Abstract
The clerodane diterpenoids are a widespread class of secondary metabolites and have been found in several hundreds of plant species from various families and in organisms from other taxonomic groups. These substances have attracted interest in recent years due to their notable biological activities, particularly insect antifeedant properties. In addition, the major active clerodanes of Salvia divinorum can be used as novel opioid receptor probes, allowing greater insight into opioid receptor-mediated phenomena, as well as opening additional areas for chemical investigation. This article provides extensive coverage of naturally occurring clerodane diterpenes discovered from 1990 until 2015, and follows up on the 1992 review by Merritt and Ley in this same journal. The distribution, chemotaxonomic significance, chemical structures, and biological activities of clerodane diterpenes are summarized. In the cases where sufficient information is available, structure activity relationship (SAR) correlations and mode of action of active clerodanes have been presented.
1. Background and introduction
1.1. The sources of clerodane diterpenes
Clerodane diterpenes are a large group of naturally occurring secondary metabolites found in several hundreds of plant species from various families and in organisms from other taxonomic groups, such as fungi, bacteria, and marine sponges. Table 1 illustrates the occurrence of clerodane diterpenes in the plant kingdom and marine animals.
Table 1.
The occurrence of clerodane diterpenes in the plant kingdom and marine animals
| Division | Class | Family | Genus | Number of species |
|---|---|---|---|---|
| Magnoliophyta (flowering plants) |
Dicotyledon | Lamiaceae |
Ajuga, Ballota, Elsholtzia, Glossocarya, Gomphostemma,
Kinostemon, Nepeta, Otostegia, Plectranthus, Salvia, Scutellaria, Teucrium |
81 |
| Verbenaceae→Lamiaceae | Callicarpa, Clerodendrum, Cornutia, Premna, Vitex | 7 | ||
| Euphorbiaceae | Aparisthmium, Cleidion, Croton, Macaranga | 27 | ||
| Compositae or Asteraceae |
Aster, Baccharis, Conyza, Haplopappus, Microglossa,
Nannoglottis, Pulicaria |
18 | ||
| Flacourtiaceae→Salicaceae | Casearia, Laetia, Zuelania | 16 | ||
| Menispermaceae | Burasaia, Fibraurea, Tinospora | 10 | ||
| Annonaceae | Polyalthia | 7 | ||
| Portulacaceae | Portulaca | 3 | ||
| Caesalpiniaceae | Detarium, Hymenaea | 2 | ||
| Meliaceae | Amoora | 2 | ||
| Araliaceae | Cussonia | 1 | ||
| Chrysobalanaceae | Licania | 1 | ||
| Combretaceae | Bucida | 1 | ||
| Loganiaceae→Stilbaceae | Nuxia | 1 | ||
| Mimosaceae | Entada | 1 | ||
| Rutaceae | Clausena | 1 | ||
| Monocotyledon | Dioscoreaceae | Dioscorea | 2 | |
| Alismataceae | Echinodorus | 1 | ||
| Hydrocharitaceae | Halophila | 1 | ||
| Pteridophyta (ferns) | Gleicheniaceae | Dicranopteris | 5 | |
| Marchantiophyta (liverworts) | Geocalycaceae | Heteroscyphus | 2 | |
| Jungermanniaceae | Jamesoniella | 2 | ||
| Scapaniaceae | Scapania | 2 | ||
| Adelanthaceae | Adelanthus | 1 | ||
| Lejeuneaceae | Thysananthus | 1 | ||
| Marine animals | Marine sponge | Agelas | 3 | |
| Marine mollusk | Syphonota | 1 | ||
Clerodane diterpenes have attracted interest in recent years as a result of their noteworthy biological activities, particularly as agents modifying the feeding behavior of many economically important insect phytophagous pests. Various genera from the plant family Lamiaceae have been identified as rich sources of antifeedant clerodanes, with species of the genus Scutellaria producing some of the most potent clerodane antifeedants known so far. In addition, the major active clerodanes of Salvia divinorum can serve as opioid receptor probes, enabling better understanding of opioid receptor-mediated phenomena, as well as providing additional areas for chemical investigation.
1.2. The basic structures of clerodane diterpenes
Clerodane diterpenes are bicyclic diterpenoids. The basic skeleton is divided into two fragments: a fused ring decalin moiety (C-1–C-10) and a six-carbon side chain at C-9 (C-11–C-16, with C-16 attached at C-13, i.e., 3-methylpentyl). The remaining four carbons (C-17–C-20) are attached at C-8, C-4, C-5, and C-9, respectively, on the decalin system as illustrated below.
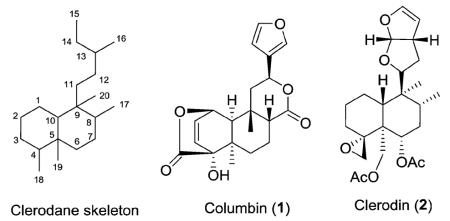
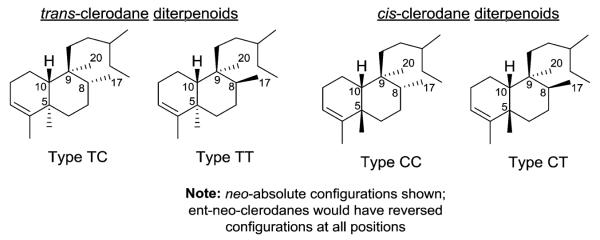
Approximately 25% of clerodanes have a 5 : 10 cis ring fusion as represented by columbin (1). This diterpenoid furanolactone has been isolated from several plants, including Sphenocentrum jollyanum Pierre (Menispermaceae) and Jateorhiza calumba Miers (Menispermaceae). It is sold in a crude drug preparation called calumbae radix or tinosporae radix. Columbin exhibited dose dependent anti-inflammatory activity as well as chemopreventative activity against colorectal cancer.1–3 The remaining 75% of clerodanes have a 5 : 10 trans ring fusion as exemplified by clerodin (2). This compound was originally isolated from Clerodendrum infortunatum L. (Lamiaceae) and has potential as a natural pesticide due to its insect antifeedant activity.4 Clerodanes with a 5 : 10 trans ring junction are characteristic of the Lamiaceae family and to a lesser extent the Compositae (Asteraceae) family, while clerodanes with a 5 : 10 cis ring junction are more commonly found in the Euphorbiaceae, Flacourtiaceae (Salicaceae), and Menispermaceae families.
In addition to the relative configuration of the trans or cis junction of the fused rings, clerodanes are further classified by their relative configurations at C-8 and C-9. Consequently, as shown in Fig. 1, four types of clerodane skeletons are defined with respect to the configuration at the ring fusion and of the substituents at C-8 and C-9: trans–cis (TC), trans–trans (TT), cis–cis (CC), and cis–trans (CT).5 In the majority of clerodanes, the C-17 and C-20 substituents on C-8 and C-9 are cis.5
Fig. 1.
Stereochemical variety in clerodane diterpenoids.
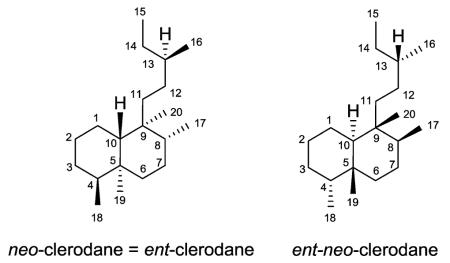
In their 1992 review, Merritt and Ley noted that confusion exists in the literature over the absolute stereochemistry of the clerodanes.6 The absolute stereochemistry of clerodin (2), the first member of the clerodane series, was revised leading to the following terminology. neo-Clerodanes (formerly ent-clerodanes) have the same absolute stereochemistry as clerodin, while entneo-clerodanes are enantiomeric to clerodin.6,7 The former compounds predominate in number over the latter compounds.5 We have used neo-clerodane in this paper, except for compound names already given with the ent-clerodane terminology.
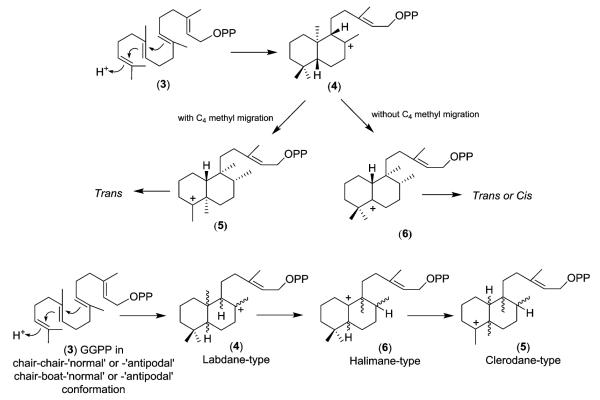
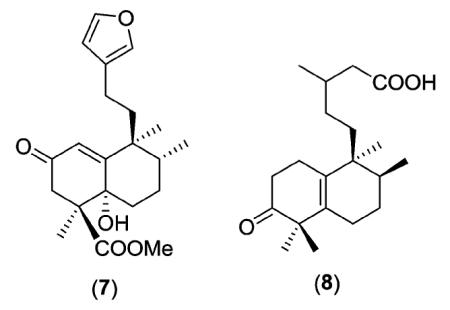
Biosynthetically, the clerodanes likely arise from geranylger-anylpyrophosphate (3) as shown in Scheme 1. However, this overall biogenetic route is simplified as many parallel pathways are needed to yield the multiplicity of clerodane natural products.8 Initially, plant cyclases catalyze a proton-initiated cationic cycloisomerization of 3 to generate a labdane-type precursor skeleton, such as 4 [one of four possible structures (‘normal’, ‘ent’, ‘syn-normal’, ‘syn–ent’), depending on the conformation of 3].8b
 Subsequently, this intermediate can undergo either a concerted or stepwise migration process of methyl and hydride shifts. A concerted process, with a C-4α to C-5α methyl group migration,9 gives clerodane-type intermediate 5 and results in only trans clerodanes. A stepwise process ‘pauses’ at a halimane-type intermediate (6) that retains both gem-dimethyl groups on C-4. Intermediate 6 can then progress to either cis or trans clerodanes. The general scheme without specific stereochemistry is also shown. Examples to support this proposed biosynthetic pathway include the isolation of the partially rearranged labdane compounds chettaphanin (7) from Adenochlaena siamensis (Compositae)10 and salmantic acid (8) from Cistus laurifolius (Cistaceae).11
Subsequently, this intermediate can undergo either a concerted or stepwise migration process of methyl and hydride shifts. A concerted process, with a C-4α to C-5α methyl group migration,9 gives clerodane-type intermediate 5 and results in only trans clerodanes. A stepwise process ‘pauses’ at a halimane-type intermediate (6) that retains both gem-dimethyl groups on C-4. Intermediate 6 can then progress to either cis or trans clerodanes. The general scheme without specific stereochemistry is also shown. Examples to support this proposed biosynthetic pathway include the isolation of the partially rearranged labdane compounds chettaphanin (7) from Adenochlaena siamensis (Compositae)10 and salmantic acid (8) from Cistus laurifolius (Cistaceae).11
Scheme 1.
1.3. The biological activities of clerodane diterpenes
The most important biological activities of clerodanes are insect antifeedant effects12 and action as novel opioid receptor probes.13
1.3.1. Insect antifeedant activity
Clerodane diterpenes are best-known and most extensively studied for their insect anti-feeding and related insecticidal properties, with an emphasis placed on the safety aspects of such natural insect antifeedants in relation to the lives of mammals and fish. To date, over 400 natural and semi-synthetic clerodanes have been examined in laboratory assays, yielding several compounds with potent antifeedant activity against various insect species.12
1.3.2. Probes in opioid pharmacology
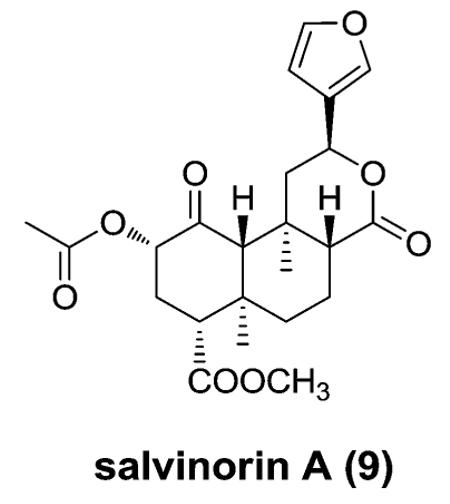
In 2002, opioid receptors were implicated in the actions of the psychoactive mint Salvia divinorum.13 The main active constituent isolated from the leaves of S. divinorum is the neoclerodane diterpene salvinorin A (9). This molecule is interesting to pharmacologists, because it is a non-serotonergic hallucinogen that lacks a basic nitrogen and is a potent and selective agonist for κ opioid receptors. Synthetic organic chemists also find it an attractive target because of its unique structure containing seven chiral centers and a diterpene scaffold.
Opioid agonists based on 9 have the potential to treat pain, cough, diarrhea, stimulant dependence, and mood disorders. Antagonists derived from 9 have potential use in treating several medical conditions, including drug dependence, depression, opioid-induced constipation, and obesity. Thus, analogues of 9 may prove to be excellent research tools and provide greater insight into opioid receptor-mediated phenomena.
1.3.3. Other bioactivities
Besides insect antifeedant activity and opioid receptor agonist effects, clerodane diterpenes can exhibit other pharmacological activities, including antitumor,14 antifungal, NGF-potentiating, antibiotic, anti-peptic ulcer, antiplasmodial, as well as hypoglycemic, hypolipidemic, and anti-thrombin inhibitory activity.
This review provides extensive coverage of naturally occurring clerodane diterpenes discovered in the last 25 years (1990–2015) along with their various bioactivities. The distribution, chemotaxonomic significance, chemical structures, and biological activities of clerodane diterpenes are summarized. In the cases where sufficient information is available, the structure activity relationship (SAR) correlations and mode of action of active clerodanes have been presented.
2. Structure classifications and sources of clerodane diterpenes
During the last 25 years, over 1300 diterpenoids and nor-diterpenoids with the clerodane carbon skeleton have been isolated. For clarity and the purposes of this review, they have been grouped together by particular structural features as described below.
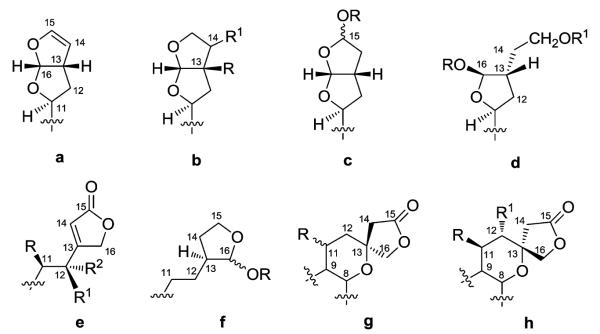
Firstly, the C-11–C-16 fragment can be acyclic or occur as several different bi- and mono-cyclic substructures (Fig. 2). Substructures a–c contain a bicyclic furofuran system, either tetrahydro (a) or hexahydro (b and c). Moreover, the two latter systems can have oxygen moieties present on C-13 and C-14 (b) or C-15 (c), forming a hemiacetal or acetal. Furthermore, when OR is methoxy or ethoxy in substructure c, the compound could be an artifact resulting from the use of methanol or ethanol using the isolation procedure. Substructure d possesses one furan ring (C-11–C-13, C-16) and a two-carbon open chain system (C-14–C-15), formally arising from the opening of the acetal moiety and subsequent reduction of the C-15 aldehyde to a primary alcohol. Alternatively, carbons C-11 and C-12 are present as a two-carbon ethyl chain, while carbons C-13–C-16 form an attached single ring, either an α,β-unsaturated-γ-lactone (e) or lactol (f). Sometimes, Δ11,12 unsaturation is present or carbons C-11 and C-12 can bear oxygenated groups. Finally, bicyclic spiro substructures can be found. The tetrahydropyran incorporates C-8 and C-9, as well as C-11–C-13, and the γ-lactone (C-13–C-16) can assume both possible configurations (g, h) at C-13.
Fig. 2.
C11–C16 moiety of clerodane diterpenoids.
Secondly, the decalin moiety contains some consistent functional features. The decalin junction is mostly trans, and the two groups (C-17 and C-20) on positions C-8 and C-9, respectively, are primarily cis, as well as α-oriented (type TC neo-clerodanes in Section 1.2). Six classifications (A–F) have been made based on the formal oxidation number of carbon C-18 in the decalin moiety (Fig. 3). Groups A and B contain a C-4α/C-18 epoxide, while C-18 is present as a hydroxymethyl in group C. Furthermore, in groups A and B, carbon C-19 is often hydroxylated, either esterified (A) or forming a hemiacetal or acetal bridge with the α-hydroxy group on carbon C-2 (B). In a few cases, carbon C-19 is a methyl or a carboxylic group. In substructure D, C-4 and C-18 form an exocyclic double bond. In this case, carbon C-19 is always a methyl. When C-18 is a methyl, C-19 is also always a methyl. Finally, carbon C-18 can have a higher oxidation number as in an aldehyde (E) or acetal (F). Again, in this case, carbon C-19 is always a methyl.
Fig. 3.
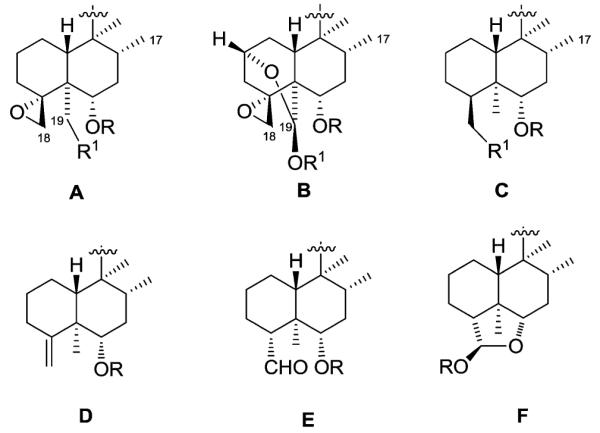
The decalin moiety of clerodane diterpenoids.
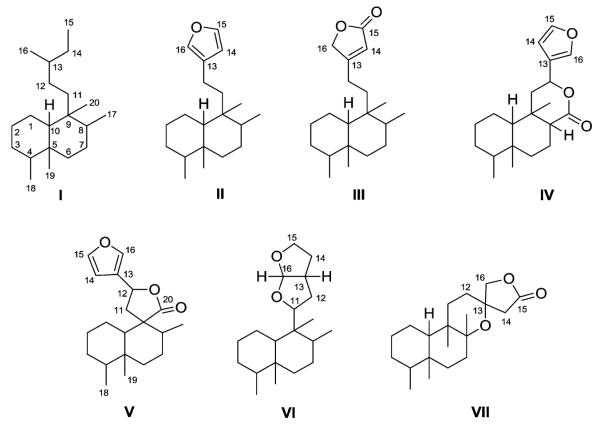
Finally, all of the natural neo-clerodanes have been classified into seven different types (I–VII, Fig. 4) on the basis of their two fragments, the C-11–C-16 moiety (a–h) and the decalin moiety (A–F). Unless otherwise indicated, the diterpenes possess the neo-clerodane absolute stereochemistry.
Fig. 4.
Basic skeletal classifications of clerodane diterpenoids.
2.1. Type I with an acyclic side chain at C-9
Type I clerodane diterpenoids are characterized firstly by having an acyclic side chain at C-9, and then are further divided into three subtypes related to the decalin system. The first subtype has an O-containing five-membered cyclic ring attached to the decalin ring A (18,19-oxide), the second subtype has a double bond between C-3 and C-4 or at another (or no) position of either decalin ring (without an 18,19-oxide), and the third subtype has an epoxy ring in the decalin system.
2.1.1. Type I subtype I with an O-containing five-membered ring at C-18 and C-19
In this subtype, most of the representative compounds are based on two derivations of the 18,19-oxide clerodane nucleus – zuelanin (double bonds at C-12/C-13 and C-14/C-15) and isozuelanin (double bonds at C-13/C-16 and C-14/C-15) (Fig. 5). Various substituents are found at C-2, C-6, C-7, C-18 and C-19, as well as sometimes at C-12, in the isozuelanin subtype, and the decalin system can be saturated. Compounds with other skeletons also exist.
Fig. 5.
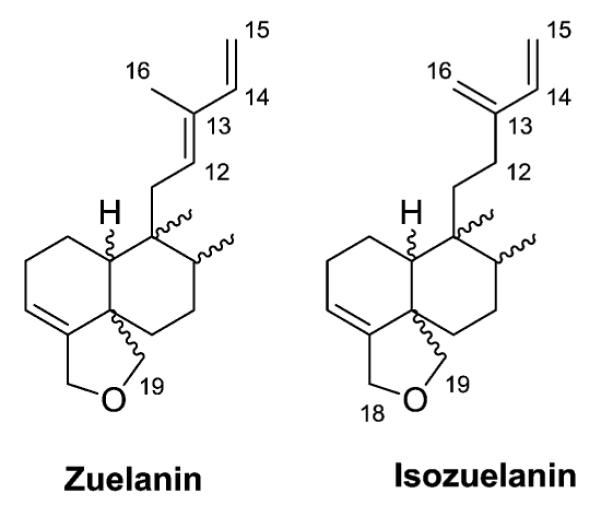
Zuelanin and isozuelanin skeletons.
2.1.1.1. Type I subtype Ia with the isozuelanin skeleton15–33 (Table 2 – compounds 10–86 found in ESI†)
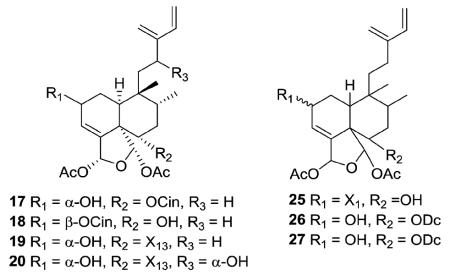
The new clerodanes from type I subtype Ia are all 5 : 10 cis and 17 : 20 trans, mostly isolated from genuses Casearia and Zuelania in the family Salicaceae. The structures of zuelaguidins A–D (17–20) from Z. guidonia are typical of this subtype.16 Corymbulosins A–C (25–27) from Laetia corymbulosa were elucidated as clerodane diterpenes unsaturated at C-3, C-13(16), and C-14.18 Corymbulosin A (25) has a decadienoate ester at C-2, while corymbulosins B and C (26–27) have a saturated decanoate ester at C-6. The latter two compounds are identical except for the configuration at C-2. As based on coupling constant and NOE data, H-2 is equatorial in the former and axial in the latter. However, the study was unable to assign the absolute or relative stereochemistry of the three compounds. Corymbulosin A was the most cytotoxic with IC50 values of 0.6 μM against SF539 human CNS tumor cells and 8 μM against the LOX melanoma cell line in two-day cytotoxicity tests.18
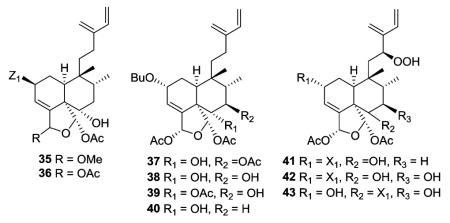
Two other compounds in this structural subtype, intrapetacins A (35) and B (36) from Licania intrapetiolaris, with a p-hydroxybenzoate group at C-2, displayed moderate cytotoxicity against KB cells, with IC50 values of 2.0 and 0.8 μg mL−1.20 Caseanigrescens A–D (37–40) from C. nigrescens showed moderate cytotoxicity against the A2780 human ovarian cancer cell line, with an IC50 range of 0.83–1.4 μM.21 Unlike most compounds in this subtype, compounds 37–39 are substituted at C-7 (37, acetoxy; 38–39, hydroxy). When 37–40 were stored in
 CDCl3 for varying times during NMR analysis, their hemiacetal resonances disappeared and aldehyde resonances appeared. This result indicated that all four compounds slowly hydrolyzed to corresponding unstable dialdehydes. The hydrolysis was likely caused by traces of acid in the specific CDCl3 used, and did not occur when the compounds were allowed to stand in a fresh sample of CDCl3.21 Argutins F–H (41–43) with a unique hydroperoxide moiety at C-12 were isolated from C. arguta.22
CDCl3 for varying times during NMR analysis, their hemiacetal resonances disappeared and aldehyde resonances appeared. This result indicated that all four compounds slowly hydrolyzed to corresponding unstable dialdehydes. The hydrolysis was likely caused by traces of acid in the specific CDCl3 used, and did not occur when the compounds were allowed to stand in a fresh sample of CDCl3.21 Argutins F–H (41–43) with a unique hydroperoxide moiety at C-12 were isolated from C. arguta.22
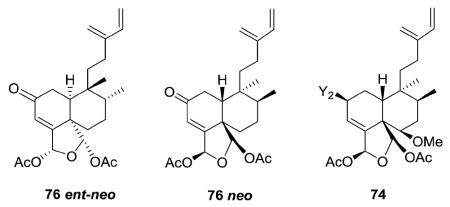
It should be noted that the clerodane absolute stereochemistry was not determined in every structural characterization study. For example, esculentin A (76) has been reported in both ent–neo16 and neo32 configurations. In addition, while caseargrewiin A (74) was shown as a neo-clerodane, caseargrewiins B–D (1256–1258, structures in Section 3.5) co-isolated from C. grewiifolia were shown as ent-neo-clerodanes. The absolute configuration of C-2 in 1258 established as R by a modified Mosher's method, and the absolute stereochemistry in the rest of the molecule based on NMR coupling constants and NOESY correlations.31 Generally, this review has focused on relative configurations only.
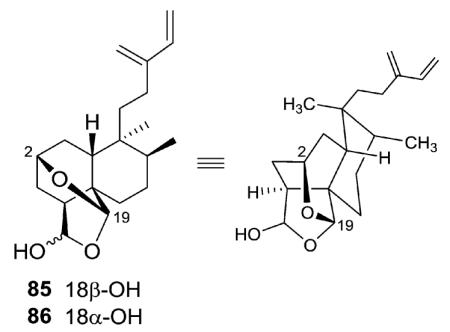
Caseabalansin A and 18-epicaseabalansin A (85–86) are the first examples of clerodane diterpenoids with an oxygen bridge between C-2 and C-19.33 They were initially isolated as an inseparable 1.3 : 1 isomeric mixture from C. balansae and identified based on NMR spectroscopy. Conversion to the 18-acetates allowed separation of the two compounds by HPLC.
2.1.1.2. Type I subtype Ib with the zuelanin skeleton14,22,23,25,28,34–40 (Table 3 – compounds 87–135 found in ESI†)
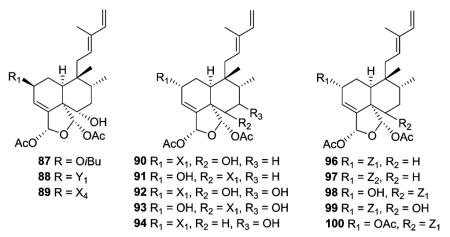
The newly reported type I subtype Ib compounds are also 5 : 10 cis and 17 : 20 trans, mostly isolated from the family Salicaceae. They are structurally similar to type I subtype Ia compounds, but with a different double bond pattern in the C-11–C-16 acyclic side chain. Casearvestrins A–C (87–89) from Casearia sylvestris show the typical zuelanin skeleton, with acetoxy groups at both C-18 and C-19, in addition to a 18,19-oxide. In these three compounds, C-2 and C-6 are also substituted with various four to six carbon esters and a hydroxy group, respectively. Compounds 87–89 displayed promising bioactivity in cytotoxicity assays against a panel of tumor cell lines and antifungal assays against Aspergillus niger in a disk diffusion assay.34 Argutins A–E (90–94) from C. arguta contain the same cyclic ether with varying combinations of decadienoyloxy, hydroxy, and hydrogen at C-2, C-6, and C-7. Among them, argutin B (91) showed the highest degree of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitization. Furthermore, the synergistic effect of 91 and TRAIL together was three-fold greater than that of 91 alone.22 Like the type I subtype Ia compounds 35–36 mentioned above, type I subtype Ib case-arborins A–E (96–100) from C. arborea contain structurally novel aromatic esters, either at C-2 or C-6. When evaluated against LOX and SF539 cell lines using a two-day cytotoxicity assay, compounds 96–100 exhibited IC50 values ranging from 0.29 to 9.7 μM.35
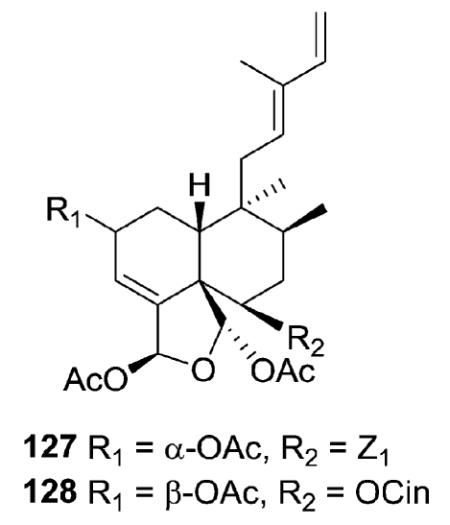
Caseobliquins A (127) and B (128) from C. obliqua have different substituents at C-6, a p-hydroxybenzoate moiety in 127 and a cinnamoate moiety in 128.39 Meanwhile, the acetoxy groups on C-18 and C-19 are trans, rather than cis as in the above-mentioned compounds.
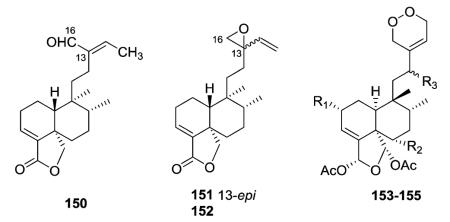
2.1.1.3. Type I subtype Ic with other skeletons16,41,42 (Table 4 – compounds 136–155 found in ESI†)
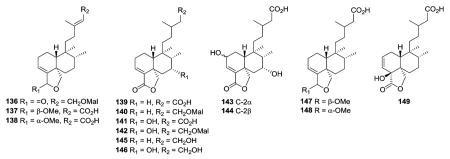
Type I subtype Ic includes compounds with acyclic side chains different from 3-methylenepent-4-ene or (E)-3-methylpenta-2,4-diene, which are found in subtypes Ia and Ib, respectively. Most of the type I subtype Ic compounds contain a 18,19-lactone ring. Compounds 136–138 from the aerial parts of Olearia teretijdia contain a Δ13E double bond, while this position is saturated in 139–149. The terminal carbon in the side chain is generally hydroxymethyl, malonyloxymethyl, or carboxy rather than methyl. Compound 149 also has a Δ2 rather than Δ3 double bond.41
Baccharis linearis was the source of three new clerodanes: baclinal (150) with a 3-formyl-3E-pentenyl side chain and epimeric baclinepoxides (151–152) with an interesting 13,16-spiro-oxirane in the C-9 side chain.42 Zuelaguidins E, G, and H (153–155), isolated from Zuelania guidonia (family Salicaceae),
 were the first reported diterpenoids containing a 3,6-dihydro-1,2-dioxin moiety. This endoperoxide may result from a Diels–Alder reaction between zuelaguidin A (17, see Section 2.1.1.1), also found in Z. guidonia, and molecular oxygen.16 Compounds 153–155 are 5 : 10 cis and 17 : 20 trans. The remaining compounds in type I subtype Ic are 5 : 10 trans and 17 : 20 cis, as typical of clerodanes isolated from the family Asteraceae.
were the first reported diterpenoids containing a 3,6-dihydro-1,2-dioxin moiety. This endoperoxide may result from a Diels–Alder reaction between zuelaguidin A (17, see Section 2.1.1.1), also found in Z. guidonia, and molecular oxygen.16 Compounds 153–155 are 5 : 10 cis and 17 : 20 trans. The remaining compounds in type I subtype Ic are 5 : 10 trans and 17 : 20 cis, as typical of clerodanes isolated from the family Asteraceae.
2.1.2. Type I subtype II with a double bond between C-3 and C-4 or another position
2.1.2.1. Type I subtype IIa with a double bond between C-3 and C-4 (ref. 14, 15, 18, 26, 41 and 43–88) (Table 5 – compounds 156–253 found in ESI†)
Type I subtype IIa compounds contain many of the same acyclic side chains as type I subtype Ia–Ic compounds. For instance, both 3-methylenepent-4-ene (156–158) and 3-methylpenta-2,4-diene (164–167) side chains are found in type I subtype IIa. Interestingly, compounds 164–166 with a Δ12Z double bond were isolated from both Schistochila acuminate and Heteroscyphus planus,45–47 while heteroscyphol (167) with an assigned Δ12E double bond was found only in Heteroscyphus planus.47
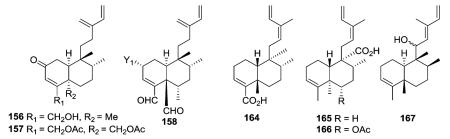
Terpenetetriene (159) was produced from a transformant of Streptomyces lividans and postulated to be a probable biosynthetic intermediate of terpentecin (160), a diterpenoid antibiotic previously isolated from the bacterium Streptomyces griseosporus.43 This study was the first to report a bacterial diterpene cyclase. Compound 159 was also isolated together with 161 from Jungermannia infusca. These two compounds have the same planar structure but different stereochemical structures. Both are 5 : 10 trans, but the former compound is 17 : 20 trans,43 while the latter is 17 : 20 cis.44
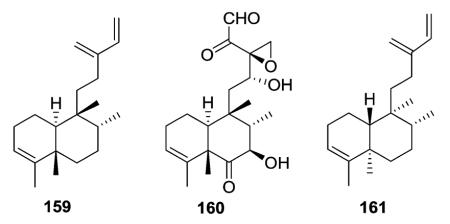
Compounds 193 and 194 have a neo-clerodane skeleton with a Δ3,4 C=C bond and a C=O group at C-2.63 Compounds 197 (2-oxokolavenic acid) and 198, isolated from different plant species, are identical, except for the orientation of the C-20 methyl group, β in the former compound and α in the latter compound.65,66 The cis-decalin (5α Me, 10α H) and trans orientation of C-8 and C-9 (17α Me, 20β Me) in 197 were confirmed by X-ray crystallographic analysis. Furthermore, 2-oxokolavenic acid with a trans-decalin (5β Me, 10α H) and cis orientation of C-8 and C-9 (17β Me, 20β Me) was co-isolated at the same time from the fruits of Detarium microcarpum, as well as previously from the bark and leaves of the same plant.65
Portulene acetal (199) with a caged hemiacetal [6.6]-ring was isolated as a minor constituent from Portulaca grandiflora.67 The structures of 200 and 201 were quite similar, except for a tigloyl group in 200 and an angeloyl group in 201.68 Seven new clerodanes, exemplified by 207, were obtained from Baccharis trinervis.72 Although no double bond is present between C-13/C-14, the stereochemistry of C-13 in these compounds and their analogue 214 from B. gaudichaudiana73 could not be deduced spectroscopically.
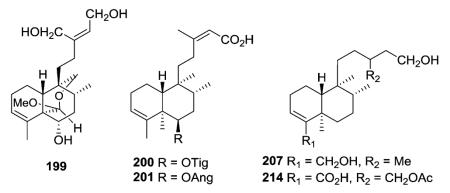
The four new clerodanes (227–230) isolated from Nuxia sphaerocephala were assigned to the ent-series (now neo-series) based on optical rotation values, plus the absolute configuration of C-13 in 227 was established as S based on its phenylglycine methyl ester (PGME) amide derivatives.78 In addition to having a free carboxylic acid rather than ethyl ester at the side chain terminus, compounds 231 and 232 have a formyloxy group and an acetyloxy group, respectively, on C-2 compared with the hydrogen in 233.79 All three compounds were isolated from Clausena dunniana.
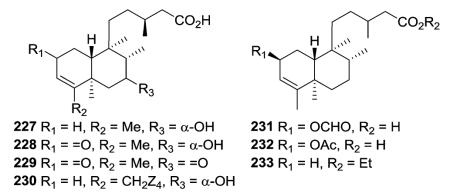
Compounds 240–242 were established as hydroxy and peroxy derivatives of the 2-oxo group 239, based on X-ray and CD analysis.81 Compounds 249–252, isolated as 3 : 1 or 4 : 1 mixtures from Linaria saxatilis, are the (12E)- and (12Z)-stereo-isomers of the Δ3-endocyclic analogues.87
2.1.2.2. Type I subtype IIb with a double bond at another (or no) position36,55,63,65,74,75,79,82,83,88–102 (Table 6 – compounds 254–289 found in ESI†)
The decalin double bonds in type I subtype IIb compounds can be either endocyclic at C-1/C-2 (e.g., 277), C-2/C-3 (276), or C-7/C-8 (272) or exocyclic at C-4/C-18 (281) or C-8/C-17 (258). Alternatively, some compounds in this subtype do not have a decalin double bond, but instead often have dihydroxy substitution (e.g., 266–267). Like in type I subtype IIa, various acyclic side chains are present.
Leojaponin A (276), characterized by a C4-C7 oxa-bridge and a double bond between C-2 and C-3, is the first clerodane diterpenoid obtained from Leonurus japonicus.96 Palmadorins A and B (288–289), from the Antarctic nudibranch Austrodoris kerguelenensis, were the first two of a new series of clerodane diterpene glycerides.88
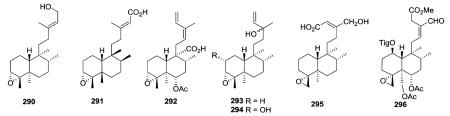
2.1.3. Type I subtype III with an epoxy ring46,65,90,99,103–105 (Table 7 – compounds 290–296 found in ESI†)
Among all compounds with an acyclic side chain, seven compounds are classified as type I subtype III with a epoxy ring either at C-3/C-4 (290–294)46,65,90,103,104 or C-4/C-18 (295–296).99,105 The β-orientations of the four methyl groups (C17–C20), as well as H-10, in 291 from Detarium microcarpum are shown as reported.65 The assignments were based on NOE correlations, but seem uncommon from a biogenetic viewpoint. Compound 292 from Heteroscyphus planus is a possible intermediate in the biosynthesis of diterpenes that have a spiro-γ-lactone group at the C-9 position.46 Compounds 293 from Jungermannia paroica and 294 from Stachys glutinosa have almost identical structures with a hydroxy group at C-13, but a hydrogen and α-hydroxy group, respectively, at C-2.103,104 The orientation of the epoxy methylene H2-18 in 295 from Polyalthia longifolia was deduced to be β, based on comparison of the chemical shifts and coupling constants with those of similar structures in the literature.99 Highly oxygenated compound 296 from Ajuga decumbens inhibited lipopolysaccharide (LPS)-induced nitric oxide production in RAW 264.7 macrophages.105
2.2. Type II with a 2-ethylfuran-based side chain at C-9
Type II clerodane diterpenoids are characterized initially by a 2-ethylfuran-based, rather than acyclic, side chain at C-9. When
 viewed simplistically, an oxygen atom has been inserted between C-15 and C-16 of a 3-methylenepent-4-ene side chain.
viewed simplistically, an oxygen atom has been inserted between C-15 and C-16 of a 3-methylenepent-4-ene side chain.
