Abstract
Largely as a result of rising obesity rates, the incidence of type 2 diabetes is escalating rapidly. Type 2 diabetes results from multi-organ dysfunctional glucose metabolism. Recent publications have highlighted hypothalamic insulin- and adipokine-sensing as a major determinant of peripheral glucose and insulin responsiveness. The preponderance of evidence indicates that the brain is the master regulator of glucose homeostasis, and that hypothalamic insulin and leptin signaling in particular play a crucial role in the development of insulin resistance. This review discusses the neuronal crosstalk between the hypothalamus, autonomic nervous system, and tissues associated with the pathogenesis of type 2 diabetes, and how hypothalamic insulin and leptin signaling are integral to maintaining normal glucose homeostasis.
Introduction
Despite over a century of research, the incidence of type 2 diabetes (T2D) continues to rise. In 2010 approximately 285 million people worldwide had diabetes, and that number could reach 438 million by 2030 (International Diabetes Federation; http://www.idf.org/). Considering the cardiovascular, renal and neurological complications associated with diabetes [1,2], these trends foretell a devastating decline in global health.
T2D is commonly thought to develop from the neck down. Impaired insulin responsiveness by peripheral tissues (adipose, skeletal muscle and liver) increases insulin release, purportedly leading to pancreatic β-cell failure in the presence of inherited β-cell abnormalities [3,4]. However, as we will describe, the central nervous system (CNS) regulates both pancreatic function and insulin sensitivity, and is therefore likely to be involved in the pathogenesis of T2D. Recent evidence suggests that the hypothalamus in particular influences autonomic systems controlling pancreatic secretion, adipose storage, thermogenesis, peripheral glucose uptake, and hepatic glucose flux. These findings emphasize a crucial role for central insulin and the adipokine leptin in the autonomic regulation of peripheral glucose and are opening a new and vital frontier for T2D research.
The progress in these areas in the past few years is largely due to new gene-targeting techniques replacing traditional reliance on pharmacological inhibitors and agonists. Pharmacological approaches had the disadvantage of being of questionable specificity, leaving their actual targets open to interpretation. However, genetic targeting has raised new concerns, including acute or developmental compensation for loss of a gene product. Occasionally, gene replacement is used to drive expression in cells that might not express a gene under normal circumstances, clouding interpretation of study results. For these reasons we examine data gathered using multiple approaches to highlight those data that have proven most robust.
Key autonomic connections contributing to peripheral glucose homeostasis
Both the hypothalamus and brainstem play a role in the regulation of glucose homeostasis. The brainstem initiates parasympathetic support for ingestion and digestion and sympathetic responses to severe energy depletion [5–7]. The hypothalamus, in turn, calibrates autonomic tone to external conditions and global homeostatic need by independently adjusting behavior, body temperature, and functions of the pancreas, liver, and cardiopulmonary system [8–10].
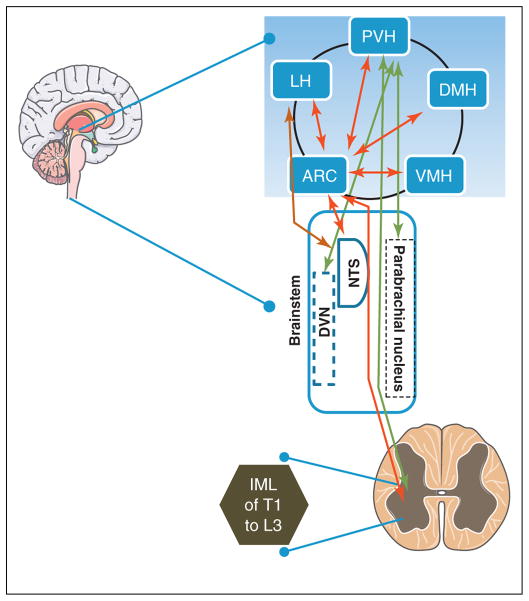
Modifications to autonomic tone are made possible by the extensive and often reciprocal connections between hypothalamic and brainstem nuclei [11–13]. The paraventricular hypothalamus (PVH) has widespread projections throughout the brainstem including to the dorsal vagal nucleus (DVN), the origin of parasympathetic preganglionic cells [14]. In addition, the PVH projects to the intermediolateral cell column of the spinal cord (IML) which contains sympathetic preganglionic fiber cell bodies [14]. Similarly, the lateral hypothalamus (LH) connects reciprocally with the nucleus tractus solitarii (NTS) and parabrachial nucleus, allowing output to sympathetic and parasympathetic systems via second-order projections. Finally, leptin-responsive proopiomelanocortin/cocaine and amphetamine-regulated transcript (POMC/CART)-expressing neurons of the arcuate nucleus (ARC), in addition to communicating with the dorsomedial hypothalamus (DMH), PVH, LH, medullary dorsal motor nucleus of the vagus (DMV), and NTS [14], project directly to the IML (Figure 1) [9,15]. Recent evidence suggests that these connections allow the hypothalamus to influence autonomic systems controlling pancreatic secretion, adipose storage, thermogenesis, peripheral glucose uptake, and hepatic glucose flux.
Figure 1. Key autonomic connections.
Previously identified intra-hypothalamic and extra-hypothalamic connections that mediate sympathetic and parasympathetic signal transduction to peripheral organs. PVH, paraventricular hypothalamus; LH, lateral hypothalamus; ARC, arcuate nucleus; VMH, ventromedial hypothalamus; DMH, dorsomedial hypothalamus; DVN, dorsal vagal nucleus; NTS, nucleus of the tractus solitarii; IML, intermediolateral cell column of the spinal cord.
Insulin and leptin signaling in the hypothalamus
That insulin has important central effects on blood glucose regulation has been recognized since the classic work of Woods and Porte [16], in which central injection of insulin in dogs was found to induce a significant increase of pancreatic insulin output. Only recently, however, have the mechanisms been explored. Whole-body insulin receptor (IR) knockdown indeed results in more pronounced hyperinsulinemia and hyperglycemia than peripheral tissue knockdown [17]. Furthermore, hypothalamic IR disruption alone is sufficient to reduce the suppression of hepatic glucose production (HGP) in response to insulin [18]. Streptozotocin (STZ)-induced diabetes causes a significant reduction in hypothalamic insulin signaling reversed by intracerebroventricular (ICV) insulin [19]. Additionally, ICV but not peripheral pretreatment with an inhibitor of PI3K (Box 1) reduced the glucose-lowering effect of peripheral insulin in STZ rats [19]. Adenovirus delivery of IRS-2 in the ARC slowed the rise in blood glucose levels in response to STZ, suggesting an acute increase in peripheral insulin sensitivity to declining insulin levels [19]. These data indicate that the hypothalamus is a significant insulin-responsive tissue and contributes to whole-body glucose homeostasis via IRS–PI3K signaling.
Box 1. Insulin and leptin signaling in brief.
