Abstract
Human mouse double minute 2 (Mdm2) plays an essential role in the regulation of the tumor suppressor p53. The G/G variant of SNP309 was shown to increase Mdm2 mRNA/protein expression and to be associated with an increased risk and earlier onset of different cancers in Asian populations. However, the frequency and impact of these G/G variants have not been studied in Caucasian renal cell carcinoma (RCC) patients. Therefore, we analyzed an unselected German cohort of 197 consecutive RCC patients and detected the G/G variant in 18 (9.1%) patients, the G/T variant in 116 (58.9%) patients and the T/T variant in 63 (32.0%) patients. Studying the association between age at tumor onset and SNP309 genotypes, no correlation was detected in the entire RCC cohort or among the male RCC patients. However, the female G/G patients (median age 59.5 years) were diagnosed 13.5 years earlier than the T/T females (median age 73 years). When separating all females into two groups at their median age (68 years), 7 and 1 patients with the G/G variant and 9 and 13 patients with the T/T variant were noted in these age groups (P=0.024). To study the age dependency of tumor onset further, a second, age-selected cohort of 205 RCC patients was investigated, which comprised especially young and old patients. Interestingly, the G/G type occurred more often at lower tumor stages and tumor grades compared with higher stages (P=0.039 and 0.004, respectively). In females, the percentage of the G/G variant was only slightly higher in the younger age group, whereas in males, the percentage of the G/G variant was remarkably higher in the younger age group (19.4% vs 8.0%). In summary, female Caucasian RCC patients with the MDM2 SNP309 G/G genotype showed significantly earlier tumor onset than patients with the wild-type T/T genotype.
Introduction
The human mouse double minute 2 (Mdm2) gene/protein interacts with the tumor suppressor p53 in a negative auto-regulatory feedback loop.1 In humans, MDM2 has been shown to be overexpressed in several tumors and is associated with accelerated cancer progression and a lack of response to therapy in some tumor entities and cancer cell lines.2, 3, 4, 5, 6 Bond and co-workers7 suggested that elevated Mdm2 RNA and protein levels may be caused by a single-nucleotide polymorphism (SNP309 T>G; rs2279744) in the P2 promoter of the Mdm2 gene, which may increase the DNA-binding affinity of the SP1 transcription factor. An eightfold higher level of MDM2 mRNA was found in G/G genotype cells compared with T/T cells in a panel of cell lines.7 Cells with homozygous genotypes (TT>GG) for MDM2 SNP309 and wild-type p53 differed by an average of fivefold in their respective drug sensitivities.8
A significant effect on age of cancer diagnosis (tumor onset) has already been shown for individuals with Li-Fraumeni syndrome in female patients with soft tissue sarcomas, diffuse large B-cell lymphomas, invasive ductal breast carcinoma and colorectal cancer.7, 9, 10 However, in several studies on breast cancer and lung cancer, MDM2 SNP309 was not associated with increased MDM2 protein expression or increased cancer risk.11, 12, 13, 14 There are two factors that can influence the effect of the SNP309G/G genotype as follows: estrogen receptor status, which will be discussed below, and ethnicity. The association among MDM2 SNP309 and breast cancer and lung cancer is modified by ethnic background, that is, the MDM2 SNP309 represents a risk factor for breast cancer in Chinese women but not in non-Chinese women15 and is associated with lung cancer risk in Asians.16 This ethnic difference can be explained at least partially by a second MDM2 SNP (SNP285 G>C; rs117039649) that can antagonize, as a C/G variant, the effect of SNP309. This SNP285C/G reduces the binding affinity of the SP1 transcription factor.17 Interestingly, the SNP285C allele is absent in Asian populations (Han Chinese and Mongolians) and African Americans but is a pan-Caucasian variant that occurs in approximately 6–8% of the Caucasian population and at lower frequencies in Finns and Sami populations.18 Knappskog and Lønning suggested that the studies performed on the SNP285C-free Asian cohorts represent the ‘true' effect of SNP309G, whereas the studies in Western Europe need to be ‘corrected' with respect to SNP285 status.17
To date, MDM2 SNP309 studies are limited to an Asian-Japanese cohort of renal cell carcinoma (RCC) patients.19 In this cohort, Hirata and co-workers found increased MDM2 protein expression in the tumors of G/G variant carriers, an elevated number of GG carriers in a young patient group compared with a young healthy control group (both ⩽64 years) and an 1.9-fold increased risk of tumor-related death for G/G genotyped patients compared with T/G+T/T patients. However, until now, the MDM2 SNP309 had not been studied in a Caucasian cohort of RCC patients.
