Abstract
We reviewed the literature from 2010 to 2016 on the relationship between posttraumatic stress disorder (PTSD) and cardiometabolic health conditions, including metabolic syndrome, coronary artery disease, stroke, and myocardial infarction, among others. Collectively, PTSD was associated with increased risk of cardiometabolic health problems, with pre-clinical and clinical studies offering evidence of behavioral (e.g., poor sleep, cigarette use, poor diet and insufficient exercise) and biological (e.g., autonomic reactivity, inflammation) mediators of these associations. We discuss the possibility that these behavioral and biological mechanisms lead to accelerated cellular aging, as regulated in the epigenome, which contributes to premature cardiometabolic health decline. This has implications for the assessment, prevention, and treatment of cardiometabolic conditions among those with PTSD. It also highlights the need to better understand the mechanisms linking PTSD to accelerated aging and to develop interventions to attenuate or reverse this phenomenon.
Research on the physical health consequences of posttraumatic stress disorder (PTSD) has increased substantially in the last decade. A recent PubMed search for the terms “PTSD” and “cardiovascular” from 2010–2015 yielded 209 entries, whereas only 102 entries were returned when restricting the search to 2004–2009. This line of research is likely to yield important discoveries concerning the neurobiological consequences of the disorder and the molecular mechanisms that maintain symptoms and promote health decline. It may also lead to new clinical guidelines regarding the assessment and treatment of medical morbidities among individuals with PTSD.
Prior reviews have summarized how PTSD relates to physical health and cardiovascular health.1,2 This article provides an updated review, focused on research from 2010 on, concerning the association between PTSD and cardiovascular conditions. We restricted our review to PTSD-related cardiovascular conditions because of the substantial prevalence of cardiovascular disease (CVD) in the PTSD population and because of the broad scope of this research, ranging from pre-clinical to epidemiological studies and inclusive of both cross-sectional and longitudinal designs. We also discuss the possibility that PTSD-related CVD and cardiometabolic problems may reflect outward manifestations of an accelerated aging process wherein the stress of PTSD alters the pace of cellular aging and leads to premature health decline.
PTSD and Cardiometabolic Conditions
A growing number of epidemiological studies point to a strong association between PTSD and risk of cardiovascular conditions. For example, in a Danish population study,3 PTSD was associated with increased incidence of myocardial infarction (MI), stroke, ischemic stroke, and venous thromboembolism, as recorded in the medical record. Standardized incidence ratios comparing those with PTSD to the general population were increased by approximately 1.4 to 2.1, depending on the condition. In a US population study of older adults, PTSD was associated with increased odds of self-reported physician-diagnosed cardiac conditions, including hypertension, angina, tachycardia, and “other heart disease,” with odds ratios ranging from 1.3 – 1.8 (across conditions) after controlling for a variety of demographic and psychiatric variables.4 However, odds of arteriosclerosis, MI, high cholesterol, and stroke were not increased in those with PTSD.4
Estimates of increased risk for MI and/or stroke as a function of both trauma exposure (without PTSD) and PTSD symptoms were obtained in a large national longitudinal study of nurses. The point estimate for relative risk of having either (or both) of these conditions was somewhat greater among those with at least four PTSD symptoms (versus no trauma exposure; 1.6 hazard ratio) as compared to those with trauma exposure but no PTSD symptoms (versus no trauma exposure; 1.45 hazard ratio), after adjusting for demographic covariates, family history of cardiac risk, and childhood body type.5 Results also suggested that this association might be mediated by health behaviors and medical risk factors, including cigarette and alcohol use, physical activity and diet, high blood pressure, type 2 diabetes, hormone therapy, and antidepressant medication use.5