Insulin
Insulin binding to IR triggers intrinsic tyrosine kinase activity resulting in tyrosine phosphorylation and activation of insulin receptor substrate (IRS) proteins. [123]. Phosphatidylinositol 3-kinase (PI3K) is sequestered to the cell membrane and is phosphorylated by IRS proteins [124]. PI3K can be composed of one of three catalytic subunits (p110α, p110β, or p110δ) and one of five regulatory subunits (p85α, p55α, p50α, p85β, or p55γ) [125]. p110/p85 heterodimers are the PI3K isoforms largely associated with insulin signaling [125,30,29,126]. PI3K phosphorylation leads to the phosphorylation and activation of protein kinase B (PKB/AKT) [127]. AKT serves as a crucial hub molecule that regulates metabolism and cell survival through its kinase activity on numerous downstream proteins in peripheral insulin-responsive tissue such as skeletal muscle [128–130] and the CNS [30,25,131]. AKT partly facilitates signal transduction through the phosphorylation and cytoplasmic sequestering of forkhead-box protein 01 (FOXO1) [130]. FOXO1 is a negative regulator of insulin signaling whose nuclear translocation has been associated with obesity and hyperphagia through altering the transcription profile of AgRP and POMC neurons [131,31,132]. Although PI3K is required for activation of the insulin signaling pathway, it questionable whether AKT is required for all downstream effects of PI3K activation; for example when activated by leptin [20,21].
Leptin
Leptin is an adipocyte-derived factor that circulates in proportion to adipose tissue mass. Leptin regulation of energy balance (calorie intake and expenditure) is mediated largely through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway [133–135]. LepR binding results in the auto-phosphorylation of JAK2 and JAK2-mediated phosphorylation of and activation of STAT3. STAT3 phosphorylation acts to regulate the transcription of neuropeptide mRNA. This leptin-mediated signaling cascade is negatively regulated by suppressor of cytokine signaling (SOCS)3 in a negative-feedback loop [136]. JAK/STAT-independent signaling, particularly leptin-induced PI3K phosphorylation, is suggested to contribute to hypothalamic-mediated insulin and glucose homeostasis [4,29,25,20,21,134,137,33].
Whereas central leptin activation of JAK/STAT signaling is well-characterized, leptin activation of classic insulin-signaling pathways, such as the PI3K–AKT pathway, could underlie many of its effects on glucose homeostasis (Box 1). For instance, ICV leptin induces PI3K assembly with IRS-1 and IRS-2 in the hypothalamus within 5 minutes of administration [20]. Furthermore, restoring leptin receptor(LepR) expression to the ARC of LepR-deficient rats with an adenovirus increased insulin sensitivity in a manner dependent on central PI3K signaling [21]. In addition, constitutive activation of AKT in the ARC produced similar increases in insulin sensitivity suggesting the involvement of PI3K-induced AKT activity [21]. More recently, ICV leptin treatment of lean rats [22] and obese, leptin-deficient (ob/ob) mice [23] improved glucose sensitivity and increased the phosphorylation of AKT in skeletal muscle, and this was dependent on hypothalamic PI3K [22] (Box 2).
Box 2. Leptin as an insulin mimetic.
Leptin can improve, and in some cases normalize, glucose levels in states of insulin-deficiency by mimicking the actions of insulin. Hyperleptinemia in insulin-deficient mice enhances phosphorylation of several protein kinases in skeletal muscle traditionally modulated by the IR (Box 1) [138]. Leptin treatment improves liver, adipose, and skeletal muscle glucose and insulin sensitivity [84,79,107,139,85]. Furthermore, treatment of diabetic rats with leptin normalizes liver protein kinase B (PKB/AKT) phosphorylation and reduces the expression of hepatic gluconeogenic genes [139]. Finally, hyperleptinemia in insulin-deficient mice restores plasma glucagon levels and normalizes the expression of genes and proteins regulating HGP [138].
Note that these actions could take place through four separate mechanisms. (i) Leptin has peripheral actions that alter insulin sensitivity and pancreatic function independent of fat mass. (ii) By acting centrally to suppress food intake and increase energy expenditure, leptin promotes decreases in fat mass and indirectly improves insulin sensitivity. (iii) Leptin also alters fat mass by influencing rates of lipolysis via autonomic pathways. (iv) Finally, leptin acts centrally to increase insulin sensitivity and glycemia independent of fat mass. Recent advances in gene-targeting techniques have begun to differentiate between these mechanisms.
Insulin and leptin could act synergistically to regulate AKT phosphorylation in the hypothalamus. ob/ob mice show a significant reduction in peripheral insulin-stimulated hypothalamic AKT phosphorylation [23]. Furthermore, leptin pretreatment enhanced the effect of insulin on hypothalamic immunostaining for AKT phosphorylation to levels close to control values [23]. To address the origin of leptin resistance in obese animals, hypothalamic AKT phosphorylation following insulin treatment was investigated in global leptin-receptor and forebrain-specific lep-tin-receptor knockout mice [23]. Both receptor mutants showed significant reductions in hypothalamic AKT phosphorylation [23]. Furthermore, ICV treatment of normal-weight rats with a leptin receptor antagonist prevented AKT phosphorylation in the ARC following central insulin treatment [23]. To study the impact of chronic leptin treatment on hypothalamic insulin signaling, metabolically normal rats were ICV infused with leptin for 14 d followed by an acute insulin ICV injection [24]. Whereas acute insulin alone increased the protein expression of IR-β and the association or IR-β with IRS-2, chronic leptin treatment abolished the acute increase in hypothalamic insulin sensitivity [24]. Furthermore, chronic leptin treatment caused an increase in IR-β assembly with SOCS-3, which was not normalized following acute insulin [24]. These data demonstrate that, in contrast to acute treatments, chronically elevated leptin levels, such as those occurring in obesity, could reduce hypothalamic insulin sensitivity.
Important hypothalamic circuits
Additional studies have begun to elucidate the specific neuronal populations through which hypothalamic insulin and leptin signaling mediate their effects on whole body glucose metabolism. Given their role in energy homeostasis, POMC and AgRP neurons have attracted attention in this regard. Xu and colleagues injected mouse lines carrying Cre recombinase under POMC or AgRP promoter control with adenovirus harboring a Cre-inducible fluorescent reporter of PI3K activity into the mediobasal hypothalamus and showed that leptin and insulin activate PI3K in POMC neurons [25]. In AgRP neurons, however, only insulin activated PI3K activity. Leptin withdrawal induced PI3K activity in orexigenic AgRP neurons, as would be expected in states of leptin deficiency or nutrient deprivation.
IR signaling in AgRP neurons appears to contribute to insulin-mediated suppression of HGP. Specific deletion of IR from AgRP neurons blunted the ability of insulin to suppress HGP during euglycemic–hyperinsulinemic clamps; however this was not observed in mice with IR deleted from POMC neurons [26]. Using a genetic knock-in approach, the selective expression of IR in AgRP neurons of L1 mice rescued insulin-mediated suppression of HGP [27]. As previously mentioned, however, this approach could induce expression of IR in neurons that would not normally express it.
