Desaphy et al., 2009, Desaphy et al., 2012). Transient transfection of tSA201 cells with 8 μg of pcDNA3.1 expression vector containing the coding region of hERG channel (a generous gift from prof. Valeria Casavola, University of Bari, Italy; originally from prof. E. Ficker, MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH) and 1 μg of a vector encoding the CD8 gene reporter was performed using the calcium-phosphate co-precipitation method (Desaphy et al., 2001). Only cells decorated with micro-beads coated with anti-CD8 antibody were used for patch-clamp 24 at 48 h after transfection.
Patch-clamp recordings were performed at ambient temperature (20–22 °C) using an Axopatch 1D amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA). Voltage clamp protocols and data acquisition were obtained with pClamp 10.2 software (Axon Instr.), through a 12-bit A-D/D-A interface (Digidata 1440, Axon Instr.). Pipettes were fabricated from Corning 7052 glass capillaries (Garner Glass, Claremont, CA, USA) using a vertical PP-82 puller (Narishige, Tokyo, Japan), to obtain tip resistance in between 1.5 and 3.5 MΩ. Currents were low-pass filtered at 2 kHz (−3 dB) and digitized at 10–20 kHz. Five minutes after establishing the whole-cell configuration, sodium or potassium currents were recorded using specific voltage-clamp protocols described in the Results section. Data were analyzed off-line using Clampfit 10.2 and SigmaPlot 10.0 (Systat Software GmbH, Erkrath, Germany).
2.2. In vivo evaluation of the antimyotonic activity of To042
Animal housing and experiments were performed in accordance with the Italian Guidelines for the use of laboratory animals, which conforms to the European Union Directive for the protection of experimental animals (2011/63/EU), and received approval from the Animal Experimentation Ethic Committee of the University of Bari (CESA prot. 7/12) and Italian Health Department (Decreto n. 91/2013-B). All efforts were made to minimize animal suffering and to reduce the number of animals used. Twelve adult Wistar rats (body weight: 350–500 g; Charles River Laboratories, Calco, Italy) were individually housed and received water and food ad libitum. Experiments were performed in 3–4 rats simultaneously as previously described (Desaphy et al., 2013b, Desaphy et al., 2014). Briefly, myotonia condition was induced in the rats by i.p. injection of 30 mg/kg body weight of 9-anthracene-carboxylic acid (9-AC), a potent blocker of skeletal muscle ClC-1 chloride channels. A few minutes after 9-AC injection, animals constantly show a manifest muscular stiffness and difficulties to move, with no noticeable side effects (Desaphy et al., 2013b). The myotonia was quantified by measuring the time of righting reflex (TRR), that is time needed by the rat to turn back on its four limbs from the supine position. Ten minutes after 9-AC injection, the rats were administrated an oral dose of drug (mexiletine or To042) or vehicle saline solution using an esophageal cannula. The TRR was determined 10 min before 9-AC injection and 30, 60, 120 and 180 min after 9-AC. At each time point, the TRR value was calculated as the mean of 7 determinations, repeated at 1-min intervals to prevent the warm-up phenomenon. For each drug dose, the experiments were repeated at least 3 times in different rats, and experimental data are given as the mean ± S.E.M.
2.3. Rotarod experiments
The well-established rotarod test was used to exclude undesired CNS effects of To042. Twelve male Wistar rats (Charles River Lab.), weighting 150–250 g, were trained daily for 5 days on the rotarod (Panlab-Harvard Apparatus, Cornella, Spain) to make the animals familiar with handling, oral administration using esophageal cannula, and rotarod hardware. The daily procedure consisted in 4 trials on the rotarod, accelerating from 4 to 40 rpm in 2 min, run 10 min before, and 10, 30, and 100 min after vehicle (sterile distilled water) administration (time 0). The rotarod performance was evaluated at each time point as the time length the rat was able to stay on the rotarod. After the training period, one half (six) rats were randomly assigned to drug group, receiving 3 mg/kg To042 per os at time 0. The test was repeated 5 times a week. Drug dose was increased to 10 mg/kg for another week, and to 30 mg/kg for the last week. Performance value was calculated at each time point as the mean ± SEM from 5 trials (days) measured in 6 rats.
2.4. In vitro cytotoxicity studies
Murine C2C12 skeletal muscle cells were cultured and maintained at 37 °C in 5% CO2/95% O2 in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. For cell viability tests, cells at 70% confluence were re-suspended in 96-well cultures at a density of ∼12.000–13000 cells per well in culture medium. After 18 h, they were incubated with tocainide or To042 for 6 h. Stock solutions of To042 were prepared by dissolving the compound in DMEM containing <0.1% dimethylsulfoxide (DMSO). DMSO at the concentration used for dilution had no effect on cell viability. Tocainide was readily soluble in DMEM. Fresh stock solutions were made daily and diluted as required. One hour before absorbance lecture, 10 μl of Cell Counting Kit-8 pure solution (CCK-8 Alexis Biochemicals) was added into each well. The CCK-8 solution contains the monosodium salt of [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8), which is reduced by cellular dehydrogenases to give a yellow colored product (formazan) absorbing at 450 nm. The quantity of formazan dye is directly proportional to the number of viable cells. Absorbance was measured at 450 nm with a microplate spectrophotometer (Victor V31420-40, PerkinElmer). Cell viability is expressed as percent with respect to cell treated with vehicle alone (0.1% DMSO), after proper subtraction of blank absorbance. The data are expressed as mean ± SEM, and differences are considered significant for p < 0.05 using unpaired Student's t-test.
2.5. Drugs and solutions
For sodium current recordings, the pipette solution contained (in mM): CsF 120, CsCl 10, NaCl 10, EGTA 5, HEPES 5; and was adjusted to pH = 7.2 with CsOH. The extracellular bath solution contained (in mM): NaCl 150, KCl 4, CaCl2 2, MgCl2 1, HEPES 5, glucose 5; and was adjusted to pH 7.4 with NaOH. For hERG potassium current recordings, the pipette solution contained (in mM) KCl 130, MgCl2 1, MgATP 5, EGTA 5, HEPES 10; and was adjusted to pH 7.2 with KOH. The extracellular bath solution contained (in mM): NaCl 123, KCl 4, CaCl2 2, MgCl2 1, 25 NaHCO3 25, NaH2PO4− 2, HEPES 5; and was adjusted to pH 7.4 with NaOH. For patch-clamp experiments, To040, To042, and mexiletine were solubilized in the bath solution supplemented with 0.2% DMSO to obtain the desired concentration. No effect of 0.2% DMSO was observed on sodium or potassium currents. The patched cell was continuously exposed to a stream of control or drug-supplemented bath solution flowing out by gravity from a plastic capillary positioned close to the cell.
For in vivo experiments, the solution of anthracene-9-carboxylic acid (2.4 g/l 9-AC) was prepared each day in sterile water supplemented with 0.3% bicarbonate and the volume for intraperitoneal injection was adjusted to obtain 30 mg/kg body weight (Desaphy et al., 2013b, Desaphy et al., 2014). The exploratory drugs were solubilized daily in physiological 0.9% NaCl saline at the desired dose for oral administration in rats with an esophageal cannula (final volume of about 1 ml).
All drugs and chemicals were purchased from Sigma-Aldrich, whereas To040 and To042 were synthesized in our laboratories as salts, as previously described (Catalano et al., 2008, Muraglia et al., 2014).