Results and discussion
In genotyping our unselected cohort of 197 Caucasian RCC patients for MDM2 SNP309, we detected the G/G variant in 18 (9.1%) patients, the G/T variant in 116 (58.9%) patients and the T/T variant in 63 (32.0%) patients (Figure 1). Among the 80 included female RCC patients, 8 (10.0%) carried the G/G variant, 50 (62.5%) carried the G/T variant and 22 (27.5%) carried the T/T variant. The 117 male RCC patients displayed the G/G variant in 10 (8.5%) patients, the G/T variant in 66 (56.4%) patients and the T/T variant in 41 (35.0%) patients (Table 1). The distribution of the genotypes did not differ among histomorphological subtypes, tumor grades or tumor stages (Table 1).
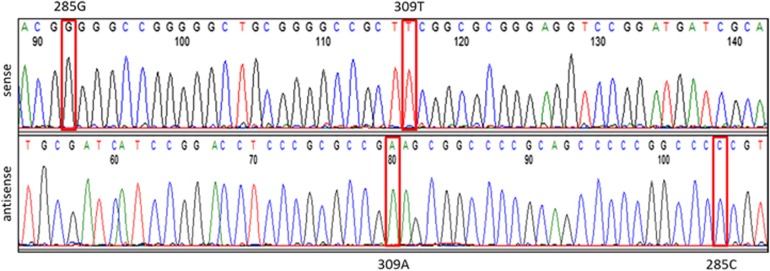
Figure 1.
Sequencing of MDM2 SNPs 285 and 309. Sequencing of MDM2 SNP 285 was performed as described in Spiegelberg et al.26 using PCR sense primer 5′-TGGACTGGGGCTAGGCAGTC-3′ and antisense primer 5′CTGAGTCAACCTGCCCACTG3′ at a 62.9 °C annealing temperature. The PCR product size was 248 bp and also encompassed the neighboring MDM2 SNP309 region. The sequencing result is shown for an RCC sample. Sequencing of SNP285 showed a G/G homozygous (sense strand) and, correspondingly, a C/C homozygous (antisense strand) genotype. Sequencing of SNP309 revealed a T/T homozygous (sense strand) and, accordingly, an A/A homozygous (antisense strand) genotype.
Table 1. Clinicopathological parameters and human MDM2 SNP309 genotypes in the unselected cohort of RCC patients.
| Clinicopathological parameters | No. of patients |
SNP309 |
P-valuea | ||
|---|---|---|---|---|---|
| Total | T/T | T/G | G/G | ||
| Total | 197 | 63 | 116 | 18 | |
| Gender | 0.534 | ||||
| Females | 80 | 22 | 50 | 8 | |
| Males | 117 | 41 | 66 | 10 | |
| Morphology | 0.453 | ||||
| Clear cell | 181 | 61 | 103 | 17 | |
| Papillary | 10 | 2 | 8 | 0 | |
| Chromophobe | 5 | 0 | 4 | 1 | |
| Unknown | 1 | 0 | 1 | 0 | |
| Age (years) | 0.760 | ||||
| ⩽67 | 101 | 30 | 61 | 10 | |
| >67 | 96 | 33 | 55 | 8 | |
| Age (years) | |||||
| Females | 80 | ||||
| ⩽68 | 43 | 9 | 27 | 7 | 0.077b |
| >68 | 37 | 13 | 23 | 1 | 0.024c |
| Males | 117 | 0.613 | |||
| ⩽67 | 62 | 21 | 37 | 4 | |
| >67 | 55 | 20 | 29 | 6 | |
| Tumor stage | 0.346 | ||||
| pTa | 1 | 0 | 1 | 0 | |
| pT1 | 108 | 32 | 70 | 6 | |
| pT2 | 24 | 7 | 14 | 3 | |
| pT3 | 59 | 22 | 28 | 9 | |
| pT4 | 2 | 0 | 2 | 0 | |
| Unknown | 3 | 2 | 1 | 0 | |
| Grouped tumor stage | |||||
| pTa+pT1+pT2 | 133 | 39 | 85 | 9 | 0.082 |
| pT3+pT4 | 61 | 22 | 30 | 9 | |
| unknown | 3 | 2 | 1 | 0 | |
| Tumor grade | 0.855 | ||||
| G1 | 30 | 9 | 19 | 2 | |
| G2 | 100 | 29 | 60 | 11 | |
| G3 | 62 | 22 | 35 | 5 | |
| Unknown | 5 | 3 | 2 | 0 | |
| Grouped tumor grade | |||||
| G1+G2 | 130 | 38 | 79 | 13 | 0.662 |
| G3 | 62 | 22 | 35 | 5 | |
| Unknown | 5 | 3 | 2 | 0 | |
Age groups were separated by the medians (67 years for all RCC patients, 68 years for female RCC patients and 67 years for male RCC patients).