We recently showed that deletion of IR from POMC neurons is not sufficient to induce obesity or overt metabolic dysfunction, in contrast to deletion of LepR from POMC neurons [28]. Furthermore, deletion of both IR and LepR from POMC neurons results in impaired glucose tolerance and insulin resistance, despite maintaining normal body weight [28]. These effects appear to be at least partially dependent on PI3K signaling [29] although others have seen no PI3K involvement [30] In addition, mice with 3-phosphoinositide dependent protein kinase-1 (PDK1) deleted specifically from POMC neurons exhibit hyperglycemia that is rescued by simultaneous expression of FOXO1 [31]. Certainly, other signaling pathways such as the JAK/STAT pathway are likely to contribute to the cumulative effects of IR and LepR deletion.
Recent patch-clamp electrophysiology and immunostaining have mapped leptin and insulin sensitivity to distinct ARC POMC cells throughout the ARC and retrochiasmatic area (RCA) demonstrating heterogeneity among POMC neurons [32]. These findings differ slightly from the electrophysiological data of Al-Qassab and colleagues [30] who found a small population of POMC neurons (three of eight) depolarized and hyperpolarized following sequential leptin and insulin treatment, respectively. That this activity was found in only a small number of neurons is supportive of segregation of leptin and insulin effects. In addition, insulin has been shown to inhibit [26] or stimulate [30,24] subpopulations of AgRP neurons. These findings could indicate another hypothalamic population with receptor heterogeneity. Future studies are needed to characterize the heterogeneity of other hypothalamic cell populations. The ability of leptin and insulin to modulate POMC neuronal excitability would be expected to alter cell firing and synaptic release, thereby modifying functions regulated by POMC neurons. However, these studies do not examine signaling leading to gene-expression changes in these neurons and therefore miss an important aspect of leptin and insulin action. Indeed, the connection between electro-physiology and whole-animal physiology needs additional exploration because altered neuronal responsiveness does not always translate into long-term effects in the animal [33].
In addition to POMC and AgRP neurons of the ARC, steroidogenic factor-1 (SF-1)-expressing neurons of the VMH have also been implicated in the regulation of glucose homeostasis [34–36]. Whereas whole-body SF-1-knockout mice are obese [36], more recent studies have found that SF-1-expressing neurons in the VMH could be vital contributors to the role of SF-1 in glucose homeostasis. Patch-clamp electrophysiological studies demonstrated that leptin depolarizes SF-1 neurons [34]. Furthermore, mice with LepR deleted from SF-1 neurons become obese on regular chow diet and have impaired energy metabolism when challenged with a high-fat diet [34]. The role of SF-1 neurons in glucose homeostasis, however, was demonstrated by specifically deleting SOCS-3, a negative regulator of leptin signaling (Box 1) from SF-1 neurons [35]. SF-1-specific SOCS-3 deletion resulted in enhanced VMH STAT-3 immunostaining, indicative of increased leptin signaling [35]. Additionally, the SOCS-3 deletion in SF-1 neurons improved glucose sensitivity and was protective against high fat diet induced hyperinsulinemia [35].
As these studies demonstrate, the hypothalamus plays a crucial role in glucose regulation, but a gap exists between these data and a clear understanding of how this regulation is accomplished. It is therefore useful to examine what is known about CNS output affecting peripheral tissues involved in glucose homeostasis. In many cases, it remains to be determined whether this output is under hypothalamic control.
Autonomic regulation of the pancreas
The pancreas contributes to peripheral glucose metabolism via secretion of the antagonistic hormones glucagon and insulin. Vagal parasympathetic outflow regulates the release of glucagon [37]. As discussed earlier, hypothalamic neurons project to areas of central parasympathetic regulation, indicating that vagal–pancreas regulation could be mediated by hypothalamic signals. The VMH in particular has been implicated. Mice lacking functional ATP-sensitive K+ channels (KATP−/−) have impaired glucose sensing in the VMH and lack the ability to detect hypoglycemia [38], suggesting that glucose sensing in the VMH contributes to the control of glucagon secretion. However, because KATP channels on pancreatic α-cells have been shown to directly regulate glucagon release [39,40], more specific VMH targeted mutations in KATP channels will be required to determine their true contribution to glucagon regulation. VMH-lesioned rats show increased parasympathetic output to the pancreas [41], suggesting that hyperactive VMH-parasympathetic stimulation contributes to the rapid onset of metabolic changes observed with VMH lesions. The VMH could also regulate pancreatic secretion by releasing glutamate, a fast-acting excitatory neurotransmitter released by VMH neurons [42]. Briefly, mice were generated that lacked Vglut2, a glutamate transporter, from SF-1 neurons [42]. These Vglut2 SF-1 mutant mice fail to increase glucose levels when challenged with acute insulin, and this was shown to be at least partly due to impaired glucagon secretion [42].
The degree of sympathetic tone is also thought to participate in managing pancreatic secretion. Early studies revealed that the VMH of obese rats had reduced levels of norepinephrine with unchanged levels of acetylcholine [43], suggesting that obesity is associated with reduced sympathetic drive, resulting in parasympathetic dominance. Rats infused with lipid or fed a high-fat diet have a significant decrease in the ratio of sympathetic to parasympathetic nerve activity and elevated glucose-stimulated insulin secretion dependent on sympathetic activation [44]. These studies suggest that a balance between sympathetic and parasympathetic output is required for normal pancreatic function. Reduced sympathetic tone could result in exaggerated parasympathetic activity and subsequent hypersecretion of pancreatic hormones. More definitive data are necessary to demonstrate which hypothalamic nuclei are involved in the orchestration of autonomic cues to the pancreas (Figure 2).
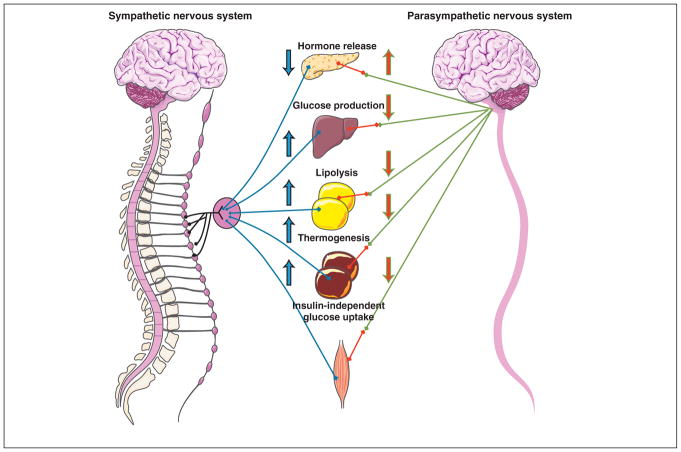
Figure 2. Autonomic regulation of key organs involved in peripheral glucose and insulin metabolism.