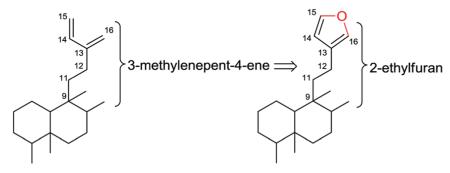
Type II compounds then are further split into various subtypes. Subtypes Ia and Ib generally have more complex or multiple O-containing rings at various positions of the decalin moiety, as compared with subtype Ic with only one simple epoxy ring on the decalin moiety. Subtypes IIa and IIb do not have an O-containing ring, but instead have one or more double bonds in the decalin moiety (IIa) or saturated decalin or oxodecalin moiety (IIb). Finally, subtype III compounds have a distinctive tetrahydrofuran rather than furan in the C-9 side chain, along with various decalin moieties.
2.2.1. Type II with various O-containing rings
2.2.1.1. Type II subtype Ia with various O-containing rings75,106–127 (Table 8 – compounds 297–323 found in ESI†)
Nasimalun A (297) from Barringtonia racemosa illustrates a type II subtype Ia clerodane with a C-18/C-19 γ-lactone ring,106 while teumassilenin B (302) from Teucrium massiliense is a type II subtype Ia with a similar γ-lactol ring.111
T. massiliense also yielded new clerodane diterpenes from two additional type II subtypes: teumassilenin C (336) (type II subtype Ib with an oxetane ring, see Section 2.2.1.2) and teumassilenin A (423) (type II subtype IIb with an oxodecalin, see Section 2.2.2.2; the first example of an 18β-aldehyde from Teucrium species).111
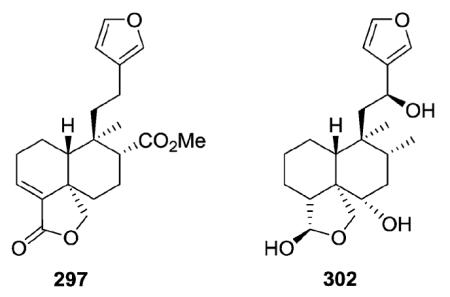
A furan ring is present between C-18 and C-6 in clerodanes 314–316,122,123 while plaunol E (306) has a γ-lactone ring at this same position, as well as a δ-lactol ring between C-20 and C-19.115 Compound 306 significantly inhibited LPS-induced NO production with an IC50 value of 2.79 μM.115
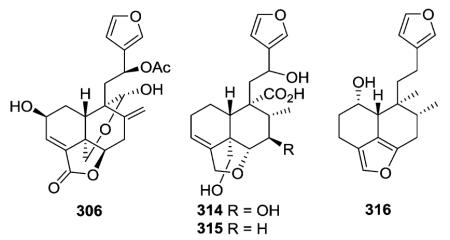
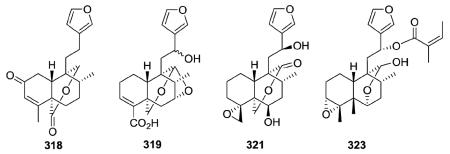
Other new clerodanes in this subtype also had oxygenated rings incorporating C-20. Sacacarin (318) from Croton cajucara has a C-19/C-20 δ-lactone ring. Compound 319 from Salvia miniata has an oxygenated structure containing C-19/C-20 and C-7/C-20 acetalic bridges.108 The C-20/C-19 δ-lactone in 321 (ref. 116) from Teucrium oxylepis and the C-20/C-6 δ-lactol ring in 323 (ref. 75) from Pteronia eenii are also accompanied by C-4/C-18 and C-3/C-4 epoxide rings, respectively.
2.2.1.2. Type II subtype Ib with other O-containing rings84,111,128–140 (Table 9 – compounds 324–355 found in ESI†)
Clerodane diterpenoids with an axial oxyfunction at the C-7 position are rare, but a few examples, such as 326–329, have been reported. The structure of 326 from Tecrium cossonii distinctly contained a 4,18-spiro-oxirane compared with those of 327–329 from Ptychopetalum olacoides.129,130 Methyl dodonates A–C (330–332), three new modified clerodanes containing a tricyclo [5.4.0.01.3]undecane ring system, were isolated from Dodonaea viscosa.131 They have been proposed as putative intermediates in the biogenetic pathway to diterpenes possessing a bicyclo[5.4.0]undecane or bicyclo[5.3.0]decane ring system.
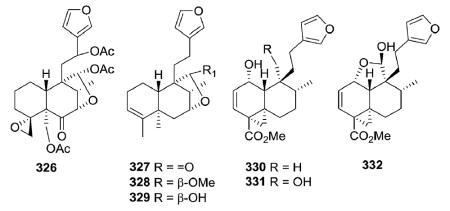
Teucrolin E (335) from Teucrium oliverianum contains an oxo group (C=O) at C-7.133,134 Its originally proposed structure also contained an oxetane ring with the oxygen connecting C-4 to C-10, leaving C-18 as a hydroxymethyl group.133 However, additional NMR analysis of the diacetate, including NOE studies, indicated that C-18 is instead part of a tetrahydrofuran ring that includes C-10 and a tertiary OH group is present at C-4.134 As mentioned previously, teumassilenin C (336) from Teucrium massiliense does possess an oxetane ring; however, the oxygen connects C-4α and C-19.111 Anastreptin (337) from Adelanthus lindenbergianus contains a cyclic ketal moiety with oxygen bridges from C-12 to C-7 and C-12 to C-8 of the decalin moiety.84 Three type II subtype Ib clerodanes (bafoudiosbulbins A, D, and E; 339–341) were isolated from Dioscorea bulbifera.135,136 Compound 339 is a stereoisomer of 340; both compounds contain a lactone bridge (OC=O) between C-2 and C-5, as well as between C-6 and C-8.135,136 Compound 341 is identical to 340, but also contains a 3α,4α-epoxide.136
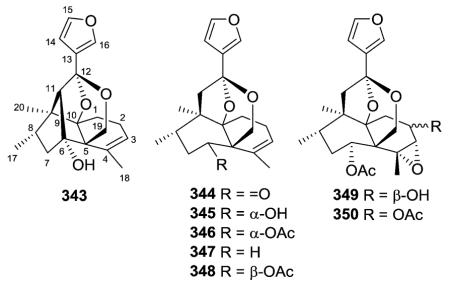
Scaparvin A (343), a novel caged cis-clerodane diterpenoid with an unprecedented C-6/C-11 bond, was isolated together with scaparvins B–E (344–346, 350), without the C-6/C-11 bond, from the Chinese liverwort Scapania parva.138 Their absolute structures were elucidated by analysis of NMR and CD data coupled with electronic circular dichroism (ECD) calculations. The authors proposed an enzymatic intramolecular aldol reaction as the key step in the biogenetic pathway of 343.138 Parvitexins A–C (347–349) from S. parvitexta were the first natural products identified with an unusual 2,8-dioxobicyclo[3.2.1] octane moiety.139
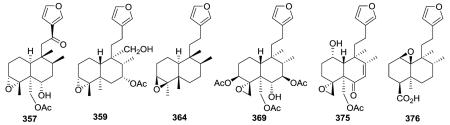
2.2.1.3. Type II subtype Ic with a simple epoxy ring123,129,139,141–148 (Table 10 – compounds 356–376 found in ESI†)
Clerodane diterpenes in this subtype generally have either a 3,4 epoxide (e.g., 357 from Scapania parvitexta,139 359 from Croton eluteria,142 364 from Thysanathus spathulistipus143) or a 4,18-spiro-oxirane (e.g., 369 from Teucrium oliverianum,146 375 from T. fruticans123). Their structural differences are mainly in the linkages between the carbocyclic and heterocyclic moieties and the functionalization of the decalin core. However, the β-oriented epoxide of phlomeoic acid (376) from Phlomis bracteosa is uniquely at C-1/C-10.148
2.2.2. Type II with or without a C=C double bond in the decalin moiety
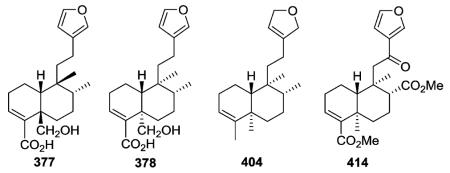
2.2.2.1. Type II subtype IIa with one or more decalin C=C double bonds74,106,110,130,149–168 (Table 11 – compounds 377–419 found in ESI†)
Vishautriwatic acid (377) from Dodonaea viscosa
 was identified with different stereochemistry at C-5 and C-9 from the known neo-clerodane hautriwatic acid (378) found in Eremocarpus setigerus.149,150 The cis-relationship of H-10 and Me-20 in 377 is uncommon compared with the trans-relationship found in 378. Crotonolide G (404, Croton laui), with a unique 2,5-dihydrofuran rather than furan in the C-9 side chain, displayed significant antibacterial activity with an MIC value of 43.4 μM against four strains of Gram-positive bacteria, including Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Micrococcus luteus ATCC 9341, and Bacillus subtilis CMCC 63501.165 Crotomembranafuran (414, Croton membranaceus), which has a less common ethanone rather than ethyl or ethylene linkage between the decalin and furan ring systems, had an IC50 value of 10.6 μM against PC-3 cells.168
was identified with different stereochemistry at C-5 and C-9 from the known neo-clerodane hautriwatic acid (378) found in Eremocarpus setigerus.149,150 The cis-relationship of H-10 and Me-20 in 377 is uncommon compared with the trans-relationship found in 378. Crotonolide G (404, Croton laui), with a unique 2,5-dihydrofuran rather than furan in the C-9 side chain, displayed significant antibacterial activity with an MIC value of 43.4 μM against four strains of Gram-positive bacteria, including Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Micrococcus luteus ATCC 9341, and Bacillus subtilis CMCC 63501.165 Crotomembranafuran (414, Croton membranaceus), which has a less common ethanone rather than ethyl or ethylene linkage between the decalin and furan ring systems, had an IC50 value of 10.6 μM against PC-3 cells.168
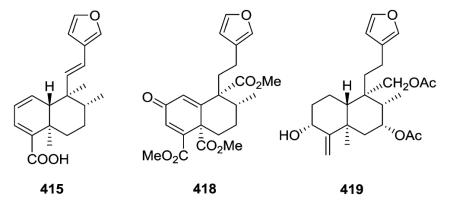
In addition to the C-3/C-4 double bond, compounds 415 and 418 found in Conyza hypoleuca also contain a second double bond (C-1/C-2 in 415;155 C-1/C-10 plus C-2 oxo in 418 (ref. 169)). Finally, eluterin B (419) from Croton eluteria contains an exocyclic C-4/C-18 double bond.142
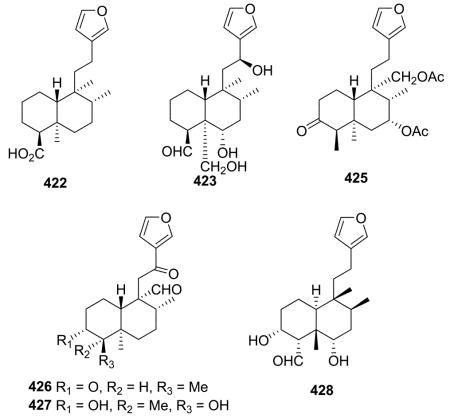
2.2.2.2. Type II subtype IIb without a decalin C=C double bond89,111,142,151,165,170–172 (Table 12 – compounds 420–428 found in ESI†)
Crolechinic acid (422) is representative of type II subtype IIb compounds and was found as a minor constituent in Croton lechleri based on TLC profiles and NMR spectra.89 Eluterin A (425) differs structurally from eluterin B (419) only in the functionalities at C-3, C-4, and C-18: oxo and β-methyl in the former, but α-hydroxy and exocyclic double bond in the latter.142 Four new tricyclic clerodane type diterpene aldehydes (423 and 426–428) were characterized through modern spectroscopic techniques and comparison with literature data.111,170–172 20α-Aldehydes are present in 426 and 427 from C. hovarum.170,171 Compounds 423 and 428 both contain a 18-aldehyde and the same relative stereochemistry, but the former was reported as a neo-clerodane from Teucrium massiliense and the latter as an ent-neo-clerodane from Nepeta juncea.111,172
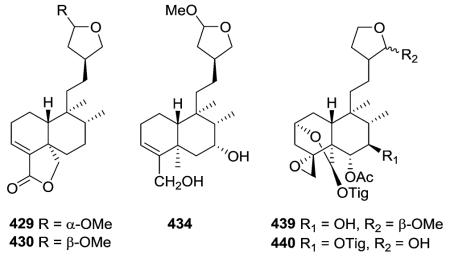
2.2.3. Type II subtype III with a tetrahydrofuran ring72,89,149,173–179 (Table 13 – compounds 429–445 found in ESI†)
Compounds 429 and 430 from Baccharis trinervis were determined as trinerolide and 15-epi-trinerolide, respectively, based on the number of split peaks and coupling constant of the acetal H at C-15.72 Compound 434 was a possible artifact from B. articulata, with the true natural product being its hemiacetal analogue.173 Two new neo-clerodane diterpenoids with multiple O-containing rings, compounds 439 and 440, were isolated from an acetone extract of the aerial parts of Scutellaria galericulata.176,177
2.3. Type III with a 3-ethyl-2-butenolide-based side chain at C-9
Type III clerodane diterpenoids bear a 3-ethyl-2-butenolide-based side chain at C-9, with various 6O-containing rings or double bonds at different positions. Comparison of the type II and type III general structures shows that the furan ring in the former has been replaced by a furan-2(5H)-one (or 2-butenolide) in the latter.
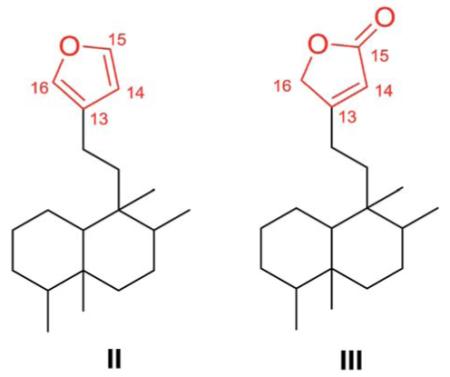
2.3.1. Type III subtype I with O-containing rings
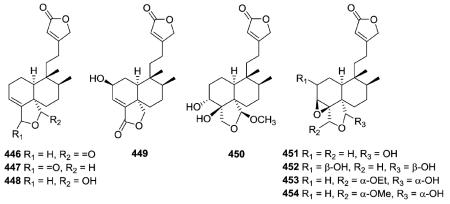
2.3.1.1. Type III subtype Ia with five-membered cyclic O-containing rings84,173,180–191 (Table 14 – compounds 446–480 found in ESI†)
Amphiacrolides A–E, I, J, L, and M (446–454), isolated from Amphiachyris dracunculoides, have an ethyl butenolide side chain attached at C-9, as supported by characteristic MS fragments (m/z 111, 98, and 97) and 1H and 13C-NMR peaks.180–182 The stereochemistries of the amphiacrolides were established from the chemical correlation of these compounds to gutierolide, a compound with absolute stereochemistry determined by X-ray analysis.180–182
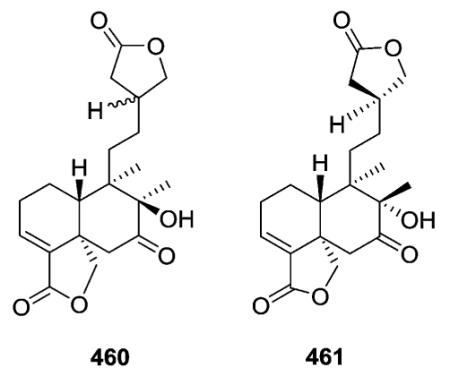
The planar structures of 460 and 461, isolated from different Baccharis species are identical, but the compounds are epimeric at C-8.173,186 The absolute stereochemistry (neo-series, 5 : 10 trans, 17 : 20 trans) of gaudichanolide A (461) from B. gaudichaudiana was established by X-ray crystallographic analysis.186
Several new clerodanes, exemplified by 463 from Cephaloziella kiaeri, have a unique unsaturated γ-lactone moiety incorporating C-18 and C-6.84,182,187–191 Three 1 : 1 mixtures (470–475) of epimeric clerodane diterpenes with a C-8/C-12 ether bridge were isolated from Adelanthus lindenbergianus.84 Structures 474–476 contain a second ether bridge between C-12 and C-7 forming a cyclic ketal at C-12.84 A C-1/C-12 ether bridge is present in 477 also from A. lindenbergianus,84 while a C-10/C-12 ether bridge is found in 478–480 from Scapania ciliata.191
2.3.1.2. Type III subtype Ib with other O-containing rings52,181,192–214 (Table 15 – compounds 481–527 found in ESI†)
Many compounds in this subtype contain the typical functional groups of neo-clerodane diterpenoids, including a C-4/C-18 (e.g., 505) or C-3/C-4 epoxide (e.g., 515, 516) and cis C-17α and C-20α methyl groups.192–209 Among them, hastifolin A (505) from Scutellaria hastifolia showed significant antifeedant activity against larvae of Spodoptera littoralis at a concentration of 100 ppm; its feeding index was 60 ± 15.2 and FI50 concentration was 45 ppm.204 Seguiniilactones A and B (515–516) from Colquhounia seguinii differ structurally only in where the butenolide ring is connected to C-12. This connection is at the β position of the
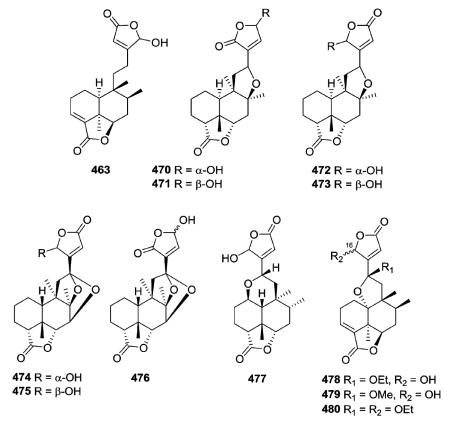 butenolide ring in the former compound and at the α position in the latter compound.209 Thus, the carbonyl moiety of the lactone ring is at C-15 (a 15,16-olide) in 515 and at C-16 (a 16,15-olide) in 516. A β-substituted α,β-unsaturated γ-lactone functionality was found to be crucial for the strong antifeedant activity of this compound class, and 515 was approximately 17-fold more potent than commercial neem oil insecticide against the generalist plant-feeding insect Spodoptera exigua.208,209
butenolide ring in the former compound and at the α position in the latter compound.209 Thus, the carbonyl moiety of the lactone ring is at C-15 (a 15,16-olide) in 515 and at C-16 (a 16,15-olide) in 516. A β-substituted α,β-unsaturated γ-lactone functionality was found to be crucial for the strong antifeedant activity of this compound class, and 515 was approximately 17-fold more potent than commercial neem oil insecticide against the generalist plant-feeding insect Spodoptera exigua.208,209
The C-2/C-19 ether bridges of amphiacrolide K (518; Amphiachyris dracunculoides) and conyzalactone (519; Conyza blinii) have opposite relative configurations, and these two compounds are also the two ent-neo-clerodane exceptions in this subtype.181,210 neo-Clerodane-type diterpenoid 520 from Ajuga decumbens has a C-1/C-19 ether bridge.211 Compounds 521–525 contain a C-20/C-7 γ-lactone/lactol bridge,52,212 compounds 526 has a C-18/C-7 δ-lactone bridge,213 and finally, microdon B (527) possesses a C-17/C-12 δ-lactone bridge.214
2.3.1.3. Type III subtype IIa with C3/C4 double bond58,70,72,155,163,164,175,183,189,213,215–228 (Table 16 – compounds 530–575 found in ESI†)
A wide range of substituents are found on the decalin and butenolide moieties in type III subtype IIa compounds. With CO2H at the decalin C-4 position and OMe or H, respectively, at the butenolide C-4 position, limbatolides B (533) and C (534) from Otostegia limbata inhibited acetylcholinesterase
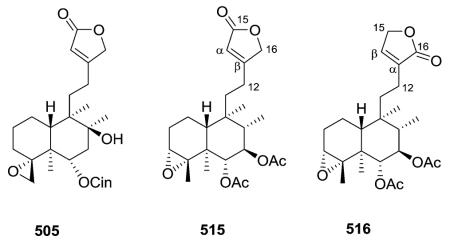
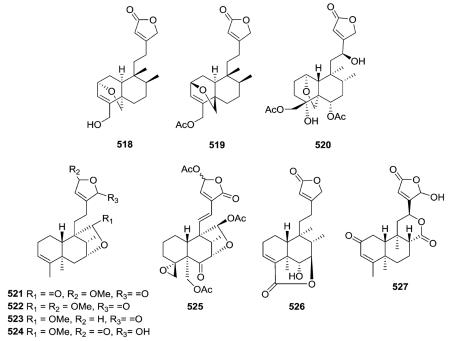 (AChE) and butyrylcholinesterase (BChE) enzymes in a concentration-dependent manner with IC50 values of 47.2, 103.7 and 17.5, 14.2 μM, respectively.189 Polylongifoliaon B (543) from Polyalthia longifolia is one of a few type III subtype IIa compounds with an α,β-unsaturated ketone in the decalin ring A.220 This compound improved the viability of human neuroblastoma cells (SK-N-MC cells) under Aβ-induced neurotoxicity with an IC50 value of 3.75 μM.220
(AChE) and butyrylcholinesterase (BChE) enzymes in a concentration-dependent manner with IC50 values of 47.2, 103.7 and 17.5, 14.2 μM, respectively.189 Polylongifoliaon B (543) from Polyalthia longifolia is one of a few type III subtype IIa compounds with an α,β-unsaturated ketone in the decalin ring A.220 This compound improved the viability of human neuroblastoma cells (SK-N-MC cells) under Aβ-induced neurotoxicity with an IC50 value of 3.75 μM.220
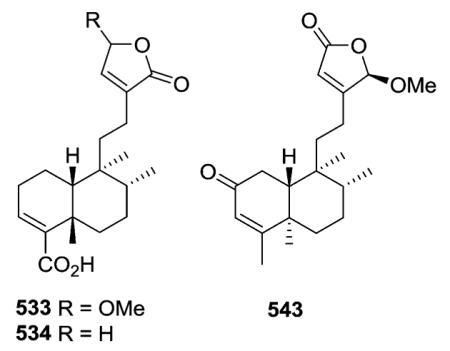
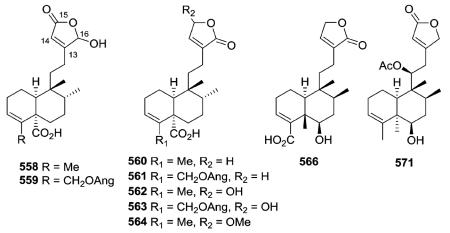
The H-10 of compounds 558–571 has the more unusual α-orientation (ent-neo-clerodane).163,164,183,213,226 Both cis–trans (e.g., 558–564), trans–cis (e.g., 566), and cis–cis (e.g., 571) configurations of the decalin ring junction and C-17/C-20 orientation, respectively, are found. Solidagoic acids C–I (558–564) from Solidago virgaurea contain a carboxylic acid at C-19, a motif that is relatively uncommon among the clerodanes.183 Compared with the standard drug deoxynojirimycin (425.6 ± 8.1 μM), compound 566 from Duranta repens showed significant α-glucosidase inhibitory activity (IC50 577.7 ± 19.0 μM).226 Structurally, it has a 6β-OH and opposite stereochemistry at C-8 to C-10 compared with 534 mentioned above.
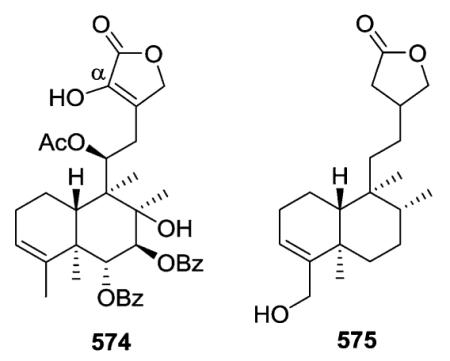
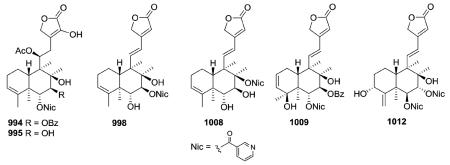
Scutebata A (574), as well as scutebatas B and C (994 and 995; see Section 2.9.1), from Scutellaria barbata possess a rare hydroxy group at the α-position of the α,β-unsaturated lactone ring. Scutebata A showed weak cytotoxic activity against SK-BR-3 cells with an IC50 value of 15.2 μM.228 Compound 575 from Baccharis trinervis has a saturated, rather than unsaturated, γ-lactone in its side chain.72
2.3.1.4. Type III subtype IIb with double bonds in other positions117,194,205,206,208,217,229–248 (Tables 17 and 18 – compounds 576–614 found in ESI†)
Compounds 577–579, 584, and 888 (see Section 2.7) from Scutellaria barbata showed significant cytotoxic activities against three human cancer lines, HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma, with IC50 values in the range of 2.0–8.1 μM.229,230,232 Compound 584 has a 2,3-epoxy-2-isopropyl-n-propoxy moiety attached at C-6, and its possible biosynthesis was proposed.230 Salvidivins C (587) and D (588) from Salvia
 divinorum are unique neo-clerodane diterpenes that possess a γ-hydroxy-α,β-unsaturated γ-lactone moiety, and are geometrical isomers at the γ-lactone moiety.235
divinorum are unique neo-clerodane diterpenes that possess a γ-hydroxy-α,β-unsaturated γ-lactone moiety, and are geometrical isomers at the γ-lactone moiety.235
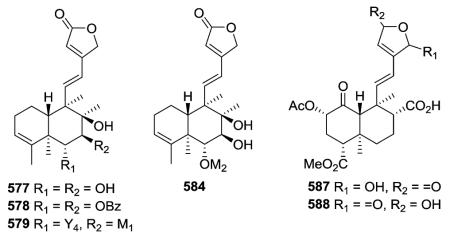
Several compounds in this subtype have a 4,18-exo-methylene group (e.g., 589, 597, 598–600),217,231,236–242 while compounds 612–614 have a unique α-oriented cyclopropane
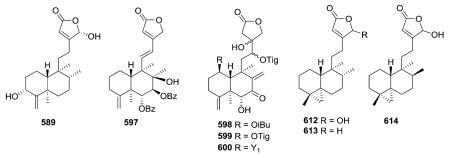 ring formed from C-4, C-5, and C-19.247,248 The latter three compounds were isolated from two different marine organisms, an Okinawa tunicate Cystodytes sp and a Formosan gorgonian coral Echinomuricea sp. Echinoclerodane A (614) was found to be the C-8 epimer of dytesinin A (612), and the chiral carbons in 614 were assigned as 4S*, 5S*, 8S*, 9S* and 10R*.248
ring formed from C-4, C-5, and C-19.247,248 The latter three compounds were isolated from two different marine organisms, an Okinawa tunicate Cystodytes sp and a Formosan gorgonian coral Echinomuricea sp. Echinoclerodane A (614) was found to be the C-8 epimer of dytesinin A (612), and the chiral carbons in 614 were assigned as 4S*, 5S*, 8S*, 9S* and 10R*.248
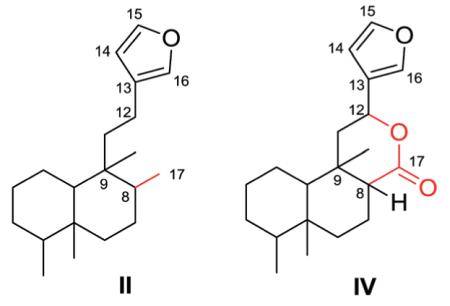
2.4. Type IV with a 5-(3-furyl)-δ-valerolactone-based side chain at C-9
Compounds in this group are characterized by a 5-(3-furyl)-δ-valerolactone-based side chain at C-9, together with lactone and epoxy rings, hydroxy and acetoxy groups, as well as double bonds. Both type II and type IV compounds contain a furan ring in the C-9 side chain but differ by the presence of a 17,12-δ-lactone ring in the latter class. Thus, as seen below, position 17 is generally a free methyl group in type II compounds, while it is incorporated into the δ-lactone ring as the carbonyl group in type IV compounds.
2.4.1. Type IV subtype I with O-containing rings108,109,113,120,249–262 (Table 19 – compounds 615–655 found in ESI†)
In addition to the 17,12-δ-lactone ring characteristic of type IV clerodanes, many new subtype I compounds from Salvia species have a 18,19-γ-lactone ring. Their decalin moieties also contain various numbers and locations of double bonds (see 615, 617, 622–624) and oxygenated groups. The 1β,10β-epoxy group of 627–630 from Salvia herbacea was deduced by analysis of spectroscopic data.249 Tehuanin G (630) exhibited anti-inflammatory activity (IC50 0.24 μM/ear) comparable to that of
 indomethacin, the reference compound.249 In contrast, the C-1(2)-epoxy group of 632–634 from S. reptans has an α-orientation.252,253 Except for the stereochemistry of the C-18–C-19 lactone ring fusion (trans in 632, cis in 633), compounds 632 and 633 have identical structures with both A/B and B/C cis ring fusions, as established by X-ray analysis.252,253 Furthermore, tehuanins A–C (635–637) from S. herbacea contain a 1,8-oxygen bridge; this unusual structural feature was confirmed by X-ray diffraction of 635.249
indomethacin, the reference compound.249 In contrast, the C-1(2)-epoxy group of 632–634 from S. reptans has an α-orientation.252,253 Except for the stereochemistry of the C-18–C-19 lactone ring fusion (trans in 632, cis in 633), compounds 632 and 633 have identical structures with both A/B and B/C cis ring fusions, as established by X-ray analysis.252,253 Furthermore, tehuanins A–C (635–637) from S. herbacea contain a 1,8-oxygen bridge; this unusual structural feature was confirmed by X-ray diffraction of 635.249
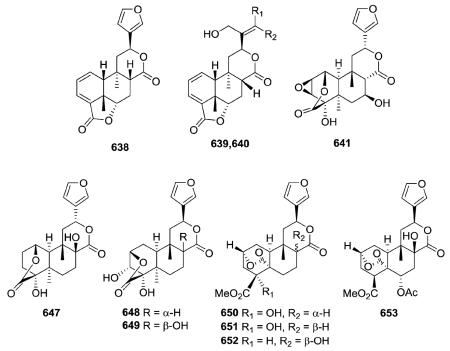
In addition to the C-12–C-17 δ-lactone ring found in the type IV class, compounds 638–640 from Jamesoniella autumnalis have a second lactone ring at C-18/C-6,120 while fibrauretin A (641) from Fibraurea tinctoria has a second lactone ring at C-1/C-18 as well as an epoxide ring at C-2/C-3.254 Compound 647, a stereoisomer of 8-hydroxycolumbin at the C-12 position, contains the same C-1(18)-lactone ring as 641 rather than the C-2(18)-lactone ring of compounds 648 and 649.258 Compounds
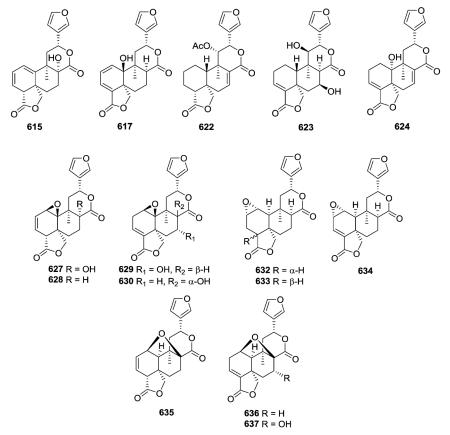 650–653 are novel furano-clerodanes from Dioscorea antaly and D. bulbifera with a second δ-lactone ring bridging carbonyl C-19 to C-2.259,260
650–653 are novel furano-clerodanes from Dioscorea antaly and D. bulbifera with a second δ-lactone ring bridging carbonyl C-19 to C-2.259,260
2.4.2. Type IV subtype II other compounds84,108,132,157,165,214,235,257,263–274 (Table 20 – compounds 656–691 found in ESI†)
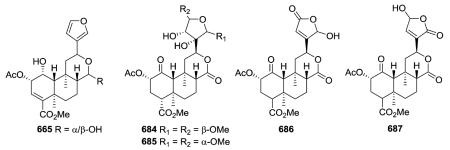
Compound 665 from Salvia divinorum is a C-17 epimeric mixture of the hemiacetal salvinorin J, and is an example of a neo-clerodane hemiacetal (lactol) susceptible to mutarotation with the formation of an equilibrium mixture of C-17 epimers.266 Salvinicins A (684) and B (685) from the same plant are unique neo-clerodanes with a 3,4-dihydroxy-2,5-dimethoxytetrahydrofuran ring.273 Their absolute stereochemistry (neo-series, A/B ring trans, B/C ring trans) was based on X-ray crystallographic analysis. Interestingly, salvinicin A (684) exhibited partial κ agonist activity with an EC50 value of 4.1 ± 0.6 μM (Emax = 80% relative to (-)-U50, 488H), while salvinicin B (685) exhibited antagonist activity at μ receptors with a Ki of >1.9 μM. This report provided a new lead in the development of opioid receptor antagonists.273 Salvidivins A (686) and B (687) are a pair of geometrical isomers of the γ-hydroxy-α,β-unsaturated γ-lactone, differing from each other in the linkage position to C-12. It appears that 686 and 687 are important precursors of 684 (a partial agonist of the κ-opioid receptor) and 685 (the first μ-opioid antagonist with a neo-clerodane skeleton).235
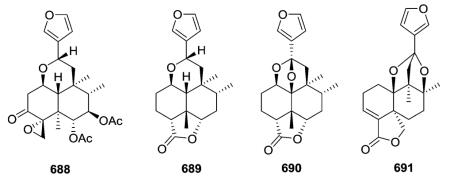
Compounds 688–691 are type IV subtype II compounds with variations on the δ-lactone structure.84,108,274 Compound 688
 from Cornutia grandifolia and 689 from Adelanthus lindenbergianus are distinguished by a unique ether linkage between C-1 and C-12.84,274 Remarkably, the structures of orcadensin (690) also from A. lindenbergianus, as well as salvianduline D (691) from Salvia miniata, contain cyclic ketal functions with two oxygen bridges from C-12 to different positions of the decalin moiety.84,108
from Cornutia grandifolia and 689 from Adelanthus lindenbergianus are distinguished by a unique ether linkage between C-1 and C-12.84,274 Remarkably, the structures of orcadensin (690) also from A. lindenbergianus, as well as salvianduline D (691) from Salvia miniata, contain cyclic ketal functions with two oxygen bridges from C-12 to different positions of the decalin moiety.84,108
2.5. Type V with an α-spiro-attached 4-(3-furyl)-γ-butyrolactone-based side chain at C-9
Clerodane diterpenoids of this group contain an unusual C-9-spiro-γ-lactone substituted at C-12 with a furan ring or are compounds arising from rearrangements of this structure. As contrasted in the below figure, the γ-lactone ring of type V compounds includes C-20 (C=O), C-9 (as the one carbon link to the decalin system), C-11, and C-12, while the δ-lactone ring of type IV compounds incorporates C-17 (C=O), C-8, C-9, C-11, and C-12. Type V compounds have a free Me-17, while type IV compounds have a free Me-20.

2.5.1. Type V subtype I with various O-containing rings75,108,113,165,187,275–281 (Table 21 – compounds 692–719 found in ESI†)
Compound 692 from Salvia fulgens exemplifies a variation on the basic type V structure. In it, C-20 is connected to C-7 as well as C-12 by oxygen atoms, forming two tetrahydrofuran rings joined at the C-20 acetal. This interesting structure was confirmed by X-ray crystallographic analysis as a dihydro derivative of the known salvifaricin, which has a double bond at C-1/C-2, as well as C-3/C-4.275 Like salvifaricin, salvifolin (695; S. tiliaefolia)276 and dugesin F (696; S. dugesii),113 also have two double bonds in the decalin A-ring. However, unlike salvifaricin and 692, the second ether bridge from C-20 to C-7 is missing in 695 and 696, and instead a cyclic hemiacetal (spiro-γ-lactol) and C-7 oxo groups are present. An A/B cis ring fusion was elucidated in 695, in comparison with linearolactone whose structure was established by X-ray diffraction analysis, while the structurally similar 696 has a trans decalin ring fusion. Dugesin F (696) exhibited an inhibitory effect on influenza virus FM1, a strain that causes a cytopathic effect (CPE) in MDCK cells.113 The results [TC50 45.67 μg mL−1, IC50 9.43 μg mL−1, therapeutic index (TI) 4.84] implied that 696 is a non-toxic antiviral compound against influenza virus FM1.
