Summary
The activation of the immune system for a selective removal of tumor cells represents an attractive strategy for the treatment of metastatic malignancies, which cannot be cured by existing methodologies. In this review, we examine the design and therapeutic potential of immunocytokines and bispecific antibodies, two classes of bifunctional products which can selectively activate the immune system at the tumor site. Certain protein engineering aspects, such as the choice of the antibody format, are common to both classes of therapeutic agents and can have a profound impact on tumor homing performance in vivo of individual products. However, immunocytokines and bispecific antibodies display different mechanisms of action. Future research activities will reveal whether an additive of even synergistic benefit can be obtained from the judicious combination of these two types of biopharmaceutical agents.
Keywords: Immunocytokines, Bispecific Antibodies, Immunotherapy of Cancer, Antibody engineering, Armed antibodies
Using the immune response to attack tumors
The pharmacotherapy of cancer has relied for many decades on chemotherapy (for the majority of solid and liquid tumors) and on hormone therapy (for certain classes of hormone-sensitive tumors). While therapeutic success has been achieved for various types of hematological malignancies and some solid tumors (e.g., metastatic testicular cancer), the majority of disseminated forms of solid cancer remain incurable. The therapeutic efficacy of conventional cancer therapeutics is often limited not only by the activity of multidrug resistance proteins and by the insurgence of mutations, but also by the inability of small organic molecules to accumulate in sufficient amounts at the site of disease (1–3). These limitations have stimulated the investigation of alternative strategies for the cancer treatment.
Due to their ability to recognize cognate antigens with exquisite specificity, monoclonal antibodies have attracted considerable interest as biopharmaceutical agents for the selective inhibition of tumor-promoting factors or for the selective ablation of cancer cells. A number of antibody products have gained marketing authorization for cancer therapy (4, 5). However, since cancer cures are rarely observed with antibody products in patients with metastatic solid malignancies, substantial research efforts have been devoted to the development of “armed” forms of antibody therapeutics, in which the antibody molecule serves as delivery vehicle for potent bioactive agents (e.g., drugs with cleavable linkers, cytokines, radionuclides) (6). In this context, the development of bispecific antibodies (capable of simultaneous recognition of a tumor cell and of a lymphocyte) and of antibody-cytokine fusion proteins (also termed “immunocytokines”) represents a promising area of antibody engineering research, with the potential to selectively modulate the activity of the immune system at the site of disease. This review focuses on these two classes of therapeutic agents, highlighting similarities and differences, opportunities and challenges.
Bispecific antibodies and immunocytokines represent only two of the many classes of biopharmaceutical agents, which are being considered for industrial applications, harnessing the activity of the patient’s immune system to fight tumors. Other classes of immunomodulatory products, including immunological checkpoint inhibitors (7, 8), recombinant cytokines (9), vaccines (10) and engineered T-cells (11) have been reviewed elsewhere and will not be discussed in this article.
Recent advances in cancer genome sequencing have provided a quantitative analysis of the somatic mutation rates in thousands of cancer specimens for various types of malignancies (12). On average, the majority of tumor cells contains more than one million mutations. As a consequence, it can be expected that some of these mutations, corresponding to expressed genes and to non-synonymous variants, may correspond to peptides, suitable for presentation in MHC class I or class II proteins.
Various lines of evidence suggest that tumor cells may be recognized and attacked by cellular components of the immune system, including lymphocytes and natural killer (NK) cells. The “immune surveillance” hypothesis (13) is supported by many observations, including the following ones:
-
(i)
mice lacking certain important components of the immune system develop tumors
-
(ii)
immunosuppressed patients (e.g., HIV patients, post-transplant patients) develop tumors more frequently compared to normal individuals
-
(iii)
certain immunostimulatory products (e.g., cytokines, cancer vaccines, antibodies against immunological checkpoint inhibitors) can induce potent therapeutic responses (and, sometimes, cures) in mouse models of cancer and in patients
-
(iv)
allogeneic bone marrow transplantation for leukemia is largely successful via the “graft versus leukemia” effect
At the same time, tumors may avoid immune recognition by a variety of mechanisms. For example, the down-regulation of peptide:MHC complexes or of co-stimulatory molecules may decrease the efficacy of T cell-based killing activity (14, 15). Many tumors create an immunosuppressive environment by a local upregulation of anti-inflammatory mediators, such as TGF-β or IL10 (16). Furthermore, tumor cells can accumulate mutations, which inhibit crucial pathways for programmed cell death, making them less susceptible to the action of death signals (e.g., FasL/Fas interaction, granzymes) (17). The relatively low density of lymphocytes at the tumor site and the relatively high proportion of immunosuppressive regulatory T cells (Treg) have been proposed as a characteristic feature of a tumor-permissive environment, which needs to be altered in successful immunotherapy (18), leading to a massive infiltration of T cells and/or NK cells into the neoplastic mass.