This diagram depicts the reciprocal nature of the autonomic nervous system in the pancreas, liver, WAT, BAT, and skeletal muscle. Pancreatic hormone release is in constant flux according to the circulating glucose and insulin levels. Hypothalamic dysfunction during states of obesity and/or insulin resistance could reduce sympathetic tone, leading to hyperactive parasympathetic outflow and a subsequent increase in pancreatic hormones. The opposite control paradigm operates in the liver where parasympathetic activation reduces hepatic glucose production. Whether increased hepatic glucose production during hepatic vagotomy is due to parasympathetic ablation or sympathetic hyperactivity is uncertain. WAT lipolysis is impaired with sympathetic denervation, whereas activation of sympathetic outflow via central melanocortin signaling enhances WAT lipolysis. BAT thermogenesis is under similar control by the autonomic nervous system. The sympathetic branch is involved in enhancing the expression of genes that promote thermogenesis. For both WAT and BAT the mechanism of reciprocity of the parasympathetic branch could operate similarly to that in the liver and pancreas. Skeletal muscle is more often associated with somatic innervation because of the voluntary nature of skeletal movement. However, sympathetic nervous system pathways play an important part in insulin-independent glucose uptake, a function that could be mediated by the VMH.
Insulin and leptin involvement
Central leptin gene therapy restores euglycemia in diabetic mice with impaired β-cell function and improves insulin production in pancreatic islets [45]. Similar findings have been observed in clinical studies when treating lypodystrophic patients with leptin [46]. By contrast, rats receiving central viral leptin gene therapy showed a suppression in circulating insulin [47]. These contrasting results could be explained by the fact that the study by Kojima and colleagues [45] and the clinical study by Ebihara and coworkers [46] utilized disease models that had a pre-existing defect in insulin secretion. On the other hand, the rats used in the study by Otukonyong and colleagues [47] were essentially physiologically normal, and the suppression in circulating insulin could have resulted from enhanced insulin sensitivity in peripheral tissues. Nonetheless, interruption of leptin-induced neural relays (by lesioning the VMH, surgically transecting descending hypothalamic tracts, or by deleting LepR in hypothalamic neurons) initiates insulin hypersecretion [48,49]. Likewise, intracerebroventricular injection of (ICV) leptin suppresses blood insulin levels and increases insulin sensitivity in diabetic rats before any discernable decrease in body weight [50,51], suggesting that leptin resistance triggers insulin hypersecretion by removing the restraint on β cells [52]. Similarly, restoring functional LepR to hypothalamic sites in LepR mutants abolishes hyperinsulinemia [53]. Finally, hyperinsulinemia and type 2 diabetes accompanied by severe leptinopenia in lypodystrophic humans and mice is completely abrogated by leptin replacement without affecting food intake and body weight [46,54–56]. These effects of central leptin on circulating insulin levels may result both from altered pancreatic function and insulin sensitivity in tissues such as skeletal muscle.
Several studies suggest that parasympathetic activity could regulate the availability of insulin-producing cells whereas the sympathetic branch modulates insulin secretion. ICV leptin infusion suppresses β-cell insulin secretion in a sympathetic nervous system (SNS)-dependent manner [57]. Parasympathetic pancreatic blockade reduces β-cell proliferation [58], however further studies are required to determine the influence of autonomic tone on β-cell survival in vivo. Central insulin also appears to act on the VMH to increase glucagon release [59], possibly as a mechanism to increase glucose levels in response to high circulating insulin. VMH–parasympathetic signaling could mediate this regulation, because others have found direct VMH–vagus connections [37,60].
Autonomic regulation of adipose tissue
Retrograde labeling of fat pads indicates that the ARC, LH, PVH, DMH, preoptic area (POA) and other areas of the hypothalamus provide sympathetic input to white adipose tissue (WAT) [61]. Sympathetic innervation of WAT is a key initiator of lipolysis [62–65], a process at least partly regulated by the melanocortin pathway [64,66–68] (Figure 2). Indeed, pharmacological melanocortin agonists promote lipolysis [67,69–73]. However, there is debate as to whether these effects are secondary to body weight or diet [73]. Nevertheless, melanocortin-induced stimulation of WAT lipolysis has been shown to involve sympathetic activation [66]. Indeed, central melanocortin agonism increases phosphorylation of perilipin A and hormone-sensitive lipase, both of which are required for lipolysis, and increases norepinephrine turnover in WAT [64]. Foster and colleagues have recently demonstrated that the PVH is not necessary for lipolysis induced by food deprivation [74]; thus, additional work is needed to clarify the contribution of other hypothalamic and extra-hypothalamic nuclei with sympathetic–WAT projections.
Brown adipose tissue (BAT), crucial for thermoregulation, is densely innervated by sympathetic neurons with strong upstream input from the mPOA and PVN and moderate input from the suprachiasmatic nucleus and LH [75]. As with WAT, pharmacological PPAR-γ activation results in increased BAT mass [62]. BAT hypertrophy is accompanied by a significant increase in uncoupling protein-1 (UCP1) mRNA, which promotes thermogenesis [62], and this regulatory pathway requires sympathetic tone [76]. Activation of hypothalamic melanocortin pathways causes an increase in BAT norepinephrine turnover [66]. In addition, a melanocortin agonist injected into the PVH results in elevated interscapular BAT temperature [77] and activates lipolytic pathways (Figure 2) [64].
Insulin and leptin involvement
Sympathetic denervation of one WAT fat pad in rats significantly reduces the effects of leptin on the intact WAT fat pads and results in WAT hypertrophy [78], indicating the actions of leptin require SNS activation. ICV leptin decreases WAT lipid storage through increasing lipolysis [79], decreasing triglyceride synthesis [80], and inhibiting lipogenesis [81]. Leptin infused into the medio-basal hypothalamus (MBH) of rats inhibits WAT lipogenesis through the activation of PI3K signaling, and this requires autonomic innervation [81]. Interestingly, VMH leptin induces apoptosis in WAT [82,83]; whether this phenomenon is related to lipolysis is unknown.
Central leptin signaling also influences BAT function. VMH or ARC infusion of leptin increases glucose uptake in BAT, an effect partially dependent on sympathetic innervation [84–86]. In addition, hypothalamic leptin transgene expression in leptin-deficient mice promotes increases in the expression of BAT genes involved in glucose uptake and thermogenesis [87].
Insulin action in the brain not only has a well-known catabolic function via control of food intake, but also an anabolic function via stimulation of lipogenesis. Brain-specific IR knockout mice develop obesity because of an increase in WAT mass [88]. Deletion of IRs from the brain in adulthood induces the loss of WAT with a concomitant increase in circulating triglyceride levels, suggesting a role for central insulin signaling in the prevention of lipodystrophy and the expansion of adipocyte size [17]. ICV insulin augments adipocyte size, fat mass and adipose lipoprotein lipase expression [17]. Finally, deletion of IRs from POMC neurons already lacking LepR results in mice with lower body weights and fat accumulation despite a reduced metabolic rate [28]. These results suggest that POMC neuronal populations can promote physiologic adaptation to a positive energy balance and elevated insulin levels by encouraging appropriate fat storage in WAT.