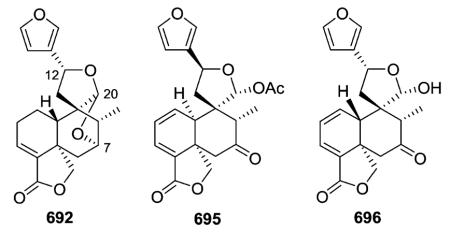
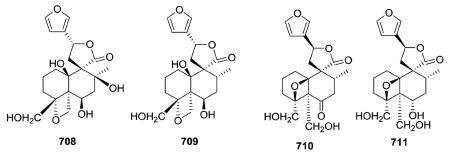
Teusandrins A–D (708–711) isolated from Teucrium sandrasicum contain a non-rearranged C-9-spiro-γ-lactone. Notably, such diterpenoids containing an oxetane ring involving positions 4α,19 (e.g., 708 and 709) and 4β,10β (e.g., 710 and 711), as well as 4β,6β (not illustrated) of the neo-clerodane skeleton are relatively frequent among the constituents of Teucrium plants.281
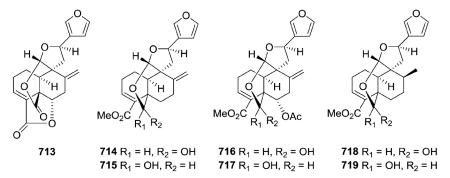
Several new clerodane diterpenoids, crotonolides A–D (713–714, 716, 718) and isocrotonolides B–D (715, 717, 719), were isolated from the aerial parts of Croton laui.165 They contain a variation on the C-9-spiro-γ-lactone with the two oxygen atoms on C-20 incorporated into both a tetrahydrofuran ring through C-12 and a six-membered lactone/lactol ring between C-19 and C-20. Crotonolide A (713) also contains a Δ3,4 double bond, a Δ8,17 exocyclic double bond, and a 18,6-γ-lactone. In 714–719, the latter lactone ring is absent, and also C-19 is hydroxylated rather than present as an oxo group. Compounds 714/715, 716/717, and 718/719 are epimeric pairs at C-19 and were obtained as 3 : 1 interconverting mixtures.
2.5.2. Type V subtype II with 4,18-; 3,4-; or 8,17-oxirane moieties75,123,126,128,142,144,145,262,280–294 (Table 22 – compounds 720–767 found in ESI†)
Most of the new compounds in this subtype have a C-12 furan, a spiro-20,12-hemiacetal function involving the C-9, C-11, C-12, and C-20 carbons, a 4α,18-oxirane, and a trans decalin ring junction. Teumassin (720) from Teucrium massiliense contains the rare feature of a C-2 hydroxy group.282 The diterpenes 729–732 isolated from T. polium possess the same absolute configuration, and belong to the neo-clerodane series.285 In this clerodane subtype, the C-12 stereocenter can have an R-configuration (e.g., 743 from T. maghrebinum), as well as an S-configuration (e.g., 729).126,144,280 New C-10 oxygenated type V subtype III neo-clerodane derivatives, sandrasin A (744) and 6-deacetylsandrasin A (745) were isolated from the aerial parts of T. sandrasicum.289 Analysis of spectroscopic data revealed ether linkages between both C4α,C18 and C19α,C20α in 750 obtained from T. abutiloides.287
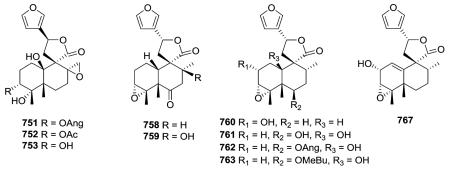
The investigation of different Pteronia species afforded 28 new diterpenes, including five cis-clerodanes in this subtype (751–753, 758–759).75 Compounds 751–753 have a 8,17-oxirane and C-10 is hydroxylated. Compounds 758–759 have a 3,4-
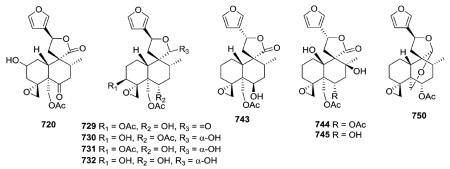 oxirane and C-10 bears a hydrogen. An extract of the aerial parts of Microglossa pyrrhopappa afforded cis-clerodanes 760–763 as well as 767 with a Δ1,10 double bond.128
oxirane and C-10 bears a hydrogen. An extract of the aerial parts of Microglossa pyrrhopappa afforded cis-clerodanes 760–763 as well as 767 with a Δ1,10 double bond.128
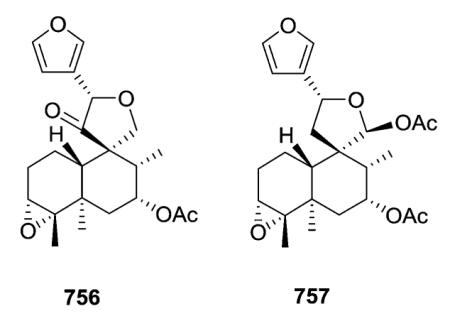
Cascarilla, the bitter bark of the South American tree Croton eluteria, is a commercially available and inexpensive source of polyfunctionalized clerodane diterpenoids. In addition to the bitter triol cascarillin, ten additional new diterpenoids, including eluterins J and I (756–757) in this subtype, were isolated and characterized.142 The structural differences among cascarilla clerodanes mainly involve the linkage between the carbocyclic and the heterocyclic moieties and the functional groups on C-3, C-4, and C-6. Although cascarillin was previously reported to be a γ-hydroxyaldehyde, this study showed that it is actually a mixture of interconverting γ-lactols.142 Compound 756 is set apart by the oxygenation of C-11, a very unusual feature in furoclerodanes.142
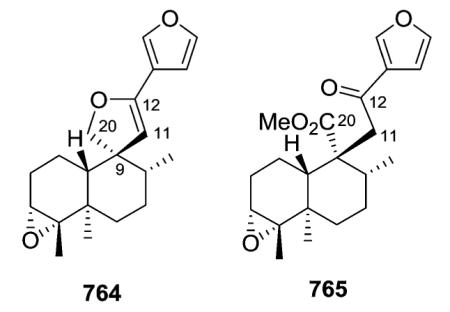
Crotonpene A (764), which has a rare 2,3-dihydrofuran ring with a spiro-carbon at C-9 and an oxygen connecting C-12 and C-20, may be formed by oxidation or enzyme catalysis of crotonpene B (765). Both compounds are found in Croton yanhuii.294
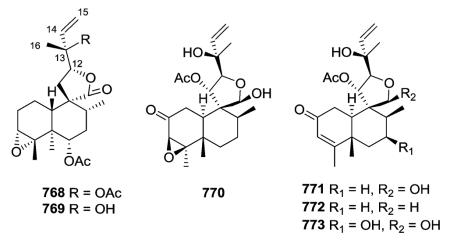
2.5.3. Type V subtype III with a C-9-spiro-γ-lactone/lactol moiety and opened furan ring47,61 (Table 23 – compounds 768–773 found in ESI†)
Clerodane-type diterpenoids (768–773) with a C-9-spiro-γ-lactone/lactol moiety bearing an opened furan ring (2-hydroxy-3-buten-2-yl) at C-12 are rare and found only in Heteroscyphus plants. In the decalin portion, compounds 768 and 769 possess a 3,4-epoxide, 770 contains a 3,4-epoxy and 2-oxo groups, and compounds 771–773 have a 3,4-double bond and 2-oxo moiety.47,61
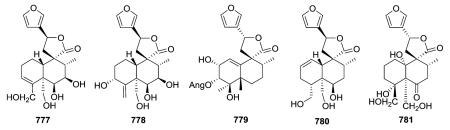
2.5.4. Type V subtype IV other compounds75,142,277,289,292,295–299 (Table 24 – compounds 774–793 found in ESI†)
Compounds in this subtype possess the furanyl substituted C-9-spiro-γ-lactone/lactol together with various substituents and unsaturation (Δ3,4
777;297
Δ4,18
778;297
Δ1,10
779;75
Δ1,2
780;277 saturated 781 (ref. 297)) in the decalin system. A small coupling constant between H-6 and H-7 proved that these protons were in α,α-equatorial positions in 777, which consequently contains a cis-6β,7β-diol. Teulolin B (778) is the first neo-clerodane diterpene with an exocyclic double bond at C-4/C-18 isolated from Teucrium species.297 In sandrasin B (781), C-4 bears α-OH and β-CH2OH groups, rather than being involved in a spiro-oxirane with C-18 as found in 744 and 745, which are type V subtype II compounds (see Section 2.5.2.) co-isolated from T. sandrasicum.289
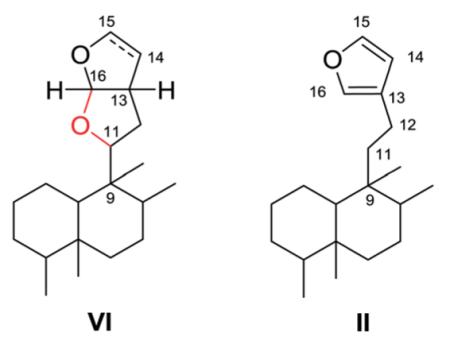
2.6. Type VI with a furofuran-based side chain at C-9
Type VI compounds contain a bicyclic furofuran system, either hexahydro or tetrahydro, attached at C-9. As contrasted below, an oxygen bridge between C-11 and C-16 differentiates the bicyclic type IV from the monocyclic type II compounds.
2.6.1. Type VI subtype I with a hexahydrofurofuran-based side chain at C-9 (ref. 176, 193, 195, 207 and 300–319) (Table 25 – compounds 794–845 found in ESI†)
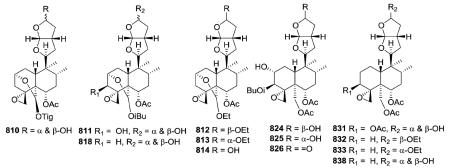
Most neo-clerodanes in this group possess a hexahydrofurofuran side chain at C-9 and a 4α,18-spiro-oxirane group, while some compounds also contain an additional C-19,2α-hemiacetal function (compare the structures of 815 and 834 obtained from Scutellaria discolor).311 Interestingly, compound 819, isolated from S. columnae, was the first neo-clerodane diterpene reported to have a hexahydrofurofuran moiety with an 11R-configuration.313
Generally, clerodin hemiacetal derivatives are found as C-15 epimeric mixtures. Scupolin K (811) from S. polyodon was found as a mixture of the C-15 epimers of the 14,15-dihydro-
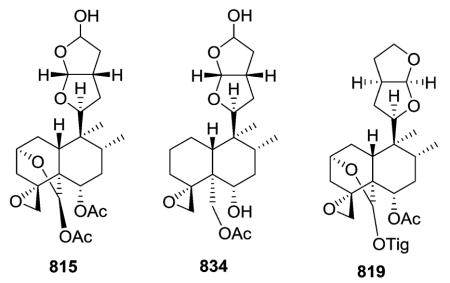 15-hydroxy derivative of scupolin J,310 and scutalsin (818) from S. altissima was also a 1 : 1 epimeric mixture of the C-15 hemiacetal function.312 Compounds 812–814 and 832–833, which have ethoxy acetal groups, are considered to be artifacts from Scutellaria discolor formed in the course of extraction or separation using ethanol.311 Compounds 824 and 825 from Ajuga salicifolia are the C-15 epimers of the 14,15-dihydro-15-hydroxy derivative of 826.316 Compound 831 from Clerodendrum inerme was assigned as a mixture of C-15 epimers of 14,15-dihydro-15-hydroxy-3-epicaryoptin.319 Two pairs of diastereomeric hemiacetals, scutecyprols A (838) and B (810), were detected in the aerial parts of S. cypria. After oxidation, they were isolated as their γ-lactone derivatives.309
15-hydroxy derivative of scupolin J,310 and scutalsin (818) from S. altissima was also a 1 : 1 epimeric mixture of the C-15 hemiacetal function.312 Compounds 812–814 and 832–833, which have ethoxy acetal groups, are considered to be artifacts from Scutellaria discolor formed in the course of extraction or separation using ethanol.311 Compounds 824 and 825 from Ajuga salicifolia are the C-15 epimers of the 14,15-dihydro-15-hydroxy derivative of 826.316 Compound 831 from Clerodendrum inerme was assigned as a mixture of C-15 epimers of 14,15-dihydro-15-hydroxy-3-epicaryoptin.319 Two pairs of diastereomeric hemiacetals, scutecyprols A (838) and B (810), were detected in the aerial parts of S. cypria. After oxidation, they were isolated as their γ-lactone derivatives.309
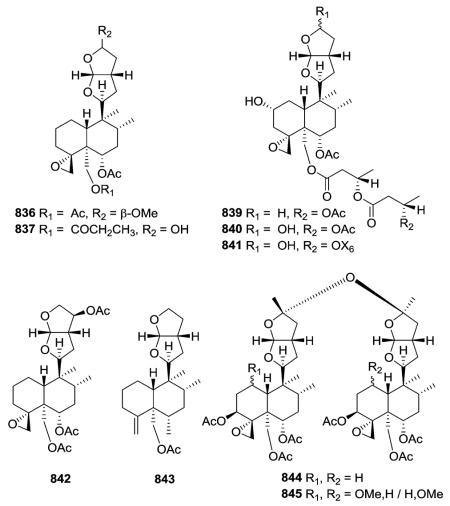
Compound 837 from S. alpine contains an isobutyroyloxy group at C-19, while a rarer propanoyloxy substituent is present in 836 from S. barbata; however, their absolute configurations were not ascertained.195,321 Scupontins C, D, and F (839–841) from S. pontica possess unusual [(3′S,3″S)-3′-[(3″-acetoxybutyryl) oxy]butyryloxy and [(3′S,3″S,3‴S)-3′-[[3‴-[(3‴-hydroxybutyryl) oxy]butyryl]oxy]butyryl]oxy substituents, respectively, attached to the C-19 position of the neo-clerodane nucleus.322 Scutalpin M (842) also from S. alpine is the first 14-oxidized hexahydrofuro-furan-neo-clerodane derivative isolated from natural sources.193 Compound 843 from A. lupulina has a C-4/18 exocyclic double bond, which is unusual in this type of clerodane diterpenes.315 Inermes A (844) and B (845) from C. inerme are dimeric neo-clerodanes with the two hexahydrofurofuran rings joined through an ether linkage at C-15, the latter compound contains a C-1 methoxy group not found in the former compound.319
2.6.2. Type VI subtype II with a tetrahydrofurofuran-based side chain at C-9 (ref. 207, 300–302, 306, 310, 311, 320 and 322–328) (Table 26 – compounds 846–874 found in ESI†)
The tetrahydrofurofuran system with a 14,15 double bond is the same in compounds of this subtype, and their clerodendrin skeletons also contain a 4α,18-spiro-oxirane. They differ in other substitutions on the decalin system. Certain compounds [e.g., jodrellins A and B (860–861) from Scutellaria species] also
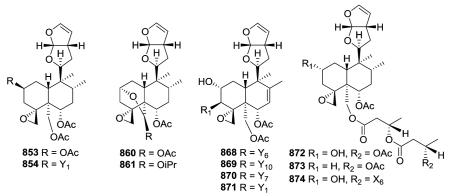 contain an additional C-19,2α-hemiacetal function,306,310–312,326–328 as found in the type VI subtype I compounds mentioned in the prior section. The spectroscopic differences observed between 853 and 854 found in S. laterifora suggested the presence of an acetoxy substituent in the former and a 2-methylbutanoyloxy group in the latter.320
Clerodendrum trichotomum yielded clerodendrins I, E, F, G (868–871) all having a double bond at C-7/C-8 in their decalin skeleton are substituted with 2α-hydroxy, 4α,18-epoxy, 6α,19-diacetoxy, 7,8-ene and 11,12,13,16-tetrahydrofurofuran functions, but with different 3β-acyloxy groups.325,328 Like 839–841, scupontins A, B, and E (872–874) from S. pontica are esterified at C-19 with diand tri-esters of 3-hydroxybutanoic acid.322
contain an additional C-19,2α-hemiacetal function,306,310–312,326–328 as found in the type VI subtype I compounds mentioned in the prior section. The spectroscopic differences observed between 853 and 854 found in S. laterifora suggested the presence of an acetoxy substituent in the former and a 2-methylbutanoyloxy group in the latter.320
Clerodendrum trichotomum yielded clerodendrins I, E, F, G (868–871) all having a double bond at C-7/C-8 in their decalin skeleton are substituted with 2α-hydroxy, 4α,18-epoxy, 6α,19-diacetoxy, 7,8-ene and 11,12,13,16-tetrahydrofurofuran functions, but with different 3β-acyloxy groups.325,328 Like 839–841, scupontins A, B, and E (872–874) from S. pontica are esterified at C-19 with diand tri-esters of 3-hydroxybutanoic acid.322
2.7. Type VII with a 13-spiro-15,16-γ-lactone moiety192,193,201,204,208,329–338 (Table 27 – compounds 875–902 found in ESI†)
The defining structural characteristics of type VII neo-clerodane structures are a 8,13-ether bridge creating a tetrahydropyran that incorporates C-8 and C-9, as well as C-11–C-13, and a 13-spiro-15,16-γ-lactone moiety. Both possible configurations are found at the spiro C-13. A comparison with type III compounds is shown below.
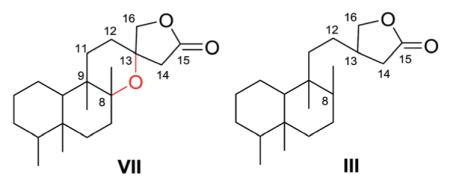
Scutorientalin C (876) is the first neo-clerodane with a free C-11 axial hydroxy group in ring C (tetrahydropyran) to be isolated from a Scutellaria species.192 The observed spectroscopic differences between 877 and 878 were consistent with the presence of a C-6α isobutyric ester in the former compound rather than the tigloyloxy group found in the latter.201,329 From a chemotaxonomic point of view, compound 879 is the first 8β,13S-epoxy-neo-clerodan-15,16-olide derivative found in European Scutellaria species, although these structural features are shown by several neo-clerodanes isolated from Asian Scutellaria species.330
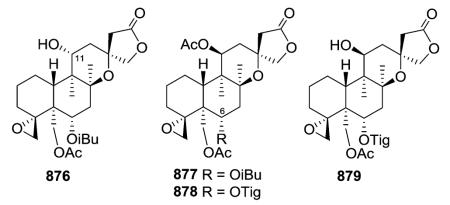
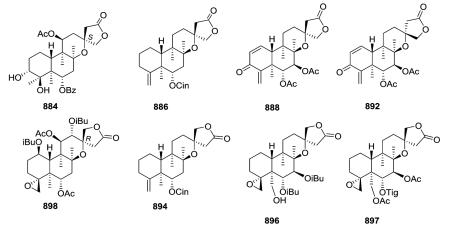
Opposite absolute configurations have been found at C-13 (e.g., 13S and 13R in 884 and 898, respectively).208,337 The aerial parts of Scutellaria hastifolia yielded several clerodanes similar structurally to the known scuteparvin, but distinguished by being trans-cinnamoyl derivatives. Some of these compounds are epimeric at C-13, and it was not possible to separate the 4 : 1 mixture of hastifolin G (886) and hastifolin F (894).204 Likewise, barbatellarine E (888) is a C-13 epimer of barbatellarine F (892), as confirmed by NOESY and optical rotation data.333,334 Comparison of spectroscopic data for 896 and 897 indicated the presence of C-6α and C-7β equatorial isobutyryloxy groups and a free C-19 hydroxy group in the former compound instead of C-6α tiglate and C-7β and C-19 acetates in the latter compound.332,336
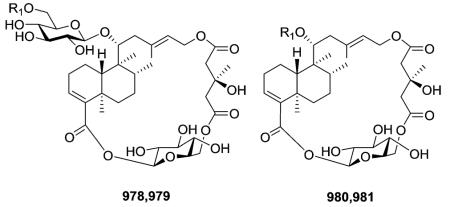
2.8. Clerodane diterpene glycosides62,102,254,255,257,268,339–361 (Table 28 – compounds 903–981 found in ESI†)
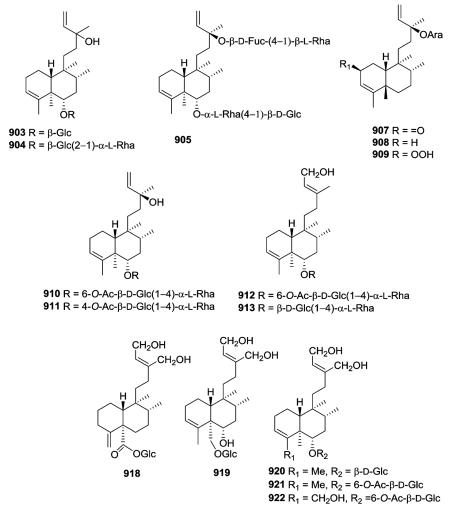
The clerodane diterpene glycosides come from many of the above types but have been placed into a separate category based on the presence of one or more sugar groups at various positions on both the decalin and C-9 side chain. Gleichenia japonica and Dicranopteris pedata yielded new glycosylated type I clerodane diterpenes with an acyclic C-9 side chain (903–904 and 905–906, 910–913, respectively).339,340 The only structural difference between 903 and 904 is the presence of only glucopyranosyl at C-6 in the former, but glucopyranosyl linked to rhamnopyranosyl in the latter. However, compound 903 inhibited the growth of lettuce, whereas 904 accelerated growth.339 The related glycoside 905 with sugars on both C-6 and C-13 also accelerated lettuce stem growth, but inhibited root growth.340 Compounds 907–909 are the first clerodane diterpenes with l-arabinoside at C-13 isolated from the family Compositae (species Nannoglottis carpesioides).341 Compounds 910–913 are monodesmosidic clerodane diterpene glycosides containing two monosaccharides, glucopyranosyl and rhamnopyranosyl.342 Compounds 918–922 possess a 1,4-dihydroxy-2-buten-2-yl-ethyl group at C-9, which is characteristic of the diterpenoids found in Portulaca and Salvia plants.62,102,344
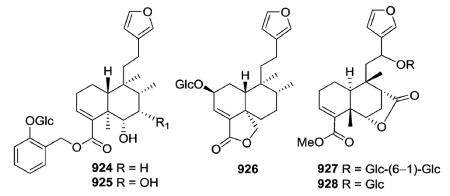
Examples of glycosylated clerodanes with type II structures are 924–925 from Elsholtzia bodinieri, 926 from Salvia amarissima, and 927–928 from Tinospora tuberculata.345–347 Amarisolide (926) was the first reported diterpene glucoside found in Salvia species.346
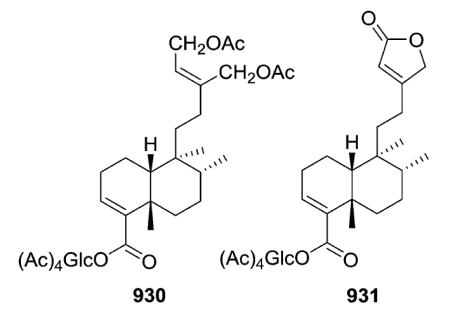
Compounds 930 and 931, both found in Baccharis sagittalis, were separated and characterized as C-18 β-d-glucopyranosyl peracetylated derivatives.349 The former compound can be classed as a type I clerodane glycoside, while the latter compound is a type III clerodane glycoside with a 3-ethyl-2-butenolide side chain.
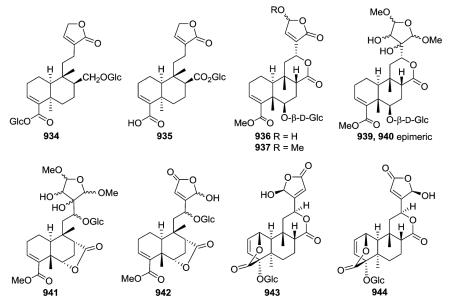
Clerodane diterpene glycosides with various five-membered O-containing rings attached at C-12 were isolated from three Tinospora species.350–352 Compounds 934 and 935 with an unsubstituted butenolide ring exhibited moderate anti-settling
 activity against the sea barnacle Balanus amphitrite, and are the first clerodane diterpenes to be reported with antifouling activity.350 Rumphiosides A and B (936 and 937) contain hydroxy- and methoxy-butenolide rings, while the tetrahydrofuran rings attached to C-12 in the epimeric 939 and 940 were possibly artifacts formed from a dialdehyde during the extraction of the plant material with methanol.351 Compounds 941 and 942 have a 17,6-γ-lactone,351 while compounds 936–937, 939–940, and 943–944 contain a 17,12-δ-lactone as well as an 18β,1β-δ-lactone. In cordifolides B and C (943 and 944) the butenolide ring located on C-12 is rotated nearly 180° from the C-12/C-13 bond, resulting in different orientations.352 Their structures were determined on the basis of spectroscopic data interpretation.352
activity against the sea barnacle Balanus amphitrite, and are the first clerodane diterpenes to be reported with antifouling activity.350 Rumphiosides A and B (936 and 937) contain hydroxy- and methoxy-butenolide rings, while the tetrahydrofuran rings attached to C-12 in the epimeric 939 and 940 were possibly artifacts formed from a dialdehyde during the extraction of the plant material with methanol.351 Compounds 941 and 942 have a 17,6-γ-lactone,351 while compounds 936–937, 939–940, and 943–944 contain a 17,12-δ-lactone as well as an 18β,1β-δ-lactone. In cordifolides B and C (943 and 944) the butenolide ring located on C-12 is rotated nearly 180° from the C-12/C-13 bond, resulting in different orientations.352 Their structures were determined on the basis of spectroscopic data interpretation.352
In addition to cordifolides B and C, Tinospora cordifolia also yielded a novel unique sulfur-containing type IV clerodane furanoditerpene glycoside, cordifolide A (948).352 Its structure and configurations at chiral centers were confirmed by single-crystal X-ray crystallographic analysis. Cordioside (949) is a 19-nor-clerodane furanoditerpene glucoside with a C-3/C-4 double bond, and hence, no C-19 carbon.354 Phytochemical investigations on
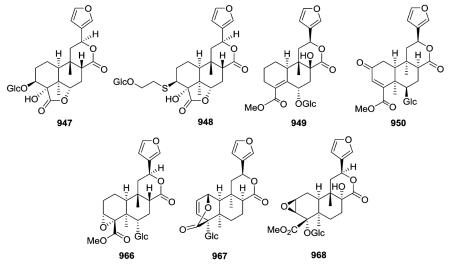
 the aerial parts of Tinospora crispa led to the isolation of several new cis-clerodane type IV furanoditerpene glycosides (e.g., 950, 966, 967). In addition, spectroscopic assignments of a previously reported compound, borapetoside A (947), were revised on the basis of HMQC and HMBC correlations.353 Type IV glucoside 968 adopted a unique all boat conformation of its tricyclic ring system, as also indicated by energy calculations.255
the aerial parts of Tinospora crispa led to the isolation of several new cis-clerodane type IV furanoditerpene glycosides (e.g., 950, 966, 967). In addition, spectroscopic assignments of a previously reported compound, borapetoside A (947), were revised on the basis of HMQC and HMBC correlations.353 Type IV glucoside 968 adopted a unique all boat conformation of its tricyclic ring system, as also indicated by energy calculations.255
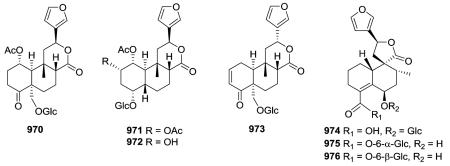
Compounds 970 and 973 are type IV 18-nor-clerodane glucosides, whereas 971 and 972 are 18,19-dinor-clerodane-type diterpene glucosides.254,357 Compounds 970–972, isolated from Tinospora sinensis, were subjected to an α-glucosidase inhibition assay, and exhibited IC50 values of 2.9, 3.8, 3.3, and 1.9 mM, respectively. Meanwhile, the positive control, acarbose, demonstrated an IC50 value of 0.84 μM.357 Compounds 974–976 are three new type V 19-nor-neo-clerodane diterpene glucosides.358
Compounds 978–981 are described by a novel macrocyclic skeleton containing an neo-clerodane diterpenoid moiety, one or two d-glucose units, and a 3-hydroxy-3-methylglutaric residue.360,361 Two long nine-atom extended strands are con nected by two “cyclohexane-chairlike” two atom junctions to create a unique three-dimensional construction. The structure and the absolute stereochemistry of 978 were elucidated through a combination of spectroscopic techniques, degradation reactions, and conformational analysis methods. Compound 978 inhibited high density induced apoptosis in several human and murine carcinoma cell lines.
2.9. Clerodane derivatives
2.9.1. N (or S or Cl)-containing derivatives218,228,230,232–234,333,338,362–379 (Table 29 – compounds 982–1054 found in ESI†)
Compounds 982 and 983 from Polyalthia longifolia contain a succinic diamide moiety attached at C-12; the former compound also has a double bond between C-13 and C-
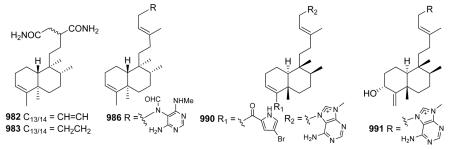 14.362 Other N-containing clerodane diterpenes have a heterocylic group attached at the terminal carbon (C-15) of a 3-methyl-3-pentenyl side chain. For example, when the marine sponge Agelas axifera was investigated for cancer cell growth inhibitory constituents, pyrimidine diterpenes (e.g., 986) were isolated.364 Other compounds from Agelas species possess both a 9 N-methyladenine moiety at C-15 as well as a 2-carboxy-4-bromopyrrole linked through an ester at C-18 (e.g., 990), whereas compound 991 with a C4/C18 exocyclic methylene has only the former moiety.365
14.362 Other N-containing clerodane diterpenes have a heterocylic group attached at the terminal carbon (C-15) of a 3-methyl-3-pentenyl side chain. For example, when the marine sponge Agelas axifera was investigated for cancer cell growth inhibitory constituents, pyrimidine diterpenes (e.g., 986) were isolated.364 Other compounds from Agelas species possess both a 9 N-methyladenine moiety at C-15 as well as a 2-carboxy-4-bromopyrrole linked through an ester at C-18 (e.g., 990), whereas compound 991 with a C4/C18 exocyclic methylene has only the former moiety.365
Scutellaria barbata is a major source of neo-clerodane diterpenoid alkaloids.228,232,233,338,366–372 Nicotinic acid (also known as niacin or vitamin B3) is a frequent N-containing component of ester groups found at various positions on the decalin, as shown in the following examples: 994–995 (mentioned in Section 2.3.1.3.), 998, 1008, 1012.
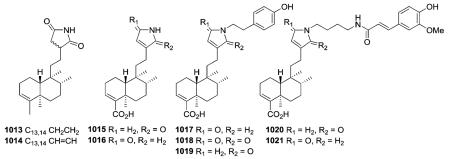
Two similar N-containing clerodanes (1013 and 1014) were isolated from P. longifolia.362 The former compound has a molecular weight two units greater than the latter, consistent with a pyrrolidine-15,16-dione in 1013 and a 1H-pyrrole-15,16-dione attached at C-12 in 1014. New clerodanes 1015–1021 with either a dihydro-2H-pyrrol-15-one, dihydro-2H-pyrrol-16-one, or 1H-pyrrole-15,16-dione at C-12 were isolated from Echinodorus macrophyllus and Casearia sylvestris.218,373,374
Compounds 1022–1024 isolated from the twigs and leaves of Cleidion brevipetiolatum have a type IV clerodane skeleton with an infrequent methylsulfinyl group present at C-3.375 Rare Cl-containing clerodanes 1025 and 1026 were isolated from Teucrium pernyi and T. racemosum, respectively.376,377 The Cl is part of a chlorohydrin in both compounds, with the CH2Cl at C-17 in 1025 and at C-18 in 1026. Because they were present in acetone extracts of the plant material, these two compounds were not regarded as artifacts of the isolation procedure.
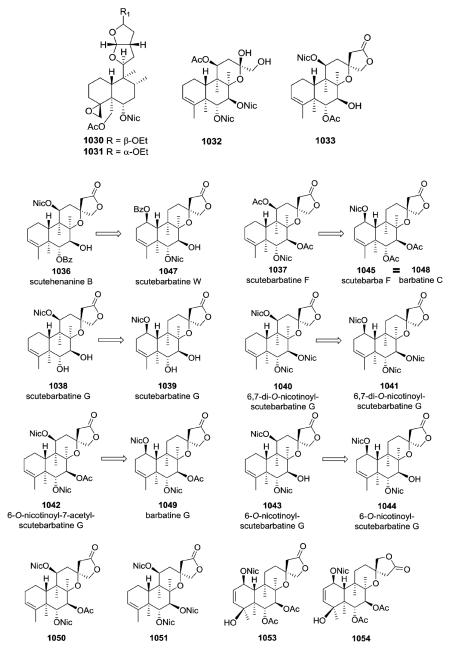
Other compounds with nicotinoyl esters were isolated from S. barbara as described below. Compound 1031, with an α-configuration of the ethoxy group, is the epimer of 1030.370 Compared with 1033, compound 1032 lacks a 13-spiro-15,16-γ-lactone moiety, as the result of oxidative cleavage between C-13 and C-14.368 NMR spectroscopy confirmed the presence of hydroxy and hydroxymethyl groups at C-13 in 1032, as well as the absence of carbon signals for C-14 and C-15. The originally reported structures of 1036–1043 were revised.366 The absolute stereochemistry 1R,5R,6R,7S,8R,9R,10R,13S was assigned to compounds 1045 (1048) and 1049, whereas compound 1050 has the same 5R,6R,7S,8R,9R,10R,11S,13R absolute configuration as
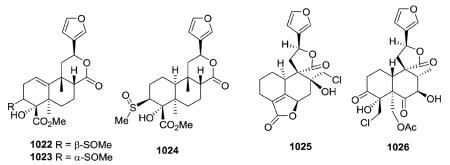 1051.379 Barbatine A (1050) also showed significant capability to protect cells against H2O2 with an ED50 value of 16.8 μM.379 Barbatellarine C (1053) is a C-13 epimer of barbatellarine D (1054), as confirmed by a NOE difference experiment and the respective NOESY spectra.333
1051.379 Barbatine A (1050) also showed significant capability to protect cells against H2O2 with an ED50 value of 16.8 μM.379 Barbatellarine C (1053) is a C-13 epimer of barbatellarine D (1054), as confirmed by a NOE difference experiment and the respective NOESY spectra.333
2.9.2. Degraded derivatives44,79,92,98,132,133,146,158,163,170,191,219,227,258,277,278,280,293,342,380–392 (Table 30 – compounds 1055–1097 found in ESI†)
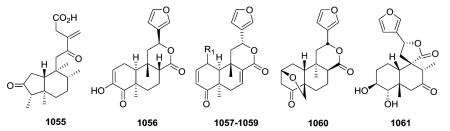
Compounds in this subtype have fewer than the normal 20 carbons of the basic clerodane skeleton. Firstly, in pentandranoic acid B (1055) from Callicarpa pentandra, a new contracted ring-A (cyclopentanone rather than cyclohexanone) is present.98 Secondly, various one-carbon substituents can be absent, primarily, but not exclusively, C-18 or C-19. Compounds 1056–1061 are rare 18-nor-clerodane diterpenoids with a C-4 oxo or hydroxy group.258,293,380,381
19-nor-Clerodanes constitute the majority of the degraded clerodanes, and the following examples come primarily from Croton and Teucrium species. One of the simplest 19-nor-clerodanes is cajucarin B (1062) isolated from Croton cajucara.158 The 19-nor-clerodane 1063 with a C-5/C-10 double bond could be formed by a retro Diels–Alder reaction.170 Except for an opened 17,12-γ-lactone ring and C-12 oxidation, compound 1064 from C. euryphyllus is quite structurally similar to 1070 and 1071, which have a butenolide moiety spanning C-19 to C-6, from Teucrium viscidum.278,382 Crassifolin H (1073) from C. crassifolius has a similar structure except for the presence of a C-5/C-10 rather than C-4/C-5 double bond.387 It demonstrated anti-angiogenic activity by reducing vessel formation to 59.3% of the control value at a concentration of 15 μg mL−1. Notably, the bioactive type V 19-nor-clerodane-type diterpenoid trans-dehydrocrotonin (1076), with a cyclohexenone decalin ring A, is one of the most investigated clerodanes in the current literature.389 Two of the three hydroxy groups in syspiresin A (1078) from T. chamaedrys are replaced by hydrogen (C-2) and a methoxy group (C-6) in teupolin IX (1079) from T. polium.92,277 Crotoeurin A (1084) from C. euryphyllus was the first nor-clerodane diterpenoid dimer connected through a unique cyclobutane ring via a [2 + 2]
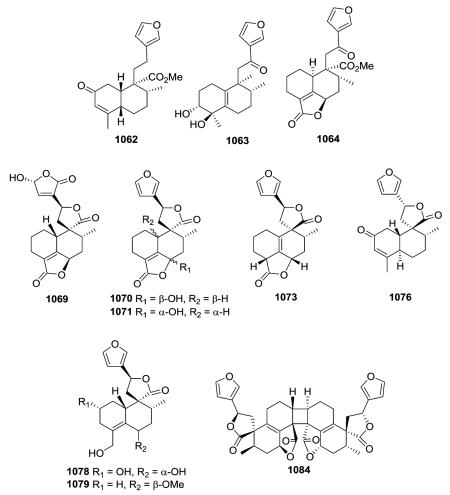
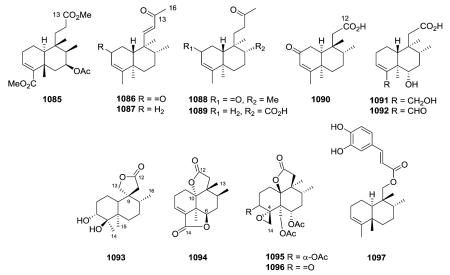 cycloaddition; its structure was confirmed by single-crystal X-ray diffraction analysis.382
cycloaddition; its structure was confirmed by single-crystal X-ray diffraction analysis.382
Compound 1085 is a 14,15,16-trinor-clerodane isolated from Sindora sumatrana.163 Isolated from several different plant species, compounds 1086–1089 are unusual 14,15-bisnor clerodanes with a C-4/C-5 double bond,44,79,219,391 and compounds 1090–1096 are 13,14,15,16-tetranor-clerodanes. Among the latter tetranor-clerodanes, C-12 is present as a free carboxylic acid (e.g., 1090–1092)227,342 or as part of a lactone ring. Croinsulactone (1093) from C. insularis contains a 12,13-γ-lactone with a spiro-carbon at C-9,132 while ciliatolide A (1094) from Scapania ciliata contains a 12,10-γ-lactone as well as a 14,6-γ-lactone (the latter ring is comparable with a 18,6-γ-lactone in a non-degraded clerodane).191 Teucrolin D (1095) and teucrolivin F (1096) from T. oliverianum also contain a 12,10-γ-lactone as well as a 4,14-spiro-oxirane (identical to a 4,18-spiro-oxirane in a non-degraded clerodane).133,146 Compound 1097 from Jamesoniella colorata is a degraded clerodane (rearranged drimane)-type sesquiterpenoid.392
2.9.3. Ring-seco derivatives75,108,119,121,128,131,144,155,187,250,252,263,270,393–398 (Table 31 – compounds 1098–1145 found in ESI†)
seco-Derivatives have an opened ring at some point in the structure, creating multiple interesting compounds, depending on which bond is broken and what additional rearrangements are present. The first examples are 9,10-seco-clerodane diterpenoids.119,128,393–395 Although the configuration of the C-8/C-9 epoxide in jamesoniellide F (1098) from the liverwort Jamiesoniella autumnalis could not be determined absolutely by NMR, this 9,10-seco-clerodane likely is a biogenetic product of cis-clerodane 1110 found in the same plant.119 Salvianduline A (1102) and pyrrhopappolide (1104) from Salvia lavanduloides and Microglossa pyrrhopappa, respectively, are both 9,10-seco-clerodanes, but contain a 17,12-δ-lactone and a 20,12-γ-lactone, respectively. Compound 1111 is a 9,10-seco-clerodane with a fully saturated
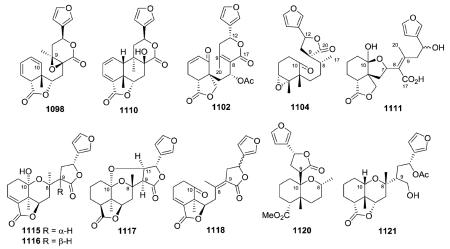 benzofuran rather than decalin bicyclic skeleton.108 A C-7/C-10 ether bridge plus a hydroxyl on C-10 forms a hemiketal. Other 9,10-seco-clerodanes contain a a C-8/C-10 ether bridge.119,187,393 Among several compounds of this type from Cephaloziella kiaeri, cephaloziellins H and I (1115–1116) are C-8/C-10 hemiketals, while one additional degree of unsaturation in cephaloziellin J (1117) was indicative of a second ether bridge between the C-10 ketal carbon and the C-11 oxygenated methine.187 Furthermore, in cephaloziellin K (1118), the hemiketal bridge found in 1115 and 1116 is absent.187 Both jamesoniellides B (1121) and A (1120), from J. autumnalis, have a C-8/C-10 ether bridge; however, the B-ring in the former compound is opened between C-9 and C-10, while that in the latter compound is fragmented between C-8 and C-9.121
benzofuran rather than decalin bicyclic skeleton.108 A C-7/C-10 ether bridge plus a hydroxyl on C-10 forms a hemiketal. Other 9,10-seco-clerodanes contain a a C-8/C-10 ether bridge.119,187,393 Among several compounds of this type from Cephaloziella kiaeri, cephaloziellins H and I (1115–1116) are C-8/C-10 hemiketals, while one additional degree of unsaturation in cephaloziellin J (1117) was indicative of a second ether bridge between the C-10 ketal carbon and the C-11 oxygenated methine.187 Furthermore, in cephaloziellin K (1118), the hemiketal bridge found in 1115 and 1116 is absent.187 Both jamesoniellides B (1121) and A (1120), from J. autumnalis, have a C-8/C-10 ether bridge; however, the B-ring in the former compound is opened between C-9 and C-10, while that in the latter compound is fragmented between C-8 and C-9.121
In various 5,10-seco-neo-clerodanes,131,155,250,263,270 the unsaturated patterns of the “opened” decalins include 2,4,10(1)-triene (cyclodeca-1,3,5-triene) (e.g.