The tumor environment is often characterized by the presence of proteins, which are typically not found in normal adult tissues and which can be used as targets for the generation of monoclonal antibodies. Indeed, both immunocytokine and bispecific antibody products typically incorporate at least one antibody moiety directed against a tumor-associated antigen, in order to mediate a preferential accumulation of the therapeutic agent at the site of disease. Many tumor-associated antigens have been proposed as possible targets for cancer therapy applications, including proteins found on the tumor cell surface (such as carcinoembryonic antigen, prostate-specific membrane antigen, A33 and various CD antigens) or components of the modified extracellular matrix (e.g., splice isoforms of fibronectins and of tenascins). As we will see later in this review, both target antigen and antibody format crucially influence pharmacokinetic behavior and therapeutic activity of the corresponding biopharmaceutical agent.
Immunocytokines
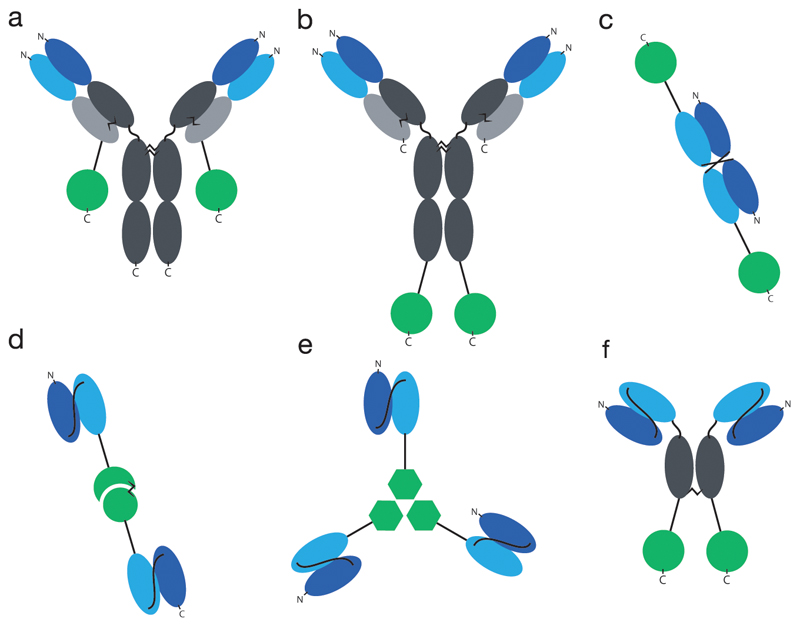
Immunocytokines are fusion proteins of antibodies and cytokines. Antibodies can be used as full IgG’s or as recombinant fragments, leading to a large variety of possible formats, some of which are depicted in Figure 1. Some cytokine payloads contain multiple subunits (e.g., those belonging to the IL12 superfamily), thus creating additional possibilities for the topological joining of antibody and cytokine moieties.
Fig. 1. Overview of common formats for immunocytokines.
a) IgG format, cytokine fused to the light chain, b) IgG format, cytokine fused to the heavy chain, c) Diabody, d) bivalent scFv format, here in fusion with heterodimeric cytokine IL12, e) trivalent scFv format, here in fusion with trimeric cytokine TNF, f) SIP format. Constant regions indicated in grey, VH indicated in dark blue, VL indicated in light blue, cytokines indicated in green (circle: monomeric cytokine like e.g. IL2, circle connected to half-circle: heterodimeric cytokine like e.g. IL12, hexagon: homotrimeric cytokine like e.g. TNF).
