Abstract
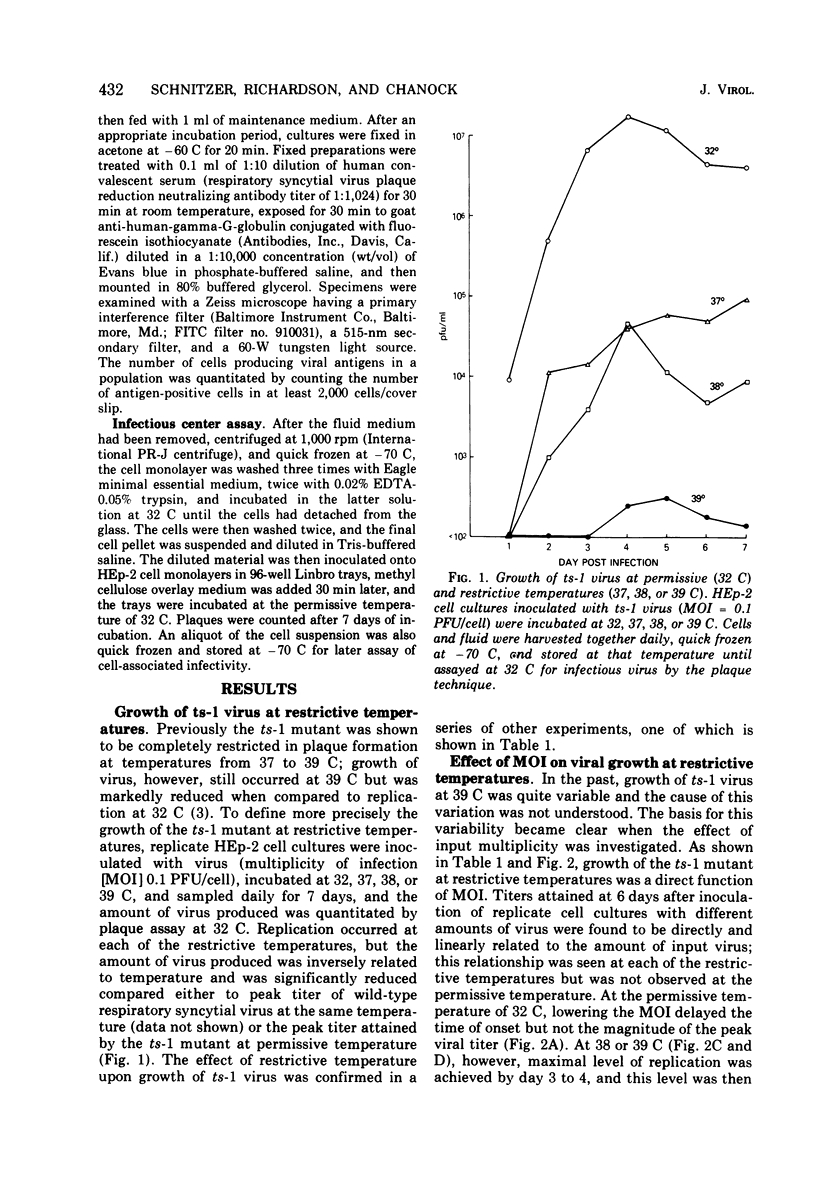
An in vitro study was performed to define in greater detail those factors which favored the growth of the ts-1 mutant of respiratory syncytial virus under restrictive conditions and the emergence of genetically altered virus with decreased temperature sensitivity. Replication of ts-1 occurred at each of the restrictive temperatures of 37, 38, and 39 C, even through plaque formation was not observed. The level of virus growth under restrictive conditions was inversely related to the incubation temperature and directly related to the multiplicity of infection. These relationships appeared to reflect the effect of restrictive temperature in reducing the quantity of virus produced and released from an infected cell. Under restrictive conditions the production of genetically altered virus which exhibited reduced temperature sensitivity was directly related to the multiplicity of infection and inversely related to temperature. Production of genetically altered virus was not observed under permissive conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLE F. E., Jr, HETRICK F. M. PERSISTENT INFECTION OF HUMAN CONJUNCTIVA CELL CULTURES BY MYXOVIRUS PARAINFLUENZA 3. Can J Microbiol. 1965 Jun;11:513–521. doi: 10.1139/m65-068. [DOI] [PubMed] [Google Scholar]
- Flamand A. Genetical behaviour of vesicular stomatitis virus during successive passages at high and low temperatures. Mutat Res. 1973 Feb;17(2):177–184. doi: 10.1016/0027-5107(73)90164-4. [DOI] [PubMed] [Google Scholar]
- Gharpure M. A., Wright P. F., Chanock R. M. Temperature-sensitive mutants of respiratory syncytial virus. J Virol. 1969 Apr;3(4):414–421. doi: 10.1128/jvi.3.4.414-421.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes D. S., Kim H. W., Parrott R. H., Camargo E., Chanock R. M. Genetic alteration in a temperature-sensitive mutant of respiratory syncytial virus after replication in vivo. Proc Soc Exp Biol Med. 1974 Apr;145(4):1158–1164. doi: 10.3181/00379727-145-37972. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Wright P. F., Hetrick F. M., Chanock R. M. Electron microscopic studies of respiratory syncytial temperature-sensitive mutants. Arch Gesamte Virusforsch. 1973;41(3):248–258. doi: 10.1007/BF01252772. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Wright P., Hodes D., Chanock R. M., Parrott R. H. Safety and antigenicity of temperature sensitive (TS) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973 Jul;52(1):56–63. [PubMed] [Google Scholar]