BAT function is also influenced by central insulin action. Infusion of insulin into the POA hyperpolarizes warm-sensitive neurons and induces BAT thermogenesis in a PI3K-dependent manner in rats [89]. This effect accompanies an increase in BAT glucose uptake and lower respiratory exchange ratio, indicating increased lipid oxidation.
Autonomic regulation of skeletal muscle
Improving skeletal muscle insulin sensitivity and non-insulin dependent glucose uptake could slow the development of T2D. VMH stimulation promotes glucose uptake in rat skeletal muscle independently of circulating insulin levels [90,91], an effect abolished by blockade of sympathetic activity [92]. Furthermore, an adrenergic agonist activates AMP-activated protein kinase (AMPK) and increases glucose uptake in myotube cultures [93,94]. AMPK is a known mediator of insulin-independent increases in glucose uptake [95] and is an attractive mechanism by which sympathetic outflow could promote glucose uptake (Figure 2).
Insulin and leptin involvement
Leptin regulates muscle metabolism via a central circuit because injections into the hypothalamus increase SNS outflow to muscle [96], thereby stimulating glucose uptake [97] and fatty acid oxidation [98,99]. Increases in glucose uptake occur in skeletal muscle when leptin is delivered specifically to the VMH but not to the ARC, and are inhibited by a melanocortin receptor antagonist [85]. Recently, the NTS and the RCA of the hypothalamus were identified as leptin-responsive sites that control CNS output to muscle [100]. The effects of hypothalamic leptin on fatty acid oxidation and glucose uptake in muscle might be due to sympathetic activation of AMPK in myocytes [94,99]. AMPK activation increases PGC1-α expression [101], a regulator of mitochondrial biogenesis and contributor to fatty acid oxidation in skeletal muscle [102,103]. Leptin injected into the lateral ventricle of rats improves tolerance to glucose, increases PGC1-α expression, and AKT, AMPK, acetyl-CoA carboxylase (ACC) and JAK2 phosphorylation in the soleus muscle [22]. These effects require hypothalamic activation of JAK2 and PI3K and are mediated by sympathetic output [22]. Therefore, central resistance to leptin could contribute to reduced AMPK and PGC1-α activation in skeletal muscle, leading to low fuel oxidation and the development of insulin resistance.
The effects of ICV insulin infusion on skeletal muscle metabolic function are under-characterized. In contrast to central infusion of leptin, ICV insulin increases insulin-stimulated muscle glycogen synthesis [104] and hypothalamic AMPK phosphorylation [89,105]. Therefore, hypothalamic AMPK could serve as a main signaling molecule mediating the differential effects of leptin and insulin on skeletal muscle glucose metabolism. Similarly, AMPK activity in POMC and AgRP neurons has contrasting effects on whole-body energy metabolism [24]. It would be beneficial for future studies to characterize the role of POMC and AgRP AMPK expression in the regulation of skeletal muscle glucose handling.
Autonomic regulation of the liver
Understanding the mechanisms involved in liver insulin responsiveness is crucial because hepatic insulin resistance increases HGP, promoting hyperglycemia. Vagal activation decreases blood glucose levels by inhibiting hepatic enzymes involved in gluconeogenesis and by activating enzymes promoting glycogen synthesis [106], effects abolished by vagotomy (Figure 2) [107,108]. Vagal activation could result from detection of (i) elevated hepatic portal glucose concentration or (ii) general hyperglycemia. The latter can activate potassium ATP-sensitive K+ (KATP) channels in the hypothalamus. KATP channel activation in rats decreases HGP, an effect mediated by vagal efferent fibers [109,110]. ICV neuropeptide Y (NPY) acutely induces hepatic insulin resistance via activation of sympathetic output to the liver [111], whereas selective parasympathetic denervation had no effect.
Insulin and leptin involvement
ICV administration of leptin acutely regulates intrahepatic glucose partitioning by simultaneously increasing gluconeogenesis but decreasing glycogenolysis [112,113]. Whereas early studies showed the net effect to be no change in HGP, under other circumstances HPG has shown overall alteration. ob/ob mice receiving ICV leptin under hyperinsulinemic–euglycemic clamps display a substantial reduction in insulin-stimulated HGP [114]. In diet-induced obese rats, ICV leptin decreases glycogenolysis without increasing gluconeogenesis, leading to a restoration of hepatic insulin sensitivity [115]. These effects appear to involve a melanocortin-dependent pathway leading to stimulation of gluconeogenesis and a mel-anocortin-independent pathway causing inhibition of glycogenolysis [116].
Of the hypothalamic nuclei, the ARC seems to play the most important role in the control of hepatic function by leptin. Restoration of leptin signaling in the ARC of LepR-deficient mice leads to a modest decrease in body weight and food intake, but it markedly improves hyperinsulinemia and blood glucose levels [117]. ARC-induced expression of the LepR is associated with reduced expression of hepatic gluconeogenic genes, together with enhanced liver insulin signaling [107]. Hepatic vagotomy abolishes these effects, providing further support for parasympathetic involvement. These effects require STAT3 signaling because hepatic insulin resistance is equally severe in s/s (lacking only leptin-induced STAT3 signaling) and db/db mice (lacking all LepR signaling), as assessed by euglycemic clamp studies [118]. However, as discussed earlier, leptin also activates the insulin-sensitive PI3K signaling cascade. This pathway is implicated in the regulatory role of central insulin on HGP, suggesting that PI3K signaling could also mediate some effects of leptin.
Insulin signaling in the hypothalamus plays a crucial role in the regulation of HGP and glucose disposal [18,119,126]. ICV insulin or an insulin mimetic suppress HGP independently of changes in circulating insulin and glucose levels in rats [119]. In addition, hepatic vagotomy and sympathectomy independently block the inhibitory effect of central insulin on HGP [109,111]. ICV insulin administered to insulin-deficient rats significantly improves liver insulin signaling but reduces HGP [120]. Finally, insulin acting in the brain leads to hepatic IL-6 induction followed by activation of STAT3, which is required for suppression of gluconeogenesis [121].
A detailed understanding of the neuronal network by which insulin controls HGP is beginning to emerge. Specific deletion of IRs from POMC and AgRP neurons has little effect on basal glucose regulation, whereas mice lacking IRs in AgRP neurons fail to suppress HGP during euglycemic–hyperinsulinemic clamps [26]. Similarly, restoration of insulin action in AgRP neurons, but not in POMC neurons, normalizes insulin suppression of HGP in mice with reduced IR expression in the hypothalamus [27]. Mice lacking IRs in AgRP neurons have reduced insulin-stimulated IL-6 and increased hepatic glucose-6-phosphatase expression [26]. Indeed, neuronal IR activation directly regulates hepatic IL-6 by a mechanism involving AgRP-expressing neurons in the hypothalamus [121].