3. Results
3.1. Dose- and use-dependent inhibition of human Nav1.4 channels by tocainide derivatives
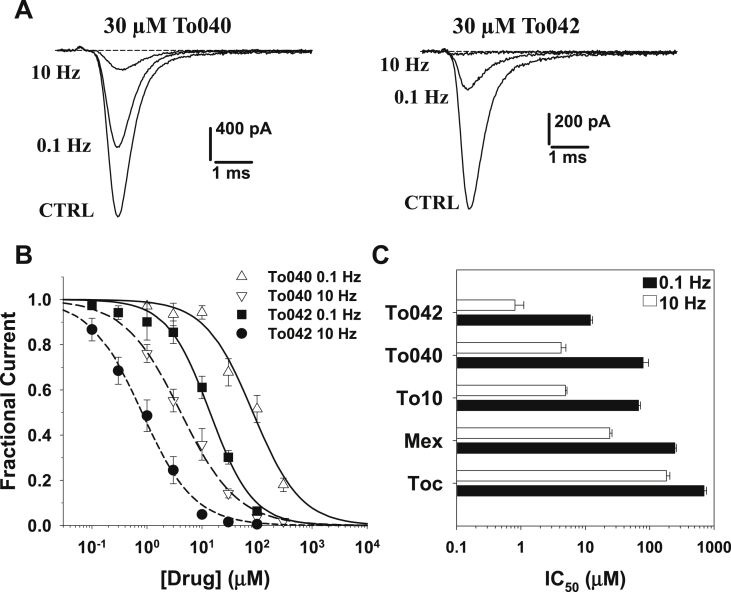
Tocainide derivatives were tested on hNav1.4 channels permanently expressed in HEK293 cells using whole-cell patch-clamp. To allow comparison with other previously-tested drugs, including tocainide, To10, and mexiletine, the stimulation protocol consisted in holding the cells at −120 mV and eliciting sodium currents by depolarizing cells at −30 mV for 25 ms. The stimulation frequency was first fixed at 0.1 Hz to determine tonic block, and then increased to 10 Hz to determine phasic block (Desaphy et al., 2009, Desaphy et al., 2013a, Desaphy et al., 2013b, Desaphy et al., 2013c). Representative effects of 30 μM To040 and To042 are shown in Fig. 2A. The concentration-response relationships obtained on hNav1.4 currents are shown in Fig. 2B. They were fitted with the standard Hill equation (1):
| (1) |
where IC50 (μM) is the half-maximum inhibitory concentration and nH is the logistic slope factor; fit values are reported in Table 1. Both the new tocainide derivatives exerted a huge dose- and use-dependent block of sodium currents, the most potent being To042. The block of sodium channels was fully reversed upon drug washout (not shown). In Fig. 2C are shown the IC50 values for To040 and To042, compared to previously published values for tocainide, To10, and mexiletine. Tocainide is the less potent blocker, followed by mexiletine. Both these drugs display antimyotonic activity in patients; while mexiletine has recently received orphan designation in myotonic syndromes, tocainide has been withdrawn because of side effects. The benzyl-β-proline tocainide derivative, To10 (also called NeP1), showed a significant improvement of sodium channel inhibition and analgesic activity in animal models (Ghelardini et al., 2010, De Luca et al., 2012). In this study, the new To040 compound showed IC50 values very similar to To10, while To042 compound further improved both tonic and phasic block. Compared to the reference antimyotonic drug, mexiletine, the IC50 values of To042 were reduced 20- and 30-fold at 0.1 and 10 Hz frequency stimulations, respectively.
Fig. 2.
Concentration-dependent effects of tocainide derivatives on hNav1.4 channels. (A) Representative traces of wild-type hNav1.4 sodium currents recorded in transfected HEK293 cells at steady-state before (CTRL) and during application of 30 μM To040 or To042 at 0.1 Hz and 10 Hz frequency stimulations. (B) Concentration-response relationships of To040 and To042 on WT hNav1.4 currents at 0.1 and 10 Hz. Each experimental point is the mean ± S.E.M. of at least 3 cells. The relationships were fit to the first-order binding equation (1) reported in the text, and fit parameter values are reported in Table 1 (C) bargraph shows IC50 values calculated as in B for To042, To040, To10, mexiletine (Mex), and tocainide (Toc).
Table 1.
Fit parameters of concentration-response relationships for Tocainide derivatives on wild-type and F1586C hNav1.4 channels.
| Tonic block (0.1 Hz) |
Phasic block (10 Hz) |
0.1-to-10 Hz ratio |
|||
|---|---|---|---|---|---|
| IC50 (μM) | nH | IC50 (μM) | nH | ||
| hNav1.4 – WT | |||||
| To040 | 79 ± 17 | 1.2 ± 0.3 | 4.2 ± 0.8 | 0.8 ± 0.1 | 18.8 |
| To042 | 12 ± 1 | 1.3 ± 0.1 | 0.81 ± 0.06 | 0.8 ± 0.1 | 14.8 |
| To10a | 67 ± 5 | 0.9 ± 0.1 | 4.9 ± 0.3 | 1.1 ± 0.1 | 13.7 |
| Tocainidea | 699 ± 69 | 1.0 ± 0.1 | 182 ± 24 | 1.1 ± 0.2 | 3.8 |
| Mexiletineb | 246 ± 15 | 0.9 ± 0.1 | 24 ± 2 | 0.8 ± 0.1 | 10.3 |
| hNav1.4 - F1586C | |||||
| To040 | 137 ± 9 | 1.1 ± 0.1 | 62 ± 4 | 1.0 ± 0.1 | 2.2 |
| To042 | 44 ± 4 | 1.7 ± 0.0 | 14 ± 2 | 1.2 ± 0.2 | 3.1 |
| Mexiletineb | 1340 ± 72 | 1.1 ± 0.1 | 1089 ± 181 | 1.0 ± 0.2 | 1.2 |
Parameter values were determined from the fit of concentration–response curves shown in Fig. 2, Fig. 3 with the standard Hill equation (1). The values are given together with the SE of the fit.
From Ghelardini et al., 2010.
From Desaphy et al., 2012.
3.2. The tocainide analogues bind to the local anesthetic receptor
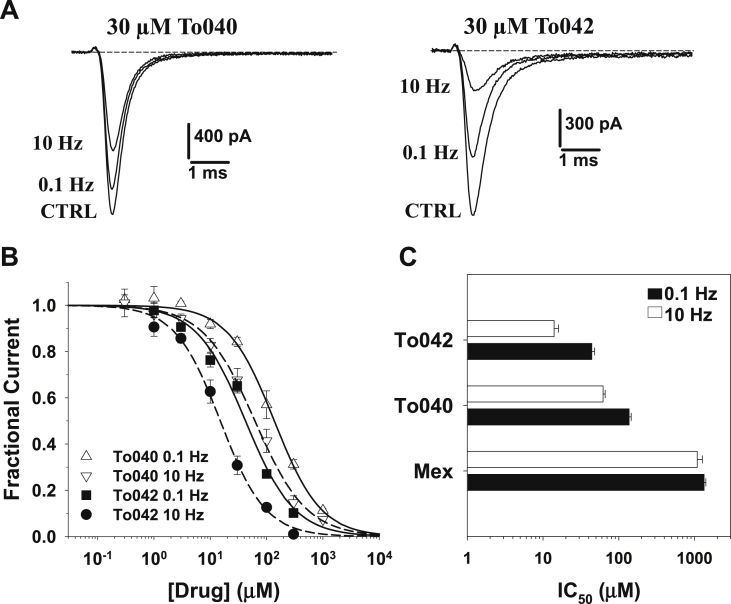
Many use-dependent sodium channel blockers bind to the local anesthetic (LA) receptor located within the ion conducting pore in the sixth transmembrane segment of domain IV of the Nav protein (Ragsdale et al., 1994, Ragsdale et al., 1996). In particular, the π-cation interaction between the charged amine group of LA-like drugs and the aromatic moiety of F1586 residue in hNav1.4 is critical for drug binding and channel use-dependent inhibition (Desaphy et al., 2012, Pless et al., 2011). Accordingly, the F1586C mutation in hNav1.4 fully zeroed the use-dependence of channel block by mexiletine (Desaphy et al., 2009, Desaphy et al., 2012). To verify whether the two tocainide derivatives also bind to the LA receptor, the compounds were tested on the F1586C hNav1.4 mutant channel. Representative F1586C current traces recorded before and after 30 μM drug application are shown in Fig. 3A; The concentration-response relationships obtained on WT channels are shown in Fig. 3B, and the fit parameters are reported in Table 1. Compared to wild-type, both tonic and phasic blocks were greatly impaired by the F1586C mutation (∼2- and ∼15-fold reduction, respectively); the 0.1-to-10 Hz IC50 ratio was greatly reduced, but use-dependence was not completely zeroed.