Results from civilian samples generalize to military veterans. In a large study of US veterans representative of those receiving outpatient VA care, PTSD was associated with an approximately 50% increased risk (hazard ratio) of new onset heart failure over the course of eight years, controlling for a variety of demographic and medical risk factors.6 A large population study comprising over 138,000 VA healthcare users aged 55 and older found that PTSD was associated with 25–50% increased incidence of CVD, congestive heart failure, MI, and peripheral vascular disease.7 Furthermore, a follow-up study from the Vietnam Era Twin Registry provided considerable evidence that PTSD is causally implicated in CVD. In an analysis of twin pairs discordant for PTSD, the incidence of CVD (defined by a variety of conditions) among twins with PTSD was nearly twice that of the co-twin control, adjusting for a variety of health behaviors and cardiac risk factors.8 The results of single-cohort studies also align well with a recent meta-analysis suggesting that PTSD was associated with a 55% increased risk for coronary heart disease.9
As would be expected, PTSD-related cardiac conditions may also lead to premature mortality. Ahmadi et al.10 found that PTSD among veterans was associated with 59% increased odds of having a biological marker of atherosclerotic coronary artery disease, and with greater than 200% increased risk for mortality over the course of, on average, 3.5 years. Although some of this increased PTSD-related mortality risk was independent of the risk associated with coronary artery disease, results also suggested a possible interaction between the conditions. Mortality risk increased as a function of greater coronary artery pathology and this pattern was accentuated for individuals with PTSD—among those with the highest levels of coronary artery-related morality risk, comorbid PTSD increased risk by 81%.10
PTSD is also linked to increased prevalence of metabolic syndrome (MetS), a cardiometabolic condition that may lead to subsequent CVD, and that may provide insight into the biological and behavioral mechanisms underlying the PTSD-CVD connection. MetS is defined by central obesity, elevated blood sugar, dyslipidemia, and hypertension.11 It is associated with increased inflammation, development of type 2 diabetes, and premature mortality (reviewed in Levine et al.12). A recent meta-analysis suggests that approximately 40% of individuals with PTSD meet criteria for MetS, which is nearly double the prevalence in population controls.13
Our research suggests that MetS is a substantial concern, even among young veterans returning from deployments to the wars in Iraq and Afghanistan. Specifically, PTSD-related MetS was associated with bilateral reductions in cortical thickness, most prominently in parietal, temporal, and frontal regions.14 Moreover, in as-of-yet unpublished work, the prevalence of MetS in a national sample of returning veterans was substantially greater among those with PTSD (~ 40%) than those without (~ 25%) and greater than a national age-matched population estimate.15 Further, PTSD predicted increased MetS severity over the course of a two-year follow-up, controlling for baseline MetS severity and demographic variables.15 Taken together, the high prevalence of MetS among individuals with PTSD and the indication that PTSD may play a causative role in the development of MetS suggests that it is critical to identify the biological and behavioral mechanisms involved and to intervene early so that MetS does not develop into CVD.
PTSD and Accelerated Aging
Of particular interest is the observation that PTSD is associated with premature onset of age-related cardiometabolic conditions (reviewed in Lohr et al.16), and possibly, with premature mortality.16,17 Although many cardiometabolic disorders tend to increase in the population with age, associations between PTSD and these disorders typically are independent of age and occur at an earlier age than expected. Miller and Sadeh,18 Lohr et al.,16 and Williamson et al.19 recently suggested that this may be because the neurobiological consequences of chronic PTSD symptoms, including alterations in metabolic, inflammatory, stress responsivity, autonomic reactivity, and oxidative stress processes, actually accelerate the cellular aging process.
There is initial molecular evidence for this. In a recent review, Lindqvist et al.20 noted several studies that suggested that PTSD was associated with shortened telomere length, although failures to replicate were also reported. Telomeres are regions of repeat DNA sequences found at the ends of chromosomes that become shorter with age. A number of psychiatric and health problems, including metabolic dysregulation, are associated with shortened telomere length.20,21 Beyond telomeres, recent advances in epigenetics have demonstrated that variation in methylation levels of select DNA loci across the genome can be aggregated into an algorithm that predicts chronological age with incredible precision, a metric referred to as “DNA methylation age”.22,23 Our research24 in a sample of Iraq and Afghanistan War veterans found that greater lifetime PTSD severity was associated with advanced DNA methylation age compared to chronological age. Moreover, advanced DNA methylation age was also reflected in reduced neural integrity in an age-sensitive region of the corpus callosum and with worse performance on neuropsychological tests of working memory.24