1122),250 10-oxo-2,4-diene (cyclodeca-3,5-dien-1-one) (e.g., 1123),250 and 1,3,5(19)-triene (5-methylenecyclodeca-1,3-diene) (e.g., 1124)263 conjugated systems. Salvimicrophyllin A (1122) from S. microphylla was unstable in solution and decomposed on exposure to light and heat.250 The relative configuration of salvimicrophyllin B (1123) was confirmed by single-crystal X-ray diffraction crystallography.250 The epimeric hemiacetals 1125/1126 and acetals 1127/1128 could not be separated when isolated from Conyza welwitschii.263 Compounds 1132 and 1135 from Conyza and Dodonaea species, respectively, have the same ethylfuran side chain at C-9 but different double bond patterns in the cyclodecane ring, 1,3,5(19) and 2,4,10(1), respectively.131,155 Tonalensin (1136) from S. tonalensis is an interesting 5,10-seco-neo-clerodane containing an acetal at C-20 with oxygens from C-7 and C-11 forming two fused tetrahydrofuran rings. Its trans,cis,cis-cyclodecatriene ring adopts a boat-chair conformation in which the Δ1,3,5-triene system is no longer coplanar.396
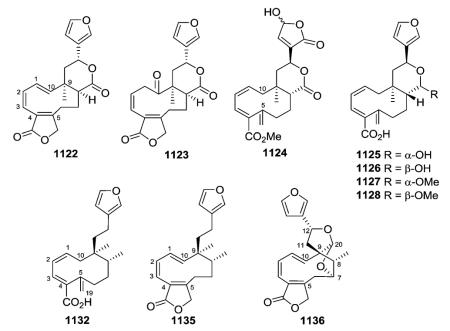
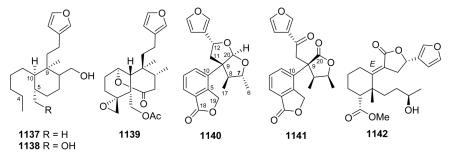
Tinosporafuranol (1137) and tinosporafuranadiol (1138), obtained from the stem bark of Tinospora cordifolia, are 4,5-seco-clerodane-type diterpenoids.397 The single-crystal X-ray diffraction analysis of 5,6-seco-compound 1139 established a 5α-configuration for the C-19 acetoxymethylene group, as well as the absolute stereochemistry. In the crystalline state, the substituted cyclohexane ring of 1139 is in a chair conformation,
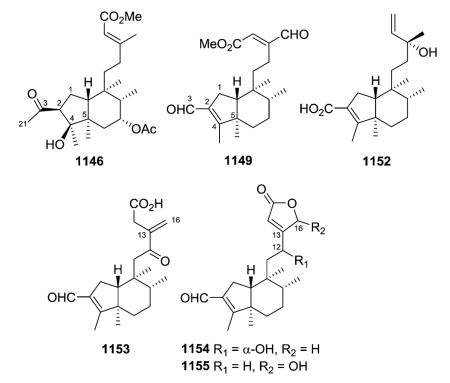 with a mean torsion angle of 57°.144 Both rhyacophiline (1140) and salvireptanolide (1141) from S. rhyacophila and S. reptans, respectively, have novel skeletons characterized by cleavage of the C-5/C-6 bond and aromatization of the A-ring.252,398 Compound 1140's structure was fully established by spectroscopic and X-ray diffraction analyses.398 Jamesoniellide J (1142) from J. autumnalis is a seco-clerodane diterpenoid cleaved between C-8 and C-9.187,393
with a mean torsion angle of 57°.144 Both rhyacophiline (1140) and salvireptanolide (1141) from S. rhyacophila and S. reptans, respectively, have novel skeletons characterized by cleavage of the C-5/C-6 bond and aromatization of the A-ring.252,398 Compound 1140's structure was fully established by spectroscopic and X-ray diffraction analyses.398 Jamesoniellide J (1142) from J. autumnalis is a seco-clerodane diterpenoid cleaved between C-8 and C-9.187,393
2.9.4. Rearranged derivatives55,75,81,98,113,128,164,187,219,220,240,271,276,290,392,399–415 (Table 32 – compounds 1146–1224 found in ESI†)
The clerodanes in this group have a vast scope of ring numbers, sizes, and fusions. The “usual” decalin systems are especially affected, but rearranged C-9 side chains also occur. In various abeoclerodane diterpenes with a (4→2) rearranged ring A moiety, C-3 is generally an aldehyde (e.g., 1149) or carboxylic acid (e.g., 1152) attached to the five-membered ring A.55,81,98,219,220,240,399 However, compound 1146 from Solidago altissima is also a homoditerpene with 21 carbons [a methyl (C-21) is attached to a C-3 oxo group] and could have resulted from reaction of a C-3 aldehyde with diazomethane used to esterify the extract.55 In addition to the rare contracted ring A, pentandranoic acid A (1153) from Callicarpa pentandra also contains an 12-oxo-13(16)-methylene in a C-9 pentanoic acid side chain rather than the C-9 ethyl-α,β-unsaturated-γ-lactone of pentandralactone (1154), also found in
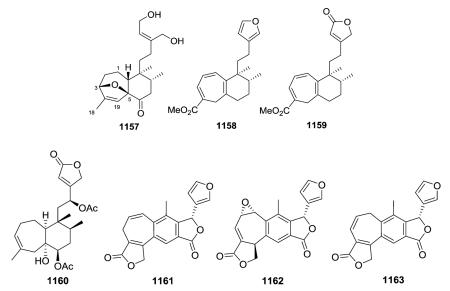 the same plant.98 Compound 1155 was isolated from Polyalthia viridis as a 1 : 1 mixture of C-16 epimers.219
the same plant.98 Compound 1155 was isolated from Polyalthia viridis as a 1 : 1 mixture of C-16 epimers.219
Compounds 1157–1160 contain an expanded seven-membered A-ring where C-19 is inserted between C-4 and C-5.164,400,401 In 1157 from Portulaca pilosa, this novel bicyclo[5.4.0] undecane trans-clerodane skeleton also has an ether bridge at C-3→C-5 in the A-ring.400 Scapanialide B (1160) from Scapania parva is the first cis-clerodane diterpenoid with a bicyclo[5.4.0] undecane skeleton isolated from liverworts.164 Compounds 1161–1163 from two Salvia species402–404 also contain a seven-membered A-ring; however, this ring includes C-6, rather than C-19. In addition, C-11 has been inserted between C-8 and C-9, creating a phenyl B-ring fused to a γ-lactone, in a tricyclic skeleton.
Notably, compounds 1164–1166 from three different Salvia species have a novel rearranged A/B ring spiro-fused neo-clerodane skeleton with the spirocyclic junction at C-10.113,402,405 The structure of spiroleucantholide (1164) might be derived biogenetically by a ring contraction of the seven-membered ring in a precursor benzocycloheptatriene skeleton to form a spiro-fused junction. This report was the first to identify a spiro-6/6 A/B ring diterpenoid derived from a neoclerodane skeleton.402
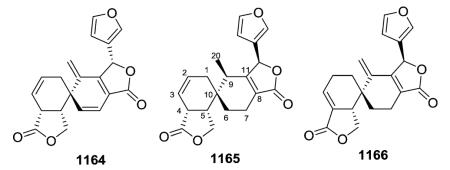
Compounds 1167–1171, also from various Saliva species, contain a salvigenane skeleton with a seven-membered B-ring, which contains C-11 inserted between C-8 and C-9.113,403,404,406,407 Like in 1161–1163, a fused γ-lactone is also present. However, the A/B ring sizes are 7/6 in 1161–1163, but 6/7 in 1167–1171.
X-ray crystal structure determination of blepharolide A (1172) showed a 5,6-unsaturated octahydro-1H-cyclopropa[α] naphthalene derivative, which presented a new rearranged clerodane structure with a C6–C6–C3 ring system. This new skeleton was named isosalvigenane. The isolation of salvigenane (blepharolide B, 1167) and isosalvigenane (blepharolide A, 1172) diterpenoids from Salvia blepharophylla suggested a botanical chemotaxonomic relation between sections Fulgentes, Albolanatae and Brandegeia of subgenus Calosphace.406 Tilifodiolide (1173) from Salvia dugesii was the first natural product with a substituted tetralin skeleton; its unusual
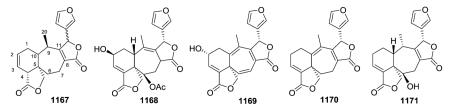 structure was confirmed by X-ray diffraction analysis.276 A biogenetic derivation of both 1173 and the co-isolated 1161 from a clerodane precursor was postulated.
structure was confirmed by X-ray diffraction analysis.276 A biogenetic derivation of both 1173 and the co-isolated 1161 from a clerodane precursor was postulated.
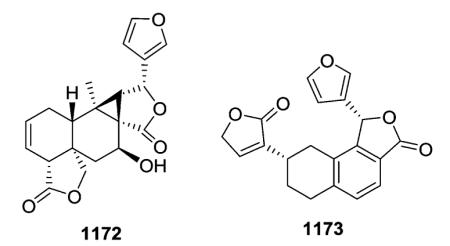
The rearranged clerodane diterpenes baenzigerides A (1174) and B (1176) and the related open-ring glucosides, baenzigerosides A (1175) and B (1177), were isolated from the stems of Tinospora baenzigeri.408,409 The novel skeleton of baenzigeride A (1174) could be produced from the cis-ent-neoclerodane epoxide (A) (Scheme 2). Rearrangement with ring-A contraction through migration of either the 1–2 or 3–4 bond would give the aldehyde (B). Reduction of B should give the primary alcohol (C), which could lactonize to 1174. Although epoxides such as (A) have not been found as natural products, the known clerodane 2,3-epoxides, e.g., jateorin, do have a 1,18-lactone group.408
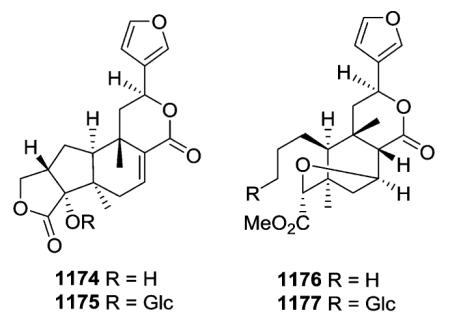
Scheme 2.
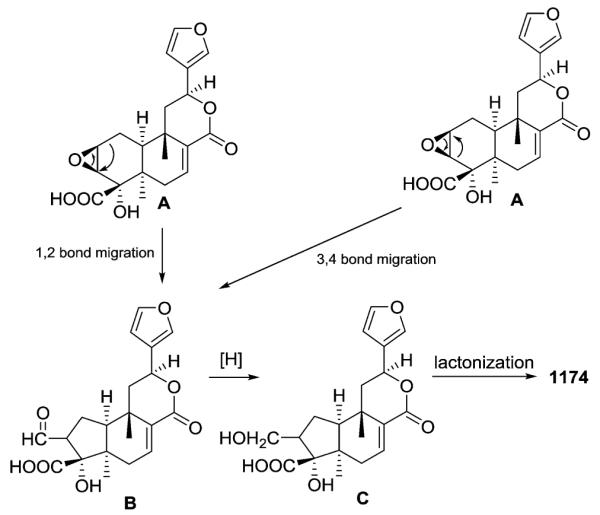
Possible biogenesis of baenzigeride A (1174).
The vapor and condensed solid resulting from heating salvinorin A (9) to 245 °C for 10 min were combined as a chloroform extract, from which two new rearranged clerodanes (1178–1179), were isolated. The major structural changes to 9 were epimerizations at C-2 and C-8, eliminations of acetoxy and methyl ester groups, and carbon–carbon rearrangements at C-1, C-2, and C-10. Compounds 1178 and 1179 are unique salvinorin derivatives with interlocking five-membered cyclopentane and six-membered anhydride rings; the 3-oxabicyclo[3.2.1]octane-2,4-dione systeme was confirmed by X-ray analysis.271
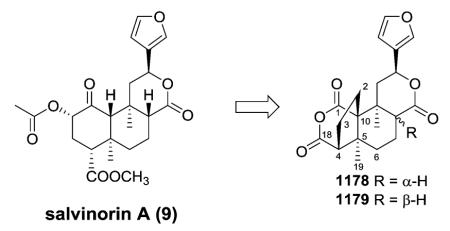
Microphyllandiolide (1180) from Salvia microphylla represents the first example of a new framework with a 9/3 bicyclic ring system (microphyllane skeleton). A plausible biogenetic pathway to this new skeleton arises from proposed transformations of other neo-clerodane diterpenes isolated from Salvia species, involving pericyclic reactions. As shown in Scheme 3, an electrocyclic opening of the decalin ring of the diene (a) would give the triene (b). Cyclopropanation of (b) would give (c), and finally, allylic oxidation of (c) would provide the C-3 hydroxy group of 1180.410
Scheme 3.
A plausible biogenetic pathway to 1180.
Compound 1181 from Teucrium betonicum is a biogenetically unexpected diterpenoid with a new 7β-homo-19(5→18)abeo-neo-clerodane skeleton.411 Its structure was established by spectroscopic means, including an X-ray diffraction analysis.
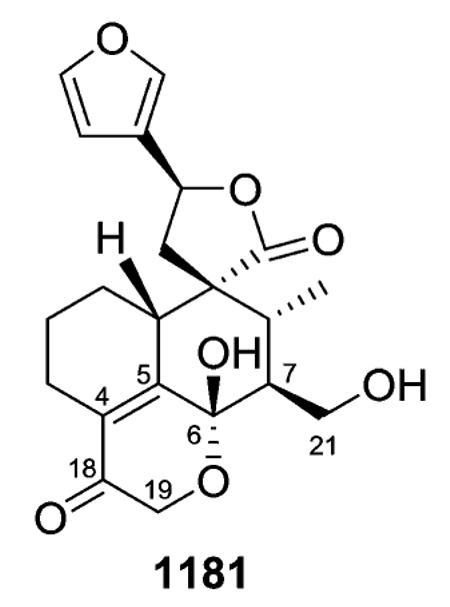
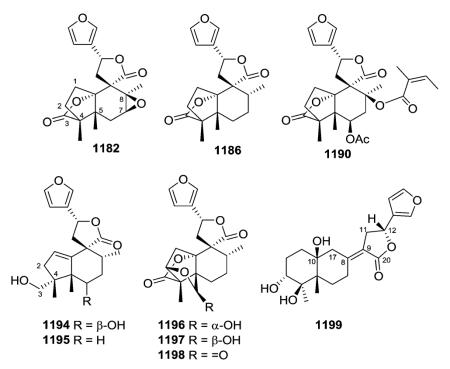
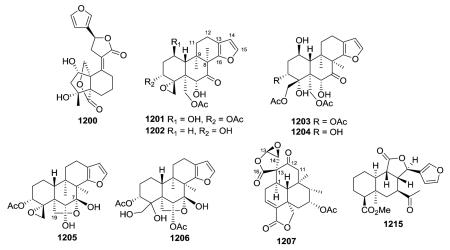
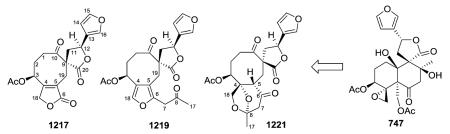
Both Microglossa pyrrhopappa (e.g., 1182, 1186) and Pteronia divaricata (e.g., 1190) provided rearranged clerodanes with a 2-oxabicyclo[2.2.1]heptan-3-one system most likely formed by Wagner–Meerwein rearrangement; compound 1182 also has a 7,8-epoxide.75,128 Ring-A of 1194 (M. pyrrhopappa) and 1195 (P. incana) is a cyclopentene ring substituted with hydroxymethyl (C-3), and the former compound has an additional hydroxy group at C-6.75,128 Pteroniatrilactone (1198) from P. eeni has the same rearranged carbon skeleton as 1186 with the addition of a third lactone between C-2 and C-19 (OC=O). An inseparable mixture of related 3 : 1 epimeric hemiacetals 19-α- and 19-β-hydroxypteronia dilactone (1196, 1197) was also isolated.75 Eeniolide (1199), also from P. eeni, was probably formed biogenetically from an 8,10-dihydroxyclerodane diterpenoid, with cleavage of the C-9/C-10 bond and formation of a C-9/C-8 double bond, followed by ring-A/B reclosure at C-17/C-10.75
Jamesoniellide C (1200) with the same side chain (furanylmethylene-dihydrofuranone) as 1199 but a 5/6 rather than 6/6 skeleton was isolated from the liverwort Jamesoniella autumnale along with the interesting 8,9 and 9,10-seco-clerodanes 1120 and 1121 (see Section 2.9.3).412 Compounds 1201–1206 with a novel rearranged tetrahydrobenzofuran fused to the decalin through a C8–C16 bond were isolated from Teucrim alyssifolium.413,414 Although 1205 and 1206 were structurally quite similar to 1201–1204, the presence of a C-7/C-19 hemiketal bridge in the former compounds was the main difference between these two related groups.413,414 Salvilanguiduline A (1207) from Salvia languidula illustrates a rearranged clerodane skeleton containing an epoxy spiro γ-lactone function and a C1–C13 bond.415 The structure of the rearranged clerodane cephaloziellin O (1215) isolated from the Chinese liverwort Cephaloziella kiaeri was confirmed by single-crystal X-ray diffraction analyses, and the absolute configurations of 16 new clerodane diterpenoids, cephaloziellins A–P, were established by comparing experimental and calculated electronic circular dichroism spectra.187
Teucrium brevifolium yielded several clerodanes containing an unusual rearranged skeleton with an eight-membered ring carbocycle (e.g., 1217, 1219, 1221).290 The ring conformation in each compound was established by exhaustive NMR spectroscopic studies as well as X-ray data on 1221. A biogenetic pathway from teubrevin D (747) was postulated to explain the formation of the 5,10-seco-9(8→19)abeo-neo-clerodane skeleton (1219, 1221) and the 7,8,17-trinor derivatives (1217).290
The leaves of Salvia xalapensis yielded two new clerodane-type diterpenoids with an opened (C5–C6) unsaturated ring-B skeleton, salvixalapadiene (1223) and isosalvixalapadiene (1224).407 Both compounds have a phenyl A-ring and two double bonds in the opened B-ring at C6-7/C8-11 and C7-8/C9-11, respectively. Their unprecedented rearranged skeleton may be derived biogenetically from a salvigenane precursor. Salvixalapoxide (1222) with a languidulane skeleton was also isolated at the same time.407
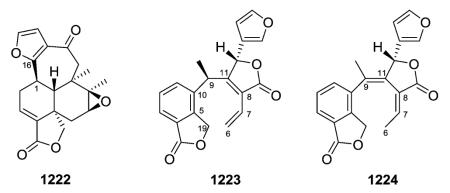
2.10. NMR features of clerodane diterpenes
NMR spectroscopy, especially 13C NMR analysis, has been widely employed for the characterization of the different skeletons of clerodane diterpenes. The assignments of carbon signals of a new clerodane diterpene by comparison with the data of known compounds require the 13C data of appropriate model compounds. It would appear to be of value to provide an easy access to an extensive list of 13C data of these diterpenes. Inspection of the chemical shifts of different types of clerodanes revealed that the main differences among the seven structural types (I–VII) are related to the number of methyl groups (C-17 or C-20 are absent, in the IV and V structural types, respectively), and chemical shifts of C-8, C-12, C-17, and C-20. The characteristic spectroscopic features associated with these seven different structural types I–VII are summarized in Table 2.
Table 2.
Characteristic 13C NMR chemical shifts of types I–VII (δC, ppm)
| I | II | III | IV | V | VI | VII | |
|---|---|---|---|---|---|---|---|
| C-5 | 37→40 | 35→55 | |||||
| C-8 | 31→38 | 31→45 | 46→52 | 35→47 | 79 | ||
| C-9 | 38→40 | 51→54 | 37 | ||||
| C-10 | 10β-H: 46 | 10β-H: 42→50 | 48→52 | ||||
| 10α-H: 50 | |||||||
| C-11 | 84→87 | ||||||
| C-12 | 32→38 | 17→27 | 70→73 | 68→75 | |||
| C-13 | 123→129 | 168→176 | 120→125 | 125→130 | 40→42 | 75→77 | |
| C-14 | 108→111 | 112→117 | 108→110 | 108→117 | |||
| C-15 | 136→146 | 172→175 | 144→146 | 138→145 | 68 | 173→176 | |
| C-16 | 137→148 | 70→74 | 138→140 | 138→145 | 102→108 | 76→80 | |
| C-17 | 16→18 | 13→18 | 169→177 | 14→20 | |||
| C-20 | 18 | 16→18 | 14→26 | 172→178 | |||
| Δ5,10: 140 | Δ7,8: 170, 100 |
Clerodanes that possess a furan ring at C-12 show carbon resonances between δC 123→132 (C-13), δC 107→114 (C-14), δC 138→146 (C-15), and δC 137→148 (C-16). In clerodane diterpenes containing a Δ3 double bond (C-3: δC 120→130; C-4: δC 130→145), the chemical shifts of these carbons are affected by the presence of the substituents at C-18. When this carbon contains a carboxyl or carboxymethyl group, C-3 (ca. δC 133) and C-4 (ca. δC 149), are deshielded. However, when C-18 is an aldehyde group, C-3 (δC 152.4) and C-4 (δC 151.7) are more deshielded. On the other hand, in clerodane diterpenes containing a Δ1,3 double bond and a carboxymethyl group at C-18, resonances occur between δC 133→134 (C-1), δC 123→125 (C-2), δC 132→136 (C-3), and δC 134→137 (C-4). In the structural type IV containing a Δ7 double bond, carbon resonances appear at δC 172 (C-7) and δC 100 (C-8). For compounds having a Δ4 double bond and C-18 as a methyl group, C-4 and C-5 resonate at δC 125.7 and 134, respectively. If C-18 is part of a lactone ring, these carbons are deshielded [δC 127.6 (C-4) and 162 (C-5)]. An epoxy ring at C-4 and C-18 leads to carbon signals at δC 63→67 (C-4) and δC 43→51 (C-18).
2.11. Abbreviation of functional groups
3. Biological activities
Clerodane secondary metabolites may benefit a plant species by acting as a chemical defense mechanism against phytophagous animals or diseases. Apart from insect antifeedant properties, several clerodane diterpenes display other effects against insects. Insecticidal activity has been reported for ajugarins I and IV.416,417 Ajugarin IV also displays insect growth regulating activity, as do 3-epi-caryoptin418 and the 19-nor-clerodanes cis-and trans-dehydrocrotonin.419 Fungicidal activity against plant pathogenic fungi has been reported for clerodin and the related jodrellins A and B.326
Besides insect antifeedant and antifungal activities, many clerodane diterpenes possess various pharmacological activities beneficial to humans, including action as opioid receptor probes, as well as NGF-potentiating, anti-ulcer, cytotoxic, anti-inflammatory, antiparasitic, and antibacterial activities.
3.1. Insect antifeedant activities
3.1.1. Insect antifeedant and related plant protective activities of clerodane diterpenes
Clerodane-type secondary metabolites have attracted considerable attention as insect antifeedants, which is by far the most extensively studied bioactivity of these diterpenes. Clerodane diterpenoids have been found in several hundreds of plant species from various families and in organisms from other taxonomic groups, such as fungi, bacteria, and marine sponges.420–422 Especially, various genera from the plant families Lamiaceae have been identified as rich sources of antifeedant clerodanes, with species of the genus Scutellaria producing some of the most potent clerodane antifeedants known so far.306,330,423–426 The most active compounds from these species against larvae of Spodoptera littoralis (cotton leafworm) were scutalpin C (497), scutecyprol B (810), jodrellin A (860), jodrellin B (861), and dihydroclerodin (1225), with corresponding feeding index (FI) values of 92 ± 8, 100 ± 0, 95 ± 16, 100 ± 0, and 97 1, respectively. In addition, 810 showed potent activity against ± several other species of Lepidoptera.426
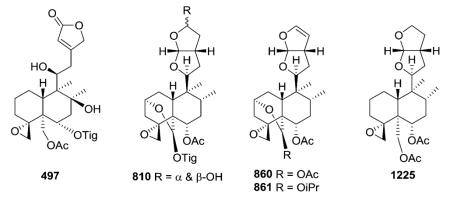
Ajubractins A–E (855–856, 795–797) and 15-epi-lupulin B (821) were isolated from a dichloromethane extract of Ajuga bracteosa.301 Among them, 795–797 and 821 showed moderately high antifeedant activities (FR = 0.14–0.15). Data analysis from the behavioral responses of S. littoralis exposed to the clerodane diterpenoids hativenes A–C (827–829), from Ajuga pseudoiva, showed that the all of the compounds tested had strong antifeedant activity at 100, 10, and 1 mg−1, which began to dissipate at 0.1 mg−1.317 Comparison of the antifeedant index at the latter two concentrations also indicated that a change in the relative configuration of carbons C-12 and C-15 did not modify the activity considerably. 14,15-Dehydroajugareptansin (859), from Ajuga reptans, had significant activity against sixth stadium larvae of Spodoptera littoralis.207 Overall, these data, compared to those of other clerodanes isolated from Ajuga and Salvia species, helped to clarify different authors' suggestions and conclusions, which related the antifeedant activity of clerodanes to the presence of a perhydrofuranofuran moiety, a trans decalin ring system bearing an epoxide, and acetate groups.
Clerodin (2), 15-methoxy-14,15-dihydroclerodin (1226), and 15-hydroxy-14,15-dihydroclerodin (1227) exhibited growth inhibitory activity against Helicoverpa armigera (cotton boll-worm) with growth inhibition index (GI50) values of 13, 21, and 11 ppm, respectively, compared to azadirachtin, the active ingredient of many pesticides, with a GI50 value of 15 ppm.427 Clerodin (2), clerodendrin B (849), 3-epicaryoptin (1228), and 15-hydroxyepicaryoptin (1229) were effective antifeedants at 10 μg cm−3 of diet against Earias vitella and at 10 μg cm−2 of leaf against Spodoptera litura.428 In addition, 2, 122, 849–850, 1229, and 2-acetoxyclerodendrin B (1230) showed good insect growth inhibitory activity, even at lower concentrations. In contrast, clerodendrin H (851), from Clerondendron trichotomum, distinctly stimulated feeding activity in adult turnip sawflies.325
Jodrellins A and B (860–861) and the reference compound clerodin (2) also reduced the growth of the plant pathogenic fungus Fusarium oxysporum f. sp. Lycopersici after 18 h, and 861 and 2 maintained growth inhibition at 50 and 100 ppm after 66 h. Compound 2 delayed germination of Verticillium tricorpus spores at 25, 50 and 100 ppm after 42 h, and 861 had similar effects even at 66 h. This study suggests that certain neo-clerodane diterpenoids may contribute to antifungal, as well as anti-insect, protection in plants.326 Clerod-14-ene-3α,4β,13ζ-triol (1231) from Viguiera tucumanensis inhibited both germination and root growth of Sorghum halepense and Chenopodium album and also slightly inhibited Ipomoea purpurea.429 Allelopathic agents with phytogrowth inhibitory effects are attractive new leads for development of agrochemicals.
3.1.2. Compilation of structure–activity relationships of clerodane insect antifeedants
To date, over 300 natural and semi-synthetic clerodanes have been examined in laboratory assays, yielding several compounds with potent antifeedant activity against various insect species, but it is not known whether this activity will be retained under field conditions. A comprehensive compilation of all test results on the insect antifeedant activity of clerodane diterpenes resulted in some interesting trends based on only the 10–20% of the most active clerodanes per insect species.12,430
(i) Active clerodanes generally have a trans-decalin neo-clerodane skeleton.
(ii) In the most potent clerodanes, the C-9 side chain fragment contains an oxygenated ring system. A furofuran-based structure appears to be most favorable for strong activity against many Lepidopteran insect species. Furan and butenolide side chains are also frequently found in the most potent clerodanes,
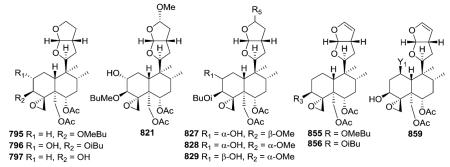
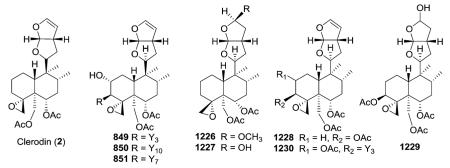
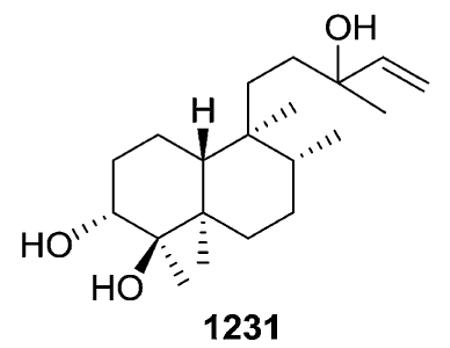 and hydrogenation of these unsaturated moieties usually results in lower potency.
and hydrogenation of these unsaturated moieties usually results in lower potency.
(iii) Structural elements from (i) and (ii) must be present simultaneously to produce high activity. However, a number of exceptions to this general observation are known. For example, while decalin 1233, which has the more common 4α-O-epoxide, but no C-9 side chain, was inactive against L. migratoria, the decalin 1232 with a 4β-O-epoxide showed equal antifeedant activity under no-choice conditions as the clerodin derivative 1234.12
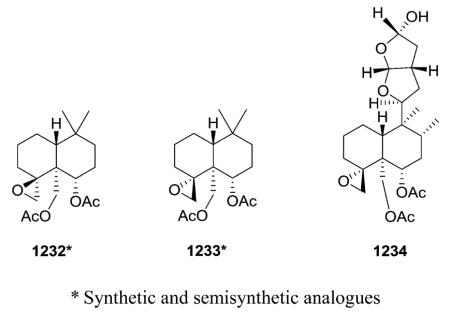
(iv) In addition to (iii), the key structural elements from (i) and (ii) must be able to adopt a certain spatial orientation for the compound to exert high activity. Indeed, the stereoelectronic factors are more important than the hydrophobic aspects as determinants of antifeedant activity. In addition, a furan ring in the side chain and a carbonyl α,β-unsaturated (or spiro-epoxide) group appear to be crucial. A conformational study indicated that the optimum interatomic distance between these two moieties ranged from 9.5 to 10.5 Å.431,432
For example, bacchotricuneatin A (1235), 7α-hydroxybacchotricuneatin A (1236), 1237, azadirachtin (1238), and the D-ring aromatic withanolide 1239 exhibited remarkable feedant-deterrent activity against T. molitor with PFI values of 22.62, 26.60, 25.03, 29.57, and 29.89, respectively.431
(v) Both rings in the decalin fragment are often substituted with hydroxy, epoxy, or ester groups, but none of these moieties can be concluded to be essential for antifeedant activity. However, slight changes in the exact identities and orientations of such groups can affect the potency of the antifeedance effect.
The general observations in (i)–(v) represent a reasonable summary of the structural features involved in the overall antifeedant activity of clerodane diterpenes. However, the underlying mechanisms of insect antifeedant activity are certainly more complex than implied by this abridged picture.
3.2. Opioid receptor agonist
3.2.1. Salvinorin A as a probe in opioid pharmacology and other salvinorin analogues
The discovery of salvinorin A (9) as the first non-nitrogenous natural product with high affinity and efficacy at the κ-opioid receptors (KORs or KOP receptors) led to a reevaluation of whether a basic nitrogen is necessary for opioid receptor affinity and efficacy. With similar potency to LSD, compound 9 is one of the most potent naturally occurring hallucinogens, and appears to have distinctive properties at KOR, including ultra-high efficacy in particular transduction
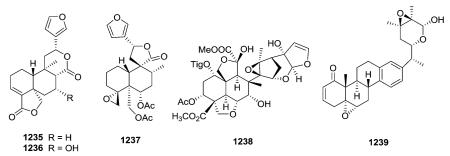 systems and a lower tendency to cause receptor desensitization.433–442 In mice, it produces antinociception that can be blocked by KOR antagonists.443,444 It also produced an aversive response in the conditioned place preference assay,445 blocked the locomotor-stimulant effects of cocaine,446 and did not exert 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM)-like effects in nonhuman primates.447 Interestingly, compound 9 has low affinity for the μ-opioid receptor (MOR), although it does show allosteric MOR modulation.448 It exhibited deleterious effects on learning and memory, acting via a KOR mechanism.448–450
systems and a lower tendency to cause receptor desensitization.433–442 In mice, it produces antinociception that can be blocked by KOR antagonists.443,444 It also produced an aversive response in the conditioned place preference assay,445 blocked the locomotor-stimulant effects of cocaine,446 and did not exert 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM)-like effects in nonhuman primates.447 Interestingly, compound 9 has low affinity for the μ-opioid receptor (MOR), although it does show allosteric MOR modulation.448 It exhibited deleterious effects on learning and memory, acting via a KOR mechanism.448–450
Phytochemical studies on S. divinorum led to the isolation of many clerodanes, including salvinorins C–J (659–662, 1240, 663–665), divinatorins A–F (405–407, 409–410, 1241), salvinicins
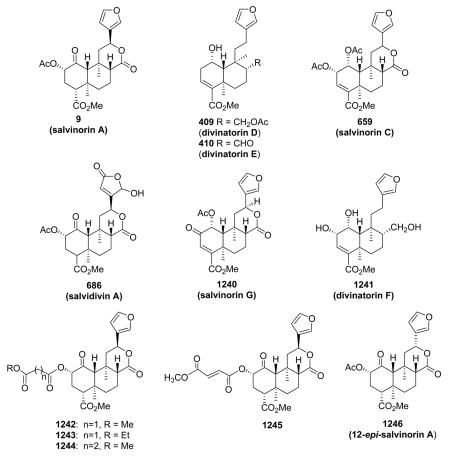 A (684) and B (685), and salvidivins A–D (686–687, 587–588).166,167,235,263–266,273,451 (The structures of 409–410, 659, 686, 1240, and 1241 are specifically shown, the remaining compounds can be found in the Structure Tables in ESI†.) In a modification study of 9, salvinorin C (659) had 250-fold lower KOR affinity compared with 9 (Ki = 1022 nM vs. Ki = 4 nM).452 Divinatorins D (409) and E (410) also had reduced KOR affinity compared with 9 (Ki = 230 nM and Ki = 418 nM, respectively, vs. Ki = 1.0 nM).167 Uniquely, salvidivin A (686) was identified as the first naturally occurring neoclerodane with KOR antagonist activity (Ke = 440 nM).453 Among dicarboxylic acid esters of 9, the methyl malonyl derivative (1242) showed the highest binding affinity (Ki = 2 nM),although analogues 1243–1245 still exhibited significant KOR affinity (Ki = 21, 36, and 39 nM).454 12-epi-Salvinorin A (1246), synthesized in four steps from 9, was a selective partial KOR agonist. It partially activated signaling through G proteins, yet acted as a full agonist in the β-arrestin 2 DiscoveRx assay.455,456
A (684) and B (685), and salvidivins A–D (686–687, 587–588).166,167,235,263–266,273,451 (The structures of 409–410, 659, 686, 1240, and 1241 are specifically shown, the remaining compounds can be found in the Structure Tables in ESI†.) In a modification study of 9, salvinorin C (659) had 250-fold lower KOR affinity compared with 9 (Ki = 1022 nM vs. Ki = 4 nM).452 Divinatorins D (409) and E (410) also had reduced KOR affinity compared with 9 (Ki = 230 nM and Ki = 418 nM, respectively, vs. Ki = 1.0 nM).167 Uniquely, salvidivin A (686) was identified as the first naturally occurring neoclerodane with KOR antagonist activity (Ke = 440 nM).453 Among dicarboxylic acid esters of 9, the methyl malonyl derivative (1242) showed the highest binding affinity (Ki = 2 nM),although analogues 1243–1245 still exhibited significant KOR affinity (Ki = 21, 36, and 39 nM).454 12-epi-Salvinorin A (1246), synthesized in four steps from 9, was a selective partial KOR agonist. It partially activated signaling through G proteins, yet acted as a full agonist in the β-arrestin 2 DiscoveRx assay.455,456
Of the derivatives of 9 reported to date, salvinorin B ethoxymethyl ether (EOM–SB) is the most potent. It exhibited ten-fold greater potency than 9
in vitro and in rodents, and had a longer duration of action in rodents.457–460 In addition, 22-thiocyanato-(RB–64) and 22-chloro-salvinorin A (RB–48) were both extremely potent and selective KOR agonists in vitro and in vivo.461
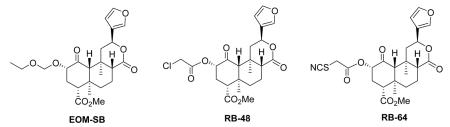
3.2.2. Structure–activity relationships of salvinorin A analogues
The general SAR studies of 9 have been performed by semi-synthetic structure modifications, and have mainly focused on 9's high affinity and selectivity for the KOP receptor. Some of these analogues have interesting pharmacological profiles, from full KOR agonist to partial δ-opioid receptor (DOR) or μ-opioid receptor (MOR) agonists and antagonists.13,448,453,462–464 The SAR is summarized in Fig. 5.13,457,465–471
Fig. 5.