Fig. 3.
Concentration-dependent effects of tocainide derivatives on F1586C hNav1.4 channel mutant. (A). Representative traces of F1586C hNav1.4 sodium currents recorded in transfected HEK293 cells at steady-state before (CTRL) and during application of 30 μM To040 or To042 at 0.1 Hz and 10 Hz frequency stimulations. (B) Concentration-response relationships of To040 and To042 on WT hNav1.4 currents at 0.1 and 10 Hz. Each experimental point is the mean ± S.E.M. of at least 3 cells. The relationships were fit to the first-order binding equation (1) reported in the text, and fit parameter values are reported in Table 1 (C) bargraph shows IC50 values calculated as in B for To042, To040, and mexiletine (Mex).
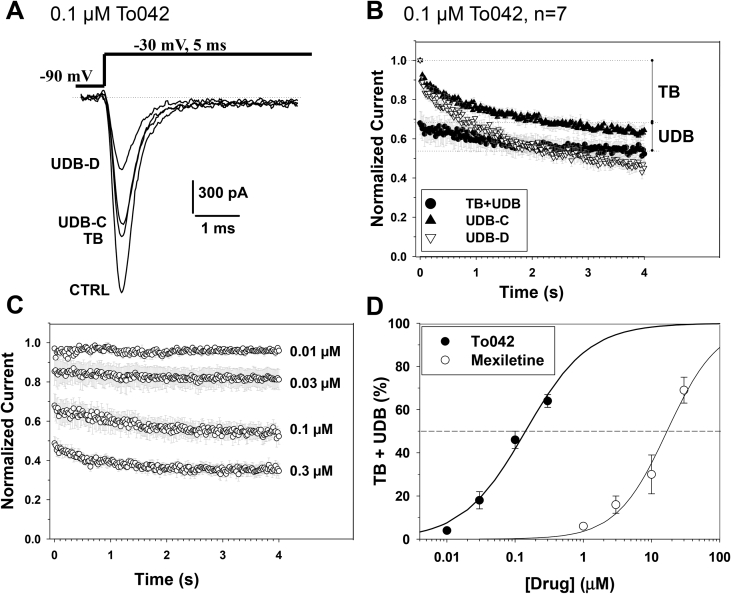
3.3. Block of hNav1.4 channels by To042 in myotonic-like conditions
The myotonic condition is characterized by action potential firing at elevated frequencies ranging from tens to hundreds of Hz. To test the most potent Toc derivative, To042, on hNav1.4 channels in a condition more similar to myotonia, we used a voltage clamp protocol consisting in depolarizing the cells from a holding potential of −90 mV (close the resting membrane potential of muscle fibers), and 5 ms-long depolarization steps applied at the stimulation frequency of 50 Hz (Desaphy et al., 2014, Desaphy et al., 2016). In absence of drug, such a stimulation induced a frequency-dependent, ∼30% reduction of sodium currents (labeled UDB-C in Fig. 4A). In presence of 0.1 μM To042, a tonic block (TB) was measured at the stimulation frequency of 0.1 Hz, and a further use-dependent block (UDB-D) was measured at 50 Hz. Fig. 4B illustrates the averaged time course of hNav1.4 peak current reduction observed in absence (UDB-C) and presence (UDB-D) of 0.1 μM To042 (n = 7). To evaluate the effect strictly related to drug, we subtracted UDB-C from UDB-D to obtain UDB and then add TB. The time course of TB + UDB was calculated at various To042 concentrations and reported in Fig. 4C, while the TB + UDB values were reported as a function of drug concentration and compared to mexiletine in Fig. 4D. The relationships were fit with equation (2):
| (2) |
and fit parameters are reported in Table 2. In a myotonia-like condition, the tocainide derivative was 120 times more potent than mexiletine.
Fig. 4.
Effects of tocainide derivatives on hNav1.4 channels in a myotonia-like condition. (A) Representative traces of wild-type hNav1.4 sodium currents recorded in transfected HEK293 cells at steady-state before (CTRL) and during application of 0.1 μM To042. The cell was depolarized to −30 mV for 5 ms (as an action potential duration) from a holding potential of −90 mV (close to the resting membrane potential of skeletal muscle fibers) at 0.1 and 50 Hz frequency stimulations (as during a myotonic run). In absence of drug, a use-dependent reduction of sodium current developed to reach a steady-state, UDB-C. In presence of drug, the tonic block (TB) exerted by To042 was measured at 0.1 Hz stimulation, while the use-dependent sodium current reduction (UDB-D) was measured at 50 Hz. (B) Time course of hNav1.4 sodium current reduction at 50 Hz in absence (UDB-C) and presence (UDB-D) of 0.1 μM To042. The quantity of block directly attributable to the drug (TB + UDB) was calculated by subtracting UDB-C to UDB-D and adding TB. (C) Concentration-dependent inhibition of hNav1.4 channels by To042 as calculated in B. (D) Concentration-response relationships for To042 and mexiletine determined as in C. Each point is the mean ± S.E.M. from at least 3 cells. The relationships were fit to the first-order binding equation (2) reported in the text and fit parameter values are reported in Table 2.
Table 2.
Fit parameters of concentration- and dose-response relationships for To042 and mexiletine for antimyotonic activity in vitro and in vivo.