The ability of systemic hyperinsulinemia to suppress HGP is impaired by the ICV infusion of either an antibody against insulin or a PI3K inhibitor [119]. Furthermore, constitutive activation of AKT in the MBH of insulin-resistant or -deficient rats results in improved insulin sensitivity [21,19]. Prolonged activation of p70S6K (downstream in the PI3K pathway) by insulin leads to inhibition of insulin signaling via negative feedback to IRS-1, and is restored by suppressing p70S6K following high-fat feeding [122].
Based on the findings that distinct POMC cells express insulin and leptin receptors [32], we and collaborators generated mice with insulin and leptin receptors deleted from POMC neurons [28]. The combined deletion resulted in greater metabolic disruption compared to mice with deletion of only LepR or IRs from POMC neurons [28]. POMC double mutants have impaired insulin tolerance and hyperinsulinemia in response to glucose challenge. In addition, they show reduced glucose infusion rates and a failure to exhibit a suppression of hepatic glucose production during hyperinsulinemic–euglycemic clamp experiments [28]. Taken together with the aforementioned studies, these results demonstrate that the combination of hypothalamic leptin and insulin signaling is required to maintain hepatic glucose control.
Concluding remarks
The studies reviewed here reveal the integral role of hypothalamic insulin and leptin signaling in the regulation of the many aspects of glucose homeostasis. These effects are probably mediated by hypothalamic projections that regulate the outflow and balance of the autonomic nervous system to peripheral tissues. Hypothalamic insulin-responsiveness is intricately linked to the regulation of HGP, skeletal muscle glycogen synthesis, BAT thermogenesis and WAT lipolysis, and pancreatic glucagon secretion. Hypothalamic leptin signaling contributes to the regulation of hepatic gluconeogenesis and insulin sensitivity, skeletal-muscle lipid oxidation and glucose uptake/utilization, BAT glucose uptake and WAT lipolysis, and pancreatic insulin secretion (Figure 3). These central effects of leptin, coupled with its peripheral insulin-sensitizing and lipolytic effects, make leptin administration a tantalizing approach for improving glucose regulation in severely insulin-resistant patients if central leptin resistance and increased adiposity can be avoided.
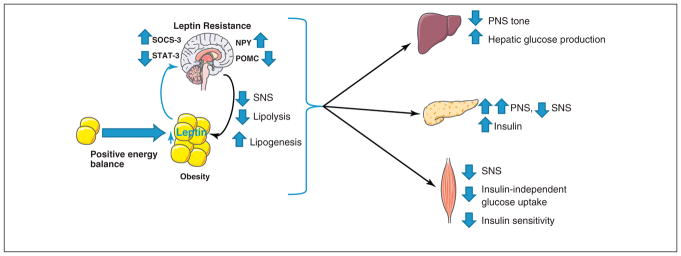
Figure 3. Proposed mechanism of hypothalamus-mediated glucose and insulin resistance.
With long-term exposure to a positive energy balance, WAT depots expand. Leptin levels increase in proportion to adipose expansion, resulting in central leptin resistance. Central leptin resistance alters the neuropeptide environment favoring orexigenic peptides such as NPY in the hypothalamus. The altered neuropeptide environment results in altered parasympathetic and sympathetic outflow: increased hepatic glucose production, increased insulin production, reduced insulin-independent glucose production and skeletal muscle insulin resistance, and reduced adipose lipolysis. As circulating insulin levels rise, adipose, liver, pancreas, and hypothalamus become insulin-resistant, and this exacerbates the state of metabolic dysfunction. PNS, parasympathetic nervous system; SNS, sympathetic nervous system; SOCS-3, suppressor of cytokine signaling-3; STAT-3, signal transducer and activator of transcription-3; NPY, neuropeptide Y; POMC, proopiomelanocortin.
The role of the brain as a master regulator of glucose homeostasis must be recognized for a complete understanding of normal and perturbed glucose homeostasis. Additional work to determine the hypothalamic contribution to the development and course of T2D is vitally needed. Future studies must focus on achieving a better understanding of the neuronal pathways that influence glucose handling by multiple tissues. Such work will facilitate the development of better-targeted pharmacological and therapeutic interventions to prevent the development of T2D and its associated comorbidities.
Acknowledgments
We would like to thank Silvana Obici, Kevin W. Williams, Thomas J. McLoughlin, Tamara Castaneda, and Sonia M. Najjar for providing comments on this manuscript while in preparation. The figures in this paper were produced using Servier Medical Art (http://www.servier.com/).
References
- 1.Fritschi C, Richlin D. The metabolic syndrome–early action to decrease risks for cardiovascular disease. AAOHN J. 2004;52:320–322. [PubMed] [Google Scholar]
- 2.Roper NA, et al. Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care. 2002;25:43–48. doi: 10.2337/diacare.25.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Ioannidis I. The road from obesity to type 2 diabetes. Angiology. 2008;59:39S–43S. doi: 10.1177/0003319708318583. [DOI] [PubMed] [Google Scholar]
- 4.Grarup NT, et al. Physiologic characterization of type 2 diabetes-related loci. Curr Diab Rep. 2010;10:485–497. doi: 10.1007/s11892-010-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes MR, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng HL, et al. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol. 2010;298:R720–728. doi: 10.1152/ajpregu.00619.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150:1705–1711. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris RB, et al. Leptin responsiveness in chronically decerebrate rats. Endocrinology. 2007;148:4623–4633. doi: 10.1210/en.2006-1565. [DOI] [PubMed] [Google Scholar]
- 9.Buijs RM, et al. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Myers MG, et al. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes MR, et al. Dorsal hindbrain 5′-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology. 2009;150:2175–2182. doi: 10.1210/en.2008-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer SE, et al. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res. 1990;534:149–169. doi: 10.1016/0006-8993(90)90125-u. [DOI] [PubMed] [Google Scholar]
- 14.Jobst EE, et al. The electrophysiology of feeding circuits. Trends Endocrinol Metab. 2004;15:488–499. doi: 10.1016/j.tem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Elias CF, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 16.Chen M, et al. Effect of cerebral intraventricular insulin on pancreatic insulin secretion in the dog. Diabetes. 1975;24:910–914. doi: 10.2337/diab.24.10.910. [DOI] [PubMed] [Google Scholar]
- 17.Koch L, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obici S, et al. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 19.Gelling RW, et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3:67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Carvalheira JB, et al. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res. 2005;13:48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]
- 21.Morton GJ, et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Roman EA, et al. Central leptin action improves skeletal muscle AKT AMPK, and PGC1 alpha activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol. 2010;314:62–69. doi: 10.1016/j.mce.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Koch C, et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci. 2010;30:16180–16187. doi: 10.1523/JNEUROSCI.3202-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgos-Ramos E, et al. Chronic central leptin infusion modifies the response to acute central insulin injection by reducing the interaction of the insulin receptor with IRS2 and increasing its association with SOCS3. J Neurochem. 2011;117:175–185. doi: 10.1111/j.1471-4159.2011.07191.x. [DOI] [PubMed] [Google Scholar]