Concepts and formats
IgG-based immunocytokines may exhibit a long circulatory half-life, as a consequence of FcRn-mediated antibody recycling (19). However, some IgG-IL2 fusion proteins have exhibited rather short and dose-dependent pharmacokinetic profiles, for reasons which are still not fully understood (20). By contrast, immunocytokine products based on smaller recombinant antibody fragments (e.g., scFv fragments) typically display faster blood clearance profiles, which may be beneficial in order to reduce side effects associated with the use of potent pro-inflammatory cytokine payloads.
Table 1 lists some of the cytokines, which have been fused to antibodies for disease-targeting applications. The table indicates the target antigen, the recombinant protein format and whether quantitative biodistribution studies have been published.
Table 1. Comprehensive overview of immunocytokines that have been studied in animal tumor models.
The name of the immunocytokine, the target antigen, the format and whether quantitative biodistribution data was published, are indicated.
| Name | Target | Format | Published quantitative biodistribution data | Reference | Name | Target | Format | Published quantitative biodistribution data | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cytokines | |||||||||
| Anti-HER2/neu IgG3-GMCSF | HER2/neu | IgG | Yes | (121) | F8-TNF | EDA | scFv | Yes | (28) |
| CLL1-GMCSF | MHC II | IgG | Yes | (122) | FAP-TNF | FAP | F(ab)2 | n.a. | (123) |
| L19-GMCSF | EDB | Db | Yes | (31) | G250-TNF | CAIX | F(ab)2 | Yes | (124) |
| 20-2b (IFNα) | CD20 | IgG | n.a. | (125) | L19-TNF | EDB | scFv | Yes | (37) |
| Anti-CD20-IFNα | CD20 | IgG | n.a. | (47) | MFE23-TNF | CEA | scFv | Yes | (126) |
| Anti-HER2/neu IgG3-IFNα | HER2/neu | IgG | n.a. | (127) | scFvMEL-TNF | gp240 | scFv | Yes | (128) |
| C2-2b-2b (IFNα) | HLA-DR | IgG | n.a. | (129) | TNF-B1 | LeY | scFv | n.a. | (130) |
| F8-IFNα | EDA | Db | Yes | (27) | TNF-FuP | EGFR | IgG | Yes | (131) |
| F8-IFNγ | EDA | Db | Yes | (30) | TNF-TNT3 | DNA | IgG | Yes | (132) |
| L19-IFNγ | EDB | scFv | Yes | (133) | ZME/TNFα | gp240 | IgG | Yes | (134) |
| TNT3-IFNγ | DNA | IgG | Yes | (46) | |||||
| F8-IL1β | EDA | Db | Yes | (25) | Chemokines | ||||
| 2aG4-IL2 | PS | IgG | n.a. | (135) | CCL5 | Db | n.a. | (136) | |
| Anti-CEA-IL2 | CEA | scFv-Fc | Yes | (137) | CCL17 | Db | n.a. | (136) | |
| Di-Leu16-IL2 | CD20 | IgG | n.a. | (138) | CCL19 | Db | Yes | (136) | |
| Anti-HER2/neu IgG3-IL2 | HER2/neu | IgG | n.a. | (139) | CCL20 | Db | Yes | (136) | |
| CEA-IL2v | CEA | IgG | n.a. | (140) | CCL21 | Db | Yes | (136) | |
| ch14.18-IL2 | GD2 | IgG | Yes | (20) | CXCL9 | Db | No | (136) | |
| ch225-IL2 | EGF | IgG | n.a. | (20) | CXCL10 | scFv | Yes | (136) | |
| CLL1-IL2 | MHC II | IgG | Yes | (122) | CXCL11 | Db | n.a. | (136) | |
| F8-IL2 | EDA | Db | Yes | (141) | ITIP | Db | n.a. | (136) | |
| F16-IL2 | Tnc A1 | Db | Yes | (69) | |||||
| FUMK1-IL2 | EpCAM | scFv | Yes | (142) | TNF superfamily members | ||||
| IL2-FuP | EGFR | IgG | Yes | (131) | TRAIL | scFv | Yes | (143) | |
| IL2-MOV19 | α-FR | scFv | Yes | (144) | TRAILtrunc | scFv | Yes | (143) | |
| KS-IL2 | EpCAM | IgG | Yes | (45) | CD40L | scFv | Yes | (143) | |
| L19-IL2 | EDB | scFv | Yes | (35) | FasL | scFv | Yes | (143) | |