| In vitro |
In vivo |
||||
|---|---|---|---|---|---|
| IC50 (μM) | nH | ED50 (mg/kg) | nH | Min (%) | |
| To042 | 0.14 ± 0.02 | 0.9 ± 0.1 | 0.07 ± 0.02 | 1.2 ± 0.3 | 30.9 ± 4.4 |
| Mexiletine | 16.9 ± 2.4 | 1.2 ± 0.2 | 7.0 ± 0.02 | 1.2 ± 0.2 | 18.0 ± 7.3 |
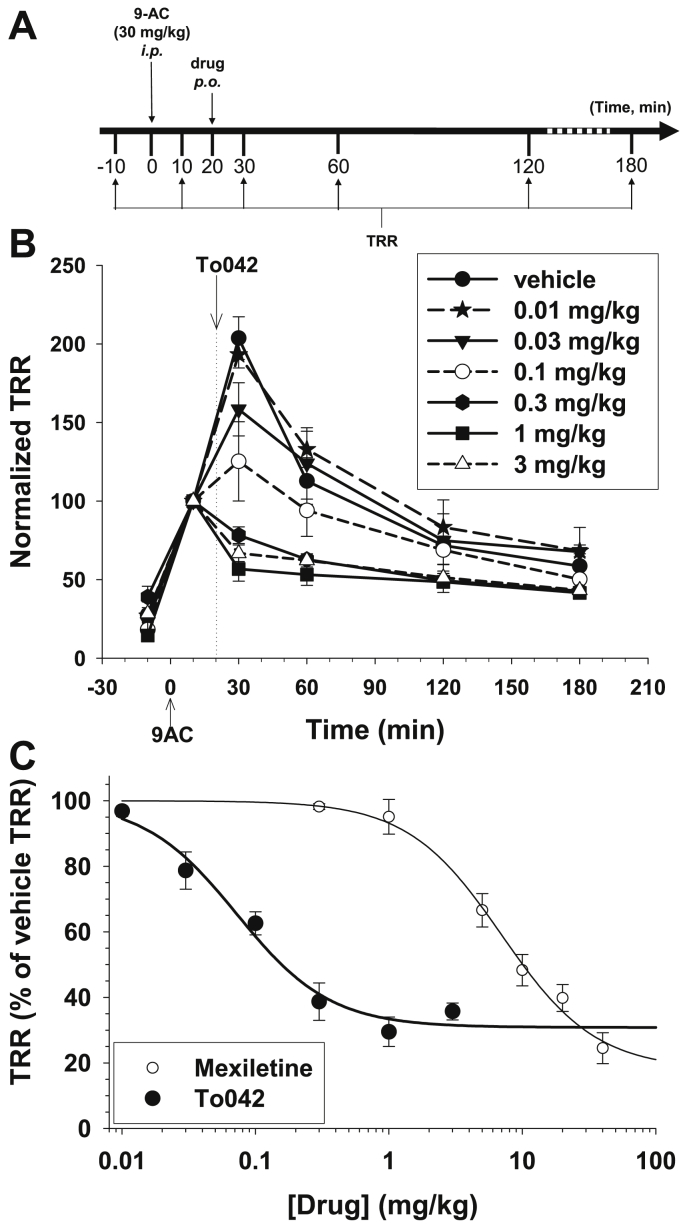
3.4. In vivo antimyotonic activity of To042 in the rat
The antimyotonic activity of To042 was assayed in vivo in a previously validated rat model of pharmacologically-induced myotonia congenita (Desaphy et al., 2013b, Desaphy et al., 2014). The intraperitoneal injection of 30 mg/kg 9-anthracene carboxylic acid (9-AC), a chloride channel blocker, mimics the genetic condition of myotonia congenita due to chloride channel loss-of-function mutation. Muscle stiffness, evaluated by measuring the time of righting reflex (TRR), is increased 10 min after 9-AC injection, reaches a maximum value after 30 min and then decreases gradually (Fig. 5A and B). An antimyotonic drug is expected to reduce the TRR; the antimyotonic effect of To042, orally-administrated 20 min after 9-AC injection, was compared to vehicle (Fig. 5B). The Toc derivative exerted antimyotonic activity at doses greater than 0.01 mg/kg and was able to exert a ∼70% reduction of TRR at doses greater than 0.3 mg/kg. The dose-antimyotonic effect relationships were drawn for To042 and mexiletine and fit with equation (3):
| (3) |
where ED50 (mg/kg) is the half-maximum effective dose, nH is the logistic slope factor, Min is the minimal TRR value, and (100-Min) corresponds to the maximal effect. Fit parameters are reported in Table 2. In vivo, To042 (ED50 = 0.07 mg/kg) resulted 100-fold more potent than mexiletine, although associated to a slightly reduced efficacy than mexiletine (maximal TRR reduction of ∼70% compared to ∼80% for Mex).
Fig. 5.
In vivo effects of tocainide derivatives in a rat model of myotonia. (A) Experimental protocol. Myotonia was induced in the rat by intraperitoneal injection of 30 mg/kg 9-anthracene carboxylic acid (9-AC) at time zero. The drug was administrated by os 20 min after 9-AC injection. The time of righting reflex (TRR) was measured at various before and after 9AC injection, as a measure of muscle stiffness. (B) Time course of the antimyotonic activity of To042 at different doses. The TRR was normalized with respect to that measured at 10 min time point. Experimental points are the means ± S.E.M. from at least 3 rats. (C) Dose-response relationship of To042, compared to the one of mexiletine, calculated as in B at 30 min time point. Each experimental point is the mean ± S.E.M. from at least 3 rats. The relationships were fit to the first-order binding equation (3) reported in the text, and fit parameter values are reported in Table 2.
Although To042 was given orally and no data is available regarding To042 bioavailability, we made an attempt to estimate the free blood concentration corresponding to the ED50. The blood volume of a rat can be estimated from body weight using a formula determined experimentally by Lee and Blaufox (1985). For a rat body weight ranging between 350 and 500 g, the estimated blood volume range is [21.8–30.8] mL. The ED50 for To042 was 0.07 mg/kg (Table 2), that is [0.025–0.035] mg To042 for [350–500] g rat body weight. Assuming a 100% bioavailability, the blood concentration of To042 would be thus [1.15–1.14] mg/L, corresponding to 3.3 μM (free base MW = 347.47). In humans, tocainide shows oral bioavailability of ∼100% and is ∼50% plasma protein bound (Lalka et al., 1976). Assuming a similar behavior of To042 in rats, the free To042 blood concentration corresponding to the ED50 would be 1.7 μM. However, binding affinity of To042 to human and rat serum albumin in vitro is by far greater than that of tocainide, most likely due to the elevated lipophilicity of the former (Pistolozzi et al., 2014). In these in vitro experiments, the free portion of To042 was approximately 20 times lower than that of tocainide. Thus with a further speculation, we may hypothesize that, at the dose of 0.07 mg/kg, the free To042 concentration in rat blood would be 0.085 μM. This value is amazingly very close to the IC50 for use-dependent inhibition of sodium channels (0.14 μM, Table 1).