DNA from part of the unselected cohort (n=137) and the age-selected cohort was isolated from kidney specimens of patients who had undergone surgery for RCC. For this purpose, normal kidney tissue of the highest purity possible was needed. Therefore, three experienced uropathologists (AH, SB and VS) marked tumor-free areas on hematoxylin and eosin staining that had been obtained from formalin-fixed and paraffin-embedded tissues. Then, 5 μm sections from the respective specimens were deparaffinized and manually microdissected after 15 s staining by 0.1% methylene blue using the marked hematoxylin and eosin staining as a template. The purity of the obtained tumor-free kidney tissue was near 100%.
DNA from normal kidney tissue (n=137) was isolated using the Maxwell 16 LEV Blood DNA Kit (Promega Corporation Mannheim, Germany) according to the manufacturer's instructions. Furthermore, we isolated DNA from the blood lymphocytes of additional RCC patients of the unselected cohort (n=60) as previously described.27
To genotype the unselected cohort (n=137) and the age-selected cohort (n=205) for MDM2 SNP309, we used a restriction fragment length polymorphism approach as summarized in Hitzenbichler et al.,28 whereby the unselected cohort was analyzed on a 2.5% agarose/TAE gel instead of a capillary sequencing system. In addition, genotyping of the other part of the unselected cohort (n=60) was performed as described previously.7
Cross tables, χ2-test, significant values are in bold face.
All genotypes.
Genotypes G/G vs T/T.
Next, we looked for an association between age at tumor onset and SNP309 genotype. We did not detect an association within the entire unselected RCC cohort or the male RCC patient subcohort (data not shown). However, female RCC patients with a G/G genotype were diagnosed 13.5 years earlier (median age 59.5 years) than those with a T/T genotype (median age 73 years; Table 2). When the female RCC patients were divided into age-related groups on the basis of median age at diagnosis (i.e., ⩽68 years vs >68 years), genotype distributions differed significantly. We found 7 G/G patients and 1 G/G patient and 9 T/T patients and 13 T/T patients in the young and the elder female groups, respectively (P=0.024; χ2-test; Table 1). This was comparable with the earlier findings of Bond et al., who described an association involving the SNP309G allele (G/C and G/G) in colorectal cancer patients, with an average age difference of 9 years at tumor diagnosis9 and a 12-year age difference at tumor onset in soft tissue sarcoma patients, which was restricted to females.7
Table 2. Gender-specific median ages and distributions of SNP309 genotypes in the unselected cohort of RCC patients.
| RCC patients | All |
SNP309 |
||
|---|---|---|---|---|
| T/T | T/G | G/G | ||
| Females | ||||
| Age in years: median (range) | 68 (48–89) | 73 (55–84) | 68 (48–89) | 59.5 (55–83) |
| Males | ||||
| Age in years: median (range) | 67 (46–91) | 73 (35–91) | 66 (39–88) | 68.5 (44–81) |
Female RCC patients with a G/G SNP309 genotype (median age 59.5 years) were diagnosed 13.5 years earlier than the T/T females (median age 73 years), a difference that was statistically significant.
The significance of different genotype distributions among the patient groups was calculated by the two-sided exact χ2-test using IBM SPSS Statistics 20 software (SPSS-Science, Chicago, IL, USA). P-values <0.05 were regarded as significant.