Related research in a civilian sample with a high prevalence of community violence failed to replicate this association between PTSD and advanced DNA methylation age.25 However, results suggested that life stress, especially among older adults, was associated with accelerated DNA methylation age and that the loci included in one of the methylation age algorithms were enriched for glucocorticoid responsivity.25 Together, these studies provide initial evidence that psychological stress and trauma-related psychiatric symptoms may accelerate the aging process at the genomic level and also raise the possibility that these epigenetic alterations may give rise to premature cardiometabolic and other age-related health problems, including neuronal decline.18
Biological and Behavioral Mechanisms Linking PTSD, Accelerated Aging, and CVD
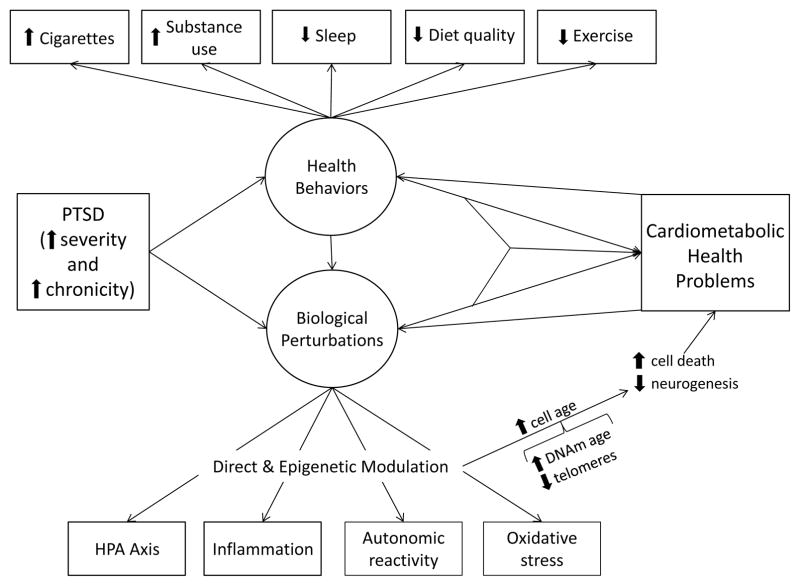
Figure 1 provides a conceptual model of the hypothesized pathways linking PTSD to the genetic and clinical manifestations of accelerated aging; we next discuss each aspect of this model in turn. With respect to biological mechanisms of PTSD-related accelerated aging, pre-clinical research suggested that socially stressed mice displayed behaviors that were analogous to PTSD and showed acute damage to cardiac tissue (that is eventually repaired)26 as well as changes in the expression of genes involved in inflammation, cell growth, and cell repair.26,27 Additional preclinical research suggested that genes related to cardiometabolic conditions (and many other biological processes, including stress responding, inflammation, neurogenesis, and circadian rhythm) were differentially expressed in the brains of mice with a putative PTSD-like phenotype. 27 This differential expression occurred at varying time points across distinct regions of the brain implicated in PTSD, including the amygdala, hippocampus, and medial prefrontal cortex. Thus, it is possible that in the face of chronic symptoms of PTSD and unmitigated biological stress responding (e.g., via dysregulation of the hypothalamic-pituitary-adrenal axis and related increases in inflammation), these epigenetic alterations and cardiac damage overwhelm repair processes and become permanent—and part of a cascade of subsequent dysregulation in systems that govern cardiac and metabolic health.
Figure 1.
provides a conceptual model of the hypothesized associations between PTSD and cardiometabolic health problems via biological and health behavior pathways. The figure also reflects the potential for these pathways to exert synergistic effects and for cardiometabolic health problems to further compromise health behaviors and biological systems.
Consistent with this possibility, Williamson et al.19 proposed that PTSD symptoms such as poor sleep, physiological and emotional reactivity, hypervigilance, and exaggerated startle might lead to autonomic problems relevant for the development of CVD, including reduced heart rate variability, increased blood pressure, insufficient cortisol responsivity, and increased excitatory neurohormone release. These conditions would be expected to negatively influence cardiometabolic health and promote accelerated cellular aging. Miller and Sadeh18 further suggested that these types of PTSD symptoms, particularly when chronic, may also lead to oxidative stress in both the periphery and central nervous system such that cell death occurs without sufficient antioxidant capacity to maintain cellular health and growth.
Behavioral mechanisms are also critical to consider as potential mediators of the association between PTSD and poor cardiometabolic health. For example, Dennis et al.28 recently demonstrated that PTSD severity was indirectly associated with reduced heart rate variability, a risk factor for CVD, via poor sleep, cigarette use, and alcohol dependence. In a separate study, the association between comorbid PTSD and depressive symptoms was related to lower high-density lipoprotein and higher triglycerides via poor sleep and cigarette use.29 Associations between PTSD and insufficient exercise and/or sedentary lifestyle and problematic eating patterns (e.g., binging, fast food consumption) represent additional mechanisms that may link PTSD with CVD.30 Use of psychotropic medication, particularly anti-psychotics such as clozapine and olanzapine, is associated with increased risk for MetS31 and may be another biobehavioral pathway from PTSD to CVD.