| NHS-IL2LT | DNA | IgG | n.a. | (52) | LIGHT | scFv | Yes | (143) | |
| F8-IL4 | EDA | Db | Yes | (41) | VEGI | scFv | Yes | (143) | |
| F8-IL6 | EDA | Db | Yes | (25) | VEGItrunc | scFv | Yes | (143) | |
| F8-IL7 | EDA | Db | Yes | (145) | LTα | scFv | Yes | (143) | |
| F8-IL9 | EDA | Db | Yes | (33) | LTβ | scFv | Yes | (143) | |
| F8-IL10 | EDA | Db | Yes | (146) | LTα1/β2 | Db | Yes | (143) | |
| L19-IL10 | EDB | Db | Yes | (147) | |||||
| BC1-IL12 | EDB | IgG | n.a. | (83) | Other payloads | ||||
| chTNT3-IL12 | DNA | IgG | Yes | (81) | B7.2 | Db | Yes | (34) | |
| F8-p35/p40-F8 | EDA | scFv | Yes | (148) | tTF | scFv | Yes | (149) | |
| IL12-L19 | EDB | SIP | Yes | (29) | TNFR | scFv | n.a. | (146) | |
| IL12-L19 | EDB | scFv | Yes | (36) | VEGF-A164 | scFv | Yes | (32) | |
| IL12-SS1 | MSLN | scFv | n.a. | (150) | VEGF-A120 | scFv | Yes | (32) | |
| KS-IL12 | EpCAM | IgG | n.a. | (151) | |||||
| KS-IL12/IL2 | EpCAM | IgG | n.a. | (44) | |||||
| L19-p35/p40-L19 | EDB | scFv | Yes | (29) | |||||
| mscIL12-her2.IgG3 | HER2/neu | IgG | n.a. | (152) | |||||
| F8-IL13 | EDA | Db | Yes | (153) | |||||
| Anti-GD2-RLI (IL15) | GD2 | IgG | n.a. | (154) | |||||
| L19-IL15 | EDB | Db | Yes | (31) | |||||
| F8-IL17/IL17-F8 | EDA | scFv-homodimeric cytokine-scFv | Yes | (155) | |||||
| Anti-CD20-IL21 | CD20 | IgG | n.a. | (156) | |||||
Our laboratory has systematically investigated the tumor-targeting properties of immunocytokines directed against the alternatively-spliced extradomain A (EDA) and extradomain B (EDB) of fibronectin, recognized by the F8 and L19 antibodies, respectively (21, 22). These targets are particularly suited for immunocytokine development activities, as they are (i) conserved from mouse to man; (ii) virtually undetectable in normal adult tissues (exception made for the endometrium in the proliferative phase and placenta), while being expressed in the majority of solid tumors and lymphomas. Analysis of biodistribution studies reveals five main types of pharmacokinetic behavior, which can be summarized as follows:
-
(a)
payloads which can be efficiently delivered at the tumor site by fusion to antibodies (e.g., IL2, IL4, IL6, IL10, IFNa, TNF) (23–28)
-
(b)
payloads which can be efficiently delivered to the tumor in some immunocytokine format but not in others (e.g., IL12) (29)
-
(c)
payloads which are trapped by receptors at low doses, but which regain tumor targeting performance at higher doses (e.g., when a cognate receptor has been saturated in vivo (e.g., IFNg, GM-CSF) (30, 31)
-
(d)
payloads which are too positively or negatively charged, or simply too large, thus preventing efficient extravasation (e.g., VEGF164 vs. VEGF120 in the mouse) (32)
-
(e)
payloads which are extensively glycosylated and which are rapidly captured by the asialoglycoprotein receptor in the liver and, as a consequence, removed from circulation (e.g., IL9 produced in certain experimental conditions, B7 proteins) (33, 34)
In general, we prefer to use antibodies in bivalent or trivalent formats, in order to ensure a high binding avidity to the target and, consequently, a long residence time at the tumor site. We will discuss these aspects when examining the various formats of bispecific antibody products.