3.5. Effects of To042 on cardiac hERG channels
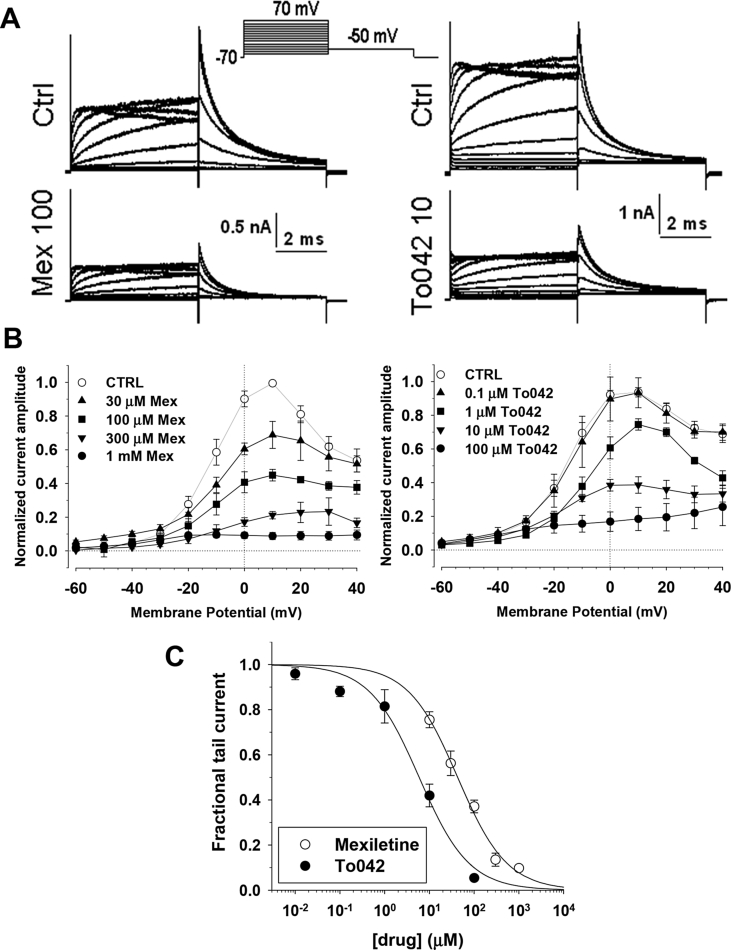
It is well known that inhibition of the cardiac delayed rectifier current (IKr) and its cloned molecular counterpart, the human Ether-a-go-go related gene (hERG) channel, mediates the life-threatening QT interval prolonging effects of a wide range of clinically used drugs, especially those containing hydrophobic groups linked to a basic nitrogen (Durdagi et al., 2010, Kannankeril et al., 2010). A number of sodium channel blockers have been proposed to exert hERG channel blockade at clinically relevant concentrations (Danielsson et al., 2003, Paul et al., 2001, Paul et al., 2002). Although the Class Ib antiarrhythmic agents, mexiletine and lidocaine, have comparatively low affinity for IKr/hERG channels (Paul et al., 2002, Wang et al., 1996), we verified whether To042 may exert significant hERG channel block in transfected tSA cells. We used a conventional voltage protocol, consisting in successive 5-s voltage steps applied every 18 s, ranging from −70 to +70 mV, in 10-mV increments, and followed by a 5-s step to −50 mV to elicit hERG tail currents (Fig. 6A). Representative examples of hERG current families recorded before and after application of 10 μM To042 or 100 μM mexiletine are illustrated. Current-voltage relationships in Fig. 6B show the dose-dependent effect of drugs on current amplitude measured at the end of first voltage step. Inhibition of hERG currents by mexiletine was observed at concentrations greater than 1 μM and was complete at 1 mM. Effect of To042 initiated at concentrations greater than 0.1 μM and was maximal at 100 μM. The concentration-response relationships were obtained from hERG tail currents elicited at −10 mV and fitted with equation (1) (Fig. 6C). The IC50 values were 42.8 ± 4.4 μM (nH = 0.79 ± 0.07) for mexiletine and 5.9 ± 1.4 μM (nH = 0.77 ± 0.13) for To042.
Fig. 6.
Comparative effects on To042 and mexiletine on hERG potassium channel. (A) Representative traces of hERG potassium currents recorded in transfected tsA201 cells at steady-state before (CTRL) and during application of 100 μM mexiletine (left panel) or 10 μM To042 (right panel). The cells were held at −70 mV and depolarized for 5 s to potentials ranging from −70 to +70 mV, applied in 10 mV increments. Tail currents were recorded at −50 mV. (B) Current-voltage relationships were drawn from steady-state current amplitudes measured at the end of the 5 s-long test pulse, before and during application of various mexiletine (left panel) and To042 concentrations (right panel). Each experimental relationship is the mean ± S.E.M. of at least 3 cells. (C) Concentration-response relationships drawn using tail current amplitudes measured at −50 mV after test depolarization at −10 mV. Each data point is the mean ± S.E.M. from at least 3 cells. The relationships were fit to the first-order binding equation (1) and fit parameter values are reported in the text.
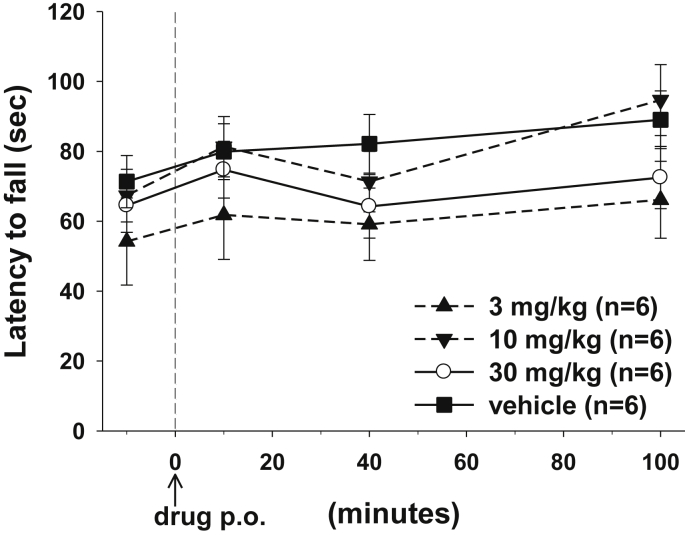
3.6. Effects of To042 on rat motor coordination
In a recent study, the rotarod test was used to compare effects of three drugs on motor coordination in adr mice, a genetic model of myotonia congenita (Novak et al., 2015). The results showed that, although the three drugs displayed similar antimyotonic activity in the TRR test, one of these was not able to improve rotarod performance, suggesting CNS toxic effects.
To exclude CNS toxicity of To042 in vivo, we evaluated rat motor coordination and balance after oral drug administration using the rotarod test in control rats. The rotarod performance was calculated as the time length the rat was able to stay on the rotarod, the speed of which accelerated from 4 to 40 rpm in 2 min. The tocainide derivative did not alter rat motor performance at dose up to 30 mg/kg p.o. (Fig. 7).
Fig. 7.
Effects of To042 on rat motor coordination measured as the latency to fall from the rotarod. Each data point is the mean ± S.E.M. from 6 rats.
Note that we did not perform the rotarod test in 9-AC treated rats, because this would not add any important information regarding drug toxicity.
3.7. Cytotoxicity
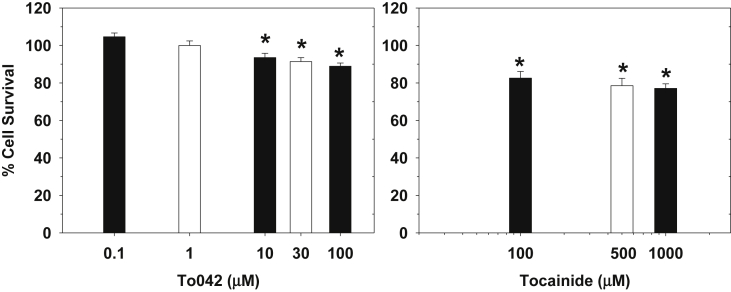
The safety of tocainide has been long questioned and its use limited in some countries due to the undesirable cytotoxic effects. We previously observed a reduction of HeLa and MRC-5 cell viability with 100 μM tocainide (Ghelardini et al., 2010). Other local anesthetic-like drugs have been shown to exert toxic effects on skeletal muscle cells, both in vivo and in vitro (Foster and Carlson, 1980, Irwin et al., 2002, Hofmann et al., 2013). Although the exact molecular mechanism for tocainide cytotoxicity is not clear yet, we evaluated the ability of tocainide and To042 to affect viability of murine C2C12 skeletal muscle cells. Cells were exposed for 6 h to increasing concentrations of drugs (Fig. 8). From 100 μM to 1 mM, tocainide significantly reduced cell viability by about 20%. The tocainide derivative had no significant effect up to 1 μM, and induced a significant reduction of cell viability between 10 and 100 μM; the reduction was less than 12% at 100 μM. These results suggest that To042 is likely less myotoxic than tocainide; Importantly, 1 μM To042 produced no myotoxic effect, while inhibiting more than 50% of hNav1.4 channels at 50 Hz stimulation (IC50 = 0.14 μM).
Fig. 8.
Citotoxic effects of tocainide and To042 on murine C2C12 cells, expressed as percentage of cell survival after 6 h of incubation with the drug. Each bar represents the mean ± SEM from 4 to 6 experiments. Statistical analysis was performed using unpaired Student's t-test and effect was considered significant for p < 0.05.
