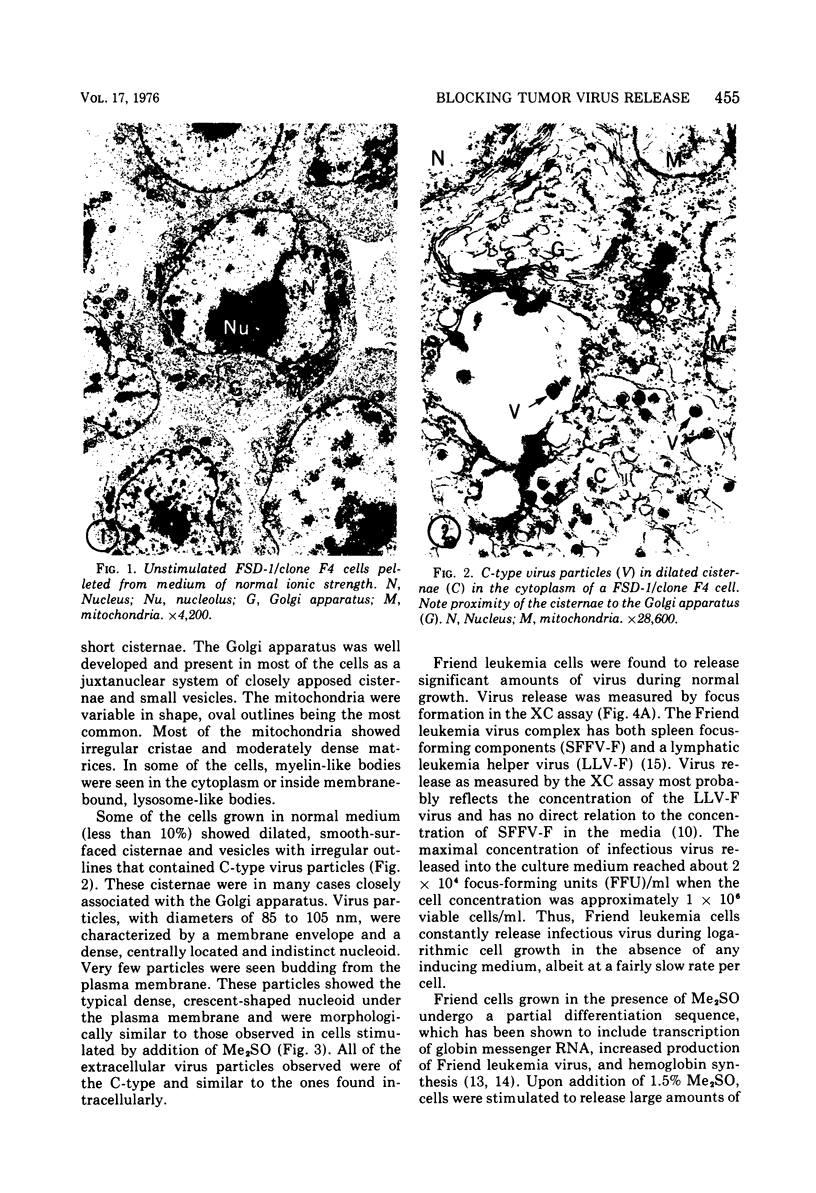
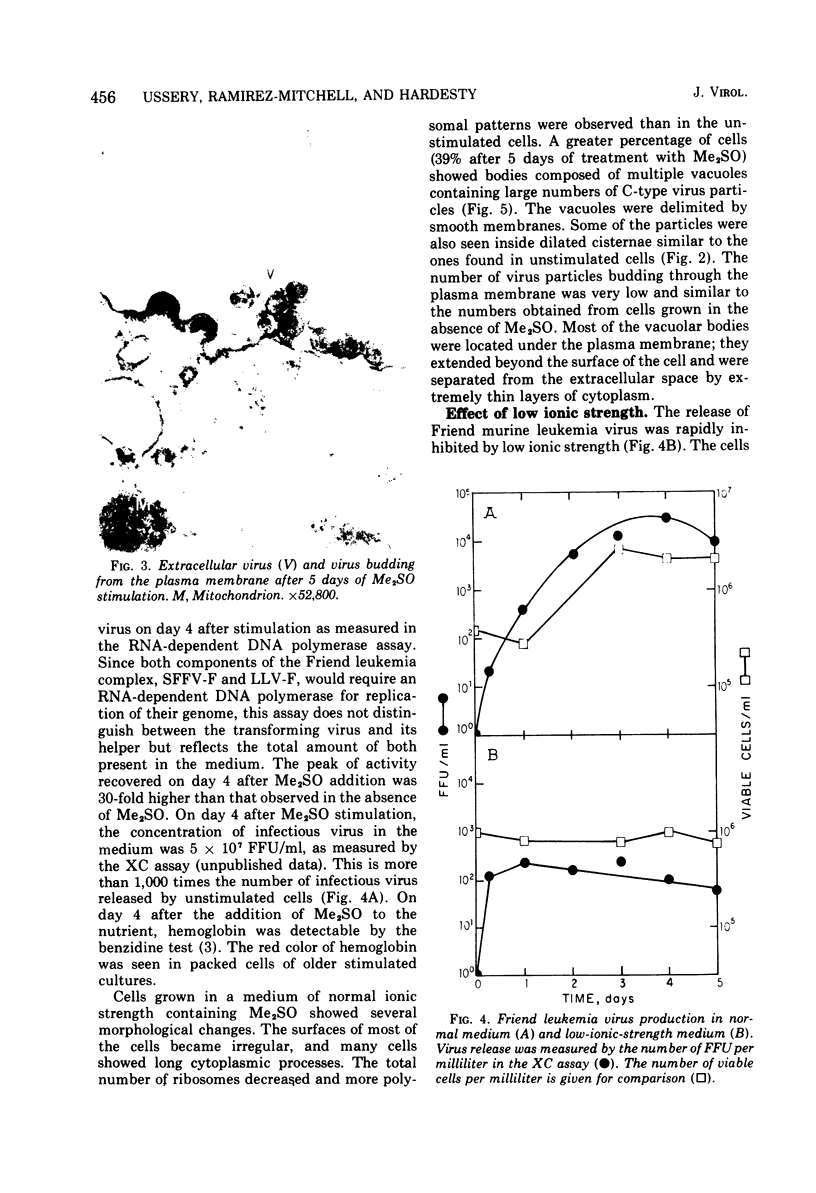
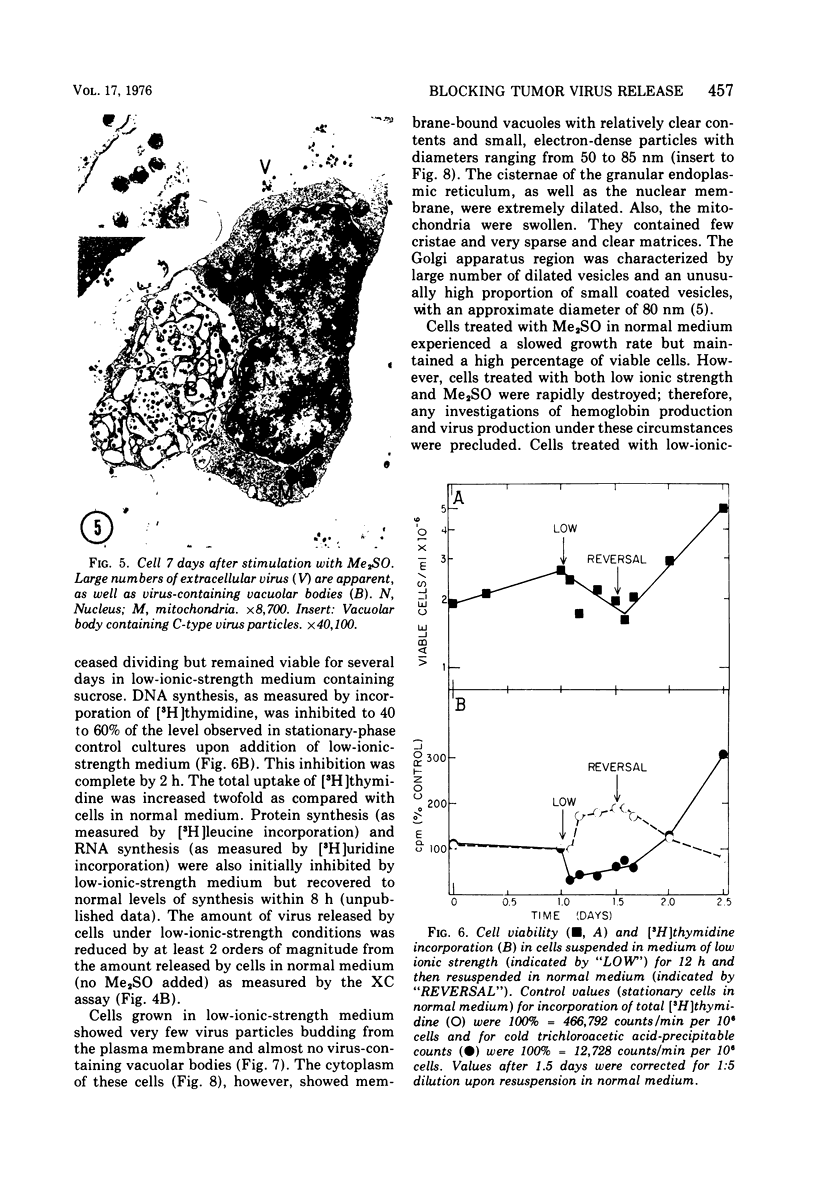
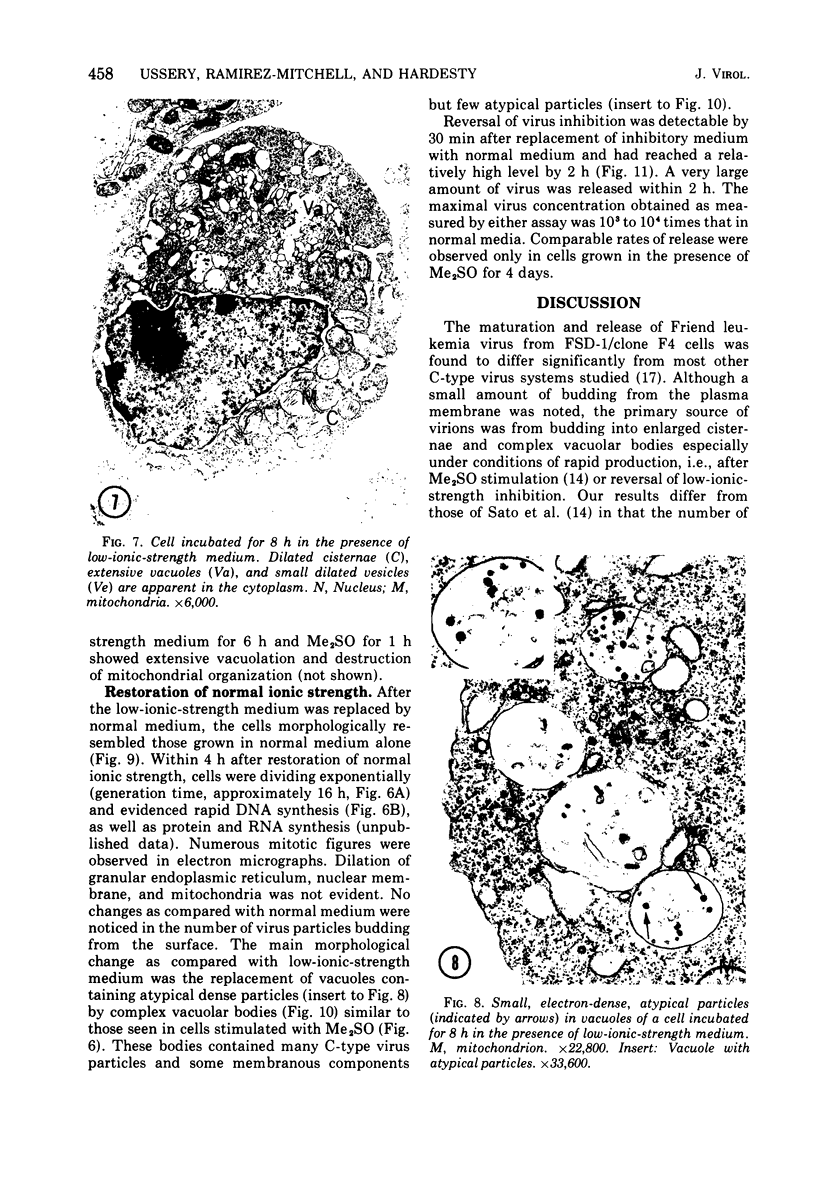
Abstract
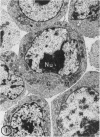
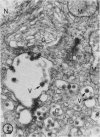
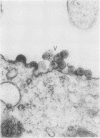
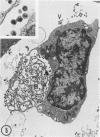
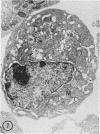
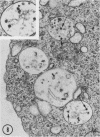
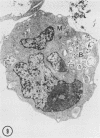
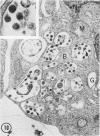
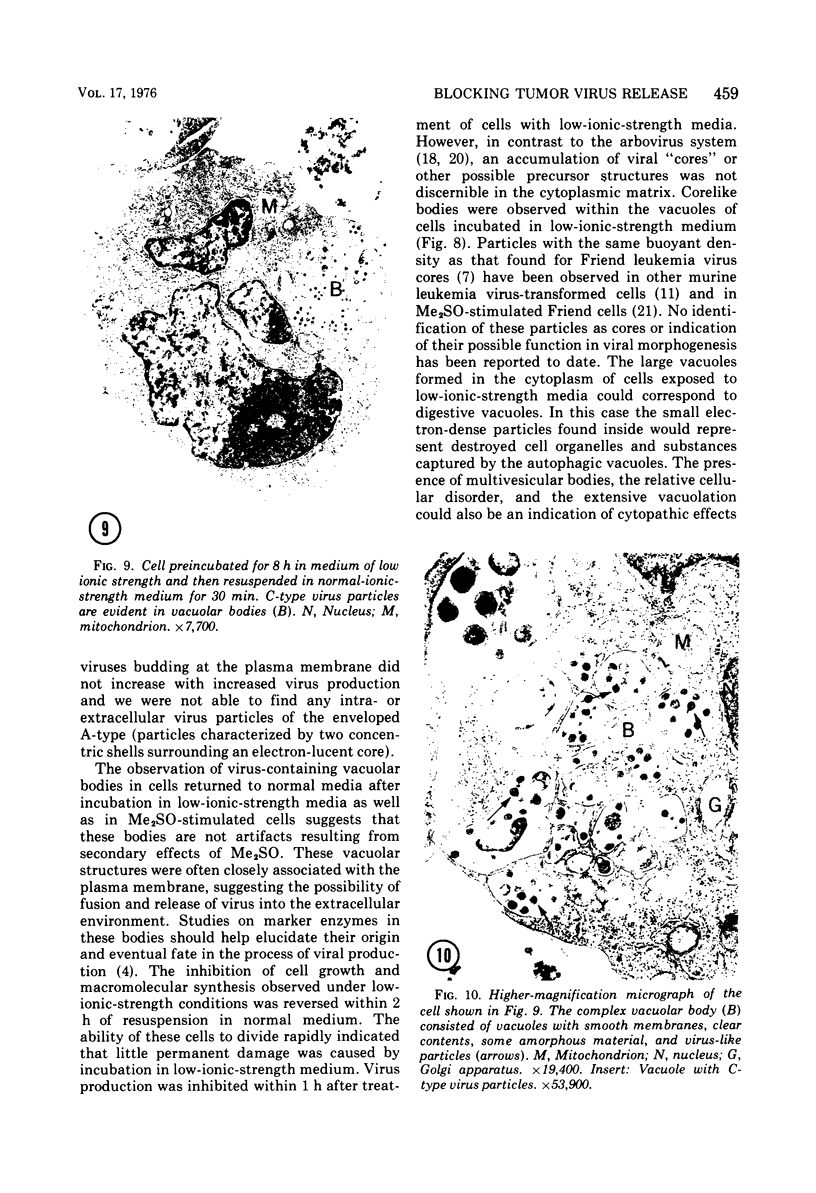
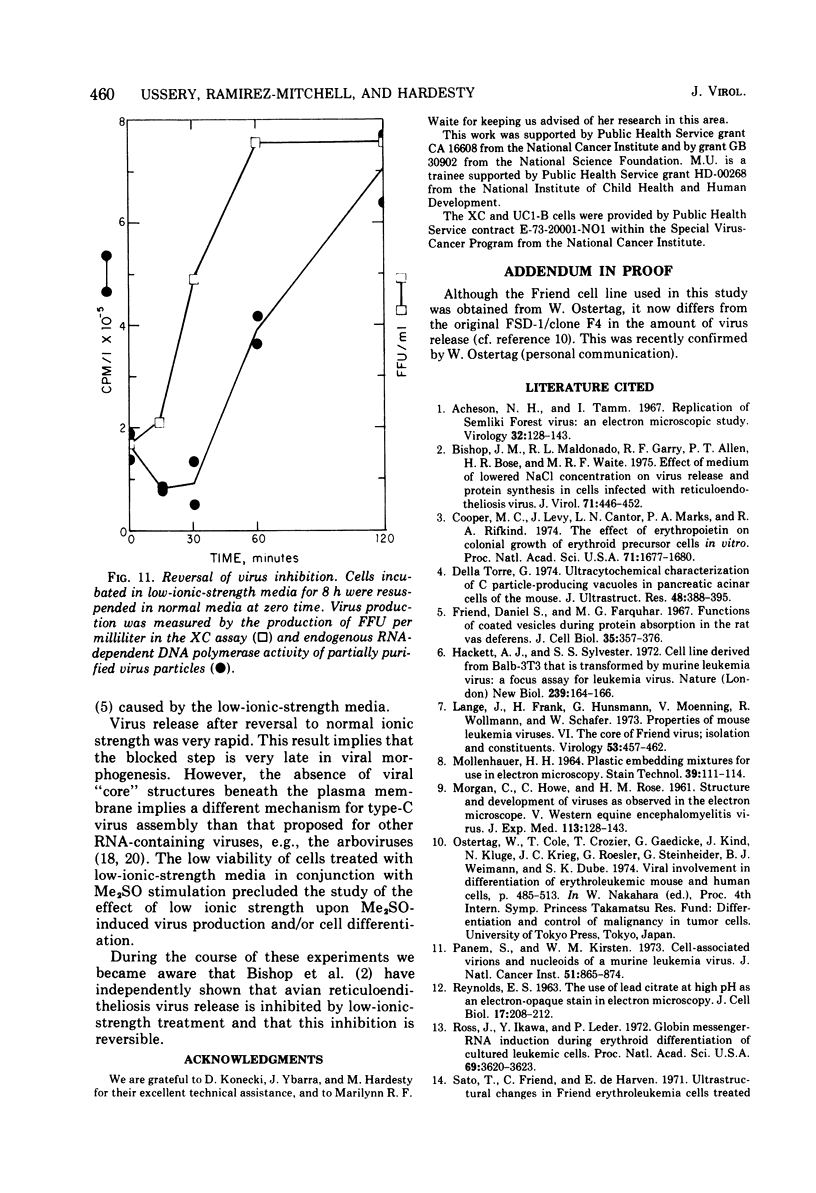
The effect of medium of low ionic strength on the release of virus from Friend leukemia cells has been studied. The release of infectious Friend leukemia virus is almost completely inhibited in medium of low ionic