Clinical Implications: Early Screening and Intervention
The accumulated findings suggest that individuals with PTSD should undergo early screening for signs of metabolic and cardiac problems. The US Preventive Services Task Force32 recommends that men age 35 and older be screened for dyslipidemia and that men and women age 20 and older be screened if they are at risk for coronary heart disease. Medical providers may want to conceptualize PTSD as a CVD risk factor and screen for PTSD so that they can determine if a patient is at elevated risk for cardiometabolic conditions. Likewise, initial signs of cardiometabolic dysfunction may need to be carefully discussed with the patient so that early intervention can be introduced. The cost/benefit analysis of psychiatric medications given weight gain side effects should also be evaluated on an ongoing basis.
The cardiometabolic health significance of PTSD warrants the development of interventions that might target both PTSD symptoms and physical health. A meta-analysis33 suggested that physical activity interventions are successful in lessening PTSD symptoms, although data were insufficient to evaluate if such interventions improve cardiometabolic disease outcomes. Pre-clinical work may shed light on this issue. In a mouse model of PTSD, stressed mice that ran on a treadmill for six weeks showed reversals in the behavioral phenotypes used to index PTSD and improvements in hypothalamic-pituitary-adrenal axis functioning.34 This suggests that exercise may lead to both symptom improvement and beneficial changes in the biological pathways affected by PTSD and that are important for cardiometabolic health.
Future Directions and Conclusions
There are many unanswered questions about PTSD-related CVD. One is whether PTSD-related accelerated cellular aging is reversible and if halting or reversing age acceleration at a cellular level leads to reductions in CVD and mortality. It is unclear which interventions might be best matched to early versus late stages of PTSD-related accelerated aging and if there are critical windows during which the stress of PTSD may exert larger or more intractable effects on subsequent aging and health. Whether indices such as telomeres and DNA methylation age capture the cellular aging process itself or are biomarkers for this is also unknown. As well, it is unclear if metrics like DNA methylation age are sensitive to the initial signs of accelerated aging and if so, if they can be used clinically to identify individuals who will develop cardiometabolic health decline, even before traditional metabolic lab panels indicate this risk.
We encourage research evaluating exercise for PTSD to examine the effects on both psychological symptoms and physical health parameters. It would also be worthwhile to evaluate whether psychotherapy for PTSD leads to long-term changes in cellular aging and health risk and if biometrics (e.g., blood pressure, glucose, cortisol reactivity) change during or after a course of psychotherapy for PTSD. Along these lines, a study evaluating electroconvulsive therapy for PTSD with comorbid depression found that it was associated with decreased PTSD and depression symptoms as well as reduced cardiac-related mortality over the course of an eight year follow-up.35 Finally, it is unclear whether PTSD is uniquely or more strongly related than other psychiatric conditions to accelerated aging and CVD and the extent to which the biological and behavioral mechanisms underlying PTSD-related health decline generalize to other psychiatric conditions.
In conclusion, a broad range of research studies have convincingly suggested that PTSD is associated with cardiometabolic conditions including MetS and its components, coronary artery disease, MI, stroke, pulmonary embolism, heart failure, angina, need for coronary bypass surgery or angioplasty, and cardiac-related mortality. The prevalence of cardiometabolic conditions is increased by roughly 40–55% among individuals with PTSD.9,13 Our review suggests that multiple biological and behavioral pathways may link PTSD to these conditions (Figure 1) and may each denote potential points of intervention to reduce PTSD-related morbidity and mortality. These cardiometabolic problems may represent the clinical expression of an underlying accelerated cellular aging process that is caused by the psychological and biological stress of PTSD symptoms, poor health behaviors, and other negative environmental experiences that tend to co-occur with PTSD. Although attempts to find the “fountain of youth” may sound fanciful, efforts to better understand the cellular aging process and halt or reverse age acceleration are likely to have real-world consequences for individuals with PTSD. We hope that research on the health consequences of PTSD will lead to discoveries that help those with PTSD live longer, healthier, and happier lives.