4. Discussion
Through a series of previous SAR studies, we obtained two tocainide analogues, namely To040 and To042, showing greatly enhanced potency and use-dependent block of sodium currents recorded in frog skeletal muscle fibers (Carrieri et al., 2009, De Luca et al., 2004, De Luca et al., 2012, Muraglia et al., 2014, Talon et al., 2001). Here we show that these two compounds are also very potent blockers of human skeletal muscle voltage-gated sodium channels. Using a holding potential of −120 mV, To040 appears 9 and 43 times more potent than tocainide in blocking hNav1.4 channels at 0.1 and 10 Hz, respectively. Yet, To040 showed very similar effects to those of a benzylated beta-proline derivative of tocainide, namely To10 or NeP1 (Ghelardini et al., 2010), indicating that the constriction of the amine group in a rigid cycle does not necessarily affect the binding of drugs to hNav1.4 channels. In contrast, with the introduction of a naphthyl in place of the benzyl group, To042 appears more potent than To10 and To040 on hNav1.4 channels at both stimulation frequencies, indicating that increased hindrance and/or lipophilia on the N moiety further improves the binding of the drug to the channel. Thus To042 was 58 (0.1 Hz) and 225 (10 Hz) times more potent than its parent compound tocainide and is, so far, the most potent synthetic compound we found to block hNav1.4 channels, being slightly more potent than lubeluzole (Desaphy et al., 2013a).
The impairment of F1586C mutant inhibition by To040 and To042 compared to WT hNav1.4 demonstrates that Phe in position 1586 is involved in the binding of tocainide analogues to the channel, as for other local anesthetic-like drugs (Desaphy et al., 2012, Pless et al., 2011, Ragsdale et al., 1994, Ragsdale et al., 1996). Nevertheless, contrarily to mexiletine but similarly to lubeluzole, the use-dependence was not fully zeroed by the mutation, suggesting that the benzyl or naphthyl group in To040 or To042 may add a third pharmacophoric interaction with the receptor that enhances high-affinity binding, as previously proposed for lubeluzole (Desaphy et al., 2013a).
The huge potency and use-dependent behavior of To042 make it an interesting candidate for treatment of myotonia, a disease characterized by high-frequency firing of action potentials in skeletal muscle fibers. Our experiments in cell and animal models of myotonia support this hypothesis. In transfected cells stimulated in a myotonic–like manner, To042 blocked hNav1.4 currents with a potency similar to lubeluzole and 120 times greater than mexiletine, the current drug of choice for myotonic syndromes (Desaphy et al., 2014). In vivo, To042 was as potent as riluzole and 100 times more potent than mexiletine in reducing the time of righting reflex in myotonic rats (Desaphy et al., 2014). Waiting for more selective blockers of sodium channel subtypes, the use-dependence behavior of sodium channel blockers is thought to represent the basis for their selective action in pathological hyper-excited tissues (England and de Groot, 2009). Thus, although To042 may not be selective for the skeletal muscle Nav1.4 channel, the huge use-dependence of the compound, as expressed by the 0.1-to-10 Hz ratio of IC50 values, suggests a likely selective action on the ill organ, such as the myotonic muscle.
The toxicological studies suggest a satisfactory safety profile for To042. Mexiletine is reputedly a weak blocker of IKr/hERG currents and shows little concern regarding the QT interval prolongation (Suetterlin et al., 2015, Wang et al., 1996). In our study, the IC50 for hERG inhibition by mexiletine was 2.5 greater than that for blocking hNav1.4 in the myotonia-like condition (43 versus 17 μM, respectively). For To042, the ratio between hERG (6 μM) and hNav1.4 (0.14 μM) inhibitions was greatly accentuated by 42 fold. Thus, it is very likely that To042 would not exert any detrimental effect on hERG potassium channels at clinically-relevant doses. In rats, we observed no toxic effects of To042 in vivo either during the myotonia assay or the rotarod assay. In addition, at the same concentrations able to block hNav1.4 channels, the compound exerted little cytotoxic effects in vitro on murine muscle cells. These preliminary observations will need to be confirmed in further preclinical studies before to test To042 in humans. For instance, a particular attention should be given to blood dyscrasias and pulmonary fibrosis, which have been associated with tocainide (Gertz et al., 1986, Soff and Kadin, 1987; Oliphant and Goddard, 1988, Feinberg et al., 1990).
In conclusion, we obtained a new tocainide derivative with greatly enhanced use-dependent block of human skeletal muscle voltage-gated sodium channels. This improvement was closely paralleled by an enhanced antimyotonic activity in vivo, while preliminary toxicological data suggest a good therapeutic index. Although mexiletine is considered as a first choice treatment in both sodium and chloride channel myotonias, 20–40% of the patients obtain unsatisfactory response (Desaphy et al., 2013c, Desaphy et al., 2016, Suetterlin et al., 2015). In this context, To042 appears as a promising candidate to help increasing therapeutic options in myotonia. We also hypothesize that To042 may be of interest for other membrane excitability diseases, such as epilepsy and chronic pain, and merits attention for further preclinical studies.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The financial contribution of Association Française contre les Myopathies (grant #19027 to JFD) and Telethon-Italy (grant #GGP14096 to DCC) is gratefully acknowledged.
References
- Carrieri A., Muraglia M., Corbo F., Pacifico C. 2D- and 3D-QSAR of tocainide and mexiletine analogues acting as Nav1.4 channel blockers. Eur. J. Med. Chem. 2009;44:1477–1485. doi: 10.1016/j.ejmech.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Catalano A., Carocci A., Corbo F., Franchini C., Muraglia M., Scilimati A., De Bellis M., De Luca A., Conte Camerino D., Sinicropi M.S., Tortorella V. Constrained analogues of tocainide as potent skeletal muscle sodium channel blockers towards the development of antimyotonic agents. Eur. J. Med. Chem. 2008;43:2535–2540. doi: 10.1016/j.ejmech.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Danielsson B.R., Lansdell K., Patmore L., Tomson T. Phenytoin and phenobarbital inhibit human HERG potassium channels. Epilepsy Res. 2003;55(1–2):147–157. doi: 10.1016/s0920-1211(03)00119-0. [DOI] [PubMed] [Google Scholar]
- De Bellis M., De Luca A., Desaphy J.-F., Carbonara R., Heiny J.A., Kennedy A., Carocci A., Cavalluzzi M.M., Lentini G., Franchini C., Conte Camerino D. Combined modifications of mexiletine pharmacophores for new lead blockers of Nav1.4 channels. Biophys. J. 2013;104(2):344–354. doi: 10.1016/j.bpj.2012.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lera Ruiz M., Kraus R.L. Voltage-gated sodium channels: structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015;58(18):7093–7118. doi: 10.1021/jm501981g. [DOI] [PubMed] [Google Scholar]