General SAR for salvinorin A activity at KOP receptors.
[Adapted from Prisinzano TE and Rothman RB, Chem Rev., 2008, 108, 1732-1743.]
At the C-1 position, the reduction or removal of the carbonyl is tolerated, and introduction of a 1,10-alkene increases the possibility of antagonist activity.
At the C-2 position, (1) the size and electronegativity of the substituent at the C-2 position is critical for activity at opioid receptors. Bioisosteric replacements of the ester moiety are tolerated. (2) α-Substituents are preferred over the corresponding β-substituents. (3) In general, small alkyl esters favor binding to KORs. The compounds with methoxymethyl (1247) and ethoxymethyl (1248) ethers at this position are among the most potent 9-derived KOR agonists reported to date, while aromatic esters favor MOR binding.13,472 Different aromatic groups attached directly to the decalin core can be allowed by KOR.473
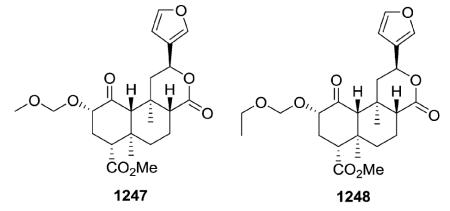
At the C-4 position, (1) small alkyl chains are preferential for KOR binding, while (2) hydrolysis or reduction of the carbomethoxy group leads to reduced KOR affinity, and (3) conversion to an amide is generally not tolerated.13,472
Furthermore, the furan ring at C-12 may be reduced or replaced, but KOR affinity is reduced. The reduction or removal of the carbonyl at C-17 and the introduction of an 8,17-alkene are tolerated.13,472
Finally, degradation of the furan ring and/or replacement with other heterocycles is tolerated. Moreover, such a modification produced the first DOR selective ligand (1249) with the scaffold of 9.13,472
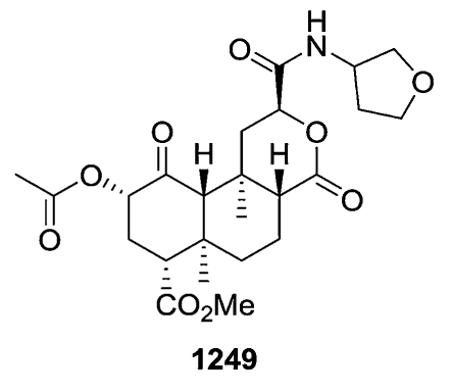
3.3. NGF-potentiating activities
With EC50 values of 20.2, 12.7, 4.0 and 2.4 μg mL−1, balanspenes D–G (13–16), which have a 18,19-expoxy clerodane-type diterpene skeleton with an additional double bond at C-3 and C-4, markedly increased the nerve growth factor NGF (20 ng mL−1)-induced quantity of neurite-bearing cells.15 An equilibrium mixture of ptychonal (380) and ptychonal hemiacetal (329), 6α,7α-dihydroxyannonene (382), and 7α,20-dihydroxyannonene (383), significantly enhanced NGF-mediated neurite outgrowth in PC12 cells at concentrations ranging from 0.1 to 50.0 μM, 0.1 to 30.0 μM, and 0.1 to 10.0 μM, respectively.130,474 At 10, 30, and 100 μmol, compounds 435 and 436 had no effect on neurite outgrowth from PC12D cells in the absence of NGF, but at 100 μmol, decidedly increased the NGF (2 ng mL−1)-induced proportion of neurite-bearing PC 12D cells by 49% and 53%, respectively.174
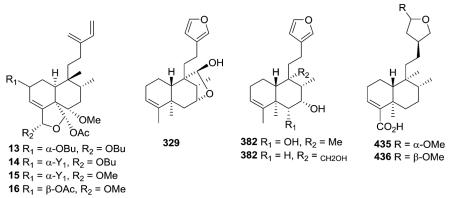
Both crotopenes A and B (764 and 765) exhibited potentiating activities on NGF-mediated neurite outgrowth from PC12 cells.294 At a concentration of 10 μM, degraded clerodane compounds 1064, 1083, and 1084 exhibited neurite outgrowth-promoting activity in NGF mediated PC12 cells.382
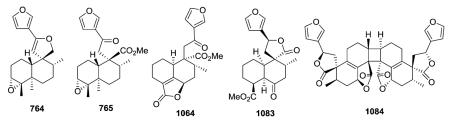
13-epi-15,16-Epoxy-15α-methoxy-ent-clerod-3-en-18-oic acid (1250) and 15,16-epoxy-7α,18-dihydroxy-15-methoxy-ent-clerod-3-ene (1251) also markedly increased the NGF (2 ng mL−1)-induced proportion of neurite-bearing cells by 49%, 53%, and 68%, respectively475
3.4. Antiulcer activities476–482
trans-Dehydrocrotonin (1076), trans-crotonin (1252), cordatin (1253), and aparisthman (1254)481 exhibited significant anti-ulcer activities, comparable with those of cimetidine. At a dose
 of 100 mg kg−1 (p.o.), the four compounds significantly reduced gastric injury induced by stress (67% or 72%), indomethacin/bethanechol (29%, 78%, 71%, 78%, respectively), ethanol (71% or 76%), pylorus ligature (30%, 35%, 59%, 50%, respectively), and hypothermic restraint (50% or 66%) in mice and rats. In the HCl/ethanol-induced gastric ulcer model, at 100 and 250 mg kg−1 (p.o.), gastric lesion formation was decreased by 48.1%, 52.3%; 50.8%, 55.8%; 70%, 59% and 77%, 66%, respectively, when compared to the control group. In the pylorus-ligature model, 1076 and 1252 (p.o.), like cimetidine, increased the volume of gastric fluid when compared to the control group, while 1253 and 1254 (p.o.) decreased the volume of gastric fluid. Compound 1076 exhibited low acute toxicity in mice (LD50 876 mg kg−1) when administered orally, however, hepatotoxicity resulted from its long-term use.
of 100 mg kg−1 (p.o.), the four compounds significantly reduced gastric injury induced by stress (67% or 72%), indomethacin/bethanechol (29%, 78%, 71%, 78%, respectively), ethanol (71% or 76%), pylorus ligature (30%, 35%, 59%, 50%, respectively), and hypothermic restraint (50% or 66%) in mice and rats. In the HCl/ethanol-induced gastric ulcer model, at 100 and 250 mg kg−1 (p.o.), gastric lesion formation was decreased by 48.1%, 52.3%; 50.8%, 55.8%; 70%, 59% and 77%, 66%, respectively, when compared to the control group. In the pylorus-ligature model, 1076 and 1252 (p.o.), like cimetidine, increased the volume of gastric fluid when compared to the control group, while 1253 and 1254 (p.o.) decreased the volume of gastric fluid. Compound 1076 exhibited low acute toxicity in mice (LD50 876 mg kg−1) when administered orally, however, hepatotoxicity resulted from its long-term use.
3.5. Cytotoxic activities
Casearupestrins A (21), B (22) and D (24) showed significant cytotoxicity against four cell lines (HL-60, HCT-8, MDA/MB-435, and SF-295), with IC50 values ranging from 0.10 to 1.3 μM.17
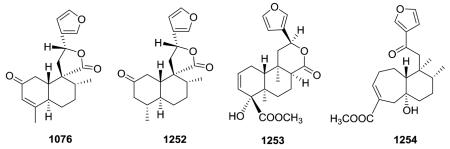 Intrapetacins A (38) and B (39) displayed moderate cytotoxicity against KB cells, with IC50 values of 2.0 and 0.8 μg mL±1.21 In addition, compound 39 caused a significant (21 mm) zone of inhibition of fungal growth. Bioassay-guided fractionation of the EtOAc extracts of Casearia membranacea afforded case-amembrins A–E (60–64), M–O (65–67), and caseamembrols A (119) and B (68) as active principles.26–28 Compounds 60 and 62–64 exhibited cytotoxic activity against PC-3 and Hep 3B, with IC50 values below 3 μM. Compounds 66 and 67 showed significant activity against KB, DLD-1, and Med tumor cell lines (1.94–8.94 μg mL−1). Compounds 119 and 68 were cytotoxic against PC-3 human prostate cancer cells with IC50 values of 2.45 and 5.66 μM, respectively.28
Intrapetacins A (38) and B (39) displayed moderate cytotoxicity against KB cells, with IC50 values of 2.0 and 0.8 μg mL±1.21 In addition, compound 39 caused a significant (21 mm) zone of inhibition of fungal growth. Bioassay-guided fractionation of the EtOAc extracts of Casearia membranacea afforded case-amembrins A–E (60–64), M–O (65–67), and caseamembrols A (119) and B (68) as active principles.26–28 Compounds 60 and 62–64 exhibited cytotoxic activity against PC-3 and Hep 3B, with IC50 values below 3 μM. Compounds 66 and 67 showed significant activity against KB, DLD-1, and Med tumor cell lines (1.94–8.94 μg mL−1). Compounds 119 and 68 were cytotoxic against PC-3 human prostate cancer cells with IC50 values of 2.45 and 5.66 μM, respectively.28
Laetiaprocerines A–D (101–103, 274) were cytotoxic toward the MCF7 human tumor cell line.36 The structurally similar zuleanin-type compound caseamembrin G (1255) was cytotoxic against KB, HeLa, and Hep59T/VGH carcinoma cell lines.483
Esculentin B (95) from Casearia esculenta and caseargrewiins A–H (74, 1256–1258, 104–107) from C. grewiifolia showed significant cytotoxicity against three cancer cell lines (KB, BC1, and NCI-H187) with IC50 values ranging from 0.1 to 8.7 μg mL−1.31,37 The most potent compounds were 104 and 106
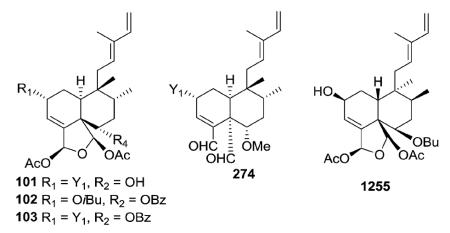 against KB (IC50 0.66, 0.67 μg mL−1), 1258, 95, 105, and 107 against BC1 (IC50 0.1, 0.17, 0.20, 0.21 μg mL−1), and 104 and 1257 against NCI-H187 (IC50 0.15, 0.3 μg mL−1), respectively, similar values to those for the control drug ellipticine. Bucidarasins A–C (133–135) showed potent cytotoxicity against nine human tumor cell lines with IC50 values ranging from 0.5 to 1.9 μM.14
against KB (IC50 0.66, 0.67 μg mL−1), 1258, 95, 105, and 107 against BC1 (IC50 0.1, 0.17, 0.20, 0.21 μg mL−1), and 104 and 1257 against NCI-H187 (IC50 0.15, 0.3 μg mL−1), respectively, similar values to those for the control drug ellipticine. Bucidarasins A–C (133–135) showed potent cytotoxicity against nine human tumor cell lines with IC50 values ranging from 0.5 to 1.9 μM.14
Compound 203 from Brazilian propolis reduced the incidence of skin tumors by inhibiting DNA synthesis via a de novo pathway, and suppressed the tumor growth by decreasing DNA synthesis via a salvage pathway.69 Premnones A–C (234–236)
 exhibited cytotoxic activity when evaluated against three human cancer cell lines (Lu1, LNCaP, and MCF-7), and one normal cell line (HUVEC) in the range of ED50 of 0.7–7.0 μg mL−1.80
exhibited cytotoxic activity when evaluated against three human cancer cell lines (Lu1, LNCaP, and MCF-7), and one normal cell line (HUVEC) in the range of ED50 of 0.7–7.0 μg mL−1.80
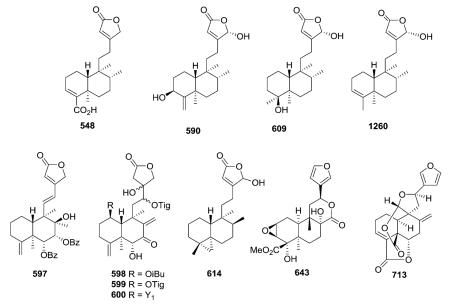 However, 234 was not active when evaluated in a follow-up in vivo hollow fiber assay at the highest dose tested (50 mg kg−1), using LNCaP, Lu1, and MCF-7 cells.
However, 234 was not active when evaluated in a follow-up in vivo hollow fiber assay at the highest dose tested (50 mg kg−1), using LNCaP, Lu1, and MCF-7 cells.
Crispene E (468) inhibited STAT3 dimerization in a cell-free fluorescent polarization assay and displayed significant toxicity against the STAT3-dependent MDA-MB 231 breast cancer cell line with selective inhibition of the expression of STAT3 and STAT3 target genes cyclin D1, fascin and bcl-2.190 Ajugalide B (507) exhibited broad spectrum antiproliferative activity against A549, AGS, HepG2, and HT29 human cancer cell lines, with GI50 values ranging from 3.18 to 5.94 μM.484 Below its cytotoxic concentration, 507 also reduced the tumorigenic and metastatic ability of A549 cancer cells by inhibiting anchorage-independent growth and cell migration; thus, 507 could be a lead for development of potential cancer chemotherapy.
Clerodermic acid (548) induced potent apoptosis against human leukemia HL60 cells.485 Compounds 590, 609, and 1260 from Polyalthia barnesii exhibited their highest potency against LNCaP and U373 cell lines, but generally showed broad spectrum cytotoxicity, with ED50 values of less than 4 μg mL−1 toward several cell lines.237 Scutebata L (597) exhibited moderate activity against several human cancer cell lines with IC50 values ranging from 12.6 to 26.1 μM.231 Calcicolins A (598) and C (600) showed significant cytotoxic effects against the
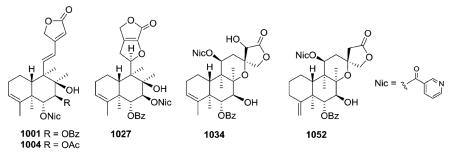 D.mel-II and HepG2 cell lines with IC50 values of 2.06, 2.10, and 9.04, 8.30 μg mL−1, respectively.242 Calcicolin B (599) also showed good toxicity against D.mel-II cells (IC50 = 3.09 μg mL−1) but was not as potent against HepG2 cells (IC50 = 16.16 μg mL−1).242 Echinoclerodane A (614) exhibited moderate cytotoxicity against MOLT-4, HL-60, DLD-1 and LoVo tumor cells and inhibited superoxide anion generation and elastase release by human neutrophils.248 Tinosporin A (643) showed low cytotoxicity against HL-60 and MCF-7 cells, with IC50 values of 18.63 and 23.58 μM, respectively.256 Crotonlide A (713) exhibited moderate cytotoxicity against HL-60 (IC50 9.42 μM) and P-388 (IC50 7.45 μM) tumor cell lines.165
D.mel-II and HepG2 cell lines with IC50 values of 2.06, 2.10, and 9.04, 8.30 μg mL−1, respectively.242 Calcicolin B (599) also showed good toxicity against D.mel-II cells (IC50 = 3.09 μg mL−1) but was not as potent against HepG2 cells (IC50 = 16.16 μg mL−1).242 Echinoclerodane A (614) exhibited moderate cytotoxicity against MOLT-4, HL-60, DLD-1 and LoVo tumor cells and inhibited superoxide anion generation and elastase release by human neutrophils.248 Tinosporin A (643) showed low cytotoxicity against HL-60 and MCF-7 cells, with IC50 values of 18.63 and 23.58 μM, respectively.256 Crotonlide A (713) exhibited moderate cytotoxicity against HL-60 (IC50 9.42 μM) and P-388 (IC50 7.45 μM) tumor cell lines.165
Many neo-clerodane diterpene alkaloids from Scutellaria barbata showed significant cytotoxic activity against three human cancer lines (HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma cells) (IC50 2.0–8.1 μM) in various studies.230,232,233,367,370,371,378 Examples are shown below (1001, 1004, 1027, 1034, 1052) as well as in prior Section 2.9.1 (998, 1008, 1009, 1012, 1030, 1031, 1036–1042).
The rearranged clerodane polylongifoliaic A (1156) exhibited potent activity against SK-N-MC human neuroblastoma cells with an IC50 value of 1.64 μM.220 16-Oxo-cleroda-3,13(14)E-dienl5-oic acid (1259)486 and polyalthialdoic acid (189) showed antiproliferative activity against human leukemia HL-60 cells, with IC50 values of 13.7 and 21.8 μM, respectively, compared with 5-fluorouracil's IC50 of 9.5 μM.60,487
Table 3 lists cytotoxicity results found for casearins A–R (1261–1278) against V-79 cells.488,489 Three compounds without an oxygenated substituent at C-6, casearins G (1267), H (1268) and I (1269) showed the highest potency (IC50 0.17–0.51 μM). Casearins J (1270) and K (1271) with a hydroxy moiety at C-6 exhibited similar or slightly weaker activity (IC50 0.52 and 1.1 μM, respectively) than 1267–1269. Casearins L (1272) and M (1273) have a hydroxy moiety at C-7 rather than C-6, and showed slightly reduced activity (IC50 1.6 and 1.8 μM, respectively). Casearin C (1263), with an interesting decanoate at C-7 exhibited significant potency (IC50 0.77 μM), while a related compound, casearin E (1265) with the same decanoate group but slightly different oxygenated substituents at C-6 and C-18 was much less potent (IC50 4.7 μM). The authors postulated that the greater hydrophobicity of 1263 might increase its affinity for the V-79 cell membrane leading to significant activity. Furthermore, casearins A (1261) and D (1264) were converted to derivatives Aa (1279), Ab (1280), Ac (1281) with oxo, propionate, and butanoate groups, respectively, at C-6 and Da (1282) with oxo groups at both C-2 and C-6. Only compound 1279 retained cytotoxic potency (IC50 0.55 μM); compounds 1280–1282 had greatly reduced activity (IC50 17, 38, 19 μM, respectively). Thus, the bulkiness of the C-6 group could greatly influence the activity.489,490 In other studies, the structurally similar casearin X (1283) and caseargrewiin F (105), which do not have an oxygenated group at C-7, showed cytotoxic activity against MOLT-4, MDA-MB-435, HCT-8, and SF-295 human cell lines, with IC50 values from 0.22 to 0.97 and 0.09 to 0.17 μM, respectively. Their lower cytotoxicity against L-929 cells (IC50 1.52 and 1.06 μM) perhaps indicated a more selective cytotoxic response to tumor cell lines.490 Other zuleanin-type clerodanes, case-arvestrins A–C (87–89), had comparable IC50 values between 0.2 and 0.8 μM against a panel of tumor cell lines, including LX-1, HCT116, and A2780.34
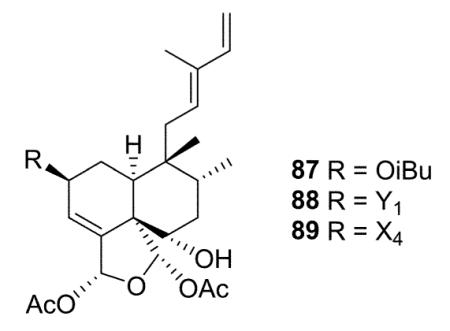
Table 3.
Structures and cytotoxic data (V-79 cells) of casearins and their derivativesa
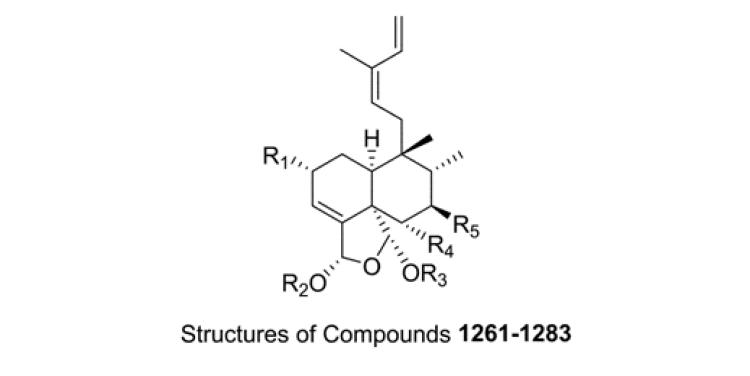
|
||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Casearin | R1 | R2 | R3 | R4 | R5 | IC50 (μmol L−1) |
| A (1261) | OCH3 | Ac | Ac | OH | OBu | 1.0 |
| B (1262) | OCH3 | Ac | Ac | OAc | OBu | 8.5 |
| C (1263) | OH | Ac | Ac | OAc | ODc | 0.77 |
| D (1264) | OH | Bu | Ac | OH | OBu | 1.8 |
| E (1265) | OH | Et | Ac | OH | ODc | 4.7 |
| F (1266) | OH | Et | Ac | OH | OBu | 29 |
| G (1267) | OCH3 | Ac | Ac | H | OBu | 0.17 |
| H (1268) | OH | Ac | Ac | H | OBu | 0.37 |
| I (1269) | OH | Ac | Bu | H | OBu | 0.51 |
| J (1270) | OCH3 | Bu | Ac | OH | OBu | 1.1 |
| K (1271) | OAc | Ac | Ac | OH | OBu | 0.52 |
| L (1272) | OCH3 | Bu | Ac | OAc | OH | 1.6 |
| M (1273) | OH | Bu | Bu | OAc | OH | 1.8 |
| N (1274) | OCH3 | Ac | Bu | OAc | OBu | 5.9 |
| O (1275) | OCH3 | Bu | Ac | OAc | OBu | 6.0 |
| P (1276) | OCH3 | Ac | Ac | OAc | OAc | 7.8 |
| Q (1277) | OH | Ac | Ac | OAc | OBu | 4.3 |
| R (1278) | =O | Ac | Ac | OH | OBu | 5.4 |
| Aa (1279) | OCH3 | Ac | Ac | =O | OBu | 0.55 |
| Ab (1280) | OCH3 | Ac | Ac | OPr | OBu | 17 |
| Ac (1281) | OCH3 | Ac | Ac | OBu | OBu | 38 |
| Da (1282) | =O | Bu | Ac | =O | OBu | 19 |
| X (1283) | Bu | Bu | Ac | OH | H | |
Ac = acetate, Bu = butanoate, Dc = decanoate.
Mechanistic investigations showed that 1264 can protect DNA against different types of damage and act as an antioxidant by inducing detoxificant enzymes in HepG2 cells; these actions give rise to interesting chemopreventive characteristics in both HepG2 cells and the Salmonella typhimurium bacterial strain.491 Finally, casearin X (1283) showed potent cytotoxic effects against CEM and HL-60 lines (IC50 0.4 μM) and PBMC cells (IC50 1.2 μM) and caused cell death via apoptotic pathways. These data further substantiated the promising antitumor-related properties of casearins.492 In addition, 1283 exhibited chemopreventive activity against DNA damage induced by the particulates formed from burning sugarcane, which implied that 1283 can act by different mechanisms to protect DNA against damage, including repairable and non-repairable damages.493
Compounds 1284–1286 showed potent and selective cytotoxicity against P-388, A-549, HT-29, Mel-28 cell lines, with IC50 values of 0.2–2.4 μM.494 (−)-Kolavenol (1287) increased lifespan (I.L.S.) in mice with IMC carcinoma, and was twice as effective (I.L.S. 98%, 41 mg per kg per day, 4 days) as 5-FU (46%, 30 mg per kg per day, 4 days).495 (+)-7β-Acetoxy-15,16-epoxycleroda-3,13(16),14-trien-18-oic acid (1288) strongly inhibited P-glyco-protein and likely has promise for development as an MDR-reversing agent.496 (5R,10R)-4R,8R-Dihydroxy-2S,3R:15,16-diepoxycleroda-13(16),17,12S:18,1S-dilactone (1289) exhibited a preventive effect against chemically-induced hepatocellular carcinoma (HCC) in rats, and could be a potent chemopreventive drug for HCC.497
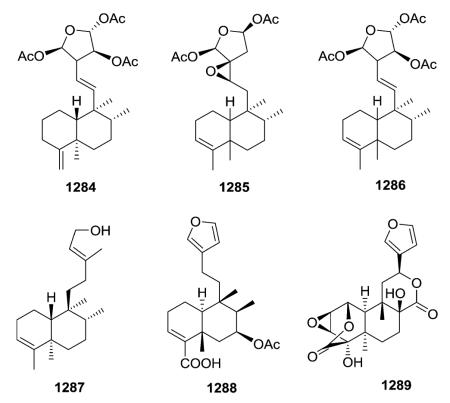
trans-Dehydrocrotonin (1076) and trans-crotonin (1252) (see structures in Section 3.4) were evaluated for their effects on the survival of mice bearing Sarcoma 180 and Ehrlich carcinoma ascitic tumors, as well as the proliferation of cultured Ehrlich cells and TNFα activity. When the mice were treated with 80 and 120 mg kg−1 of 1076 or 38 mg kg−1 of 5-FU, substantial antitumor activity was observed (%T/C 128–140). Both compounds showed a cytotoxic value of 16 μM against Ehrlich carcinoma in 48 h cell culture. In vitro electrophoresis of DNA extracted from the treated tumor cells showed no apoptosis. But significant TNFα activity was detected in Ehrlich tumor-bearing mice treated with 1076, likely due to enhanced immune function.498
3.6. Anti-inflammatory activities
The hallucinogenic compound salvinorin A (9) (see structure in Section 3.2) is a potent KOR agonist. However, its multiple pharmacological effects are likely due to action on other targets, such as the cannabinoid CB1 receptor (CB1R). For instance, it exerted potent anti-inflammatory and antinociceptive effects, as mediated by KOR and CB1R,499 and thus, is an interesting new lead compound for the development of novel anti-inflammatory agents targeting KOR and CB1R. This observation also encourages further studies on 9 leading to the development of novel peripherally restricted derivatives with similar potency and selectivity. Thus, 9-related compounds may be useful future drugs, especially in patients with inflammatory bowel disease, in which pain is the most pronounced symptom during maintenance of remissions.500
Compound 485 inhibited LPS-induced NO production in BV-2 cells dose-dependently with an IC50 value of 28.6 ± 2.6 μM.194 Tinospinosides B (956) and C (957) also showed inhibitory effects against NO production.355
E-Isolinaridial (1290) and E-isolinaridial methylketone (1291) inhibited human synovial sPLA2 in a concentration-dependent manner with IC50 values of 0.20 and 0.49 μM, respectively, similar to that of scalaradial, an anti-inflammatory marine natural product that selectively inhibits 14 kDa type II phospholipase A2(PLA2).501 In addition, these compounds reduced cell-free 5-lipoxygenase activity and A23187-induced neutrophil LTB4 biosynthesis, and significantly decreased receptor-mediated degranulation. 6-Oxocleroda-3,13(14)E-dien-15-oic acid methyl ester (1292) and 16-hydroxycleroda-3,13(14) E-dien-15-oic acid (1293), displayed significant activity against fMLP/CB induced superoxide generation by neutrophils with IC50 values of 0.6 ± 0.09 and 1.49 ± 0.28 μg mL−1, respectively.246,436 The suppressive effects of 1293 on human neutrophil respiratory burst and degranulation were due, at least partially, to inhibition of calcium, AKT, and p38 signaling pathways.457,502 3β,4β:15,16-Diepoxy-13(16),14-clerodadiene (1294) and thysaspathone (365) inhibited NO production in LPS-stimulated RAW 264.7 cells with IC50 values of 20.1 and 11.6 μM, respectively.143,438
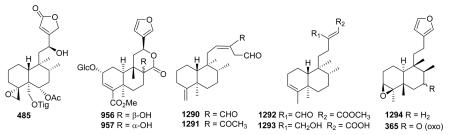
3.7. Antiparasitic/antiprotozoal activities
Caseargrewiins A–D (74, 1256–1258, see structures in Section 3.5) were active in vitro against Plasmodium falciparum in vitro, with respective IC50 values of 2.9, 2.4, 3.0, and 3.3 μg mL−1.31 Clerodane diterpenoids 227–230 also inhibited the growth of the chloroquine-resistant strain FeB1, with IC50 values between 4.3 and 14.6 μg mL−1.78 Penianthic acid (1295) and epicordatine (1296) exhibited weak activity against chloroquine-resistant strain K1.503 Casearlucine A (1297), caseamembrol A (119, see structure in Section 3.5), and laetiaprocerines A–D (101–103, 274, see structures in Section 3.5) displayed activity against P. falciparum with IC50 values as low as 0.5 μM against FeB1 and F-32 strains.36 In addition, compounds 119 and 1297 also showed activity against Leishmania amazonensis amastigote axenic stages and promastigote.36 Ajugarin-1 (1298) showed moderate in vivo antiplasmodial activity, with an IC50 of 23.0 ± 3.0 μM, against FCA 20/GHA P. falciparum.504,505 At a dose of 200 mg kg−1 in mice, the clerodane diterpenoid gomphostenin-A (1299) exhibited an impressive 93% chemosuppression against P. berghei.506
Clerodane 182, a diastereoisomer of kolavenol, exhibited trypanocidal activity (IC50 2.5 μg mL−1) against Trypanosoma brucei rhodesiense, the parasitic cause of acute human African trypanosomiasis (sleeping sickness).56 This compound was isolated from the root bark of Entada abyssinica, which is used by traditional healers in Uganda to treat sleeping sickness.
Infuscatin (626) and sepulturins A, C, D and E (1300, 1302–1304) showed antiprotozoal activity against clinically isolated strains of Entamoeba histolytica and Giardia lamblia with similar potency to (+)-catechin and tyramine, but much less than metronidazole. Sepulturins A–F (1300–1305) were isolated from Saliva shannoni J.D. Smith, which is used as a traditional medicine in El Salvador against malaria.507 The structure of 626 (ref. 251) was also revised based on NOESY data and structural similarity to 1302. These new clerodane diterpenes contain a tertiary hydroxy group at C-8 or C-10 or both positions.
3.8. Antifungal and antibacterial activities
A fruit pulp extract of Detarium microcarpum inhibited the growth of the plant pathogenic fungus Cladosporium cucumerinum and of the enzyme acetylcholinesterase, which has been
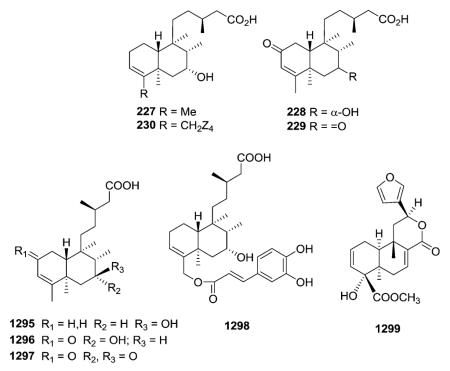
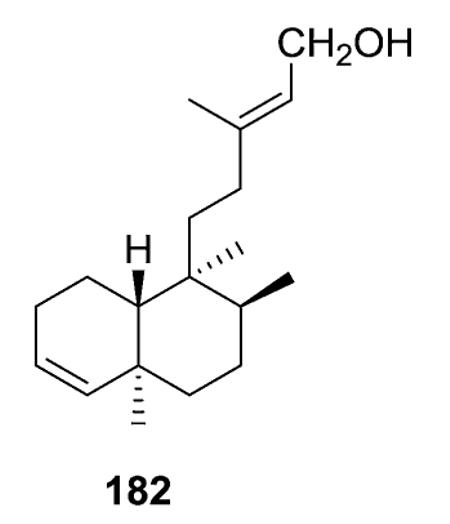
 implicated in Alzheimer's disease. Fractionation of this extract led to the isolation of four new clerodane diterpenes, 197 (see Section 2.1.2.1), 266–267 (see Section 2.1.2.2), and 291 (see Section 2.1.3). Their structures were elucidated from spectroscopic data and X-ray crystallography of 197 and 266. Compounds 197, 267, and 291 showed both antifungal activity and inhibition of acetylcholinesterase.65
implicated in Alzheimer's disease. Fractionation of this extract led to the isolation of four new clerodane diterpenes, 197 (see Section 2.1.2.1), 266–267 (see Section 2.1.2.2), and 291 (see Section 2.1.3). Their structures were elucidated from spectroscopic data and X-ray crystallography of 197 and 266. Compounds 197, 267, and 291 showed both antifungal activity and inhibition of acetylcholinesterase.65
Caseanigrescen C (39) caused a significant (21 mm) zone of inhibition of fungal growth,21 and 16-oxo-cleroda-3,13(E)-diene-15-oic acid (204) also exhibited high antifungal activities, with MIC values of 25 to 50 μg, as compared with fungicide Dithane M-45.70 Caseargrewiins A–D (74, 1256–1258, see Section 3.5) exhibited moderate activity against Mycobacterium tuberculosis, with MIC values of 12.5, 12.5, 25.0, and 12.5 μg mL−1, respectively.31
Compounds 205 and 206 were isolated as active antibacterial principles from Haplopappus foliosus.71 They were active against six Gram-positive bacteria, but inactive against five Gram-negative bacteria. 18-Acetoxy-cis-cleroda-3-en-15-oic acid (226) also showed antibacterial activity against five Gram-positive bacteria.77 16α-Hydroxy-cleroda-3,13(Z)-diene-15,16-olide (551) was highly active against Gram-negative bacteria, including Escherichia coli, Klebsiella aerogenes, and Pseudomonas species, with MICs in the range 0.78–1.5 mg.70 Among Gram-positive bacteria, compound 551 was also highly active against Bacillus species with MIC of less than 2 μg. Interestingly, against
 Gram-positive methicillin-resistant Staphylococcus aureus, compound 551 showed both in vivo efficacy in infected mice due to bacterial membrane disruption as well as synthergistic activity with clinically used antibiotics, such as tetracycline, linezolid, and daptomycin.508
Gram-positive methicillin-resistant Staphylococcus aureus, compound 551 showed both in vivo efficacy in infected mice due to bacterial membrane disruption as well as synthergistic activity with clinically used antibiotics, such as tetracycline, linezolid, and daptomycin.508
Furthermore, neo-clerodanes-(5R,8R,9S,10R)-15,16-diol-15,16-dihydrohardwickiic acid (1306) and (2S,5R,8R,9S,10R)-2β-hydroxy-16-oxo-15,16-dihydrohardwickiic acid (1307) from Salvia adenophora showed strain-dependent activity against Gram-positive Staph. epidermidis.509 In contrast, lupulin A (822) showed clear activity against Gram-negative Ps. aeruginosa and E. coli (inhibitory zone 3–5 mm),315 and hativenes A–C (827–829) showed potent activity against three Gram-negative rods (E. coli, Ps. aeruginosa and Salmonella typhimurium).510 Lupulin F (794) also showed antibacterial activity against Ps. aeruginosa and E. coli.300
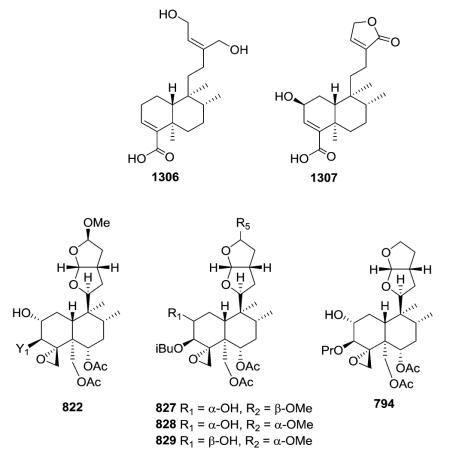
3.9. Other bioactivities
trans-Dehydrocrotonin (t-DCTN, 1076, see structure in Section 3.4) exhibits multiple biological effects, including antitumor, antiulcerogenic, hypolipidaemic, antiatherogenic, anti-oestrogen, antigenotoxicity, anti-inflammatory, and insect growth inhibitory property activities.511–518 The anti-hyperglycemic potential of this compound was supported by its reported hypoglycemic effect, which was almost comparable to that produced by glibenclamide (2 mg kg−1), a clinically useful drug.519 Compound 1076 can also be used as a potent analgesic agent in case of peripheral algesia, without CNS effects.520 The hypotensive and bradycardia effects of 1076 are possibly related to some extent to the release of nitric oxide as well as direct effects on vascular smooth muscle, and cardiac pacemaker activity.521
Casearinols A (1308) and B (1309) and casearinones A (1310) and B (1311), inhibited the binding of T-cell leukocyte function antigen 1 to intercellular adhesion molecule 1, with an IC50 of 50 μM.522 This report is the first to link this diterpene class with immunomodulatory activity. Diterpene 1312, an active principle of Baccharis trimeta, blocked vascular smooth muscle contractions induced by extracellular Ca2+ in KCl-depolarized preparations.523
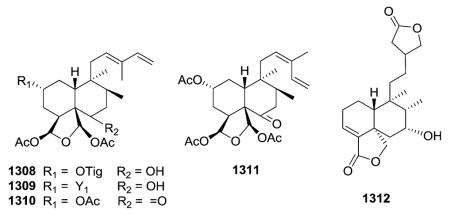
Interestingly, 16α-hydroxycleroda-3,13Z-dien-15,16-olide (551, see structure in Section 3.8) is the first clerodane diterpene reported to have potential as a lipid lowering agent. It represents a new structural class of HMG-CoA reductase inhibitor.524
3.10. Toxicity
Some neo-clerodane diterpenoids, especially furan-containing diterpenoids, are highly hepatotoxic in mice, causing mid zonal hepatic necrosis. They have also been alleged to cause fulminant hepatitis, chronic hepatitis, or cirrhosis in humans.