Acknowledgments
This work was supported by a Career Development Award to Erika J. Wolf from the United States (U.S.) Department of Veterans Affairs, Clinical Sciences Research and Development Program. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.Schnurr PP, Green BL. Understanding relationships among trauma, post-traumatic stress disorder, and health outcomes. Adv Mind Body Med. 2004;20:18–29. [PubMed] [Google Scholar]
- 2.Kubzansky LD, Koenen KC. Is posttraumatic stress disorder related to development of heart disease? An update. Cleve Clin J Med. 2009;76(Suppl 2):S60–S65. doi: 10.3949/ccjm.76.s2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gradus JL, Farkas DK, Svensson E, et al. Associations between stress disorders and cardiovascular disease events in the Danish population. BMJ Open. 2015;5:e009334. doi: 10.1136/bmjopen-2015-009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Physical health conditions associated with posttraumatic stress disorder in U.S. older adults: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Am Geriatr Soc. 2012;60:296–303. doi: 10.1111/j.1532-5415.2011.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumner JA, Kubzansky LD, Elkind MS, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. Am J Public Health. 2015;105:757–763. doi: 10.2105/AJPH.2014.302342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beristianos MH, Yaffe K, Cohen B, Byers AL. PTSD and risk of incident cardiovascular disease in aging veterans. Am J Geriatr Psychiatry. 2014 doi: 10.1016/j.jagp.2014.12.003. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62:970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166:806–814. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108:29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program [NCEP] Executive summary of the third report of the National Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127:1–19. doi: 10.1159/000354910. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, Vancampfort D. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism. 2015;64:926–933. doi: 10.1016/j.metabol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Wolf EJ, Sadeh N, Leritz EC, et al. PTSD as a catalyst for the association between metabolic syndrome and reduced cortical thickness. Biological Psychiatry. doi: 10.1016/j.biopsych.2015.11.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf EJ, Bovin MJ, Green JD, et al. Posttraumatic stress disorder longitudinally predicts increasing metabolic syndrome severity. Under review. [Google Scholar]
- 16.Lohr JB, Palmer BW, Eidt CA, et al. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am J Geriatr Psychiatry. 2015;23:709–725. doi: 10.1016/j.jagp.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlenger WE, Corry NH, Williams CS, et al. A prospective study of mortality and trauma-related risk factors among a nationally representative sample of Vietnam veterans. Am J Epidemiol. 2015;182:980–990. doi: 10.1093/aje/kwv217. [DOI] [PubMed] [Google Scholar]
- 18.Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19:1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson JB, Porges EC, Lamb DG, Porges SW. Maladaptive autonomic regulation in PTSD accelerates physiological aging. Front Psychol. 2015;5:1571. doi: 10.3389/fpsyg.2014.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindqvist D, Epel ES, Mellon SH, et al. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epel ES. Psychologic and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 22.Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf EJ, Logue MW, Hayes JP, et al. Accelerated DNA methylation age: associations with PTSD and neural integrity. Psychoneuroendocrinology. 2016;63:155–162. doi: 10.1016/j.psyneuen.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zannas AS, Arloth J, Carrillo-Roa T, et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015;16:266. doi: 10.1186/s13059-015-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JH, Lee I, Hammamieh R, et al. Molecular evidence of stress-induced acute heart injury in a mouse model simulating posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2014;111:3188–3193. doi: 10.1073/pnas.1400113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhie S, Gautam A, Meyerhoff J, Chakraborty N, Hammamieh R, Jett M. Brain transcriptome profiles in mouse model simulating features of post-traumatic stress disorder. Mol Brain. 2015;8:14. doi: 10.1186/s13041-015-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis PA, Watkins LL, Calhoun PS, et al. Posttraumatic stress, heart rate variability, and the mediating role of behavioral health risks. Psychosom Med. 2014;76:629–637. doi: 10.1097/PSY.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennis PA, Ulmer CS, Calhoun PS, et al. Behavioral health mediators of the link between posttraumatic stress disorder and dyslipidemia. J Psychosom Res. 2014;77:45–50. doi: 10.1016/j.jpsychores.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall KS, Hoerster KD, Yancy WS., Jr Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev. 2015;37:103–115. doi: 10.1093/epirev/mxu011. [DOI] [PubMed] [Google Scholar]
- 31.Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14:339–347. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helfand M, Carson S. Evidence Synthesis No. 49. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Screening for lipid disorders in adults: selective update of 2001 US preventive services task force review. Report No. 08-05114-EF-1. Available at: http://www.ahrq.gov/downloads/pub/prevent/pdfser/lipidser.pdf. [PubMed] [Google Scholar]
- 33.Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of post-traumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230:130–136. doi: 10.1016/j.psychres.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Liu Y, Yin S, et al. Long-term effects of early adolescent stress: dysregulation of hypothalamic-pituitary-adrenal axis and central corticoptropin releasing factor receptor 1 expression in adult male rats. Behav Brain Res. 2015;288:39–49. doi: 10.1016/j.bbr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Ahmadi N, Moss L, Simon E, Nemeroff CB, Atre-Vaidya N. Efficacy and long-term clinical outcome of comorbid posttraumatic stress disorder and major depressive disorder after electroconvulsive therapy. Depress Anxiety. 2015 doi: 10.1002/da.22451. epub ahead of print. [DOI] [PubMed] [Google Scholar]