- De Luca A., De Bellis M., Corbo F., Franchini C., Muraglia M., Catalano A., Carocci A., Conte Camerino D. Searching for novel anti-myotonic agents: pharmacophore requirement for use-dependent block of skeletal muscle sodium channels by N-benzylated cyclic derivatives of tocainide. Neuromuscul. Disord. 2012;22:56–65. doi: 10.1016/j.nmd.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A., Natuzzi F., Desaphy J.-F., Loni G., Lentini G., Franchini C., Tortorella V., Conte Camerino D. Molecular determinants of mexiletine structure for potent and use-dependent block of skeletal muscle sodium channels. Mol. Pharmacol. 2000;57:268–277. [PubMed] [Google Scholar]
- De Luca A., Pierno S., Liantonio A., Desaphy J.-F., Natuzzi F., Didonna M.P., Ferrannini E., Jockusch H., Franchini C., Lentini G., Corbo F., Tortorella V., Conte Camerino D. New potent mexiletine and tocainide analogues evaluated in vivo and in vitro as antimyotonic agents on myotonic ADR mouse. Neuromuscul. Disord. 2004;14:405–416. doi: 10.1016/j.nmd.2004.04.006. [DOI] [PubMed] [Google Scholar]
- De Luca A., Pierno S., Natuzzi F., Franchini C., Duranti A., Lentini G., Tortorella V., Jockusch H., Camerino D.C. Evaluation of the antimyotonic activity of mexiletine and some new analogs on sodium currents of single muscle fibers and on the abnormal excitability of the myotonic ADR mouse. J. Pharmacol. Exp. Ther. 1997;282(1):93–100. [PubMed] [Google Scholar]
- De Luca A., Talon S., De Bellis M., Desaphy J.-F., Franchini C., Lentini G., Catalano A., Corbo F., Tortorella V., Conte Camerino D. Inhibition of skeletal muscle sodium currents by mexiletine analogues: specific hydrophobic interactions rather than lipophilia per se account for drug therapeutic profile. Naunyn-Schmied. Arch. Pharmacol. 2003;367:318–327. doi: 10.1007/s00210-002-0669-0. [DOI] [PubMed] [Google Scholar]
- De Luca A., Talon S., De Bellis M., Desaphy J.-F., Lentini G., Corbo F., Scilimati A., Franchini C., Tortorella V., Conte Camerino D. Optimal requirements for high affinity and use-dependent block of skeletal muscle sodium channel by N-benzyl analogs of tocainide-like compounds. Mol. Pharmacol. 2003;64:932–945. doi: 10.1124/mol.64.4.932. [DOI] [PubMed] [Google Scholar]
- Desaphy J.-F., Camerino D.C., Tortorella V., De Luca A. Effect of mexiletine on sea anemone toxin-induced non-inactivating sodium channels of rat skeletal muscle: a model of sodium channel myotonia. Neuromuscul. Disord. 1999;9(3):182–189. doi: 10.1016/s0960-8966(98)00115-1. [DOI] [PubMed] [Google Scholar]
- Desaphy J.-F., Carbonara R., D'Amico A., Modoni A., Roussel J., Imbrici P., Pagliarani S., Lucchiari S., Lo Monaco M., Conte Camerino D. Translational approach to address therapy in myotonia permanens due to a new SCN4A mutation. Neurology. 2016;86(22):2100–2108. doi: 10.1212/WNL.0000000000002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J.-F., Carbonara R., Costanza T., Conte Camerino D. Preclinical evaluation of marketed sodium channel blockers in a rat model of myotonia discloses promising antimyotonic drugs. Exp. Neurol. 2014;255:96–102. doi: 10.1016/j.expneurol.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J.-F., Carbonara R., Costanza T., Lentini G., Cavalluzzi M.M., Bruno C., Franchini C., Conte Camerino D. Molecular dissection of lubeluzole use–dependent block of voltage-gated sodium channels discloses new therapeutic potentials. Mol. Pharmacol. 2013;83:406–415. doi: 10.1124/mol.112.080804. [DOI] [PubMed] [Google Scholar]
- Desaphy J.-F., Costanza T., Carbonara R., Conte Camerino D. In vivo evaluation of antimyotonic efficacy of β-adrenergic drugs in a rat model of myotonia. Neuropharmacology. 2013;65:21–27. doi: 10.1016/j.neuropharm.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J.-F., De Luca A., Didonna M.P., George A.L., Jr., Conte Camerino D. Different flecainide sensitivity of hNav1.4 channels and myotonic mutants explained by state-dependent block. J. Physiol. 2004;554:321–334. doi: 10.1113/jphysiol.2003.046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J.-F., De Luca A., Tortorella P., De Vito D., George A.L., Jr., Conte Camerino D. Gating of myotonic Na channel mutants defines the response to mexiletine and a potent derivative. Neurology. 2001;57:1849–1857. doi: 10.1212/wnl.57.10.1849. [DOI] [PubMed] [Google Scholar]
- Desaphy J.-F., Dipalma A., Costanza T., Carbonara R., Dinardo M.M., Catalano A., Carocci A., Lentini G., Franchini C., Conte Camerino D. Molecular insights into the local anesthetic receptor within voltage-gated sodium channels using hydroxylated analogs of mexiletine. Front. Pharmacol. 2012;3:17. doi: 10.3389/fphar.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J.-F., Dipalma A., De Bellis M., Costanza T., Gaudioso C., Delmas P., George A.L., Jr., Conte Camerino D. Involvement of voltage-gated sodium channels blockade in the analgesic effects of orphenadrine. Pain. 2009;142:225–235. doi: 10.1016/j.pain.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Desaphy J.F., Modoni A., Lomonaco M., Camerino D.C. Dramatic improvement of myotonia permanens with flecainide: a two-case report of a possible bench-to-bedside pharmacogenetics strategy. Eur. J. Clin. Pharmacol. 2013;69(4):1037–1039. doi: 10.1007/s00228-012-1414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdagi S., Subbotina J., Lees-Miller J., Guo J., Duff H.J., Noskov S.Y. Insights into the molecular mechanism of hERG1 channel activation and blockade by drugs. Curr. Med. Chem. 2010;17:3514–3532. doi: 10.2174/092986710792927886. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency . 2013. Orphan Designation (EU/3/13/1126). Mexiletine Hydrochloride for the Treatment of Non-dystrophic Myotonia.http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2013/06/WC500144749.pdf [Google Scholar]
- European Medicines Agency . 2013. Orphan Designation (EU/3/13/1189). Mexiletine Hydrochloride for the Treatment of Myotonic Disorders.http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2013/10/WC500153466.pdf [Google Scholar]
- European Medicines Agency . 2014. Orphan Designation (EU/3/14/1353). Mexiletine Hydrochloride for the Treatment of Myotonic Disorders.http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2015/01/WC500180387.pdf [Google Scholar]
- England S., de Groot M.J. Subtype-selective targeting of voltage-gated sodium channels. Br. J. Pharmacol. 2009;437:1413–1425. doi: 10.1111/j.1476-5381.2009.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg L., Travis W.D., Ferrans V., Sato N., Bernton H.F. Pulmonary fibrosis associated with tocainide: report of a case with literature review. Am. Rev. Respir. Dis. 1990;141(2):505–508. doi: 10.1164/ajrccm/141.2.505. [DOI] [PubMed] [Google Scholar]
- Foster A.H., Carlson B.M. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth. Analg. 1980;59(10):727–736. [PubMed] [Google Scholar]
- Gertz M.A., Garton J.P., Jennings W.H. Aplastic anemia due to tocainide. N. Engl. J. Med. 1986;314(9):583–584. doi: 10.1056/NEJM198602273140917. [DOI] [PubMed] [Google Scholar]