At the site of disease, pro-inflammatory cytokines can mediate various types of biological activity. For example, IL2, IL12 and TNF payloads mediate a massive infiltration of leukocytes (particularly T cells and NK cells) into the tumor mass, which may be responsible for the therapeutic activity of the products (35–38). Certain cytokines (most notably, TNF and IL2) activate the endothelium at the site of disease, favoring an increased uptake of therapeutic agents within the tumor mass (38, 39). The mechanism of antitumor activity for immunocytokine products can be difficult to establish, even though depletion experiments in immunocompetent mice facilitate the task to quantitatively assess the contribution of CD4+ T cells, CD8+ T cells and NK cells (40, 41). Alternative views on the contribution of tumor targeting to therapeutic activity have recently been proposed for IgG-based immunocytokines (42).
Not all cytokines that can be efficiently delivered to the tumor display a potent anti-cancer activity, at least not in all models. For example, murine IFNa and IL6 could efficiently be delivered to tumors but did not exhibit therapeutic activity when fused to antibodies specific to splice isoforms of fibronectin (25, 27). Interestingly, a xenograft model of U266 tumors in NSG mice showed prolonged survival in mice treated with IgG-IFNa fusion proteins, as compared to the control group (43).
The seminal work of the groups of Reisfeld, Gillies, Morrison and Epstein with IgG-based immunocytokines has revealed potent therapeutic activities for products based on IL2, IL12, GM-CSF, IFNa and IFNg in immunocompetent mice with various types of tumors, including Lewis Lung Carcinoma, CT26 colon carcinoma, MAD109 lung carcinoma, B78D14 melanoma and B cell lymphoma (44–48). This work is reviewed in (49–51) and will not be analyzed in detail here. It is important to mention, though, that in some tumor models T cells appeared to play a crucial role in the anticancer activity of the immunocytokine product (52, 53), while in other models NK cells appeared to be more important (35).
In our experience, immunocytokines are typically not curative in mice when used systemically at doses, which cause less than 5% loss of body weight. However, some tumor models are more sensitive than others and can be cured with immunocytokine monotherapy (e.g., TNF-based immunocytokines in mouse models of sarcoma) (28). Interestingly, cured mice may gain protective immunity, which makes them resistant against subsequent challenges with homologous or heterologous tumor cells (41).
Various types of anti-cancer therapeutic agents have been found to synergize with immunocytokine products, including external beam radiation (54–57), certain cytotoxic drugs (28, 58, 59), immunological checkpoint inhibitors (60), anti-cancer immunoglobulins acting via antibody-dependent cell-mediated cytoxicity (ADCC) mechanisms (61) and other immunocytokine products (38, 41, 62). This is an area of intense pharmaceutical research and we expect, in the coming years, to learn more about those products that can be potentiated by immunocytokines, and viceversa.
Table 2 lists immunocytokine products which have been studied in clinical trials in cancer patients.
Table 2. Immunocytokines in clinical trials.
The immunocytokine, the target antigen, the format, the indications, phases of clinical studies and the developing company are indicated.