Diosbulbin D (1313, DBD) was the first hepatotoxic furano norclerodane diterpenoid isolated from Dioscorea bulbifera.525,526 The effects of DBD on the growth of normal human liver L-02 cells may be due to its induction of cell apoptosis, which may also explain the toxicity observed with plants containing furano clerodane diterpenoids.527 Diosbulbin B (1314, DB) is another hepatotoxic compound found in high quantity in
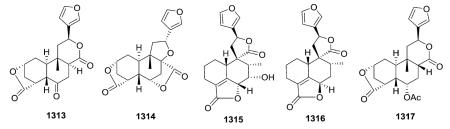 D. bulbifera.528,529 The hepatotoxic effects of the ornamental and medicinal germander plant (Teucrium chamaedrys) were attributed to the furano neo-clerodanes teucrin A (1315) and teuchamaedryn A (1316).530 Plants in the genus Teucrium have been used as erroneously “safe” herbal hypoglycemic and slimming aids.530–532 Other furanoclerodane diterpenoids have not been assayed for hepatotoxicity, with more attention put on other pharmacological properties. Determination of toxicity is an important criterium for both consumer safety and clinical candidacy. For instance, the possible development of 8-epidiosbulbin E acetate (1317) (with a δ-lactone cis-fused to the decalin system rather than trans-fused as in the hepatotoxic 1313, DBD)526 as a potential plasmid-curing agent against multidrug-resistant bacteria, which pose a tremendous current health challenge.533
D. bulbifera.528,529 The hepatotoxic effects of the ornamental and medicinal germander plant (Teucrium chamaedrys) were attributed to the furano neo-clerodanes teucrin A (1315) and teuchamaedryn A (1316).530 Plants in the genus Teucrium have been used as erroneously “safe” herbal hypoglycemic and slimming aids.530–532 Other furanoclerodane diterpenoids have not been assayed for hepatotoxicity, with more attention put on other pharmacological properties. Determination of toxicity is an important criterium for both consumer safety and clinical candidacy. For instance, the possible development of 8-epidiosbulbin E acetate (1317) (with a δ-lactone cis-fused to the decalin system rather than trans-fused as in the hepatotoxic 1313, DBD)526 as a potential plasmid-curing agent against multidrug-resistant bacteria, which pose a tremendous current health challenge.533
4. Conclusion
In this review, discoveries of clerodane diterpenes from 1990–2015 were categorized by their chemical structures. During the last 25 years, over 1300 diterpenoids and nor-diterpenoids with the clerodane carbon skeleton have been isolated. These natural neo-clerodanes have been classified into seven different groups on the basis two fragments, the C-11–C-16 moiety and the decalin moiety. In addition, clerodane-type diterpene glycosides and clerodane derivatives, including N-containing derivatives, degraded derivatives, ring-seco derivatives, and rearranged derivatives reported in the literature were considered in this review. Although their insect antifeedant activity and opioid receptor agonist effects are generally considered most important, clerodane diterpenes exhibit many other pharmacological activities. The distribution, chemotaxonomic significance, biological activity, structure activity relationship correlations, and modes of action of active clerodanes have also been summarized.
The body of knowledge on the chemistry, biological activity, and pharmacology of clerodane diterpenes continues to grow rapidly. Despite such advances, continued investigations into the biological mechanisms involved in insect antifeedant activity, as well as extended research into the chemistry and pharmacology of opioid receptor ligands, such as salvinorin A (9) or other natural products, may provide the means to differentiate among different types of antifeedants and give better defined sets of clerodanes with a distinct mode of action from which more detailed structure–activity relationships can be deduced, and may yet yield the holy grail of opioids.
Supplementary Material
Acknowledgements
We are grateful for the financial support from Natural Science Foundation of China (21572082, 21262021) and the State Key Laboratory of Phytochemistry and Plant Resources in West China (No. P2015-KF01). This work was supported in part by National Cancer Institute Grant CA177584 awarded to K. H. Lee. We would like to thank Dr Yan-Hong Li and Mr Shi-Li Yan at Kunming University of Science and Technology for their contributions to the preparation and proofreading of the manuscript.
Biography
 Rong-Tao Li received her Ph.D at Kunming Institute of Botany, Chinese Academy of Science (CAS) in 2004, under the guidance of Prof. Han-Dong Sun. She worked with Prof. Kuo-Hsiung Lee from March 2013 to June 2014, as a Visiting Professor at the UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill. She has been awarded several prizes conferred by national, CAS, provincial, or ministerial authorities, including the “National Excellent Doctoral Dissertation of P.R. China” in 2006, the “New Century Excellent Talents on University” in 2006, the “Young Academic and Technical Leader of Yunnan Province” in 2009, and the “Excellent Doctoral Dissertation of the Chinese Academy of Science” and “CPA-Servier Yong Investigator Award in Medicinal Chemistry” 2005. She has performed phytochemical studies on about 50 plants, published 12 patents, and over 130 scientific papers. She is now Professor, Kunming University of Science and Technology (2005-present). Her current research interests involve the activity-directed isolation, structure elucidation and the structure–activity relationships of natural products from medically important plants.
Rong-Tao Li received her Ph.D at Kunming Institute of Botany, Chinese Academy of Science (CAS) in 2004, under the guidance of Prof. Han-Dong Sun. She worked with Prof. Kuo-Hsiung Lee from March 2013 to June 2014, as a Visiting Professor at the UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill. She has been awarded several prizes conferred by national, CAS, provincial, or ministerial authorities, including the “National Excellent Doctoral Dissertation of P.R. China” in 2006, the “New Century Excellent Talents on University” in 2006, the “Young Academic and Technical Leader of Yunnan Province” in 2009, and the “Excellent Doctoral Dissertation of the Chinese Academy of Science” and “CPA-Servier Yong Investigator Award in Medicinal Chemistry” 2005. She has performed phytochemical studies on about 50 plants, published 12 patents, and over 130 scientific papers. She is now Professor, Kunming University of Science and Technology (2005-present). Her current research interests involve the activity-directed isolation, structure elucidation and the structure–activity relationships of natural products from medically important plants.
 Susan L. Morris-Natschke received her B.S. in chemistry from the University of Maryland-College Park in 1975 and her Ph.D. in organic chemistry from the University of North Carolina-Chapel Hill (UNC-CH) in 1982. She is currently Research Professor in the Division of Chemical Biology and Medicinal Chemistry, UNC Eshelman School of Pharmacy, UNC-CH, where she has been on the faculty since 1983. Her interests include scientific writing/editing, as well as the synthesis and structure-activity relationships of bioactive natural products.
Susan L. Morris-Natschke received her B.S. in chemistry from the University of Maryland-College Park in 1975 and her Ph.D. in organic chemistry from the University of North Carolina-Chapel Hill (UNC-CH) in 1982. She is currently Research Professor in the Division of Chemical Biology and Medicinal Chemistry, UNC Eshelman School of Pharmacy, UNC-CH, where she has been on the faculty since 1983. Her interests include scientific writing/editing, as well as the synthesis and structure-activity relationships of bioactive natural products.
 Kuo-Hsiung Lee received his B.S. in pharmacy from Kaohsiung Medical University, Taiwan (1961), M.S. in pharmaceutical chemistry from Kyoto University, Japan (1965), and Ph.D. in medicinal chemistry from University of Minnesota, Minneapolis (1968). He joined the faculty of UNC Eshelman School of Pharmacy, University of North Carolina-Chapel Hill, in 1970 and is now Kenan Distinguished Professor of Medicinal Chemistry and Director of the Natural Products Research Laboratories. He has published over 848 research articles, been granted over 114 patents, and received numerous awards, including most recently, the Third Cheung On Tak International Award for Outstanding Achievement in Chinese Medicine from Hong Kong Baptist University, School of Chinese Medicine in 2016.
Kuo-Hsiung Lee received his B.S. in pharmacy from Kaohsiung Medical University, Taiwan (1961), M.S. in pharmaceutical chemistry from Kyoto University, Japan (1965), and Ph.D. in medicinal chemistry from University of Minnesota, Minneapolis (1968). He joined the faculty of UNC Eshelman School of Pharmacy, University of North Carolina-Chapel Hill, in 1970 and is now Kenan Distinguished Professor of Medicinal Chemistry and Director of the Natural Products Research Laboratories. He has published over 848 research articles, been granted over 114 patents, and received numerous awards, including most recently, the Third Cheung On Tak International Award for Outstanding Achievement in Chinese Medicine from Hong Kong Baptist University, School of Chinese Medicine in 2016.
Footnotes
Electronic supplementary information (ESI) available: Tables 2–32: compound structures arranged by chemical classifications structures of clerodane diterpenes arranged by source. See DOI: 10.1039/c5np00137d
6. References
- 1.Kohno H, Maeda M, Tanino M, Tsukio Y, Ueda N, Wada K, Sugie S, Mori H, Tanaka T. Cancer Lett. 2002;183:131–139. doi: 10.1016/s0304-3835(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 2.Moody JO, Robert VA, Connolly JD, Houghton PJ. J. Ethnopharmacol. 2006;104:87–91. doi: 10.1016/j.jep.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 3.Shi Q, Liang M, Zhang W, Zhang C, Liu R, Shen Y, Li H, Wang X, Wang X, Pan Q, Chen C. Biomed. Chromatogr. 2007;21:642–648. doi: 10.1002/bmc.802. [DOI] [PubMed] [Google Scholar]
- 4.Lallemand J-Y, Six Y, Ricard L. Eur. J. Org. Chem. 2002;3:503–513. [Google Scholar]
- 5.Tokoroyama T. Synthesis. 2000;5:611–633. [Google Scholar]
- 6.Merritt AT, Ley SV. Nat. Prod. Rep. 1992;9:243–287. doi: 10.1039/np9920900243. [DOI] [PubMed] [Google Scholar]
- 7.Rogers D, Ünal GG, Williams DJ, Ley SV, Sim GA, Joshi BS. J. Chem. Soc., Chem. Commun. 1979;3:97–99. [Google Scholar]
- 8 (a).Wilson SR, Neubert LA, Huffman JC. J. Am. Chem. Soc. 1976;98:3669–3674. doi: 10.1021/ja00428a047. [DOI] [PubMed] [Google Scholar]; (b) Peters RJ. Nat. Prod. Rep. 2010;27:1521–1530. doi: 10.1039/c0np00019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gayen AK. Ph.D. thesis. University of Jadaupar; 1984. [Google Scholar]
- 10.Sato A, Kurabayashi M, Nagahori H, Ogiso A, Mishima H. Tetrahedron Lett. 1970;13:1095–1098. doi: 10.1016/s0040-4039(01)97917-1. [DOI] [PubMed] [Google Scholar]
- 11.Teresa JP, Urones JG, Marcos IS, Bermejo F, Basabe P. Phytochemistry. 1983;22:2783–2785. [Google Scholar]
- 12.Gebbinck E. A. Klein, Jansen BJM, de Groot A. Phytochemistry. 2002;61:737–770. doi: 10.1016/s0031-9422(02)00174-7. [DOI] [PubMed] [Google Scholar]
- 13.Prisinzano TE, Rothman RB. Chem. Rev. 2008;108:1732–1743. doi: 10.1021/cr0782269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi K, Nakanishi Y, Bastow KF, Cragg G, Nozaki H, Lee KH. Bioorg. Med. Chem. Lett. 2002;12:345–348. doi: 10.1016/s0960-894x(01)00742-9. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Zhang Q, Wang M, Ren Q, Sun Y, Jin D-Q, Xie C, Chen H, Ohizumi Y, Guo Y. J. Nat. Prod. 2014;77:2182–2189. doi: 10.1021/np5003516. [DOI] [PubMed] [Google Scholar]
- 16.Calderón C, De Ford C, Castro V, Merfort I, Murillo R. J. Nat. Prod. 2014;77:455–463. doi: 10.1021/np400672g. [DOI] [PubMed] [Google Scholar]
- 17.Vieira-Júnior GM, Dutra LA, Ferreira PM, de Moraes MO, Lotufo L. V. Costa, de Ó Pessoa C, Torres RB, Boralle N, Bolzani V. da S., Cavalheiro AJ. J. Nat. Prod. 2011;74:776–781. doi: 10.1021/np100840w. [DOI] [PubMed] [Google Scholar]
- 18.Beutler JA, McCall KL, Herbert K, Johnson T, Shoemaker RH, Boyd MR. Phytochemistry. 2000;55:233–236. doi: 10.1016/s0031-9422(00)00281-8. [DOI] [PubMed] [Google Scholar]
- 19.Khan MR, Gray AI, Sadler IH, Waterman PG. Phytochemistry. 1990;29:3591–3595. [Google Scholar]
- 20.Oberlies NH, Burgess JP, Navarro HA, Pinos RE, Soejarto DD, Farnsworth NR, Kinghorn AD, Wani MC, Wall ME. J. Nat. Prod. 2001;64:497–501. doi: 10.1021/np0005006. [DOI] [PubMed] [Google Scholar]
- 21.Williams RB, Norris A, Miller JS, Birkinshaw C, Ratovoson F, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2007;70:206–209. doi: 10.1021/np0605034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitson EL, Thomas CL, Henrich CJ, Sayers TJ, McMahon JB, McKee TC. J. Nat. Prod. 2010;73:2013–2018. doi: 10.1021/np1004455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan MR, Gray AI, Waterman PG. Phytochemistry. 1990;29:2939–2942. [Google Scholar]
- 24.Gibbons S, Gray AI, Waterman PG. Phytochemistry. 1996;41:565–570. [Google Scholar]
- 25.Prakash C. V. Sai, Hoch JM, Kingston DGI. J. Nat. Prod. 2002;65:100–107. doi: 10.1021/np010405c. [DOI] [PubMed] [Google Scholar]
- 26.Shen YC, Wang CH, Cheng YB, Wang LT, Guh JH, Chien CT, Khalil AT. J. Nat. Prod. 2004;67:316–321. doi: 10.1021/np0303658. [DOI] [PubMed] [Google Scholar]
- 27.Shen YC, Cheng YB, Ahmed AF, Lee CL, Chen SY, Chien CT, Kuo YH, Tzeng GL. J. Nat. Prod. 2005;68:1665–1668. doi: 10.1021/np058063o. [DOI] [PubMed] [Google Scholar]
- 28.Shen YC, Wang LT, Wang CH, Khalil AT, Guh JH. Chem. Pharm. Bull. 2004;52:108–110. doi: 10.1248/cpb.52.108. [DOI] [PubMed] [Google Scholar]
- 29.Mosaddik MA, Forster PI, Booth R, Waterman PG. Nat. Prod. Commun. 2006;1:441–448. [Google Scholar]
- 30.Mosaddik MA, Forster PI, Booth R, Waterman PG. Biochem. Syst. Ecol. 2007;35:631–633. [Google Scholar]
- 31.Kanokmedhakul S, Kanokmedhakul K, Kanarsa T, Buayairaksa M. J. Nat. Prod. 2005;68:183–188. doi: 10.1021/np049757k. [DOI] [PubMed] [Google Scholar]
- 32.Vijayakumar EKS, Bal-Tembe S, Joshi KS, Deore VB. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2002;41:2706–2708. [Google Scholar]
- 33.Wang B, Wang XL, Wang SQ, Shen T, Liu YQ, Yuan HQ, Lou HX, Wang XN. J. Nat. Prod. 2013;76:1573–1579. doi: 10.1021/np400212d. [DOI] [PubMed] [Google Scholar]
- 34.Oberlies NH, Burgess JP, Navarro HNA, Pinos RE, Fairchild CR, Peterson RW, Soejarto DD, Farnsworth NR, Kinghorn AD, Wani MC, Wall ME. J. Nat. Prod. 2002;65:95–99. doi: 10.1021/np010459m. [DOI] [PubMed] [Google Scholar]
- 35.Beutler JA, McCall KL, Herbert K, Herald DL, Pettit GR, Johnson T, Shoemaker RH, Boyd MR. J. Nat. Prod. 2000;63:657–661. doi: 10.1021/np990553r. [DOI] [PubMed] [Google Scholar]
- 36.Jullian V, Bonduelle C, Valentin A, Acebey L, Duigou A-G, Prévostb M-F, Sauvain M. Bioorg. Med. Chem. Lett. 2005;15:5065–5070. doi: 10.1016/j.bmcl.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 37.Kanokmedhakul S, Kanokmedhakul K, Buayairaksa M. J. Nat. Prod. 2007;70:1122–1126. doi: 10.1021/np070083y. [DOI] [PubMed] [Google Scholar]
- 38.Khan MR, Gray AI, Sadler IH, Reed DR, Waterman PG. Phytochemistry. 1990;29:1609–1614. [Google Scholar]
- 39.Vieira GM, Jr, de Oliveira Gonçalves T, Regasini LO, Ferreira P. M. Pinheiro, do Ó Pessoa C, Lotufo L. V. Costa, Torres RB, Boralle N, da Silva Bolzani V, Cavalheiro AJ. J. Nat. Prod. 2009;72:1847–1850. doi: 10.1021/np9004079. [DOI] [PubMed] [Google Scholar]
- 40.Gibbons S, Gray AI, Waterman PG. Phytochemistry. 1996;43:635–638. [Google Scholar]
- 41.Zdero C, Bohlmann F, King RM. Phytochemistry. 1992;31:1703–1711. [Google Scholar]
- 42.He K, Montenegro G, Hoffmann JJ, Timmermann BN. Phytochemistry. 1996;41:1123–1127. [Google Scholar]
- 43.Dairi T, Hamano Y, Kuzuyama T, Itoh N, Furihata K, Seto H. J. Bacteriol. 2001;183:6085–6094. doi: 10.1128/JB.183.20.6085-6094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagashima F, Takaoka S, Asakawa Y. Phytochemistry. 1998;49:601–608. [Google Scholar]
- 45.Chen JA, Huang CD, Wu CL. J. Chin. Chem. Soc. 1992;39:263–266. [Google Scholar]
- 46.Nabeta K, Oohata T, Izumi N, Katoh K. Phytochemistry. 1994;37:1263–1268. [Google Scholar]
- 47.Hashimoto T, Nakamura I, Tori M, Takaoka S, Asakawa Y. Phytochemistry. 1995;38:119–127. [Google Scholar]
- 48.Nagashina F, Sekiguchi T, Takaoka S, Asakawa Y. Chem. Pharm. Bull. 2004;52:556–560. doi: 10.1248/cpb.52.556. [DOI] [PubMed] [Google Scholar]
- 49.Kijjoa A, Pinto MMM, Pinho PMM, Herz W. Phytochemistry. 1993;34:457–460. [Google Scholar]
- 50.de Souza Moreira CP, de Oliveira DM, dos Santos CN, Zani CL, de Almeida Alves TM. Tetrahedron Lett. 2014;55:4898–4900. [Google Scholar]
- 51.Nagashima F, Suzuki M, Takaoka S, Asakawa Y. Chem. Pharm. Bull. 2000;48:1818–1821. doi: 10.1248/cpb.48.1818. [DOI] [PubMed] [Google Scholar]
- 52.Tang W, Harada K, Kubo M, Hioki H, Fukuyama Y. Nat. Prod. Commun. 2011;6:327–332. [PubMed] [Google Scholar]
- 53.Tazaki H, Hayashida T, Furuki T, Nabeta K. Phytochemistry. 1999;52:1551–1553. [Google Scholar]
- 54.Tojoa E, Rial ME, Urzua A, Mendoza L. Phytochemistry. 1999;52:1531–1533. [Google Scholar]
- 55.Tori M, Katto A, Sono M. Phytochemistry. 1999;52:487–493. [Google Scholar]
- 56.Freiburghaus F, Steck A, Pfander H, Brun R. J. Ethnopharmacol. 1998;61:179–183. doi: 10.1016/s0378-8741(98)00035-x. [DOI] [PubMed] [Google Scholar]
- 57.Zdero C, Bohlmann F, King RM. Phytochemistry. 1992;31:213–216. [Google Scholar]
- 58.Rustaiyan A, Habibi Z, Zdero C. Phytochemistry. 1990;29:985–987. [Google Scholar]
- 59.Jorge B, Arlett M, Samuel P, Glauco LLAM, Oscar W, Ivan B. Bol. Soc. Chil. Quim. 1995;40:157–162. [Google Scholar]
- 60.Zhao G, Jung JH, Smith DL, Wood KV, McLaughlin JL. Planta Med. 1991;57:380–383. doi: 10.1055/s-2006-960122. [DOI] [PubMed] [Google Scholar]
- 61.Nabeta K, Oohata T, Ohkubo S, Sato T, Katoh K. Phytochemistry. 1996;41:581–587. [Google Scholar]
- 62.Ohsaki A, Kasetani Y, Asaka Y, Shibata K, Tokoroyamat T, Kubota T. Phytochemistry. 1991;30:4075–4077. [Google Scholar]
- 63.Yang SM, Wu SH, Qin XD, Luo XD, Wu DG. Helv. Chim. Acta. 2004;87:1279–1286. [Google Scholar]
- 64.Nogueira RT, Shepherd GJ, Laverde A, Jr, Marsaioli AJ, Imamura PM. Phytochemistry. 2001;58:1153–1157. doi: 10.1016/s0031-9422(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 65.Cavin AL, Hay AE, Marston A, Stoeckli-Evans H, Scopelliti R, Diallo D, Hostettmann K. J. Nat. Prod. 2006;69:768–773. doi: 10.1021/np058123q. [DOI] [PubMed] [Google Scholar]
- 66.Avila D, Medina JD. Phytochemistry. 1991;30:3474–3475. [Google Scholar]
- 67.Ohsaki A, Asaka Y, Kubota T, Shibata K, Tokoroyama T. J. Nat. Prod. 1997;60:912–914. [Google Scholar]
- 68.Zeng ZX, Ma WH, Li YL, Han T, Zheng CJ, Qin LP. Helv. Chim. Acta. 2012;95:1121–1125. [Google Scholar]
- 69.Mitamura T, Matsuno T, Sakamoto S, Maemura M, Kudo H, Suzuki S, Kuwa K, Yoshimura S, Sassa S. Anticancer Res. 1996;16:2669–2672. [PubMed] [Google Scholar]
- 70.Murthy MM, Subramanyam M, Bindu MH, Annapurna J. Fitoterapia. 2005;76:336–339. doi: 10.1016/j.fitote.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Urzúa A, Torres R, Mendoza L, Monache F. Delle. Planta Med. 2003;69:675–677. doi: 10.1055/s-2003-41118. [DOI] [PubMed] [Google Scholar]
- 72.Kuroyanagi M, Uchida K, Ueno A, Satake M, Shimomura K. Phytochemistry. 1993;34:1377–1384. [Google Scholar]
- 73.Guo Y, Li Y, Xu J, Li N, Yamakuni T, Ohizumi Y. Chem. Pharm. Bull. 2007;55:1532–1534. doi: 10.1248/cpb.55.1532. [DOI] [PubMed] [Google Scholar]
- 74.Zdero C, Bohlmann F. Phytochemistry. 1991;30:607–609. [Google Scholar]
- 75.Zdero C, Jakupovic J, Bohlmann F. Phytochemistry. 1990;29:1231–1245. doi: 10.1016/0031-9422(90)85198-o. [DOI] [PubMed] [Google Scholar]
- 76.Leitão GG, Kaplan MAC, Galefft C. Phytochemistry. 1992;31:3277–3279. [Google Scholar]
- 77.Urzua A, Jara F, Tojob E, Wilkens M, Mendoza L, Rezende MC. J. Ethnopharmacol. 2006;103:297–301. doi: 10.1016/j.jep.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 78.Mambu L, Grellier P, Florent L, Joyeau R, Ramanitrahasimbola D, Rasoanaivo P, Frappier F. Phytochemistry. 2006;67:444–451. doi: 10.1016/j.phytochem.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 79.He HP, Shen YM, Zuo GY, Yang XS, Hao XJ. Helv. Chim. Acta. 2003;86:3187–3193. [Google Scholar]
- 80.Chin YW, Jones WP, Mi Q, Rachman I, Riswan S, Kardono LBS, Chai HB, Farnsworth NR, Cordell GA, Swanson SM, Cassady JM, Kinghorn AD. Phytochemistry. 2006;67:1243–1248. doi: 10.1016/j.phytochem.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Bomm MD, Zukerman-Schpector J, Lopes LMX. Phytochemistry. 1999;50:455–461. [Google Scholar]
- 82.Fazio C, Paternostro MP, Passannanti S, Piozzi F. Phytochemistry. 1994;37:501–503. [Google Scholar]
- 83.Fazio C, Assannanti S, Paternostro MP, Piozzi F. Phytochemistry. 1992;31:3147–3149. [Google Scholar]
- 84.Bläs B, Zapp J, Becker H. Phytochemistry. 2004;65:127–137. doi: 10.1016/s0031-9422(03)00387-x. [DOI] [PubMed] [Google Scholar]
- 85.Furlan M, Lopes MN, Fernandes JB, Pirani JR. Phytochemistry. 1996;41:1159–1161. [Google Scholar]
- 86.Nagashima F, Suzuki M, Takaoka S, Asakawa Y. J. Nat. Prod. 2001;64:1309–1317. doi: 10.1021/np010223i. [DOI] [PubMed] [Google Scholar]
- 87.San Feliciano A, Gordaliza M, del Corral J. M. Miguel, de la Puente ML. Phytochemistry. 1993;33:631–633. [Google Scholar]
- 88.Diyabalanage T, Iken KB, McClintock JB, Amsler CD, Baker BJ. J. Nat. Prod. 2010;73:416–421. doi: 10.1021/np900617m. [DOI] [PubMed] [Google Scholar]
- 89.Cai Y, Chen ZP, Phillipson JD. Phytochemistry. 1993;32:755–760. [Google Scholar]
- 90.Nagashima F, Tanaka H, Kan Y, Huneck S, Asakawa Y. Phytochemistry. 1995;40:209–212. [Google Scholar]
- 91.Avila D, Medina JD, Deeming AJ. J. Nat. Prod. 1992;55:845–850. [Google Scholar]
- 92.Çalis I, Bedir E. J. Nat. Prod. 1996;59:457–460. [Google Scholar]
- 93.Fiorentino A, D'Abrosca B, Pacifico S, Izzo A, D'Angelo G, Monaco P. Nat. Prod. Commun. 2010;5:1539–1542. [PubMed] [Google Scholar]
- 94.Avila D, Medina JD. J. Nat. Prod. 1993;56:1586–1589. [Google Scholar]
- 95.Nagashima F, Tori M, Asakawa Y. Phytochemistry. 1991;30:849–851. [Google Scholar]
- 96.Liu Z-K, Wu D-R, Shi Y-M, Zeng T, Liu S-H, Dua X, Dang Y-J, Xiao W-L, Sun H-D. Chin. Chem. Lett. 2014;25:677–679. [Google Scholar]
- 97.Faheem A, Mohd A, Prawez A. Nat. Prod. Res. 2010;24:926–934. [Google Scholar]
- 98.Xu J, Harrison LJ, Vittal JJ, Xu Y-J, Goh S-H. J. Nat. Prod. 2000;63:1062–1065. doi: 10.1021/np990584m. [DOI] [PubMed] [Google Scholar]
- 99.Lee TH, Wang MJ, Chen PY, Wu TY, Wen WC, Tsai FY, Lee CK. J. Nat. Prod. 2009;72:1960–1963. doi: 10.1021/np900207z. [DOI] [PubMed] [Google Scholar]
- 100.Hao XJ, Yang XS, Zhang Z, Shang LJ. Phytochemistry. 1995;39:447–448. [Google Scholar]
- 101.Wijerathne E. M. Kithsiri, Dd Silva LB, Tezuka Y, Kikuchi T. Phytochemistry. 1995;39:443–445. [Google Scholar]
- 102.Ohsaki A, Ogawa M, Imai Y, Shinzato T, Shigemori H, Kobayashi JI. J. Nat.Prod. 2001;64:804–805. doi: 10.1021/np010105v. [DOI] [PubMed] [Google Scholar]
- 103.Harrison LJ, Connolly JD, Rycroft DS. Phytochemistry. 1992;31:1420–1421. [Google Scholar]
- 104.Ruiu S, Anzani N, Orru A, Floris C, Caboni P, Alcaro S, Maccioni E, Distinto S, Cottiglia F. J. Nat. Prod. 2015;78:69–76. doi: 10.1021/np500671v. [DOI] [PubMed] [Google Scholar]
- 105.Lv H, Luo J, Kong L. Nat. Prod. Res. 2014;28:196–200. doi: 10.1080/14786419.2013.866114. [DOI] [PubMed] [Google Scholar]
- 106.Hasan CM, Khan S, Jabbar A, Rashid MA. J. Nat. Prod. 2000;63:410–411. doi: 10.1021/np990488l. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez AA, Esquivel B, Ramamoorthy TP, Rodriguea-Hahn L. Phytochemistry. 1995;38:171–174. [Google Scholar]
- 108.Bisio A, Damonte G, Fraternale D, Giacomelli E, Salis A, Romussi G, Cafaggi S, Ricci D, Tommasi ND. Phytochemistry. 2011;72:265–275. doi: 10.1016/j.phytochem.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 109.Almanza G, Balderrama L, Labbé C, Lavaud C, Massiot G, Nuzillard J-M, Connolly JD, Farrugia LJ, Rycroft DS. Tetrahedron. 1997;53:14719–14728. [Google Scholar]
- 110.West LM, Northcote PT, Battershill CN. Aust. J. Chem. 1998;51:1097–1102. [Google Scholar]
- 111.Fontana G, Paternostro MP, Savona G, Rodríguez B, de la Torre MC. J. Nat. Prod. 1998;61:1242–1247. doi: 10.1021/np980137r. [DOI] [PubMed] [Google Scholar]
- 112.Fullas F, Hussain RA, Chai H-B, Pezzuto JM, Soejarto DD, Kinghorn AD. J. Nat. Prod. 1994;57:801–807. doi: 10.1021/np50108a017. [DOI] [PubMed] [Google Scholar]
- 113.Xu G, Zhao F, Yang X-W, Zhou J, Yang L-X, Shen X-L, Hu Y-J, Zhao Q-S. Nat. Prod. Bioprospect. 2011;1:81–86. [Google Scholar]
- 114.Aravind S, Balachandran J, Ganesh MR, Kumari GNK. Nat. Prod. Res. 2010;24:7–12. doi: 10.1080/14786410801940265. [DOI] [PubMed] [Google Scholar]
- 115.Premprasert C, Tewtrakul S, Plubrukarn A, Wungsintaweekul J. J. Nat. Med. 2013;67:174–181. doi: 10.1007/s11418-012-0668-5. [DOI] [PubMed] [Google Scholar]
- 116.Cuadrado MJS, de la Torre MC, Rodríguez B, Bruno M, Piozzi F, Savona G. Phytochemistry. 1991;30:4079–4082. [Google Scholar]
- 117.Ragasa CY, Cruz MC, Gula R, Rideout JA. Org. Biomol. Chem. 2015;13:3882–3886. [Google Scholar]
- 118.Nagashima F, Asakawa Y. Phytochemistry. 1990;29:3229–3231. [Google Scholar]
- 119.Tazaki H, Zapp J, Becker H. Phytochemistry. 1995;39:859–868. [Google Scholar]
- 120.Tazaki H, Nabeta K, Becker H. Phytochemistry. 1998;48:681–685. [Google Scholar]
- 121.Blechschmidt M, Becker H. J. Nat. Prod. 1992;55:111–121. [Google Scholar]
- 122.Galstyan AM, Shashkov AS, Oganesyan GB, Mnatsakanyan VA, Serebryakov EP. Khim. Prir. Soedin. 1992;5:503–508. [Google Scholar]
- 123.Coll J, Tandrón Y. Phytochemistry. 2005;66:2298–2303. doi: 10.1016/j.phytochem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Moustapha C, Hasen T, Waleed M, Sadaka M. Jordan J. Chem. 2011;6:339–345. [Google Scholar]
- 125.Maciel MAM, Pinto AC, Brabo SN, Da Silva MN. Phytochemistry. 1998;49:823–828. [Google Scholar]
- 126.Sattar EA, Mossa JS, Muhammad I, El-Feraly FS. Phytochemistry. 1995;40:1737–1741. [Google Scholar]
- 127.Malakov PY, Papanov GY, Boneva IM. Phytochemistry. 1992;31:4029–4030. [Google Scholar]
- 128.Zdero C, Bohlmann F, Mungai GM. Phytochemistry. 1990;29:3233–3241. doi: 10.1016/0031-9422(90)85198-o. [DOI] [PubMed] [Google Scholar]
- 129.Alcázar R, de la Torre MC, Rodríguez RB, Bruno M, Piozzi F, Savona G, Arnold NA. Phytochemistry. 1992;31:3957–3960. [Google Scholar]
- 130.Tang W, Hioki H, Harada K, Kubo M, Fukuyama Y. J. Nat. Prod. 2008;71:1760–1763. doi: 10.1021/np8004002. [DOI] [PubMed] [Google Scholar]
- 131.Ortega A, Garcia PE, Cárdenas J, Mancera C, Marquina S, del Carmen Garduño ML, Maldonado E. Tetrahedron. 2001;57:2981–2989. [Google Scholar]
- 132.Graikou K, Aligiannis N, Chinou I, Skaltsounis A-L, Tillequin F, Litaudon M. Helv. Chim. Acta. 2005;88:2654–2660. [Google Scholar]
- 133.Al-Yahya MA, Muhmmad I, Mirza HH, El-Feraly FS. J. Nat. Prod. 1993;56:830–842. [Google Scholar]
- 134.Al-Yahya MA, El-Feraly FS, Dunbar DC, Muhmmad I. Phytochemistry. 2002;59:409–414. doi: 10.1016/s0031-9422(01)00396-x. [DOI] [PubMed] [Google Scholar]
- 135.Teponno RB, Tapondjou AL, Gatsing D, Djoukeng JD, Mansour EA, Tabacchi R, Tane P, Evans HS, Lontsi D. Phytochemistry. 2006;67:1957–1963. doi: 10.1016/j.phytochem.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 136.Teponnoa RB, Tapondjou A, Jung HJ, Nam JH, Tane P, Park HJ. Helv. Chim. Acta. 2007;90:1599–1605. [Google Scholar]
- 137.Naengchomnong W, Pinho PM, Kijjoa A, Sawangwong P, Gonzalez MJ, Silva AMS, Eaton G, Herz W. Phytochemistry. 2006;67:1029–1033. doi: 10.1016/j.phytochem.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 138.Guo DX, Zhu RX, Wang XN, Wang LN, Wang SQ, Lin ZM, Lou HX. Org. Lett. 2010;12:4404–4407. doi: 10.1021/ol1019458. [DOI] [PubMed] [Google Scholar]