- 25.Xu AW, et al. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konner AC, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Lin HV, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes. 2010;59:337–346. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill JW, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill JW, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150:4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Qassab H, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belgardt BF, et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Williams KW, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill JW, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, et al. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149:5654–5661. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majdic G, et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 37.Rossi J, et al. Parasympathetic innervation and function of endocrine pancreas requires the glial cell line-derived factor family receptor alpha2 (GFRalpha2) Diabetes. 2005;54:1324–1330. doi: 10.2337/diabetes.54.5.1324. [DOI] [PubMed] [Google Scholar]
- 38.Miki T, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald PE, et al. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gromada J, et al. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse alpha-cells. Diabetes. 2004;53(Suppl 3):S181–189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- 41.Paes AM, et al. Acetylcholinesterase activity changes on visceral organs of VMH lesion-induced obese rats. Int J Neurosci. 2006;116:1295–1302. doi: 10.1080/00207450600920910. [DOI] [PubMed] [Google Scholar]
- 42.Tong Q, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi A, et al. Decrease of norepinephrine and preservation of acetylcholine in the hypothalamus of VMH obese rats. Brain Res Bull. 1995;36:97–99. doi: 10.1016/0361-9230(94)00173-x. [DOI] [PubMed] [Google Scholar]
- 44.Magnan C, et al. Lipid infusion lowers sympathetic nervous activity and leads to increased beta-cell responsiveness to glucose. J Clin Invest. 1999;103:413–419. doi: 10.1172/JCI3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojima S, et al. Central leptin gene therapy, a substitute for insulin therapy to ameliorate hyperglycemia and hyperphagia, and promote survival in insulin-deficient diabetic mice. Peptides. 2009;30:962–966. doi: 10.1016/j.peptides.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Ebihara K, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92:532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 47.Otukonyong EE, et al. Central leptin differentially modulates ultradian secretory patterns of insulin, leptin and ghrelin independent of effects on food intake and body weight. Peptides. 2005;26:2559–2566. doi: 10.1016/j.peptides.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Dube MG, et al. Disruption in neuropeptide Y and leptin signaling in obese ventromedial hypothalamic-lesioned rats. Brain Res. 1999;816:38–46. doi: 10.1016/s0006-8993(98)00985-8. [DOI] [PubMed] [Google Scholar]
- 49.Bagnasco M, et al. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin. Obes Res. 2003;11:1463–1470. doi: 10.1038/oby.2003.196. [DOI] [PubMed] [Google Scholar]
- 50.Hidaka S, et al. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J. 2002;16:509–518. doi: 10.1096/fj.01-0164com. [DOI] [PubMed] [Google Scholar]
- 51.Lin CY, et al. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282:E1084–1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- 52.Van Heek M, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keen-Rhinehart E, et al. AAV-mediated leptin receptor installation improves energy balance and the reproductive status of obese female Koletsky rats. Peptides. 2005;26:2567–2578. doi: 10.1016/j.peptides.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 54.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 55.Petersen KF, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi ZQ, et al. Intracerebroventricular administration of leptin markedly enhances insulin sensitivity and systemic glucose utilization in conscious rats. Metabolism. 1998;47:1274–1280. doi: 10.1016/s0026-0495(98)90336-5. [DOI] [PubMed] [Google Scholar]
- 57.Park S, et al. Central infusion of leptin improves insulin resistance and suppresses beta-cell function, but not beta-cell mass, primarily through the sympathetic nervous system in a type 2 diabetic rat model. Life Sci. 2010;86:854–862. doi: 10.1016/j.lfs.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Lausier J, et al. Vagal control of pancreatic beta-cell proliferation. Am J Physiol Endocrinol Metab. 2010;299:E786–793. doi: 10.1152/ajpendo.00202.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paranjape SA, et al. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes. 2010;59:1521–1527. doi: 10.2337/db10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, et al. Hypothalamic regulation of pancreatic secretion is mediated by central cholinergic pathways in the rat. J Physiol. 2003;552:571–587. doi: 10.1113/jphysiol.2003.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bamshad M, et al. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 62.Festuccia WT, et al. Peroxisome proliferator-activated receptor-gamma-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology. 2008;149:2121–2130. doi: 10.1210/en.2007-1553. [DOI] [PubMed] [Google Scholar]
- 63.Giordano A, et al. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem. 2005;53:679–687. doi: 10.1369/jhc.4A6566.2005. [DOI] [PubMed] [Google Scholar]
- 64.Shrestha YB, et al. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2010;299:R140–149. doi: 10.1152/ajpregu.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song CK, et al. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R501–511. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brito MN, et al. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 67.Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song CK, et al. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 69.Calton MA, et al. Association of functionally significant Melanocortin-4 but not Melanocortin-3 receptor mutations with severe adult obesity in a large North American case-control study. Hum Mol Genet. 2009;18:1140–1147. doi: 10.1093/hmg/ddn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chai B, et al. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides. 2009;30:1098–1104. doi: 10.1016/j.peptides.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong JZ, et al. Novel melanocortin-4 receptor agonists that decrease food intake and body weight. Adv Exp Med Biol. 2009;611:485–486. doi: 10.1007/978-0-387-73657-0_209. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, et al. Melanocortin potentiates leptin-induced STAT3 signaling via MAPK pathway. J Neurochem. 2009;110:390–399. doi: 10.1111/j.1471-4159.2009.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutton GM, et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster MT, et al. Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 2010;18:682–689. doi: 10.1038/oby.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bamshad M, et al. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 76.Festuccia WT, et al. Basal adrenergic tone is required for maximal stimulation of rat brown adipose tissue UCP1 expression by chronic PPAR-gamma activation. Am J Physiol Regul Integr Comp Physiol. 2010;299:R159–167. doi: 10.1152/ajpregu.00821.2009. [DOI] [PubMed] [Google Scholar]
- 77.Song CK, et al. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008;295:R417–428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rooks CR, et al. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol. 2005;289:R92–102. doi: 10.1152/ajpregu.00858.2004. [DOI] [PubMed] [Google Scholar]
- 79.Tajima D, et al. Acute central infusion of leptin modulates fatty acid mobilization by affecting lipolysis and mRNA expression for uncoupling proteins. Exp Biol Med (Maywood) 2005;230:200–206. doi: 10.1177/153537020523000306. [DOI] [PubMed] [Google Scholar]
- 80.Lin J, et al. CNS melanocortin and leptin effects on stearoyl-CoA desaturase-1 and resistin expression. Biochem Biophys Res Commun. 2003;311:324–328. doi: 10.1016/j.bbrc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Buettner C, et al. Leptin controls adipose tissue lipogenesis via central STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gullicksen PS, et al. Adipose tissue cellularity and apoptosis after intracerebroventricular injections of leptin and 21 days of recovery in rats. Int J Obes Relat Metab Disord. 2003;27:302–312. doi: 10.1038/sj.ijo.0802205. [DOI] [PubMed] [Google Scholar]