Bond et al.10 developed a model of the gender-related effect of SNP309G/G based on estrogen receptor α (ERα) expression. It is known that ERα induces MDM2 transcription.20 Interestingly, ERα binds close to SNP309 in the MDM2 P2 promoter. This binding is directly affected by the binding of the Sp1 transcription factor. This leads to the model in which the G-allele of SNP309 can accelerate tumorigenesis in the presence of an active estrogen signaling pathway. The model is supported by the finding that the G-allele of SNP309 is only associated with an earlier age of onset in high ER-positive but not ER-negative intraductal breast cancer patients.10
To study the age-related effect on tumor onset in more detail, we investigated a second age-selected cohort of 205 Caucasian RCC patients. This cohort encompassed a high proportion of patients who had contracted RCC at an extraordinary young or old age (<45 or >75 years, respectively). The G/G variant was detected in 31 (15.1%) patients, the G/T, in 85 (41.5%) patients, and the T/T, in 89 (43.4%) patients (Table 3). Among the 83 female RCC patients, the G/G variant was detected in 13 (15.7%) patients, the G/T variant, in 36 (43.3%) patients, and the T/T variant, in 34 (41%) patients; in the 122 male RCC patients, the G/G variant was found in 18 (14.7%) patients, the G/T variant in 49 (40.2%) patients, and the T/T variant in 55 (45.1%) patients (Table 3). Interestingly, in this selected patient cohort, we found significant differences in SNP309 genotype distribution between the lower tumor stages (pTa+pT1+pT2) and the higher tumor stage (pT3) and lower tumor grades (G1+G2) and the higher tumor grades (G3+G4) (P=0.039 and 0.004, respectively). Notably, the G/G genotype occurred more frequently in the lower tumor grade and lower tumor stage RCC patients (Table 3). However, the percentage of G/G variants was only slightly elevated in the younger age group (⩽68 years) compared with the elder group (>68 years) of female patients, at 18.8% in the younger age group vs 14.9% in the elder group. We most likely did not see an accumulation of younger female patients in the G/G genotype group because in this cohort, only 28 females were included in the younger age group (⩽68 years), and only 5 females carried the G/G genotype. However, remarkably, the percentage of the G/G genotype was greater in the male patients in the younger age group (⩽67 years) compared with the elder age group (>67 years), at 19.4% for males in the younger age group vs 8.0% for males in the elder age group. This result is somewhat surprising because, until now, only an age-related effect in female tumor patients has been described in relation to elevated ERα expression in G/G genotype female patients. Our finding could be associated with a relatively high number of male RCC patients (N=100) below the age median of 67 years, but further studies in RCC and other male tumor patients are necessary to confirm this.
Table 3. Clinicopathological parameters and human MDM2 SNP309 genotypes in an age-selected second cohort of RCC patients.
| Clinicopathological parameters | No. of patients |
SNP309 |
P-valuea | ||
|---|---|---|---|---|---|
| Total | T/T | T/G | G/G | ||
| Total | 205 | 89 | 85 | 31 | |
| Gender | 0.884 | ||||
| Females | 83 | 34 | 36 | 13 | |
| Males | 122 | 55 | 49 | 18 | |
| Morphology | 0.287 | ||||
| Clear cell | 99 | 37 | 44 | 18 | |
| Papillary | 29 | 15 | 11 | 3 | |
| Chromophobe | 64 | 29 | 25 | 10 | |
| Othersb | 13 | 8 | 5 | 0 | |
| Age (years) | 0.473 | ||||
| ⩽67 | 100 | 42 | 39 | 19 | |
| >67 | 105 | 47 | 46 | 12 | |
| Age (years) | |||||
| Females | 83 | ||||
| ⩽68 | 28 | 12 | 11 | 5 | 0.848 |
| >68 | 55 | 22 | 25 | 8 | |
| Males | 122 | 0.861 | |||
| ⩽67 | 72 | 30 | 28 | 14 | |
| >67 | 50 | 25 | 21 | 4 | |
| Tumor stage | 0.421 | ||||
| pT1 | 122 | 45 | 55 | 22 | |
| pT2 | 30 | 12 | 13 | 5 | |
| pT3 | 49 | 28 | 17 | 4 | |
| Unknown | 4 | 4 | 0 | 0 | |
| Grouped tumor stage | |||||
| pTa+pT1+pT2 | 152 | 57 | 68 | 27 | 0.039 |
| pT3 | 49 | 28 | 17 | 4 | |
| Tumor grade | 0.052 | ||||
| G1 | 48 | 16 | 23 | 9 | |
| G2 | 124 | 50 | 54 | 20 | |
| G3 | 27 | 18 | 7 | 2 | |
| G4 | 6 | 5 | 1 | 0 | |
| Grouped tumor grade | |||||
| G1+G2 | 172 | 66 | 77 | 29 | 0.004 |
| G3+G4 | 33 | 23 | 8 | 2 | |
The age groups were separated by the medians (67 years for all RCC patients, 68 years for female RCC patients and 67 years for male RCC patients).