- Ghelardini C., Desaphy J.-F., Muraglia M., Corbo F., Matucci R., Dipalma A., Bertucci C., Pistolozzi M., Nesi M., Norcini M., Franchini C., Conte Camerino D. Effects of a potent analog of tocainide on hNav1.7 sodium channels and in vivo pain models: a potential candidate for treatment of neuropathic pain. Neuroscience. 2010;169:863–873. doi: 10.1016/j.neuroscience.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Hoffman E.P., Kaminski H.J. Mexiletine for treatment of myotonia: a trial triumph for rare disease networks. JAMA. 2012;308(13):1377–1378. doi: 10.1001/jama.2012.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P., Metterlein T., Bollwein G., Gruber M., Plank C., Graf B.M., Zink W. The myotoxic effect of bupivacaine and ropivacaine on myotubes in primary mouse cell culture and an immortalized cell line. Anesth. Analg. 2013;117(3):634–640. doi: 10.1213/ANE.0b013e31829e4197. [DOI] [PubMed] [Google Scholar]
- Imbrici P., Liantonio A., Camerino G.M., De Bellis M., Camerino C., Mele A., Giustino A., Pierno S., De Luca A., Tricarico D., Desaphy J.F., Conte D. Therapeutic approaches to genetic ion channelopathies and perspectives in drug discovery. Front. Pharmacol. 2016;7:121. doi: 10.3389/fphar.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin W., Fontaine E., Agnolucci L., Penzo D., Betto R., Bortolotto S., Reggiani C., Salviati G., Bernardi P. Bupivacaine myotoxicity is mediated by mitochondria. J. Biol. Chem. 2002;277(14):12221–12227. doi: 10.1074/jbc.M108938200. [DOI] [PubMed] [Google Scholar]
- Kannankeril P., Roden D.M., Darbar D. Drug-induced long QT syndrome. Pharmacol. Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalka D., Meyer M.B., Duce B.R., Elvin A.T. Kinetics of the oral antiarrhythmic lidocaine congener, tocainide. Clin. Pharmacol. Ther. 1976;19(6):757–766. doi: 10.1002/cpt1976196757. [DOI] [PubMed] [Google Scholar]
- Lee H.B., Blaufox M.D. Blood volume in the rat. J. Nucl. Med. 1985;26(1):72–76. [PubMed] [Google Scholar]
- Lo Monaco M., D'Amico A., Luigetti M., Desaphy J.F., Modoni A. Effect of mexiletine on transitory depression of compound motor action potential in recessive myotonia congenita. Clin. Neurophysiol. 2015;126(2):399–403. doi: 10.1016/j.clinph.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Matthews E., Hanna M.G. Repurposing of sodium channel antagonists as potential new anti-myotonic drugs. Exp. Neurol. 2014;261:812–815. doi: 10.1016/j.expneurol.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Muraglia M., De Bellis M., Catalano A., Carocci A., Franchini C., Carrieri A., Fortugno C., Bertucci C., Desaphy J.-F., De Luca A., Conte Camerino D., Corbo F. N-Aryl-2,6-dimethylbenzamides, a new generation of tocainide analogues as blockers of skeletal muscle voltage-gated sodium channels. J. Med. Chem. 2014;57:2589–2600. doi: 10.1021/jm401864b. [DOI] [PubMed] [Google Scholar]
- Novak K.R., Norman J., Mitchell J.R., Pinter M.J., Rich M.M. Sodium channel slow inactivation as a therapeutic target for myotonia congenita. Ann. Neurol. 2015;77(2):320–332. doi: 10.1002/ana.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant L.D., Goddard M. Tocainide-associated neutropenia and lupus-like syndrome. Chest. 1988;94(2):427–428. doi: 10.1378/chest.94.2.427. [DOI] [PubMed] [Google Scholar]
- Paul A.A., Witchel H.J., Hancox J.C. Inhibition of HERG potassium channel current by the Class 1a antiarrhythmic agent disopyramide. Biochem. Biophys. Res. Commun. 2001;280:1243–1250. doi: 10.1006/bbrc.2001.4269. [DOI] [PubMed] [Google Scholar]
- Paul A.A., Witchel H.J., Hancox J.C. Inhibition of the current of heterologously expressed HERG potassium channels by flecainide and comparison with quinidine, propafenone and lignocaine. Br. J. Pharmacol. 2002;136:717–729. doi: 10.1038/sj.bjp.0704784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistolozzi M., Fortugno C., Franchini C., Corbo F., Muraglia M., Roy M., Félix G., Bertucci C. Species-dependent binding of tocainide analogues to albumin: affinity chromatography and circular dichroism study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;968:69–78. doi: 10.1016/j.jchromb.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Pless S.A., Galpin J.D., Frankel A., Ahern C.A. Molecular basis for class Ib anti-arrhythmic inhibition of cardiac sodium channels. Nat. Commun. 2011;2:351. doi: 10.1038/ncomms1351. [DOI] [PubMed] [Google Scholar]
- Ragsdale D.S., McPhee J.C., Scheuer T., Catterall W.A. Molecular determinants of state-dependent block of Na channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale D.S., McPhee J.C., Scheuer T., Catterall W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. U. S. A. 1996;93(17):9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soff G.A., Kadin M.E. Tocainide-induced reversible agranulocytosis and anemia. Arch. Intern. Med. 1987;147(3):598–599. [PubMed] [Google Scholar]
- Statland J., Phillips L., Trivedi J.R. Muscle channelopathies. Neurol. Clin. 2014;32(3):801–815. doi: 10.1016/j.ncl.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Statland J.M., Bundy B.N., Wang Y., Rayan D.R., Trivedi J.R., Sansone V.A., Salajegheh M.K., Venance S.L., Ciafaloni E., Matthews E., Meola G., Herbelin L., Griggs R.C., Barohn R.J., Hanna M.G., Consortium for Clinical Investigation of Neurologic Channelopathies Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. JAMA. 2012;308(13):1357–1365. doi: 10.1001/jama.2012.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streib E.W. Successful treatment with tocainide of recessive generalized congenital myotonia. Ann. Neurol. 1986;19(5):501–504. doi: 10.1002/ana.410190515. [DOI] [PubMed] [Google Scholar]
- Streib E.W. Paramyotonia congenita: successful treatment with tocainide. Clinical and electrophysiologic findings in seven patients. Muscle Nerve. 1987;10(2):155–162. doi: 10.1002/mus.880100209. [DOI] [PubMed] [Google Scholar]
- Suetterlin K., Männikkö R., Hanna M.G. Muscle channelopathies: recent advances in genetics, pathophysiology and therapy. Curr. Opin. Neurol. 2014;27(5):583–590. doi: 10.1097/WCO.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Suetterlin K.J., Bugiardini E., Kaski J.P., Morrow J.M., Matthews E., Hanna M.G., Fialho D. Long-term safety and efficacy of mexiletine for patients with skeletal muscle channelopathies. JAMA Neurol. 2015;72(12):1531–1533. doi: 10.1001/jamaneurol.2015.2338. [DOI] [PubMed] [Google Scholar]
- Talon S., De Luca A., De Bellis M., Desaphy J.F., Lentini G., Scilimati A., Corbo F., Franchini C., Tortorella P., Jockusch H., Conte Camerino D. Increased rigidity of the chiral centre of tocainide favours stereoselectivity and use-dependent block of skeletal muscle Na+ channels enhancing the antimyotonic activity in vivo. Br. J. Pharmacol. 2001;134:1523–1531. doi: 10.1038/sj.bjp.0704366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile J.W., Cummins T.R. Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain syndromes. Front. Pharmacol. 2011;2:54. doi: 10.3389/fphar.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . 2010. Orphan Designation. Mexiletine for the Treatment of Nondystrophic Myotonia.https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=314410 [Google Scholar]
- Wang D.W., Kiyosue T., Sato T., Arita M. Comparison of the effects of Class-I antiarrhythmic drugs, cibenzoline, mexiletine and flecainide, on the delayed rectifier K current of Guinea-pig ventricular myocytes. J. Mol. Cell. Cardiol. 1996;28:893–903. doi: 10.1006/jmcc.1996.0084. [DOI] [PubMed] [Google Scholar]