- 139.Katayama K, Shimazaki K, Tazaki H, Hasa Y, Miyoshi M, Koshino H, Furuki T, Nabeta K. Biosci., Biotechnol., Biochem. 2007;71:2751–2758. doi: 10.1271/bbb.70360. [DOI] [PubMed] [Google Scholar]
- 140.Li RJ, Sun Y, Sun B, Wang XN, Liu SS, Zhou JC, Ye JP, Zhao Y, Liu L, Lee KH, Lou H-X. Phytochemistry. 2014;105:85–91. doi: 10.1016/j.phytochem.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 141.Addae-Mensah I, Achenbach H, Thoithi GN, Waibel R, Mwangi JW. Phytochemistry. 1992;31:2055–2058. [Google Scholar]
- 142.Fattorusso E, Taglialatela-Scafati O, Campagnuolo C, Santelia FU, Appendino G, Spagliardi P. J. Agric. Food Chem. 2002;50:5131–5138. doi: 10.1021/jf0203693. [DOI] [PubMed] [Google Scholar]
- 143.Harinantenaina L, Takahara Y, Nishizawa T, Kohchi C, Soma G-I, Asakawa Y. Chem. Pharm. Bull. 2006;54:1046–1049. doi: 10.1248/cpb.54.1046. [DOI] [PubMed] [Google Scholar]
- 144.Bruno M, Alcazar R, de la Torre MC, Piozzi F, Rodriguez B, Savona G, Perales A, Arnold NA. Phytochemistry. 1992;31:3531–3534. [Google Scholar]
- 145.Bruno M, Domínguez G, Lourenço A, Piozzi F, Rodríguez B, Savona G, de la Torre MC, Arnold NA. Phytochemistry. 1991;30:3693–3697. [Google Scholar]
- 146.de la Torre MC, Bruno M, Piozzi F, Savona G, Rodríguez B, Omar AA. Phytochemistry. 1991;30:1603–1606. [Google Scholar]
- 147.Coll J, Tandrón Y. Phytochemistry. 2004;65:387–392. doi: 10.1016/j.phytochem.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 148.Hussain J, Ullah R, Khan A, Khan FU, Muhammad Z, Shah MR. Nat. Prod. Commun. 2011;6:171–173. [PubMed] [Google Scholar]
- 149.Huang Z, Jiang M-Y, Zhou Z-Y, Xu D. Z. Naturforsch., B: J. Chem. Sci. 2010;65:83–86. [Google Scholar]
- 150.Zaman SS, Barua NC, Sharma RP. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1991;30:1054–1055. [Google Scholar]
- 151.Appendino G, Borrelli F, Capasso R, Campagnuolo C, Fattorusso E, Petrucci F, Taglialatela-Scafati O. J. Agric. Food Chem. 2003;51:6970–6974. doi: 10.1021/jf034780h. [DOI] [PubMed] [Google Scholar]
- 152.Tang W, Kubo M, Harada K, Hioki H, Fukuyama Y. Bioorg. Med. Chem. Lett. 2009;19:882–886. doi: 10.1016/j.bmcl.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 153.Monti H, Tiliacos N, Faure R. Phytochemistry. 1996;42:1653–1656. [Google Scholar]
- 154.Silveira ER, McChesney JD. Phytochemistry. 1994;36:1457–1463. [Google Scholar]
- 155.Zdero C, Bohlmann F, Mungal GM. Phytochemistry. 1991;30:575–581. [Google Scholar]
- 156.Simpson BS, Claudie DJ, Gerber JP, Pyke SM, Wang J, McKinnon RA, Semple SJ. J. Nat. Prod. 2011;74:650–657. doi: 10.1021/np100701s. [DOI] [PubMed] [Google Scholar]
- 157.Peres MTLP, Pizzolatti MG, Yunes RA, Monache F. Delle. Phytochemistry. 1998;49:171–174. [Google Scholar]
- 158.Itokawa H, Ichihara Y, Shimizu M, Takaya K, Motidome M. Chem. Pharm. Bull. 1990;38:701–705. [Google Scholar]
- 159.Amaral ACF, Barnes RA. Nat. Prod. Lett. 1998;12:41–46. [Google Scholar]
- 160.Zou G-A, Zhang H-W, Aisa HA, Yang J-S, Peng C-Z, Zou Z-M. J. Nat. Med. 2011;65:391–394. doi: 10.1007/s11418-010-0503-9. [DOI] [PubMed] [Google Scholar]
- 161.Anis I, Anis E, Ahmed S, Mustafa G, Malik A, Amtul Z, Rahman A.-ur. Helv. Chim. Acta. 2001;84:649–655. [Google Scholar]
- 162.Liu C-M, Zhu R-L, Liu R-H, Li H-L, Shan L, Xu X-K, Zhang W-D. Planta Med. 2009;75:1597–1601. doi: 10.1055/s-0029-1185837. [DOI] [PubMed] [Google Scholar]
- 163.Heymann H, Tezuka Y, Kikuchi T, Supriyatna S. Chem. Pharm. Bull. 1994;42:1202–1207. [Google Scholar]
- 164.Guo D-X, Wang X-N, Zhu R-X, Liu N, Zhou J-C, Yu W-T, Lou H-X. Phytochem. Lett. 2012;5:535–540. [Google Scholar]
- 165.Liu C-P, Xu J-B, Zhao J-X, Xu C-H, Dong L, Ding J, Yue J-M. J. Nat. Prod. 2014;77:1013–1020. doi: 10.1021/np500042c. [DOI] [PubMed] [Google Scholar]
- 166.Bigham AK, Munro TA, Rizzacasa MA, Robins-Browne RM. J. Nat. Prod. 2003;66:1242–1244. doi: 10.1021/np030313i. [DOI] [PubMed] [Google Scholar]
- 167.Lee DYW, Ma Z, Liu-Chen L-Y, Wang Y, Chen Y, Carlezon WA, Jr, Cohen B. Bioorg. Med. Chem. 2005;13:5635–5639. doi: 10.1016/j.bmc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 168.Bayor MT, Ayim JSK, Marston G, Phillips RM, Shnyder SD, Wheelhouse RT, Wright CW. Nat. Prod. Commun. 2008;3:1875–1878. [Google Scholar]
- 169.Abega DF, Kapche DWFG, Ango PY, Mapitse R, Yeboah SO, Ngadjui BT. Z. Naturforsch., C: J. Biosci. 2014;69:181–185. doi: 10.5560/znc.2013-0207. [DOI] [PubMed] [Google Scholar]
- 170.Krebs HC, Ramiarantsoa H. Phytochemistry. 1997;45:379–381. [Google Scholar]
- 171.Krebs HC, Ramiarantsoa H. Phytochemistry. 1996;41:561–563. [Google Scholar]
- 172.Hussain J, Jamila N, Khan FU, Devkota KP, Shah MR, Anwar S. Magn. Reson. Chem. 2009;47:625–627. doi: 10.1002/mrc.2439. [DOI] [PubMed] [Google Scholar]
- 173.Dai J, Suttisri R, Bordas E, Soejarto DD, Kinghorn AD. Phytochemistry. 1993;34:1087–1090. [Google Scholar]
- 174.Guo Y, Li Y, Xu J, Watanabe R, Oshima Y, Yamakuni T, Ohizumi Y. J. Nat. Prod. 2006;69:274–276. doi: 10.1021/np050276q. [DOI] [PubMed] [Google Scholar]
- 175.Mostafa AE, El-Hela AA, Mohammad A-EI, Jacob M, Cutler SJ, Ross SA. Phytochem. Lett. 2014;8:10–15. [Google Scholar]
- 176.Rodriguez B, de la Torre MC, Rodriguez B, Bruno M, Piozzi F, Savona G, Simmonds MSJ, Blaney WM, Perales A. Phytochemistry. 1993;33:309–315. [Google Scholar]
- 177.Rodríguez B, de la Torre MC, Rodríguez B, Gómez-Serranillos P. Phytochemistry. 1996;41:247–253. [Google Scholar]
- 178.San Feliciano A, Gordaliza M, de Corral J. M. Miguel, de la Puente ML, García-Granda S, Salvado MA. Tetrahedron. 1993;49:9067–9078. [Google Scholar]
- 179.Gordallza M, del Corral J. M. Miguel, Mahiques MM, Maria AC, San Feliciano A. Phytochemistry. 1995;40:1307–1309. [Google Scholar]
- 180.Harraz FM, Doskotch RW. J. Nat. Prod. 1990;53:1312–1326. doi: 10.1021/np50071a027. [DOI] [PubMed] [Google Scholar]
- 181.Harraz FM, Doskotch RW. J. Nat. Prod. 1996;59:463–468. doi: 10.1021/np9601263. [DOI] [PubMed] [Google Scholar]
- 182.Harraz FM, Pcolinski MJ, Doskotch RW. J. Nat. Prod. 1996;59:5–14. doi: 10.1021/np9601263. [DOI] [PubMed] [Google Scholar]
- 183.Starks CM, Williams RB, Goering MG, O'Neil-Johnson M, Norman VL, Hu J-F, Garo E, Hough GW, Rice SM, Eldridge GR. Phytochemistry. 2010;71:104–109. doi: 10.1016/j.phytochem.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 184.Omosa LK, Midiwo JO, Derese S, Yenesew A, Peter MG, Heydenreich M. Phytochem. Lett. 2010;3:217–220. [Google Scholar]
- 185.Cenal JP, Giordano OS, Rossomando PC, Tonn CE. J. Nat. Prod. 1997;60:490–492. [Google Scholar]
- 186.Hayashi K-I, Kanamori T, Yamazoe A, Yamada M, Nozaki H. J. Nat. Prod. 2005;68:1121–1124. doi: 10.1021/np050081i. [DOI] [PubMed] [Google Scholar]
- 187.Li R-J, Zhu R-X, Li Y-Y, Zhou J-C, Zhang J-Z, Wang S, Ye J-P, Wang Y-H, Morris-Natschke SL, Lee K-H, Lou H-X. J. Nat. Prod. 2013;76:1700–1708. doi: 10.1021/np400426a. [DOI] [PubMed] [Google Scholar]
- 188.Ahmad VU, Farooq U, Abbaskhan A, Hussain J, Abbasi MA, Nawaz SA, Choudhary MI. Helv. Chim. Acta. 2004;87:682–689. [Google Scholar]
- 189.Ahmad VU, Khan A, Farooq U, Kousar F, Khan SS, Nawaz SA, Abbasi MA, Choudhary MI. Chem. Pharm. Bull. 2005;53:378–381. doi: 10.1248/cpb.53.378. [DOI] [PubMed] [Google Scholar]
- 190.Mantaj J, Rahman SMA, Bokshi B, Hasan CM, Jackson PJM, Parsons RB, Rahman KM. Org. Biomol. Chem. 2015;13:3882–3886. doi: 10.1039/c5ob00052a. [DOI] [PubMed] [Google Scholar]
- 191.Liu N, Zhu RX, Wang S, Zhang JZ, Lin ZM, Li RJ, Lou HX. Chem. Biodiversity. 2013;10:1606–1612. doi: 10.1002/cbdv.201200433. [DOI] [PubMed] [Google Scholar]
- 192.Malakov PY, Papanov GY. Phytochemistry. 1996;43:173–178. [Google Scholar]
- 193.de la Torre MC, Rodriguez B, Bruno M, Piozzi F, Savona G, Vassallo N, Servettaz O. Phytochemistry. 1995;38:181–187. [Google Scholar]
- 194.Guo P, Li Y, Jin D-Q, Xu J, He Y, Zhang L, Guo Y. Phytochem. Lett. 2012;5:563–566. [Google Scholar]
- 195.Malakov PY, Papanov GY. Phytochemistry. 1998;49:2449–2452. [Google Scholar]
- 196.Shen X, Isogai A, Furihata K, Sun H, Suzuki A. Phytochemistry. 1993;34:1091–1094. [Google Scholar]
- 197.Shen X, Isogai A, Furihata K, Sun H, Suzuki A. Phytochemistry. 1993;33:887–889. [Google Scholar]
- 198.Amano T, Nishida R, Kuwahara Y. Biosci., Biotechnol., Biochem. 1997;61:1518–1522. [Google Scholar]
- 199.Liu Z, Li ZQ, Zhang CX, Chen RY, Zhang YG, Qiu YT, Zhao SH. Chin. Chem. Lett. 1995;6:581–582. [Google Scholar]
- 200.Miyaichi Y, Kizu H, Yamaguchi Y, Tsuyoshi T. Yakugaku Zasshi. 1994;114:264–271. doi: 10.1248/yakushi1947.114.4_264. [DOI] [PubMed] [Google Scholar]
- 201.de la Torre MC, Rodriguez B, Bruno M, Malakov PY, Papanov GY, Piozzi F, Savona G. Phytochemistry. 1993;34:1589–1594. [Google Scholar]
- 202.de la Torre MC, Rodríguez B, Bruno M, Vassallo N, Bondí ML, Piozzi F, Servettaz O. J. Nat. Prod. 1997;60:1229–1235. [Google Scholar]
- 203.Malakov PY, Bozov PI, Papanov GY. Phytochemistry. 1997;46:587–589. [Google Scholar]
- 204.Raccuglia RA, Bellone G, Loziene K, Piozzi F, Rosselli S, Maggio A, Bruno M, Simmonds MSJ. Phytochemistry. 2010;71:2087–2091. doi: 10.1016/j.phytochem.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 205.Chan YY. Chem. Pharm. Bull. 2005;53:164–167. doi: 10.1248/cpb.53.164. [DOI] [PubMed] [Google Scholar]
- 206.Min ZD, Mizuno M, Wang SQ, Iinuma M, Tanaka T. Chem. Pharm. Bull. 1990;38:3167–3168. [Google Scholar]
- 207.Bremner PD, Simmonds MSJ, Blaney WM, Veitch NC. Phytochemistry. 1998;47:1227–1232. [Google Scholar]
- 208.Kizu H, Imoto Y, Tomimori T, Kikuchi T, Kadota S, Tsubono K. Chem. Pharm. Bull. 1997;45:152–160. doi: 10.1248/cpb.35.1656. [DOI] [PubMed] [Google Scholar]
- 209.Li CH, Liu Y, Hua J, Luo SH, Li SH. J. Integr. Plant Biol. 2014;56:928–940. doi: 10.1111/jipb.12242. [DOI] [PubMed] [Google Scholar]
- 210.Xu L, Guo D, Liu J, Zheng J, Koike K, Jia Z, Nikaido T. Heterocycles. 1999;51:605–609. [Google Scholar]
- 211.Sun Z, Li Y, Jin D-Q, Guo P, Song H, Xu J, Guo Y, Zhang L. Fitoterapia. 2012;83:1409–1414. doi: 10.1016/j.fitote.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 212.Rodriguez B, de la Torre MC, Bruno M, Piozzi F, Vassallo N, Ciriminna R, Servettaz O. Phytochemistry. 1997;45:383–385. [Google Scholar]
- 213.Venkateswarlu K, Satyalakshmi G, Suneel K, Reddy TS, Raju TV, Das B. Helv. Chim. Acta. 2008;91:2081–2088. [Google Scholar]
- 214.Zhang RT, Feng T, Cai XH, Luo XD. Z. Naturforsch., B: J. Chem. Sci. 2009;64:443–446. [Google Scholar]
- 215.Tchinda AT, Mouokeu SR, Ngono RAN, Ebelle MRE, Mokale ALK, Nono DK, Frederich M. Nat. Prod. Res. 2015;29:1990–1994. doi: 10.1080/14786419.2015.1022541. [DOI] [PubMed] [Google Scholar]
- 216.Krishna V, Singh P. Phytochemistry. 1999;52:1341–1343. [Google Scholar]
- 217.Jones WP, Lobo-Echeverri T, Mi Q, Chai H-B, Soejarto DD, Cordell GA, Swanson SM, Kinghorn D. J. Nat. Prod. 2007;70:372–377. doi: 10.1021/np060534z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang W, Ali Z, Li XC, Smillie TA, Guo DA, Khan IA. Fitoterapia. 2009;80:404–407. doi: 10.1016/j.fitote.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 219.Kijjoa A, Pinto MMM, Pinho PMM, Tantisewie B, Herz W. Phytochemistry. 1990;29:653–655. [Google Scholar]
- 220.Wu TH, Cheng YY, Liou JR, Way TD, Chen CJ, Chen YH, Kuo SC, El-Shazly M, Chang FR, Wu YC, Liaw CC. RSC Adv. 2014;4:23707–23712. [Google Scholar]
- 221.Achari B, Chaudhuri C, Saha CR, Dutta PK, Pakrashi SC. Phytochemistry. 1990;29:3671–3673. [Google Scholar]
- 222.Geis W, Buschauer B, Becker H. Phytochemistry. 1999;51:643–649. [Google Scholar]
- 223.Chakrabarty M, Nath AC. J. Nat. Prod. 1992;55:256–258. [Google Scholar]
- 224.Maldonado E, Ortega A. Phytochemistry. 1997;46:1249–1254. [Google Scholar]
- 225.Wang LM, Guo SJ, Jia ZJ, Cheng DL. Chin. Chem. Lett. 1996;7:619–620. [Google Scholar]
- 226.Iqbal K, Malik A, Mukhtar N, Anis I, Khan SN, Choudhary MI. Chem. Pharm. Bull. 2004;52:785–789. doi: 10.1248/cpb.52.785. [DOI] [PubMed] [Google Scholar]
- 227.Pinto AC, Garcez WS, Queiroz PPS, Fiorani NG. Phytochemistry. 1994;37:1115–1117. [Google Scholar]
- 228.Zhu F, Di YT, Liu LL, Zhang Q, Fang X, Yang TQ, Hao XJ, He HP. J. Nat. Prod. 2010;73:233–236. doi: 10.1021/np900309p. [DOI] [PubMed] [Google Scholar]
- 229.Dai SJ, Shen L, Ren Y. J. Integr. Plant Biol. 2008;50:699–702. doi: 10.1111/j.1744-7909.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- 230.Dai SJ, Qu GW, Yu QY, Zhang DW, Li GS. Fitoterapia. 2010;81:737–741. doi: 10.1016/j.fitote.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 231.Zhu F, Di YT, Li XY, Liu LL, Zhang Q, Li Y, Hao XJ, He HP. Planta Med. 2011;77:1536–1541. doi: 10.1055/s-0030-1270797. [DOI] [PubMed] [Google Scholar]
- 232.Dai SJ, Tao JY, Liu K, Jiang YT, Shen L. Phytochemistry. 2006;67:1326–1330. doi: 10.1016/j.phytochem.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 233.Dai SJ, Sun JY, Ren Y, Liu K, Shen L. Planta Med. 2007;73:1217–1220. doi: 10.1055/s-2007-990215. [DOI] [PubMed] [Google Scholar]
- 234.Qu GW, Yue XD, Li GS, Yu QY, Dai SJ. J. Asian Nat. Prod. Res. 2010;12:859–864. doi: 10.1080/10286020.2010.507546. [DOI] [PubMed] [Google Scholar]
- 235.Shirota O, Nagamatsu K, Sekita S. J. Nat. Prod. 2006;69:1782–1786. doi: 10.1021/np060456f. [DOI] [PubMed] [Google Scholar]
- 236.Sashidhara KV, Singh SP, Sarkar J, Sinha S. Nat. Prod. Res. 2010;24:1687–1694. doi: 10.1080/10236240902765301. [DOI] [PubMed] [Google Scholar]
- 237.Ma X, Lee IS, Chai HB, Zaw K, Farnsworth NR, Soejarto DD, Cordell GA, Pezzuto JM, Kinghorn AD. Phytochemistry. 1994;37:1659–1662. doi: 10.1016/s0031-9422(00)89587-4. [DOI] [PubMed] [Google Scholar]
- 238.Hara N, Asaki H, Fujimoto Y, Gupta YK, Singh AK, Sahai M. Phytochemistry. 1995;38:189–194. [Google Scholar]
- 239.Hasan CM, Islam MO, Rashid MA. Pharmazie. 1995;50:227–228. [Google Scholar]
- 240.Wu TH, Cheng YY, Chen CJ, Ng LT, Chou LC, Huang LJ, Chen YH, Kuo S-C, El-Shazly M, Wu YC, Chang FR, Liaw CC. Molecules. 2014;19:2049–2060. doi: 10.3390/molecules19022049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hussein AA, de la Torre MC, Jimeno M-L, Rodríguez B, Bruno M, Piozzi F, Servettaz O. Phytochemistry. 1996;43:835–837. [Google Scholar]
- 242.Rasikari HL, Leach DN, Waterman PG, Spooner-Hart RN, Basta AH, Banbury LK, Winter KM, Forster PI. Phytochemistry. 2005;66:2844–2850. doi: 10.1016/j.phytochem.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 243.Guo P, Li Y, Xu J, Guo Y, Jin DQ, Gao J, Hou W, Zhang T. Fitoterapia. 2011;82:1123–1127. doi: 10.1016/j.fitote.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 244.Guo P, Li Y, Xu J, Liu C, Ma Y, Guo Y. J. Nat. Prod. 2011;74:1575–1583. doi: 10.1021/np2001557. [DOI] [PubMed] [Google Scholar]
- 245.Sun Z, Li Y, Jin DQ, Guo P, Xu J, Guo Y, Zhang L. Planta Med. 2012;78:1579–1583. doi: 10.1055/s-0032-1315215. [DOI] [PubMed] [Google Scholar]
- 246.Chang FR, Hwang TL, Yang YL, Li CE, Wu CC, Issa HH, Hsieh WB, Wu YC. Planta Med. 2006;72:1344–1347. doi: 10.1055/s-2006-951691. [DOI] [PubMed] [Google Scholar]
- 247.Shimbo K, Tsud M, Fukushi E, Kawabata J, Kobayashi JI. Tetrahedron. 2000;56:7923–7926. [Google Scholar]
- 248.Cheng CH, Chung HM, Hwang TL, Lu MC, Wen ZH, Kuo YH, Wang WH, Sung P-J. Molecules. 2012;17:9443–9450. doi: 10.3390/molecules17089443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bautista E, Maldonado E, Ortega A. J. Nat. Prod. 2012;75:951–958. doi: 10.1021/np3001464. [DOI] [PubMed] [Google Scholar]
- 250.Bautista E, Toscano RNA, Ortega A. J. Nat. Prod. 2014;77:1088–1092. doi: 10.1021/np4009893. [DOI] [PubMed] [Google Scholar]
- 251.Ortega A, Maldonado E, Jankowski CK, Calsteren MRV, Diaz E. Phytochem. Anal. 1994;5:302–304. [Google Scholar]
- 252.Esquivel B, Esquivel O, Cardenas J, Sanchez AA, Ramamoorhy TP, Toscano RA, Rodriguez-Hahn L. Phytochemistry. 1991;30:2335–2338. [Google Scholar]
- 253.Maldonado E, Ortega A. Phytochemistry. 2000;53:103–109. doi: 10.1016/s0031-9422(99)00466-5. [DOI] [PubMed] [Google Scholar]
- 254.Su CR, Ueng YF, Dung NX, Reddy MVB, Wu TS. J. Nat. Prod. 2007;70:1930–1933. doi: 10.1021/np0704248. [DOI] [PubMed] [Google Scholar]
- 255.Huang C, Li W, Ma F, Li Q, Asada Y, Koike K. Chem. Pharm. Bull. 2012;60:1324–1328. doi: 10.1248/cpb.c12-00482. [DOI] [PubMed] [Google Scholar]
- 256.Qin NB, Wang A-L, Li DH, Wang KB, Lin B, Li ZL, Hua H-M. Phytochem. Lett. 2015;12:173–176. [Google Scholar]
- 257.Achenbach H, Hemrich H. Phytochemistry. 1991;30:1957–1962. [Google Scholar]
- 258.Su CR, Chen YF, Liou MJ, Tsai HY, Chang WS, Wu T-S. Bioorg. Med. Chem. 2008;16:9603–9609. doi: 10.1016/j.bmc.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 259.Rakotobe L, Mambua L, Deville A, Dubost L, Jeannoda V, Rakoto D, Bodo B. Phytochemistry. 2010;71:1007–1013. doi: 10.1016/j.phytochem.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 260.Teponno RB, Tapondjou AL, Abou-Mansour E, Stoeckli-Evans H, Tane P, Barboni L. Phytochemistry. 2008;69:2374–2379. [Google Scholar]
- 261.Pudhom K, Sommit D. Phytochem. Lett. 2011;4:147–150. [Google Scholar]
- 262.Qin H-L, Li Z-H. Phytochemistry. 2004;65:2533–2537. doi: 10.1016/j.phytochem.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 263.Zdero C, Bohlmann F, Mungai GM. Phytochemistry. 1990;29:2247–2252. doi: 10.1016/0031-9422(90)85198-o. [DOI] [PubMed] [Google Scholar]
- 264.Valdés LJ, Chang HM, Visger DC, Koreeda M. Org. Lett. 2001;3:3935–3937. doi: 10.1021/ol016820d. [DOI] [PubMed] [Google Scholar]
- 265.Munro TA, Rizzacasa MA. J. Nat. Prod. 2003;66:703–705. doi: 10.1021/np0205699. [DOI] [PubMed] [Google Scholar]
- 266.Kutrzeba LM, Ferreira D, Zjawiony JK. J. Nat. Prod. 2009;72:1361–1363. doi: 10.1021/np900181q. [DOI] [PubMed] [Google Scholar]
- 267.Brasil DSB, Müller AH, Guilhon GMSP, Alves CN, Peris G, Llusar R, Moliner V. J. Braz. Chem. Soc. 2010;21:731–739. [Google Scholar]
- 268.Song C, Xu R. Chin. Chem. Lett. 1992;3:185–188. [Google Scholar]
- 269.Sivasubramanian A, Narasimha KKG, Rathnasamy R, Campos AMFO. Nat. Prod. Res. 2013;27:1431–1436. doi: 10.1080/14786419.2012.722088. [DOI] [PubMed] [Google Scholar]
- 270.Murillo RM, Jakupovic J. Ing. Cienc. Quim. 2000;19:68–73. [Google Scholar]
- 271.Ma Z, Deng G, Lee DYW. Tetrahedron Lett. 2010;51:5207–5209. doi: 10.1016/j.tetlet.2010.07.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Pizzolatti MG, Bortoluzzi AJ, Brighente IMC, Zuchinalli A, Carvalho FK, Candido ACS, Peres MTLP. J. Braz. Chem. Soc. 2013;24:609–614. [Google Scholar]
- 273.Harding WW, Tidgewell K, Schmidt M, Shah K, Dersch CM, Snyder J, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. Org. Lett. 2005;7:3017–3020. doi: 10.1021/ol0510522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chen TB, Galinis DL, Wiemer DF. J. Org. Chem. 1992;57:862–866. [Google Scholar]
- 275.Narukawa Y, Fukui M, Hatano K, Takeda T. J. Nat. Med. 2006;60:58–63. doi: 10.1007/s11418-006-0037-3. [DOI] [PubMed] [Google Scholar]
- 276.Rodríguez-Hahn L, O'Reilly R, Esquivel B, Maldonado E, Ortega A, Cárdenas J, Toscano RA, Chan T-M. J. Org. Chem. 1990;55:3522–3525. [Google Scholar]
- 277.Fiorentino A, D'Abrosca B, Pacifico S, Scognamiglio M, D'Angelo G, Gallicchio M, Chambery A, Monaco P. Phytochemistry. 2011;72:2037–2044. doi: 10.1016/j.phytochem.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 278.Lv H, Luo J, Zhu M, Shan S, Kong L. Chem. Pharm. Bull. 2014;62:472–476. doi: 10.1248/cpb.c13-00990. [DOI] [PubMed] [Google Scholar]
- 279.Xie N, Min Z, Zhao S, Wu B, Zheng Q, Zhang P. Phytochemistry. 1991;30:1963–1966. [Google Scholar]
- 280.Bruno M, Bondì ML, Rosselli S, Piozzi F, Al-Hillo MRY, Lamara K, Ladjel S. J. Nat. Prod. 2000;63:1029–1031. doi: 10.1021/np990510a. [DOI] [PubMed] [Google Scholar]
- 281.de la Torre MC, Rodríguez B, Bruno M, Fazio C, Baser KHC, Duman H. Phytochemistry. 1997;45:1653–1662. [Google Scholar]
- 282.Bruno M, Piozzi F, Rodriduez B, Savona G, de la Torre MC, Servettaz O. Phytochemistry. 1992;31:4366–4367. [Google Scholar]
- 283.de la Torre MC, Bruno M, Savona G, Piozzi F, Rodriguez B, Orietta O. Phytochemistry. 1990;29:988–989. [Google Scholar]
- 284.Davies-Coleman MT, Hanson JR, Rivett DEA. Phytochemistry. 1994;36:1549–1550. [Google Scholar]
- 285.Ladjel S, Laamara K, Al-Hillo MRY, Paĭs M. Phytochemistry. 1994;37:1663–1666. [Google Scholar]
- 286.Gallardo OV, Tonn CE, Nieto M, Morales GB, Giordano OS. Nat. Prod. Res. 1996;8:189–197. [Google Scholar]
- 287.de la Torre MC, Rodríguez B, Piozzia F, Savonaa G, Bruno M, Carreirasa MC. Phytochemistry. 1990;29:579–584. [Google Scholar]
- 288.Rodríguez B, de la Torre MC, Bruno M, Piozzi F, Vassallo N, Ciriminna R, Serettaz O. Phytochemistry. 1996;43:435–438. [Google Scholar]
- 289.Topcu G, Eris C, Che C-T, Ulubelen A. Phytochemistry. 1996;42:775–778. [Google Scholar]
- 290.Rodriguez B, de la Torre MC, Jimeno ML, Bruno M, Fazio C, Piozzi F, Savoa G, Perales A. Tetrahedron. 1995;51:837–848. [Google Scholar]
- 291.de la Torre MC, Rodríguez B, Bruno M, Savona G, Piozzi F, Perales A, Torres MR, Servettaz O. Phytochemistry. 1990;29:2229–2233. [Google Scholar]
- 292.Cai Y, Chen ZP, Phillipson JD. Phytochemistry. 1993;34:265–268. [Google Scholar]
- 293.Zdero C, Bohlmann F, Niemeyer HM. Phytochemistry. 1990;29:1227–1230. doi: 10.1016/0031-9422(90)85198-o. [DOI] [PubMed] [Google Scholar]
- 294.Sun Y, Wang M, Ren Q, Li S, Xu J, Ohizumi Y, Xie C, Jin D-Q, Guo Y. Fitoterapia. 2014;95:229–233. doi: 10.1016/j.fitote.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 295.Tchissambou L, Chiaroni A, Riche C, Khuong-Huu F. Tetrahedron. 1990;46:5199–5202. [Google Scholar]
- 296.Sun D, Li G. Phytochemistry. 1993;33:716–717. [Google Scholar]
- 297.Bedir E, Tasdemir D, Çalis I, Zerbe O, Sticher O. Phytochemistry. 1999;51:921–925. [Google Scholar]
- 298.Sivasubramanian A, Rathnasamy R, Muthuraman MS. Pharm. Lett. 2014;6:295–298. [Google Scholar]
- 299.Ali M. Phytochemistry. 1992;31:2173–2175. [Google Scholar]
- 300.Chen H, Liu DQ, Li XZ, Xia ZH, Li Y, Liu ZL, Tan RX. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1999;38:743–745. [Google Scholar]
- 301.Castro A, Coll J, Arfan M. J. Nat. Prod. 2011;74:1036–1041. doi: 10.1021/np100929u. [DOI] [PubMed] [Google Scholar]
- 302.Malakov PY, Papanov GY. Phytochemistry. 1998;49:2443–2447. [Google Scholar]
- 303.Malakov PY, Papanov GY, de la Torre MC, Rodríguez B. Phytochemistry. 1991;30:4083–4085. [Google Scholar]
- 304.de la Torre MC, Rodríguez B, Bruno M, Piozzi F, Vassallo N, Bondí ML, Servettaz O. Phytochemistry. 1997;45:121–123. [Google Scholar]
- 305.Boneva IM, Malakov PY, Papanov GY. Phytochemistry. 1998;47:303–305. [Google Scholar]
- 306.Cole MD, Anderson JC, Blaney WM, Fellows LE, Ley SV, Sheppard RN, Simmonds MSJ. Phytochemistry. 1990;29:1793–1796. [Google Scholar]
- 307.Bruno M, de la Torre MC, Piozzi F, Rodriguez B, Savona G, Arnold NA. Phytochemistry. 1993;33:931–932. [Google Scholar]
- 308.de la Torre MC, Bruno M, Piozzi F, Rodriguez B, Savona G, Servetaz O. Phytochemistry. 1992;31:3639–3641. [Google Scholar]
- 309.Bruno M, Fazio C, Arnold NA. Phytochemistry. 1996;42:555–557. [Google Scholar]
- 310.Bruno M, Bondì ML, Rosselli S, Piozzi F, Servettaz O. J. Nat. Prod. 2000;63:1032–1034. doi: 10.1021/np990624m. [DOI] [PubMed] [Google Scholar]
- 311.Ohno A, Kizu H, Tomimori T. Chem. Pharm. Bull. 1996;44:1540–1545. [Google Scholar]
- 312.Bruno M, Piozzi F, Rodríguez B, de la Torre MC, Vassallo N, Servettaz O. Phytochemistry. 1996;42:1059–1064. [Google Scholar]
- 313.Malakov PY, Papanov GY. Phytochemistry. 1997;46:955–958. [Google Scholar]
- 314.Malakov PY, Papanov GY, Deltchev VB. Phytochemistry. 1998;49:811–815. [Google Scholar]
- 315.Chen H, Tan RX, Liu ZL, Zhang Y. J. Nat. Prod. 1996;59:668–670. doi: 10.1021/np960385s. [DOI] [PubMed] [Google Scholar]
- 316.Bozov PI, Papanov GY, Malakov PY, de la Torre MC, Rodriguez B. Phytochemistry. 1993;34:1173–1175. [Google Scholar]
- 317.Ben Jannet H, Harzallah-Skhiri F, Mighri Z, Simmonds MSJ, Blaney WM. Fitoterapia. 2000;71:105–112. doi: 10.1016/s0367-326x(99)00146-x. [DOI] [PubMed] [Google Scholar]
- 318.Achari B, Giri C, Saha CR, Dutta PK, Pakrashi SC. Phytochemistry. 1992;31:338–340. [Google Scholar]
- 319.Pandey R, Verma RK, Gupta MM. Phytochemistry. 2005;66:643–648. doi: 10.1016/j.phytochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 320.Bruno M, Cruciata M, Bondi ML, Piozzi F, de la Torre MC, Rodríguez B, Servettaz O. Phytochemistry. 1998;48:687–691. [Google Scholar]
- 321.Zhu F, Liu L, Di Y, Hao X, He H. Yunnan Zhiwu Yanjiu. 2009;31:474–476. [Google Scholar]
- 322.Rodríguez B, de la Torre MC, Jimeno M-L, Bruno M, Vassallo N, Bondí M, Piozzi F, Servettaz O. J. Nat. Prod. 1997;60:348–355. [Google Scholar]
- 323.Boneva IM, Mikhova BP, Malakov PY, Papanov GY, Duddeck H, Spassov SL. Phytochemistry. 1990;29:2931–2933. [Google Scholar]
- 324.Rao LJM, Pereira J, Gurudutt KN. Phytochemistry. 1993;34:572–574. [Google Scholar]
- 325.Kawai K, Amano T, Nishida R, Kuwahara Y, Fukami H. Phytochemistry. 1998;49:1975–1980. [Google Scholar]
- 326.Cole MD, Bridge PD, Dellar JE, Fellows LE, Cornish MC, Anderson JC. Phytochemistry. 1991;30:1125–1127. [Google Scholar]
- 327.Ohno A, Kizu H, Tomimori T. Chem. Pharm. Bull. 1997;45:1097–1100. [Google Scholar]
- 328.Kawai K, Nishida R, Fukami H. Biosci., Biotechnol., Biochem. 1999;63:1795–1797. doi: 10.1271/bbb.63.1795. [DOI] [PubMed] [Google Scholar]
- 329.Malakov PY, Papanov GY, Spassov SL. Phytochemistry. 1997;44:121–124. [Google Scholar]
- 330.Muñoz DM, de la Torre MC, Rodríguez B, Simmonds MSJ, Blaney WM. Phytochemistry. 1997;44:593–597. [Google Scholar]
- 331.Bozov PI, Malakov PY, Papanov GY, de la Torre MC, Rodriguez B, Perales A. Phytochemistry. 1993;34:453–456. [Google Scholar]
- 332.Bozov PI, Papanov GY, Malakov PY. Phytochemistry. 1994;35:1285–1288. [Google Scholar]
- 333.Lee H, Shim SH. Helv. Chim. Acta. 2011;94:643–649. [Google Scholar]
- 334.Shim SH. Chem. Nat. Compd. 2014;50:256–257. [Google Scholar]
- 335.Esquivel B, Calderón JS, Flores E. Phytochemistry. 1998;47:135–137. [Google Scholar]
- 336.Ezer N, Akcos Y, Rodríguez B. Phytochemistry. 1998;49:1825–1827. doi: 10.1016/s0031-9422(98)00291-x. [DOI] [PubMed] [Google Scholar]
- 337.Esquivel B, Dominguez RM, Martinez MA, Espinosa-Pérez G. Heterocycles. 1997;45:2247–2252. [Google Scholar]
- 338.Lee H, Kim Y, Choi I, Min BS, Shim SH. Bioorg. Med. Chem. Lett. 2010;20:288–290. doi: 10.1016/j.bmcl.2009.10.116. [DOI] [PubMed] [Google Scholar]
- 339.Siddiqui HL, Munesada K, Suga T. Chem. Lett. 1991;4:701–704. [Google Scholar]
- 340.Aoki T, Ohro T, Hirage Y, Suga T, Uno M, Ohta S. Phytochemistry. 1997;46:839–844. doi: 10.1016/s0031-9422(97)00377-4. [DOI] [PubMed] [Google Scholar]
- 341.Meng XH, Zou CZ, Jin XJ, Huang GD, Yang YJ, Zou XY, Yao XJ, Zhu Y. Fitoterapia. 2014;93:39–46. doi: 10.1016/j.fitote.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 342.Li XL, Yang LM, Zhao Y, Wang RR, Xu G, Zheng YT, Tu L, Peng LY, Cheng X, Zhao QS. J. Nat. Prod. 2007;70:265–268. doi: 10.1021/np0603166. [DOI] [PubMed] [Google Scholar]
- 343.Harinantenaina LRR, Kasai R, Yamasaki K. Phytochemistry. 2002;60:339–343. doi: 10.1016/s0031-9422(02)00064-x. [DOI] [PubMed] [Google Scholar]
- 344.Kawahara N, Tamura T, Inoue M, Hosoe T, Kawai K-I, Sekita S, Satake M, Goda Y. Phytochemistry. 2004;65:2577–2581. doi: 10.1016/j.phytochem.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 345.Hu H, Cao H, Jian YF, Zheng XD, Liu JX. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2008;47:166–170. [Google Scholar]
- 346.Maldonado E, Cárdenas J, Bojórquze H, Escamilla EM, Ortega A. Phytochemistry. 1996;42:1105–1108. [Google Scholar]
- 347.Fukuda N, Yonemitsu M, Kimura T. Liebigs Ann. Chem. 1993:491–495. [Google Scholar]
- 348.Martin TS, Ohtani K, Kasai R, Yamasaki K. Phytochemistry. 1996;42:153–158. doi: 10.1016/0031-9422(95)00902-7. [DOI] [PubMed] [Google Scholar]
- 349.Cifuente DA, Borkowski EJ, Sosa ME, Gianello JC, Giordano OS, Tonn CE. Phytochemistry. 2002;61:899–905. doi: 10.1016/s0031-9422(02)00437-5. [DOI] [PubMed] [Google Scholar]
- 350.Jiang ZY, Li WJ, Jiao LX, Guo JM, Tian K, Yang CT, Huang XZ. Planta Med. 2014;80:419–425. doi: 10.1055/s-0034-1368252. [DOI] [PubMed] [Google Scholar]