DNA from the age-selected cohort (n=205) was isolated using the High Pure PCR Template Preparation Kit (Roche Diagnostics Mannheim, Germany).
Cross tables, χ2-test, significant values are in bold face.
The group others comprises malignant tumors with undifferentiated, mixed, spindle cell morphology or Ductus-Bellini carcinoma.
Next, we determined if the above-mentioned age-related effect on tumor onset in the female RCC patients was affected by the SNP285 genotype. For both cohorts, we studied the SNP285 genotype in 18 female RCC patients with the G/G SNP309 genotype. However, in all cases studied, we detected the wild-type homozygous G/G genotype. Therefore, we cannot comment on the effect of the SNP285C/G genotype on the age of tumor onset in German RCC patients with the SNP309G/G genotype. However, our finding of the wild-type SNP285 in conjunction with the SNP309G/G genotype could be displaying the ‘true' effect of SNP309G/G and explain the significant age difference in tumor onset between the female G/G genotype patients and the female T/T genotype patients, as Knappskog and Lønning suggested.17 Future studies including SNP285C/G genotyped female RCC patients could clarify the effect of SNP285 in RCC patients.
Finally, what role could human MDM2 SNP309 play for RCC patients? An eightfold higher MDM2 expression level was reported in cancer cell lines by comparing SNP309G/G with SNP309T/T carrying cell lines.7 Several articles described that MDM2 upregulation is associated with decreased disease-specific survival in RCC patients.21 Roberts et al.22 showed that in clear cell RCC, after the loss of the von Hippel-Lindau tumor suppressor protein, hypoxia-inducible factor 2alpha accumulated and led to the MDM2-mediated suppression of p53. Accordingly, Nutlin 3, a small molecular inhibitor of the MDM2–p53 interaction, restored p53 function, induced growth arrest and senescence in RCC cells and reversed the resistance of RCC cells to Fas-mediated and chemotherapy-induced cell death.22, 23 In an RCC xenograft mice model, the concurrent administration of the MDM2 (+MDM4) antagonist MI-319 and sunitinib markedly increased the antitumor and antiangiogenic activities of sunitinib, resulting in sustained p53-dependent gene expression and significantly reduced tumor growth.24 The authors suggested that treatment with the MDM2 antagonist MI-319 could be an effective means of delaying or preventing the development of sunitinib therapy resistance in RCC.24 We suggest analyzing the MDM2 SNP309 genotype for an effect on sunitinib treatment in RCC patients in future studies. Sunitinib treatment alone can increase p53 activity in xenotransplants.24 The increased p53 activity may be affected by different MDM2 expression levels depending on the SNP309 genotype.
Furthermore, the gender-specific effect of the SNP309G/G variant may also have implications for estrogen signaling manipulation in female RCC patients. An increase in estrogen levels in postmenopausal women with the G/G variant could increase their risk for other cancers such as soft tissue sarcomas, colorectal cancers and ERα-positive invasive ductal breast cancer,20 as well as RCC. On the other hand, female patients with these cancers could benefit from a reduction in ERα levels, as was reported for invasive ductal breast cancer patients with high ERα, for whom a decrease in ERα resulted in decreased tumor growth and longer overall survival.20 However, the consequences of estrogen receptor modulation have to be considered carefully, as suggested by previous studies in breast cancer.25
The SNP309 has been studied in an Asian-Japanese RCC patient group.19 The authors showed a significant increase in the GG genotype of the MDM2 SNP309 in RCC patients (31%) compared with healthy controls (20%). However, we could not detect significant differences in the occurrence of the GG genotype between the RCC patients and the healthy controls, nor could we detect such a difference by separating RCC patients and healthy controls by gender (the latter based on data from the literature; Supplementary Table S1). However, the differences in the findings of our study compared with the results of Hirata et al. could be explained by ethnic differences between the Asian and Caucasian populations. By comparing two age groups separated by a median (⩽64 vs ⩾65 years), Hirata et al. reported a higher number of GG genotype carriers in the younger patient group (30%) than in the younger healthy control group (12%); however, this was not the case when the patient group was compared with the control group in the elder group. However, the authors did not see significant differences in the occurrence of the G/G genotype between the younger and elder RCC patient groups without distinguishing between female and male patients. We noted a significantly higher rate of G/G vs T/T genotypes in the younger females (⩽68 years) compared with the elder female RCC patients (>68 years) (P=0.024; χ2-test). However, we would have missed detecting this difference by comparing G/G vs T/T genotype in a joint female and male RCC cohort for both age groups (data not shown), which may explain why our findings were different from those of Hirata and co-workers. We analyzed overall survival data for 189 RCC patients from the unselected study cohort and did not detect a correlation between SNP309 genotype and survival, nor was a correlation found when all RCC patients were separated by gender (data not shown). Hirata et al. found a significant correlation between cancer-specific survival and the SNP309 genotype for the RCC patients in their study (N=176) and noted a worse prognosis for the G/G genotype patients vs the T/G+T/T genotype patients (P=0.0139). However, in our age-selected RCC patient cohort the SNP309G allele occurred more frequently in the lower tumor grade and the lower tumor stage groups than in higher tumor grade and tumor stage groups. Whereas the latter groups have a poorer prognosis (data not shown), which may indicate that the G-allele is not associated with a worse outcome. However, we suggest that the correlation between cancer-specific survival and SNP309 genotypes in a Caucasian population of RCC patients needs to be analyzed further in future studies.