strength, as measured by a focus-forming assay (XC assay), by endogenous RNA-dependent DNA polymerase activity of released virus particles, and by electron microscope studies of the production of C-type particles. Friend leukemia virus-transformed proerythroblasts undergo extensive morphological changes in low-ionic-strength medium. The cells are viable in this medium, but they can no longer be stimulated with dimethyl sulfoxide to produce hemoglobin and increase virus production. Infectious virus is released between 30 and 120 min of resuspension of inhibited cells in normal medium. The rate of virus release after reversal of the inhibition is much greater than the rate of virus release during normal cell growth. The morphological changes occurring after dimethyl sulfoxide stimulation of Friend leukemia cells are compared with those resulting from resuspension in normal medium of cells inhibited by low ionic strength.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Cooper M. C., Levy J., Cantor L. N., Marks P. A., Rifkind R. A. The effect of erythropoietin on colonial growth of erythroid precursor cells in vitro. Proc Natl Acad Sci U S A. 1974 May;71(5):1677–1680. doi: 10.1073/pnas.71.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S., Farquhar M. G. Functions of coated vesicles during protein absorption in the rat vas deferens. J Cell Biol. 1967 Nov;35(2):357–376. doi: 10.1083/jcb.35.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A. J., Sylvester S. S. Cell line derived from Balb-3T3 that is transformed by murine leukaemia virus: a focus assay for leukaemia virus. Nat New Biol. 1972 Oct 11;239(93):164–166. doi: 10.1038/newbio239164a0. [DOI] [PubMed] [Google Scholar]