- 83.Hamrick MW, et al. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007;327:133–141. doi: 10.1007/s00441-006-0312-3. [DOI] [PubMed] [Google Scholar]
- 84.Kamohara S, et al. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 85.Toda C, et al. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58:2757–2765. doi: 10.2337/db09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haque MS, et al. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706. [DOI] [PubMed] [Google Scholar]
- 87.Ueno N, et al. Leptin transgene expression in the hypothalamus enforces euglycemia in diabetic, insulin-deficient nonobese Akita mice and leptin-deficient obese ob/ob mice. Peptides. 2006;27:2332–2342. doi: 10.1016/j.peptides.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Bruning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 89.Sanchez-Alavez M, et al. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59:43–50. doi: 10.2337/db09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimazu T, et al. Role of the hypothalamus in insulin-independent glucose uptake in peripheral tissues. Brain Res Bull. 1991;27:501–504. doi: 10.1016/0361-9230(91)90149-e. [DOI] [PubMed] [Google Scholar]
- 91.Sudo M, et al. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am J Physiol. 1991;261:E298–303. doi: 10.1152/ajpendo.1991.261.3.E298. [DOI] [PubMed] [Google Scholar]
- 92.Minokoshi Y, et al. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res. 1994;649:343–347. doi: 10.1016/0006-8993(94)91085-5. [DOI] [PubMed] [Google Scholar]
- 93.Tanishita T, et al. The beta3-adrenergic agonist BRL37344 increases glucose transport into L6 myocytes through a mechanism different from that of insulin. J Biochem. 1997;122:90–95. doi: 10.1093/oxfordjournals.jbchem.a021744. [DOI] [PubMed] [Google Scholar]
- 94.Hutchinson DS, Bengtsson T. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells: mediation by alpha1-adrenoceptors causing glucose uptake. Diabetes. 2006;55:682–690. doi: 10.2337/diabetes.55.03.06.db05-0901. [DOI] [PubMed] [Google Scholar]
- 95.Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes. 2009;58:1096–1104. doi: 10.2337/db08-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dunbar JC, et al. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 97.Minokoshi Y, et al. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 98.Akasaka Y, et al. Chronic leptin treatment stimulates lipid oxidation in immortalized and primary mouse skeletal muscle cells. Biochim Biophys Acta. 2009;1791:103–109. doi: 10.1016/j.bbalip.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 99.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 100.Babic T, et al. Innervation of skeletal muscle by leptin receptor-containing neurons. Brain Res. 2010;1345:146–155. doi: 10.1016/j.brainres.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Irrcher I, et al. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296:C116–123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 102.Jager S, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rohas LM, et al. A fundamental system of cellular energy homeostasis regulated by PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:7933–7938. doi: 10.1073/pnas.0702683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perrin C, et al. Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, controls muscle glycogen synthesis. Endocrinology. 2004;145:4025–4033. doi: 10.1210/en.2004-0270. [DOI] [PubMed] [Google Scholar]
- 105.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 106.Shimazu T. Regulation of glycogen metabolism in liver by the autonomic nervous system. V. Activation of glycogen synthetase by vagal stimulation. Biochim Biophys Acta. 1971;252:28–38. doi: 10.1016/0304-4165(71)90089-4. [DOI] [PubMed] [Google Scholar]
- 107.German J, et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150:4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsuhisa M, et al. Important role of the hepatic vagus nerve in glucose uptake and production by the liver. Metabolism. 2000;49:11–16. doi: 10.1016/s0026-0495(00)90538-9. [DOI] [PubMed] [Google Scholar]
- 109.Pocai A, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 110.Acosta-Martinez M, Levine JE. Regulation of KATP channel subunit gene expression by hyperglycemia in the mediobasal hypothalamus of female rats. Am J Physiol Endocrinol Metab. 2007;292:E1801–1807. doi: 10.1152/ajpendo.00700.2006. [DOI] [PubMed] [Google Scholar]
- 111.van den Hoek AM, et al. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304–2310. doi: 10.2337/db07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu L, et al. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem. 1998;273:31160–31167. doi: 10.1074/jbc.273.47.31160. [DOI] [PubMed] [Google Scholar]
- 113.Rossetti L, et al. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272:27758–27763. doi: 10.1074/jbc.272.44.27758. [DOI] [PubMed] [Google Scholar]
- 114.van den Hoek AM, et al. Leptin deficiency per se dictates body composition and insulin action in ob/ob mice. J Neuroendocrinol. 2008;20:120–127. doi: 10.1111/j.1365-2826.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- 115.Pocai A, et al. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- 116.Gutierrez-Juarez R, et al. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004;279:49704–49715. doi: 10.1074/jbc.M408665200. [DOI] [PubMed] [Google Scholar]
- 117.Coppari R, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 118.Buettner C, et al. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Obici S, et al. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 120.Park S, et al. Long-term intracerebroventricular infusion of insulin, but not glucose, modulates body weight and hepatic insulin sensitivity by modifying the hypothalamic insulin signaling pathway in type 2 diabetic rats. Neuroendocrinology. 2009;89:387–399. doi: 10.1159/000197974. [DOI] [PubMed] [Google Scholar]
- 121.Inoue H, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Ono H, et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 124.Backer JM, et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vanhaesebroeck B, et al. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 126.Tups A, et al. Both p110alpha and p110beta isoforms of phosphatidylinositol 3-OH-kinase are required for insulin signalling in the hypothalamus. J Neuroendocrinol. 2010;22:534–542. doi: 10.1111/j.1365-2826.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 127.Giraud J, et al. Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J Biol Chem. 2004;279:3447–3454. doi: 10.1074/jbc.M308631200. [DOI] [PubMed] [Google Scholar]
- 128.Sarbassov DD, et al. Phosphorylation and regulation of Akt/ PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 129.Southgate RJ, et al. FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem. 2007;282:21176–21186. doi: 10.1074/jbc.M702039200. [DOI] [PubMed] [Google Scholar]
- 130.Stitt TN, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 131.Iskandar K, et al. PDK-1/FoxO1 pathway in POMC neurons regulates Pomc expression and food intake. Am J Physiol Endocrinol Metab. 2010;298:E787–798. doi: 10.1152/ajpendo.00512.2009. [DOI] [PubMed] [Google Scholar]
- 132.Kitamura T, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 133.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 134.Jiang L, et al. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J Biol Chem. 2008;283:28066–28073. doi: 10.1074/jbc.M805545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morton GJ, et al. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab. 2009;297:E202–210. doi: 10.1152/ajpendo.90865.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ernst MB, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bates SH, et al. Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 138.Yu X, et al. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.German JP, et al. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626–1634. doi: 10.2337/db09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]