In summary, female RCC patients of Caucasian origin with the G/G variant of SNP309 in the human Mdm2 gene showed a significantly earlier age of tumor onset than female RCC patients with the wild-type T/T genotype. The frequency of the SNP309 genotype should be tested, as should its association with tumor onset and prognosis in additional RCC patient populations of different ethnic origins. We suggest that the SNP309 genotype can be considered useful for diagnostic and therapeutic approaches for female RCC patients in the future.
Acknowledgments
We would like to thank American Journal Experts for editing this manuscript. HT and EN were supported by the Rudolf und Irmgard Kleinknecht-Stiftung and the Dr Robert Pfleger-Stiftung and Movember Foundation/Förderverein Hilfe beim Prostatakrebs e.V.
Glossary
- MDM2
murine double minute 2
- RCC
renal cell carcinoma
- SNP
single-nucleotide polymorphism
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis)
Supplementary Material
References
- Wu X, Bayle H, Olson D, Levine AJ. The p53-mdm2 autoregulatory feedback loop. Genes Dev 1993; 7: 1126–1132. [DOI] [PubMed] [Google Scholar]
- Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci 1999; 55: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onel K, Cordon-Cardo C. MDM2 and prognosis. Mol Cancer Res 2004; 2: 1–8. [PubMed] [Google Scholar]
- Cronauer MV, Schulz WA, Burchardt T, Ackermann R, Burchardt M. Inhibition of p53 function diminishes androgen receptor-mediated signaling in prostate cancer cell lines. Oncogene 2004; 23: 3541–3549. [DOI] [PubMed] [Google Scholar]
- Ohnstad HO, Castro R, Sun J, Heintz KM, Vassilev LT, Bjerkehagen B et al. Correlation of TP53 and MDM2 genotypes with response to therapy in sarcoma. Cancer 2013; 119: 1013–1022. [DOI] [PubMed] [Google Scholar]
- Fornari F, Milazzo M, Galassi M, Callegari E, Veronese A, Miyaaki H et al. p53/mdm2 feedback loop sustains miR-221 expression and dictates the response to anticancer treatments in hepatocellular carcinoma. Mol Cancer Res 2014; 12: 203–216. [DOI] [PubMed] [Google Scholar]
- Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004; 119: 591–602. [DOI] [PubMed] [Google Scholar]
- Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov 2008; 7: 979–987. [DOI] [PubMed] [Google Scholar]
- Bond GL, Menin C, Bertorelle R, Alhorpuro P, Aaltonen LA, Levine AJ. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet 2006. a; 43: 950–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond GL, Hirshfield KM, Kirchhoff T, Alexe G, Bond EE, Robins H et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res 2006. b; 66: 5104–5110. [DOI] [PubMed] [Google Scholar]
- Millikan RC, Heard K, Winkel S, Hill EJ, Massa B, Mayes L et al. No association between the MDM2-309 T/G promoter polymorphism and breast cancer in African-Americans or Whites. Cancer Epidemiol Biomarkers Prev 2006; 15: 175–177. [DOI] [PubMed] [Google Scholar]
- Pine SR, Mechanic LE, Bowman ED, Welsh JA, Chanock SC, Shields PG et al. MDM2 SNP309 and SNP354 are not associated with lung cancer risk. Cancer Epidemiol Biomarkers Prev 2006; 15: 1559–1561. [DOI] [PubMed] [Google Scholar]
- Krekac D, Brozkova K, Knoflickova D, Hrstka R, Muller P, Nenutil R et al. MDM2SNP309 does not associate with elevated MDM2 protein expression or breast cancer risk. Oncology 2008; 74: 84–87. [DOI] [PubMed] [Google Scholar]