- Lange J., Frank H., Hunsmann G., Moennig V., Wollmann R., Schäfer W. Properties of mouse leukemia viruses. VI. The core of Friend virus; isolation and constituents. Virology. 1973 Jun;53(2):457–462. doi: 10.1016/0042-6822(73)90225-0. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Panem S., Kirsten W. H. Cell-associated virions and nucleoids of a murine leukemia virus. J Natl Cancer Inst. 1973 Sep;51(3):865–874. doi: 10.1093/jnci/51.3.865. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Ikawa Y., Leder P. Globin messenger-RNA induction during erythroid differentiation of cultured leukemia cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3620–3623. doi: 10.1073/pnas.69.12.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Friend C., De Harven E. Ultrastructural changes in Friend erythroleukemia cells treated with dimethyl sulfoxide. Cancer Res. 1971 Oct;31(10):1402–1417. [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Bennett M., Mirand E. A., Trudel P. J. Isolation and characterization of a lymphatic leukemia virus in the Friend virus complex. J Natl Cancer Inst. 1971 Jun;46(6):1209–1217. [PubMed] [Google Scholar]
- Syrewicz J. J., Naso R. B., Wang C. S., Arlinghaus R. B. Purification of large amounts of murine ribonucleic acid tumor viruses produced in roller bottle cultures. Appl Microbiol. 1972 Sep;24(3):488–494. doi: 10.1128/am.24.3.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Brown D. T., Pfefferkorn E. R. Inhibition of Sindbis virus release by media of low ionic strength: an electron microscope study. J Virol. 1972 Sep;10(3):537–544. doi: 10.1128/jvi.10.3.537-544.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Effect of altered osmotic pressure on the growth of Sindbis virus. J Virol. 1968 Jul;2(7):759–760. doi: 10.1128/jvi.2.7.759-760.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Inhibition of Sindbis virus production by media of low ionic strength: intracellular events and requirements for reversal. J Virol. 1970 Jan;5(1):60–71. doi: 10.1128/jvi.5.1.60-71.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann B. J., Schmidt J., Wolfrum D. I. RNA-dependent DNA polymerase and ribonuclease H from Friend virions. FEBS Lett. 1974 Jul 1;43(1):37–44. doi: 10.1016/0014-5793(74)81100-2. [DOI] [PubMed] [Google Scholar]