- 351.Martin TS, Ohtani K, Kasai R, Yamasaki K. Phytochemistry. 1995;40:1729–1736. [Google Scholar]
- 352.Pan L, Terrazas C, Lezama-Davila CM, Rege N, Gallucci JC, Satoskar AR, Kinghorn AD. Org. Lett. 2012;14:2118–2121. doi: 10.1021/ol300657h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Choudhary MI, Ismail M, Shaari K, Abbaskhan A, Sattar SA, Lajis NH, Rahman A.-ur. J. Nat. Prod. 2010;73:541–547. doi: 10.1021/np900551u. [DOI] [PubMed] [Google Scholar]
- 354.Wazir V, Maurya R, Kapil RS. Phytochemistry. 1995;38:447–449. [Google Scholar]
- 355.Li W, Huang C, Li S, Ma F, Li Q, Asada Y, Koike K. Planta Med. 2012;78:82–85. doi: 10.1055/s-0031-1280163. [DOI] [PubMed] [Google Scholar]
- 356.Kiem PV, Minh CV, Dat NT, Kinh LV, Hang DT, Nam NH, Cuong NX, Huong HT, Lau TV. Fitoterapia. 2010;81:485–489. [Google Scholar]
- 357.Li W, Wei K, Fu H, Koike K. J. Nat. Prod. 2007;70:1971–1976. doi: 10.1021/np070367i. [DOI] [PubMed] [Google Scholar]
- 358.Fiorentino A, D'Abrosca B, Ricci A, Pacifico S, Piccolella S, Monaco P. Magn. Reson. Chem. 2009;47:1007–1012. doi: 10.1002/mrc.2499. [DOI] [PubMed] [Google Scholar]
- 359.Malakov PY, Papanov GY, Boneva IM, de la Torre MC, Rodriguez B. Phytochemistry. 1993;34:1095–1098. [Google Scholar]
- 360.Gavagnin M, Carbone M, Amodeo P, Mollo E, Vitale RM, Roussis V, Cimino G. J. Org. Chem. 2007;72:5625–5630. doi: 10.1021/jo0704917. [DOI] [PubMed] [Google Scholar]
- 361.Carbone M, Gavagnin M, Mollo E, Bidello M, Roussis V, Cimino G. Tetrahedron. 2008;64:191–196. [Google Scholar]
- 362.Annan K, Ekuadzi E, Asare C, Sarpong K, Pistorius D, Oberer L, Gyan BA, Ofori M. Phytochem. Lett. 2015;11:28–31. [Google Scholar]
- 363.Appenzeller J, Mihci G, Martin MT, Gallard JF, Menou JL, Esnault NB, Hooper J, Petek S, Chevalley S, Valentin A, Zaparucha A, Mourabit AA, Debitus C. J. Nat. Prod. 2008;71:1451–1454. doi: 10.1021/np800212g. [DOI] [PubMed] [Google Scholar]
- 364.Pettit GR, Tang Y, Zhang Q, Bourne GT, Arm CA, Leet JE, Knight JC, Pettit RK, Chapuis J-C, Doubek DL, Ward FJ, Weber C, Hooper JNA. J. Nat. Prod. 2013;76:420–424. doi: 10.1021/np300828y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kubota T, Iwai T, Takahashi-Nakaguchi A, Fromont J, Gonoi T, Kobayashi JI. Tetrahedron. 2012;68:9738–9744. [Google Scholar]
- 366.Wang F, Ren FC, Li Y-J, Liu JK. Chem. Pharm. Bull. 2010;58:1267–1270. doi: 10.1248/cpb.58.1267. [DOI] [PubMed] [Google Scholar]
- 367.Dai SJ, Peng WB, Zhang DW, Shen L, Wang WY, Ren Y. J. Nat. Prod. 2009;72:1793–1797. doi: 10.1021/np900362z. [DOI] [PubMed] [Google Scholar]
- 368.Nie XP, Qu GW, Yue XD, Li GS, Dai SJ. Phytochem. Lett. 2010;3:190–193. [Google Scholar]
- 369.Wang ZQ, Xu FM, Yan XZ, Zhu Y. Chin. Chem. Lett. 1996;7:333–334. [Google Scholar]
- 370.Dai SJ, Liang DD, Ren Y, Liu K, Shen L. Chem. Pharm. Bull. 2008;56:207–209. doi: 10.1248/cpb.56.207. [DOI] [PubMed] [Google Scholar]
- 371.Dai SJ, Chen M, Liu K, Jiang YT, Shen L. Chem. Pharm. Bull. 2006;54:869–872. doi: 10.1248/cpb.54.869. [DOI] [PubMed] [Google Scholar]
- 372.Dai SJ, Peng WB, Shen L, Zhang DW, Ren Y. J. Asian Nat. Prod. Res. 2009;11:451–456. doi: 10.1080/10286020902845652. [DOI] [PubMed] [Google Scholar]
- 373.Kobayashi JI, Sekiguchi M, Shimamoto S, Shigemori H, Ohsaki A. J. Nat. Prod. 2000;63:1576–1579. doi: 10.1021/np000355w. [DOI] [PubMed] [Google Scholar]
- 374.Kobayashi JI, Sekiguchi M, Shigemori H, Ohsaki A. Tetrahedron Lett. 2000;41:2939–2943. [Google Scholar]
- 375.Cheng YY, Li SF, Zhang Y, Tang GH, Di YT, Hao XJ, Li SL, He H-P. Fitoterapia. 2012;83:1100–1104. doi: 10.1016/j.fitote.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 376.Xie N, Min ZD, Zhao S-X, Lu Y, Zheng QT, Wang C, Mizuno M, Iinuma M, Tanaka T. Chem. Pharm. Bull. 1992;40:2193–2195. [Google Scholar]
- 377.Bruno M, Fazio C, Piozzi F, Sovona G, Rodríguez B, de la Torre MC. Phytochemistry. 1995;40:505–507. [Google Scholar]
- 378.Dai SJ, Wang GF, Chen M, Liu K, Shen L. Chem. Pharm. Bull. 2007;55:1218–1221. doi: 10.1248/cpb.55.1218. [DOI] [PubMed] [Google Scholar]
- 379.Nguyen VH, Pham VC, Nguyen TTH, Tran VH, Doan TMH. Eur. J. Org. Chem. 2009:5810–5815. [Google Scholar]
- 380.Huang XZ, Dai CMC, Fu GM, Guo JM, Yin Y, Liang H. Molecules. 2010;15:8360–8365. doi: 10.3390/molecules15118360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Tane P, Wabo HK, Ayafor JF, Sterner O. Phytochemistry. 1997;46:165–167. doi: 10.1016/s0031-9422(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 382.Pan Z, Ning D, Wu X, Huang S, Li D, Lv S. Bioorg. Med. Chem. Lett. 2015;25:1329–1332. doi: 10.1016/j.bmcl.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 383.Kidyu K, Thaisuchat H, Meepowpan P, Sukdee S, Nuntasaen N, Punyanitya S, Pompimon W. Nat. Prod. Commun. 2011;6:1069–1072. [PubMed] [Google Scholar]
- 384.Zakaria HM, Kenne F, Musa CC, Modest CK, Joseph JM, Mainen MM, Filip L, Kees G, Jan F, René P, Arnold V, Sandra A, Luc P. Planta Med. 2009;75:262–267. [Google Scholar]
- 385.Wang GC, Li JG, Li GQ, Xu JJ, Wu X, Ye WC, Li YL. J. Nat. Prod. 2012;75:2188–2192. doi: 10.1021/np300636k. [DOI] [PubMed] [Google Scholar]
- 386.Min Z-D, Xie N, Zhang P, Zhao S-X, Wang C-S, Zheng Q-T. Phytochemistry. 1991;30:4175–4177. [Google Scholar]
- 387.Huang W-H, Li GQ, Li JG, Wu X, Ge W, Chung HY, Ye WC, Li YL, Wang G-C. Heterocycles. 2014;89:1585–1593. [Google Scholar]
- 388.Ichihara Y, Takey K, Hitotsuyanai Y, Morita H, Okuyama S, Suganuma M, Fujiki H, Motidome M, Itokawa H. Planta Med. 1992;58:549–551. doi: 10.1055/s-2006-961547. [DOI] [PubMed] [Google Scholar]
- 389.Soares BA, Firme CL, Maciel MAM, Kaiser CR, Schilling E, Bortoluzzi AJ. J. Braz. Chem. Soc. 2014;25:629–638. [Google Scholar]
- 390.Bruno M, Fazio C, Piozzi F, Rodríguez B, de la Torre MC. Phytochemistry. 1995;40:1481–1483. [Google Scholar]
- 391.Kabir S, Rahman MS, Chowdhury AMS, Hasan CM, Rashid MA. Nat. Prod. Commun. 2010;5:1543–1546. [PubMed] [Google Scholar]
- 392.Toyota M, Omatsu I, Sakata F, Braggins J, Asakawa Y. Nat. Prod. Commun. 2010;5:999–1003. [PubMed] [Google Scholar]
- 393.Tazaki H, Becker H, Nabeta K. Phytochemistry. 1999;51:743–750. [Google Scholar]
- 394.Hertewich UM, Zapp J, Becker H. Phytochemistry. 2003;63:227–233. doi: 10.1016/s0031-9422(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 395.Ortega A, Cárdenas J, Toscano A, Maldonado E, Aumelas A, Calsteren MRV, Jankowskq C. Phytochemistry. 1991;30:3357–3360. [Google Scholar]
- 396.Toscano RA, Maldonado E, Ortega A. J. Chem. Crystallogr. 1996;26:239–242. [Google Scholar]
- 397.Ahmad F, Ali M, Alam P. Nat. Prod. Res. 2010;24:926–9354. doi: 10.1080/14786410802435679. [DOI] [PubMed] [Google Scholar]
- 398.del Carmen Fernández M, Esquivel B, Cárdenas J, Sánchez AA, Toscano RA, Rodríguez-Hahn L. Tetrahedron. 1991;47:7199–7208. [Google Scholar]
- 399.He HP, Shen YM, Hong X, Zhao YB, Zhou J, Hao XJ. J. Nat. Prod. 2002;65:392–394. doi: 10.1021/np0103517. [DOI] [PubMed] [Google Scholar]
- 400.Ohsaki A, Kasetani Y, Asaka Y, Shibata K, Tokoroyama T, Kubota T. Phytochemistry. 1995;40:205–207. [Google Scholar]
- 401.Niu HM, Zeng DQ, Long CL, Peng YH, Wang YH, Luo JF, Wang HS, Shi YN, Tang GH, Zhao FW. J. Asian Nat. Prod. Res. 2010;12:7–14. doi: 10.1080/10286020903407379. [DOI] [PubMed] [Google Scholar]
- 402.Narukawa Y, Hatano K, Takeda T. J. Nat. Med. 2006;60:206–209. doi: 10.1007/s11418-006-0037-3. [DOI] [PubMed] [Google Scholar]
- 403.Esquivel B, Domínguez RM, Ortega SH, Toscano RA, Hahn LR. Tetrahedron. 1994;50:11593–11600. [Google Scholar]
- 404.Xu G, Peng L, Niu X, Zhao Q, Li R, Sun H. Helv. Chim. Acta. 2004;87:949–955. [Google Scholar]
- 405.Jaime-Vasconcelos MÁ, Frontana-Uribe BA, José Antonio M-S, Salmón M, Cárdenas J. Molecules. 2011;16:9109–9115. doi: 10.3390/molecules16119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Bisio A, Fontana N, Romussi G, Ciarallo G, Tommasi ND, Pizza C, Mugnoli A. Phytochemistry. 1999;52:1535–1540. [Google Scholar]
- 407.Esquivel B, Tello R, Sánchez AA. J. Nat. Prod. 2005;68:787–790. doi: 10.1021/np050041e. [DOI] [PubMed] [Google Scholar]
- 408.Tuntiwachwuttikul P, Boonrasria J. B. B. Nicha, Taylor WC. Phytochemistry. 1999;52:1335–1340. [Google Scholar]
- 409.Tuntiwachwuttikul P, Taylor WC. Chem. Pharm. Bull. 2001;49:854–857. doi: 10.1248/cpb.49.854. [DOI] [PubMed] [Google Scholar]
- 410.Bautista E, Toscano RA, Ortega A. Org. Lett. 2013;15:3210–3213. doi: 10.1021/ol401022c. [DOI] [PubMed] [Google Scholar]
- 411.Gaspar H, Palma FMSB, de la Torre MC, Rodrígnez B, Perales A. Tetrahedron. 1995;51:2363–2368. [Google Scholar]
- 412.Tazaki H, Blechschmidt M, Huch V, Veith M, Becker H. Phytochemistry. 1994;37:491–494. [Google Scholar]
- 413.Topcu G, Eris C, Ulubelen A, Krawiec M, Watson WH. Tetrahedron. 1995;51:11793–11800. [Google Scholar]
- 414.Topcu G, Eris C. J. Nat. Prod. 1997;60:1045–1047. [Google Scholar]
- 415.Cárdenas J, Pavón T, Esquivel B, Toscano A, Rodríguez-Hahn L. Tetrahedron Lett. 1992;33:581–584. [Google Scholar]
- 416.Kubo I, Nakanishi K. Adv. Pestic. Sci., Plenary Lect. Symp. Pap. Int. Congr. Pestic. Chem., 4th. 1979;2:284–294. [Google Scholar]
- 417.Kubo l., Klocke JA, Miura l., Fukuyama Y. J. Chem. Soc., Chem. Commun. 1982;11:618–619. [Google Scholar]
- 418.Pereira J, Gurudutt KN. J. Chem. Ecol. 1990;16:2297–2306. doi: 10.1007/BF01026939. [DOI] [PubMed] [Google Scholar]
- 419.Kubo I, Asaka Y, Shibata K. Phytochemistry. 1991;30:2545–2546. [Google Scholar]
- 420.Rodriguez-Hahn L, Esquivel B, Cardenas J. Prog. Chem. Org. Nat. Prod. 1994;63:107–196. [Google Scholar]
- 421.Piozzi F. Heterocycles. 1994;37:603–626. [Google Scholar]
- 422.Piozzi F, Bruno M, Rosselli S. Heterocycles. 1998;48:2185–2203. [Google Scholar]
- 423.Anderson JC, Blaney WM, Cole MD, Fellows LL, Ley SV, Sheppard RN, Simmonds MSJ. Tetrahedron Lett. 1989;30:4737–4740. [Google Scholar]
- 424.Bruno M, Vassallo N, Simmonds MSJ. Phytochemistry. 1999;50:973–976. [Google Scholar]
- 425.Bruno M, Ciriminna R, Piozzi F, Rosselli S, Simmonds MSJ. Phytochemistry. 1999;52:1055–1058. [Google Scholar]
- 426.Bruno M, Piozzi F, Rosselli S. Nat. Prod. Rep. 2002;19:357–378. doi: 10.1039/b111150g. [DOI] [PubMed] [Google Scholar]
- 427.Abbaszadeh G, Srivastava C, Walia S. Natl. Acad. Sci. Lett. 2012;35:457–464. [Google Scholar]
- 428.Kumari GNK, Balachandran J, Aravind S, Ganesh MR. J. Agric. Food Chem. 2003;51:1555–1559. doi: 10.1021/jf025920a. [DOI] [PubMed] [Google Scholar]
- 429.Vaccarin CE, Palacios SM, Meragelman KM, Sosa VE. Phytochemistry. 1999;50:227–230. [Google Scholar]
- 430.Gonzalez-Coloma A, Gutierrez C, del Corral J. M. Miguel, Gordaliza M, de la. Puente ML, San Feliciano A. J. Agric. Food Chem. 2000;48:3677–3681. doi: 10.1021/jf990843d. [DOI] [PubMed] [Google Scholar]
- 431.Enriz RD, Baldoni HA, Zamora MA, Jauregui EA, Sosa ME, Tonn CE, Luco JM, Gordaliza M. J. Agric. Food Chem. 2000;48:1384–1392. doi: 10.1021/jf990006b. [DOI] [PubMed] [Google Scholar]
- 432.Enriz RD, Baldoni HA, Jauregui EA, Sosa ME, Tonn CE, Giordano OS. J. Agric. Food Chem. 1994;42:2958–2963. [Google Scholar]
- 433.Ortega A, Blount JF, Manchand PS. J. Chem. Soc., Perkin Trans. 1. 1982;10:2505–2508. [Google Scholar]
- 434.Leander I, Valdes J, Butler WM, Hatfield GM, Paul AG, Koreeda M. J. Org. Chem. 1984;49:4716–4720. [Google Scholar]
- 435.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Siebert DJ. J. Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 437.Sheffler DJ, Roth BL. Trends Pharmacol. Sci. 2003;24:107–109. doi: 10.1016/S0165-6147(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 438.Rees DC, Hunter JC. In: Comprehensive Medicinal Chemistry. Emmett JC, editor. Vol. 3. Pergamon, Oxford: 1990. pp. 805–846. [Google Scholar]
- 439.Cunningham CW, Rothman RB, Prisinzano TE. Pharmacol. Rev. 2011;63:316–347. doi: 10.1124/pr.110.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. J. Pharmacol. Exp. Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- 441.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DYW, Huang P, Li J-G, Cowan A, Liu-Chen L-Y. J. Pharmacol. Exp. Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 442.Walentiny DM, Vann RE, Warner JA, King LS, Seltzman HH, Navarro HA, Twine CE, Jr, Thomas BF, Gilliam AF, Gilmour BP, Carroll FI, Wiley JL. Psychopharmacology. 2010;210:275–284. doi: 10.1007/s00213-010-1827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.John TF, French LG, Erlichman JS. Eur. J. Pharmacol. 2006;545:129–133. doi: 10.1016/j.ejphar.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 444.McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Pharmacol., Biochem. Behav. 2006;83:109–113. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 445.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Psychopharmacology. 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 446.Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Li JX, Rice KC, France CP. J. Pharmacol. Exp. Ther. 2008;324:827–833. doi: 10.1124/jpet.107.130625. [DOI] [PubMed] [Google Scholar]
- 448.Rothman RB, Murphy DL, Xu H, Godin JA, Dersch CM, Partilla JS, Tidgewell K, Schmidt M, Prisinzano TE. J. Pharmacol. Exp. Ther. 2007;320:801–810. doi: 10.1124/jpet.106.113167. [DOI] [PubMed] [Google Scholar]
- 449.Waldhoer M, Bartlett SE, Whistler JL. Annu. Rev. Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 450.Braida D, Donzelli A, Martucci R, Capurro V, Sala M. Int. J. Toxicol. 2011;30:650–661. doi: 10.1177/1091581811418538. [DOI] [PubMed] [Google Scholar]
- 451.Ma Z, Lee DYW. Tetrahedron Lett. 2007;48:5461–5464. doi: 10.1016/j.tetlet.2007.05.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Munro TA, Rizzacasa MA, Roth BL, Toth BA, Yan F. J. Med. Chem. 2005;48:345–348. doi: 10.1021/jm049438q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Simpson DS, Katavic PL, Lozama A, Harding WW, Parrish D, Deschamps JR, Dersch CM, Partilla JS, Rothman RB, Navarro H, Prisinzano TE. J. Med. Chem. 2007;50:3596–3603. doi: 10.1021/jm070393d. [DOI] [PubMed] [Google Scholar]
- 454.Polepally PR, White K, Vardy E, Roth BL, Ferreira D, Zjawiony JK. Bioorg. Med. Chem. Lett. 2013;23:2860–2862. doi: 10.1016/j.bmcl.2013.03.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Béguin C, Potuzak J, Xu W, Liu-Chen L-Y, Streicher JM, Groer CE, Bohn LM, Carlezon WA, Jr, Cohen BM. Bioorg. Med. Chem. Lett. 2012;22:1023–1026. doi: 10.1016/j.bmcl.2011.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Béguin C, Duncan KK, Munro TA, Ho DM, Xu W, Liu-Chen L-Y, Carlezon WA, Jr, Cohen BM. Bioorg. Med. Chem. 2009;17:1370–1380. doi: 10.1016/j.bmc.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Munro TA, Duncan KK, Xu W, Wang Y, Liu-Chen L-Y, Carlezon WA, Jr, Cohen BM, Béguin C. Bioorg. Med. Chem. 2008;16:1279–1286. doi: 10.1016/j.bmc.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Hooker JM, Munro TA, Béguin C, Alexoff D, Shea C, Xu Y, Cohen BM. Neuropharmacology. 2009;57:386–391. doi: 10.1016/j.neuropharm.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Baker LE, Panos JJ, Killinger BA, Peet MM, Bell LM, Haliw LA, Walker SL. Psychopharmacology. 2009;203:203–211. doi: 10.1007/s00213-008-1458-3. [DOI] [PubMed] [Google Scholar]
- 460.Wang Y, Chen Y, Xu W, Lee DYW, Ma Z, Rawls SM, Cowan A, Liu-Chen L-Y. J. Pharmacol. Exp. Ther. 2008;324:1073–1083. doi: 10.1124/jpet.107.132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Yan F, Bikbulatov RV, Mocanu V, Dicheva N, Parker CE, Wetsel WC, Mosier PD, Westkaemper RB, Allen JA, Zjawiony JK, Roth BL. Biochemistry. 2009;48:6898–6908. doi: 10.1021/bi900605n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J. Med. Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 463.Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Dersch CM, Rothman RB, Prisinzano TE. Bioorg. Med. Chem. Lett. 2006;16:3170–3174. doi: 10.1016/j.bmcl.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 464.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. Mol. Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Fichna J, Lewellyn K, Yan F, Roth BL, Zjawiony JK. Bioorg. Med. Chem. Lett. 2011;21:160–163. doi: 10.1016/j.bmcl.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Lee DYW, Karnati VVR, He M, Liu-Chen L-Y, Kondaveti L, Ma Z, Wang Y, Chen Y, Béguin C, William J, Carlezon A, Cohen B. Bioorg. Med. Chem. Lett. 2005;15:3744–3747. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 467.Béguin C, Richards MR, Wang Y, Chen Y, Liu-Chen L-Y, Ma Z, Lee DYW, Carlezon WA, Cohen BM. Bioorg. Med. Chem. Lett. 2005;15:2761–2765. doi: 10.1016/j.bmcl.2005.03.113. [DOI] [PubMed] [Google Scholar]
- 468.Béguin C, Richards MR, Li J-G, Wang Y, Xu W, Liu-Chen L-Y, Carlezon WA, Cohen BM. Bioorg. Med. Chem. Lett. 2006;16:4679–4685. doi: 10.1016/j.bmcl.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 469.Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. J. Nat. Prod. 2006;69:914–918. doi: 10.1021/np060094b. [DOI] [PubMed] [Google Scholar]
- 470.Stewart DJ, Fahmy H, Roth BL, Yan F, Zjawiony JK. Arzneim. Forsch. 2006;56:269–275. doi: 10.1055/s-0031-1296720. [DOI] [PubMed] [Google Scholar]
- 471.Lee DYW, Yang L, Xu W, Deng G, Guo L, Liu-Chen L-Y. Bioorg. Med. Chem. Lett. 2010;20:5749–5752. doi: 10.1016/j.bmcl.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Lovell KM, Prevatt-Smith KM, Lozama A, Prisinzano TE. Top. Curr. Chem. 2011;299:141–185. doi: 10.1007/128_2010_82. [DOI] [PubMed] [Google Scholar]
- 473.Lovell KM, Vasiljevik T, Araya JJ, Lozama A, Prevatt-Smith KM, Day VW, Dersch CM, Rothman RB, Butelman ER, Kreek MJ, Prisinzano TE. Bioorg. Med. Chem. 2012;20:3100–3110. doi: 10.1016/j.bmc.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Tang W, Kubo M, Harada K, Hioki H, Fukuyama Y. Bioorg. Med. Chem. Lett. 2009;19:882–886. doi: 10.1016/j.bmcl.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 475.Guo Y, Li Y, Xu J, Li N, Yamakuni T, Ohizumi Y. Chem. Pharm. Bull. 2007;55:1532–1534. doi: 10.1248/cpb.55.1532. [DOI] [PubMed] [Google Scholar]
- 476.Brito A. R. M. Souza, Rodriguez JA, Hiruma-Lima CA, Haun M, Nunes DS. Planta Med. 1998;64:126–129. doi: 10.1055/s-2006-957388. [DOI] [PubMed] [Google Scholar]
- 477.Hiruma-Lima CA, Spadari-Bratfisch RC, Kassisse DMG, Brito A. R. M. Souza. Planta Med. 1999;65:325–330. doi: 10.1055/s-1999-13995. [DOI] [PubMed] [Google Scholar]
- 478.Rodriguez JA, Hiruma-Lima CA, Brito A. R. Souza. Hum. Exp. Toxicol. 2004;23:455–461. doi: 10.1191/0960327104ht473oa. [DOI] [PubMed] [Google Scholar]
- 479.Hiruma-Lima CA, Toma W, Gracioso JDS, Almeida ABAD, Batista LM, Magri L, Paula ACBD, Soares FR, Nunes DS, Brito A. R. M. Souza. Biol. Pharm. Bull. 2002;25:452–456. doi: 10.1248/bpb.25.452. [DOI] [PubMed] [Google Scholar]
- 480.Hiruma-Lima CA, Gracioso JDS, Toma W, Paula ACBD, Almeida ABAD, Brasil DDSB, Muller AH, Brito A. R. M. Souza. Biol. Pharm. Bull. 2000;23:1465–1469. doi: 10.1248/bpb.23.1465. [DOI] [PubMed] [Google Scholar]
- 481.Hiruma-Lima CA, Gracioso JS, Toma W, Almeida AB, Paula AC, Brasil DSB, Muller AH, Brito A. R. M. Souza. Phytomedicine. 2001;8:94–100. doi: 10.1078/0944-7113-00017. [DOI] [PubMed] [Google Scholar]
- 482.Brasil DSB, Moreira RYO, Muller AH, Alves CN. Int. J. Quantum Chem. 2006;106:2706–2713. [Google Scholar]
- 483.Shen YC, Lee CL, Khalil AT, Cheng YB, Chien CT, Kuo YH. Helv. Chim. Acta. 2005;88:68–77. [Google Scholar]
- 484.Chiou C-T, Kuo Y-H, Chan Y-Y, Juang S-H, Chan H-H, Wue T-S. Phytochemistry. 2012;80:64–69. doi: 10.1016/j.phytochem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 485.Efdi M, Itoh T, Akao Y, Nozawa Y, Koketsu M, Ishihara H. Bioorg. Med. Chem. 2007;15:3667–3671. doi: 10.1016/j.bmc.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 486.Phadnis AP, Patwarshan SA, Dhaneshwar NN, Tavale SS, Row T. N. Guru. Phytochemistry. 1988;27:2899–2901. [Google Scholar]
- 487.Akihisa T, Kikuchi T, Nagai H, Ishii K, Tabata K, Suzuki T. J. Oleo Sci. 2011;60:71–77. doi: 10.5650/jos.60.71. [DOI] [PubMed] [Google Scholar]
- 488.Itokawa H, Totsuka N, Morita H, Takeya K, Iitaka Y, Schenkel EP, Motidome M. Chem. Pharm. Bull. 1990;38:3384–3388. doi: 10.1248/cpb.38.3384. [DOI] [PubMed] [Google Scholar]
- 489.Morita H, Nakayama M, Kojima H, Takeya K, Itokawa H, Schenkel EP, Motidome M. Chem. Pharm. Bull. 1991;39:693–697. doi: 10.1248/cpb.39.693. [DOI] [PubMed] [Google Scholar]
- 490.dos Santos AG, Ferreira PMP, Júnior G. M. Vieira, Perez CC, Tininis AG, Silva GH, da Silva Bolzani V, Costa-Lotufo LV, do Ó Pessoa C, Cavalheiro AJ. Chem. Biodiversity. 2010;7:205–215. doi: 10.1002/cbdv.200800342. [DOI] [PubMed] [Google Scholar]
- 491.Prieto AM, dos Santos AG, Oliveira APS, Cavalheiro AJ, Silva DHS, Bolzani VS, Varanda EA, Soares CP. Food Chem. Toxicol. 2013;53:153–159. doi: 10.1016/j.fct.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 492.Ferreira PMP, Santos AG, Tininis AG, Costa PM, Cavalheiro AJ, Bolzani VS, Moraes MO, Costa-Lotufo LV, Montenegro RC, Pessoa C. Chem.-Biol. Interact. 2010;188:497–504. doi: 10.1016/j.cbi.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 493.Prieto AM, Santos AG, Csipak AR, Caliri CM, Silva IC, Arbex MA, Silva FS, Marchi MRR, Cavalheiro AJ, Silva DHS, Bolzani VS, Soares CP. Toxicol. Appl. Pharmacol. 2012;265:368–372. doi: 10.1016/j.taap.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 494.Gordaliza M, del Corral J. M. Miguel, de la Puente ML, Garcia-Grávalos MD, San. Feliciano A. Bioorg. Med. Chem. Lett. 1997;7:1649–1654. [Google Scholar]
- 495.Ohsaki A, Yan LT, Ito S, Edatsugi H, Iwata D, Komoda Y. Bioorg. Med. Chem. Lett. 1994;4:2889–2892. [Google Scholar]
- 496.Jung HJ, Chung SY, Nam J-W, Chae SW, Lee Y-J, Seo E-K, Lee HJ. Chem. Biodiversity. 2010;7:2095–2101. doi: 10.1002/cbdv.201000010. [DOI] [PubMed] [Google Scholar]
- 497.Dhanasekaran M, Baskar A-A, Ignacimuthu S, Agastian P, Duraipandiyan V. Invest. New Drugs. 2009;27:347–355. doi: 10.1007/s10637-008-9181-9. [DOI] [PubMed] [Google Scholar]
- 498.Grynberg NF, Echevarria A, Lima JE, Pamplona SSR, Pinto AC, Maciel MAM. Planta Med. 1999;65:687–689. doi: 10.1055/s-1999-14042. [DOI] [PubMed] [Google Scholar]
- 499.Aviello G, Borrelli F, Guida F, Romano B, Lewellyn K, Chiaro MD, Luongo L, Zjawiony JK, Maione S, Izzo AA, Capasso R. J. Mol. Med. 2011;89:891–902. doi: 10.1007/s00109-011-0752-4. [DOI] [PubMed] [Google Scholar]
- 500.Fichna J, Dicay M, Lewellyn K, Janecka A, Zjawiony JK, MacNaughton WK, Storr MA. Inflammatory Bowel Dis. 2012;18:1137–1145. doi: 10.1002/ibd.21873. [DOI] [PubMed] [Google Scholar]
- 501.Benrezzouk R, Terencio MC, Ferrándiz ML, San Feliciano A, Gordaliza M, del Corral J. M. Miguel, de la Puente ML, Alcaraz MJ. Life Sci. 1999;64:205–211. doi: 10.1016/s0024-3205(99)00119-8. [DOI] [PubMed] [Google Scholar]
- 502.Chang HL, Chang FR, Chen JS, Wang HP, Wu YH, Wang CC, Wu YC, Hwang TL. Eur. J. Pharmacol. 2008;586:332–339. doi: 10.1016/j.ejphar.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 503.Mambu L, Ramanandraibe V, Martin MT, Blond A, Grellier P, Frappier F. Planta Med. 2002;68:377–379. doi: 10.1055/s-2002-26748. [DOI] [PubMed] [Google Scholar]
- 504.Kuria KAM, Chepkwony H, Govaerts C, Roets E, Busson R, Witte P. d., Zupko I, Hoornaert G, Quirynen L, Maes L, Janssens L, Hoogmartens J, Laekeman G. J. Nat. Prod. 2002;65:789–793. doi: 10.1021/np0104626. [DOI] [PubMed] [Google Scholar]
- 505.Coll J, Tandrón YA. Phytochem. Rev. 2008;7:25–49. [Google Scholar]
- 506.Sathe M, Ghorpade R, Srivastava AK, Kaushik MP. J. Ethnopharmacol. 2010;130:171–174. doi: 10.1016/j.jep.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 507.Bautista E, Toscano A, Calzada F, Díaz E, Yépez-Mulia L, Ortega A. J. Nat. Prod. 2013;76:1970–1975. doi: 10.1021/np400606g. [DOI] [PubMed] [Google Scholar]
- 508.Gupta VK, Verma S, Pal A, Srivastava SK, Srivastava PK, Darokar MP. Appl. Microbiol. Biotechnol. 2013;97:9121–9131. doi: 10.1007/s00253-013-5154-9. [DOI] [PubMed] [Google Scholar]
- 509.Bisio A, Schito AM, Ebrahimi SN, Hamburger M, Mele G, Piatti G, Romussi G, Dal Piaz F, De Tommasi N. Phytochemistry. 2015;110:120–132. doi: 10.1016/j.phytochem.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 510.Ben Jannet H, Chaari A, Mighri Z, Martin MT, Loukaci A. Phytochemistry. 1999;52:1541–1545. [Google Scholar]
- 511.Maciel MAM, Pinto AC, Arruda AC, Pamplona SGSR, Vanderlinde FA, Lapa AJ, Echevarria A, Grynberg NF, Colus IMS, Farias RAF, Costa AML, Rao VSN. J. Ethnopharmacol. 2000;70:41–55. doi: 10.1016/s0378-8741(99)00159-2. [DOI] [PubMed] [Google Scholar]
- 512.Maciel MAM, Dantas TNC, Pinto AC, Veiga VF, Grymberg NF, Echevarria A. Curr. Top. Phytochem. 2005;7:73–88. [Google Scholar]
- 513.Maciel MAM, Dantas TNC, Câmara JKP, Pinto AC, Veiga VF, Kaiser CR, Pereira NA, Carneiro CMTS, Vanderlinde FA, Lapa AJ, Agner AR, Cóllus IMS, Echevarria-Lima J, Grynberg NF, Esteves-Souza A, Pissinate K, Echevarria A. Adv. Phytomed. 2006;2:225–253. [Google Scholar]
- 514.Maciel MAM, Cortez JKPC, Gomes FES. Rev. Fitos. 2006;2:54–73. [Google Scholar]
- 515.Costa MPD, Magalhães NSS, Gomes FES, Maciel MAM. Rev. Bras. Farmacogn. 2007;17:275–286. [Google Scholar]
- 516.Kubo I, Klocke JA, Asano S. J. Insect Physiol. 1983;29:307–316. [Google Scholar]
- 517.Costa AML, Silva JCR, Campos AR, Rao VSN, Maciel MAM, Pinto AC. Phytother. Res. 1999;13:689–691. doi: 10.1002/(sici)1099-1573(199912)13:8<689::aid-ptr532>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 518.Carvalho JT, Silva MFC, Maciel MAM, Pinto AC, Nunes DS, Lima RM, Bastos JK, Sarti SJ. Planta Med. 1996;62:402–404. doi: 10.1055/s-2006-957925. [DOI] [PubMed] [Google Scholar]
- 519.Farias RAF, Rao VSN, Viana GSB, Silveira ER, Maciel MAM, Pinto AC. Planta Med. 1997;63:558–560. doi: 10.1055/s-2006-957766. [DOI] [PubMed] [Google Scholar]
- 520.Khan MTH, Ather A, Pinto AC, Maciel MAM. Rev. Bras. Farmacogn. 2009;19:7–13. [Google Scholar]
- 521.Silva RM, Oliveira FA, Cunha KMA, Maia JL, Maciel MAM, Pinto AC, Nascimento NRF, Santos FA, Rao VSN. Vasc. Pharmacol. 2005;43:11–18. doi: 10.1016/j.vph.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 522.Hunter MS, Corley DG, Carron CP, Rowold E, Kilpatrick BF, Durley RC. J. Nat. Prod. 1997;60:894–899. doi: 10.1021/np970141n. [DOI] [PubMed] [Google Scholar]
- 523.Torres LMB, Gamberini MT, Roque NF, Lima-Landman MT, Souccar C, Lapa AJ. Phytochemistry. 2000;55:617–619. doi: 10.1016/s0031-9422(00)00223-5. [DOI] [PubMed] [Google Scholar]
- 524.Sashidhara KV, Singh SP, Srivastava A, Puri A, Chhonker YS, Bhatta RS, Shah P, Siddiqi MI. Eur. J. Med. Chem. 2011;46:5206–5211. doi: 10.1016/j.ejmech.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 525.Kawasaki T, Komori T, Setoguchi S. Chem. Pharm. Bull. 1968;16:2430–2435. [Google Scholar]
- 526.Murray RDH, Jorge ZD, Khan NH, Shahjahan M, Quaisuddin M. Phytochemistry. 1984;23:623–625. [Google Scholar]
- 527.Ma M, Jiang Z, Ruan J, Tan X, Liu J, Wang C, Zha XM, Zhang L. Exp. Toxicol. Pathol. 2012;64:611–618. doi: 10.1016/j.etp.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 528.Wang J, Liang Q, Ji L, Liu H, Wang C, Wang Z. Hum. Exp. Toxicol. 2010;30:1333–1341. doi: 10.1177/0960327110389926. [DOI] [PubMed] [Google Scholar]
- 529.Ma Y, Niu C, Wang J, Ji L, Wang Z. Hum. Exp. Toxicol. 2014;33:729–736. doi: 10.1177/0960327113506232. [DOI] [PubMed] [Google Scholar]
- 530.Gori L, Galluzzi P, Mascherini V, Gallo E, Lapi F, Menniti-Ippolito F, Raschetti R, Mugelli A, Vannacci A, Firenzuoli F. Basic Clin. Pharmacol. Toxicol. 2011;109:521–526. doi: 10.1111/j.1742-7843.2011.00781.x. [DOI] [PubMed] [Google Scholar]
- 531.Starakis I, Siagris D, Leonidou L, Mazokopakis E, Tsamadas A, Karatza C. Eur. J. Gastroenterol. Hepatol. 2006;18:681–6823. doi: 10.1097/00042737-200606000-00016. [DOI] [PubMed] [Google Scholar]
- 532.Chitturi S, Farrell GC. Eur. J. Gastroenterol. Hepatol. 2008;23:366–373. doi: 10.1111/j.1440-1746.2008.05310.x. [DOI] [PubMed] [Google Scholar]
- 533.Shriram V, Jahagirdar S, Latha C, Kumar V, Puranik V, Rojatkar S, Dhakephalkar PK, Shitole MG. Int. J. Antimicrob. Agents. 2008;32:405–410. doi: 10.1016/j.ijantimicag.2008.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.