| Compound | Target antigen | Format | Indications | Clinical phase | Developer |
|---|---|---|---|---|---|
| Di-Leu16-IL2 | CD20 | IgG | CD20+ Non-Hodgkin Lymphoma | Phase I/II | Alopexx Oncology, LLC |
| CEA-IL2v | CEA | IgG | Solid CEA+ cancers | Phase Ib | Roche Glycart |
| ch14.18-IL2 | GD2 | IgG | Melanoma, neuroblastoma | Phase I/II | Merck KGaA |
| F16-IL2 | TnC A1 | Db | AML, lung cancer | Phase II | Philogen |
| KS-IL2 | EpCAM | IgG | Ovarian cancer, colorectal cancer, NSCL carcinoma, prostate cancer | Phase I | Merck KGaA |
| L19-IL2 | EDB | Db | Melanoma, pancreas cancer, DLBCL | Phase II/III | Philogen |
| NHS-IL2LT | DNA | IgG | Non-Hodgkin lymphoma, NSCL cancer, melanoma | Phase I | Merck KGaA |
| F8-IL4 | EDA | Db | Oncology/Autoimmune diseases | In preparation | Philogen |
| BC1-IL12 | EDB | IgG | Melanoma, renal Cancer | Phase I/II | Antisoma |
| NHS-IL12 | DNA | IgG | Metastatic Solid Tumors | Phase I | Merck KGaA |
| L19-TNF | EDB | Trimeric scFv | Melanoma, sarcoma | Phase I/II | Philogen |
Below, we briefly summarize the main clinical findings for some of these products.
Selected examples
L19-TNF is a homotrimeric fusion protein, consisting of the L19 antibody in scFv format, fused to human TNF. The product has been shown to be well tolerated up to 13 µg/Kg in a monotherapy dose escalation trial, in which a Maximal Tolerated Dose was not established (63). Currently, the product is being investigated in combination with doxorubicin for the treatment of patients with metastatic soft tissue sarcoma, based on strong preclinical and clinical findings (28).
L19-IL2 is a fusion protein, consisting of the L19 antibody in diabody format, fused to human IL2. The product has been shown to be well tolerated at doses up to 22.5 Million IU IL2 equivalents, both when used as monotherapy (64) and in combination with dacarbazine (65). Initial signs of activity have been reported in patients with metastatic melanoma. The product is currently being investigated in combination with rituximab, for the treatment of patients with refractory/relapsed DLBCL. Furthermore, potent therapeutic activity has been reported for the intralesional treatment of Stage III melanoma lesions, both as monotherapy (66) and in combination with L19-TNF (67).
Similar to L19-IL2, also the F16-IL2 immunocytokine product is based on a diabody format, but the F16 antibody recognizes the alternatively-spliced A1 domain of tenascin-C (68, 69). Based on encouraging preclinical findings (23, 69, 70), the product has been studied in combination with paclitaxel or with doxorubicin for the treatment of patients with various types of malignancies (71), or in combination with low-dose cytarabine for the treatment of patients with acute myeloid leukemia (23, 72).
Hu14.18-IL2 was the first immunocytokine to enter clinical trials and is based on a IgG format. It is targeting the disialoganglioside GD2 (73), abundant in tumors of neuroectodermal origin, including melanoma. In preclinical findings, the immunocytokine’s murine analogue ch14.18-Il2 could eradicate metastases (20, 74) and could induce a tumor-specific protective immunity in syngeneic mouse models (75). Hu14.18-IL2 was studied in pediatric neuroblastoma patients (76) as well as in adult patients with melanoma (77). In a phase II study in pediatric patients with relapsed/refractory neuroblastoma, 21.7% of patients with a low tumor burden experienced a complete response (78). In a phase I/II study in adult melanoma patients no objective tumor regressions could be observed (79).
NHS-IL2LT is a fusion protein of the antibody NHS76, which targets nucleic acids in the necrotic core of tumors (80), and a mutant form of IL2 (IL2LT) with a lower toxicity profile (52). In syngeneic mouse tumor models of neuroblastoma and non-small cell lung cancer, NHS-IL2LT showed a substantial reduction of metastatic load in the lung and in the liver. In a phase I dose-escalation study, disease stabilizations over long periods were reported, but no objective tumor responses.
With anti-CEA-IL2v, Roche is developing a new class of immunocytokine products. The antibody GA504 targets carcinoembryonic antigen (CEA) and features an engineered heterodimeric Fc portion, which is devoid of FcγR and C1q binding. A single IL2 variant (IL2v), which was engineered not to bind to CD25, is appended at C-terminus of one of the two asymmetric antibody heavy chains.
IL12 based immunocytokines include NHS-IL12, an IL12 fusion protein based on the antibody chTNT3 in IgG format, which targets necrosis in tumors, and BC1-IL12, an IL12 fusion protein with the BC1 antibody, which recognizes an epitope on domain 7 of fibronectin. Both antibodies are fused to the p35 subunit of IL12 at the C-terminus of the heavy chain, with the p40 subunit forming a disulfide-linked heterodimer, while being expressed separately.