- Liu G, Wheatley-Price P, Zhou W, Park S, Heist RS, Asomaning K et al. Genetic polymorphisms of MDM2, cumulative cigarette smoking and nonsmall cell lung cancer risk. Int J Cancer 2008; 122: 915–918. [DOI] [PubMed] [Google Scholar]
- Economopoulos KP, Sergentanis TN. Differential effects of MDM2 SNP309 polymorphism on breast cancer risk along with race: a meta-analysis. Breast Cancer Res Treat 2010; 120: 211–216. [DOI] [PubMed] [Google Scholar]
- Gui XH, Qiu LX, Zhang HF, Zhang DP, Zhong WZ, Li J et al. MDM2 309 T/G polymorphism is associated with lung cancer risk among Asians. Eur J Cancer 2009; 45: 2023–2026. [DOI] [PubMed] [Google Scholar]
- Knappskog S, Lønning PE. Effects of the MDM2 promoter SNP285 and SNP309 on Sp1 transcription factor binding and cancer risk. Transcription 2011; 2: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappskog S, Gansmo LB, Dibirova K, Metspalu A, Cybulski C, Peterlongo P et al. Population distribution and ancestry of the cancer protective MDM2 SNP285 (rs117039649). Oncotarget 2014; 5: 8223–8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Hinoda Y, Kikuno N, Kawamoto K, Suehiro Y, Tanaka Y et al. MDM2 SNP309 polymorphism as risk factor for susceptibility and poor prognosis in renal cell carcinoma. Clin Cancer Res 2007; 13: 4123–4129. [DOI] [PubMed] [Google Scholar]
- Bond GL, Levine AJ. A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene 2007; 26: 1317–1323. [DOI] [PubMed] [Google Scholar]
- Noon AP, Vlatković N, Polański R, Maguire M, Shawki H, Parsons K et al. p53 and MDM2 in renal cell carcinoma: biomarkers for disease progression and future therapeutic targets? Cancer 2010; 116: 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AM, Watson IR, Evans AJ, Foster DA, Irwin MS, Ohh M. Suppression of hypoxia-inducible factor 2alpha restores p53 activity via Hdm2 and reverses chemoresistance of renal carcinoma cells. Cancer Res 2009; 69: 9056–9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polański R, Noon AP, Blaydes J, Phillips A, Rubbi CP, Parsons K et al. Senescence induction in renal carcinoma cells by Nutlin-3: a potential therapeutic strategy based on MDM2 antagonism. Cancer Lett 2014; 353: 211–219. [DOI] [PubMed] [Google Scholar]
- Panka DJ, Liu Q, Geissler AK, Mier JW. Effects of HDM2 antagonism on sunitinib resistance, p53 activation, SDF-1 induction, and tumor infiltration by CD11b+/Gr-1+ myeloid derived suppressor cells. Mol Cancer 2013; 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 2004; 5: 207–213. [DOI] [PubMed] [Google Scholar]
- Spiegelberg C, Giedl J, Gaisa NT, Rogler A, Riener MO, Filbeck T et al. Frequency of activating mutations in FGFR2 exon 7 in bladder tumors from patients with early-onset and regular-onset disease. Int J Clin Exp Pathol 2014; 7: 1708–1713. [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Taubert H, Lange H, Kriese K, Schmitt WD, Hoffmann S et al. Small polydispersed circular DNA contains strains of mobile genetic elements and occurs more frequently in permanent cell lines of malignant tumors than in normal lymphocytes. Oncol Rep 2009; 22: 393–400. [PubMed] [Google Scholar]
- Hitzenbichler F, Stoehr CG, Rogenhofer M, Wieland WF, Ruemmele P, Hartmann A et al. Mdm2 SNP309 G-variant is associated with invasive growth of human urinary bladder cancer. Pathobiology 2014; 81: 53–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.