- Kimura Y., Ito Y., Shimokata K., Nishiyama Y., Nagata I. Temperature-sensitive virus derived from BHK cells persistently infected with HVJ (Sendai virus). J Virol. 1975 Jan;15(1):55–63. doi: 10.1128/jvi.15.1.55-63.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P., Duff R., Rapp F. Latency of human measles virus in hamster cells. J Virol. 1972 Nov;10(5):995–1001. doi: 10.1128/jvi.10.5.995-1001.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Arbeter A. M., Stahl M. K., Orr I. A., Hodes D. S., Ellis E. F. Attenuated respiratory syncytial virus vaccines in asthmatic children. Pediatr Res. 1974 Jul;8(7):689–696. doi: 10.1203/00006450-197407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Selection of temperature-sensitive mutants during persistent infection: role in maintenance of persistent Newcastle disease virus infections of L cells. J Virol. 1973 Sep;12(3):481–491. doi: 10.1128/jvi.12.3.481-491.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Koshelnyk K. A., Stollar V. Temperature-sensitive virus from Aedes albopictus cells chronically infected with Sindbis virus. J Virol. 1974 Feb;13(2):439–447. doi: 10.1128/jvi.13.2.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O. Adventitious viruses in cell cultures. Prog Med Virol. 1970;12:302–336. [PubMed] [Google Scholar]
- Valentine R. C., Ward R., Strand M. The replication cycle of RNA bacteriophages. Adv Virus Res. 1969;15:1–59. doi: 10.1016/s0065-3527(08)60873-8. [DOI] [PubMed] [Google Scholar]
- WALKER D. L., HINZE H. C. A carrier state of mumps virus in human conjunctiva cells. I. General characteristics. J Exp Med. 1962 Nov 1;116:739–750. doi: 10.1084/jem.116.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L. THE VIRAL CARRIER STATE IN ANIMAL CELL CULTURES. Prog Med Virol. 1964;6:111–148. [PubMed] [Google Scholar]
- Wright P. F., Gharpure M. A., Hodes D. S., Chanock R. M. Genetic studies of respiratory syncytial virus temperature-sensitive mutants. Arch Gesamte Virusforsch. 1973;41(3):238–247. doi: 10.1007/BF01252771. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Woodend W. G., Chanock R. M. Temperature-sensitive mutants of respiratory syncytial virus: in-vivo studies in hamsters. J Infect Dis. 1970 Dec;122(6):501–512. doi: 10.1093/infdis/122.6.501. [DOI] [PubMed] [Google Scholar]
- Wright P. F., v Mills J., Chanock R. M. Evaluation of a temperature-sensitive mutant of respiratory syncytial virus in adults. J Infect Dis. 1971 Nov;124(5):505–511. doi: 10.1093/infdis/124.5.505. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Bogomolova N. N., Gavrilov V. I., Andzhaparidze O. G., Deryabin P. G., Astakhova A. N. Infectious DNA of tick-borne encephalitis virus. Arch Gesamte Virusforsch. 1974;45(3):215–224. doi: 10.1007/BF01249684. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Parfanovich M. I. Integration of measles virus nucleic acid into the cell genome. Arch Gesamte Virusforsch. 1974;45(3):225–234. doi: 10.1007/BF01249685. [DOI] [PubMed] [Google Scholar]