In a human PBL/SCID mouse model carrying DU145 prostatic carcinoma, a 44% reduction in tumor growth could be observed upon treatment with NHS-IL12 (81). NHS-IL12 was reported to induce a partial response in 2 out of 11 canine patients with spontaneously developed tumors (82). A phase I dose-determining study in humans was started in July 2011 but, to our knowledge, no results have been published so far.
BC1-IL12 showed initial signs of activity in various tumor models in SCID mice (83) and was well tolerated (MTD of 15 µg/kg) in patients with malignant melanoma and metastatic renal cell carcinoma (84).
Bispecific Antibodies
Bispecific antibodies are biopharmaceutical products, containing two antigen-binding specificities. This property can be achieved by the association of two antibodies or antibody fragments into a single molecular entity. For most therapeutic applications, one antibody moiety serves as pharmacodelivery vehicle (e.g., targeting a tumor-associated antigen), while the second antibody moiety is used to recruit and activate a suitable leukocyte (e.g., T cell recruitment, by a binding interaction with the CD3 membrane protein).
Concepts and formats
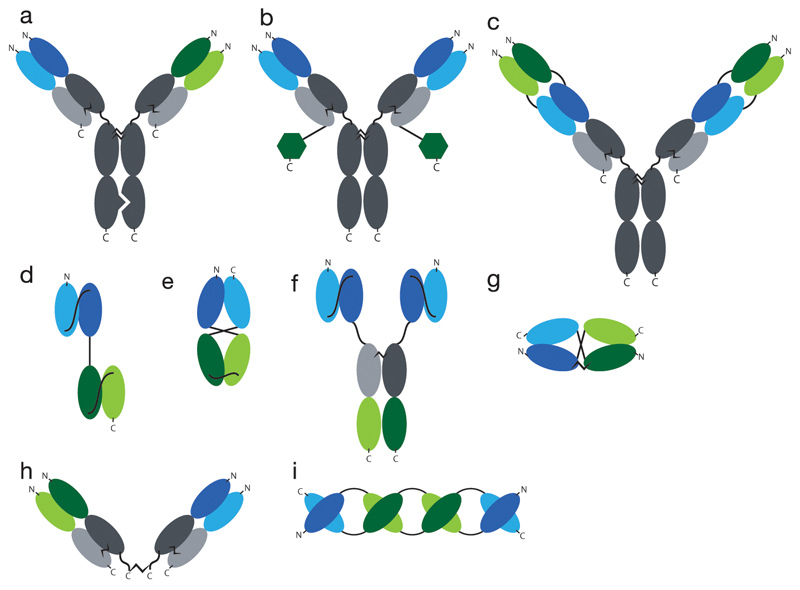
Two major classes of bispecific antibodies can be defined: (i) those featuring a full antibody format (consisting, however, of two different heavy chains and two different light chains), and (ii) those based on the assembly of two distinct antibody fragments. Some of the most commonly used bispecific antibody formats are shown in Figure 2.
Fig. 2. Overview of common formats for bispecifc antibodies.
a) knobs-into-hole bispecific antibody, like it is used in the Duobody™ format, b) globular domain proteins fused to IgGs, e.g. FynomAbs™, c) double-variable-domains Immunoglobulins (DVD-IgGs™), d) Bispecific T Cell Engager (BiTE™), e) single-chain diabody (scDb), f) Tribody, g) DART™, h) chemical cross-linking of two Fab fragments, i) TandAb™. Constant regions indicated in grey, VH1 indicated in dark blue, VL1 in light blue, VH2 in dark green and VL2 in light green. Globular domain proteins indicated as dark green hexagons.
Bispecific antibodies in IgG format have traditionally been generated by the fusion of two different hybridoma cells, resulting in a “quadroma” cell line (85). However, the combinatorial assembly process for heavy and light chains determines that only a small portion of the resulting antibody molecules have the desired functionality. In order to favor the formation of homogenous preparations of IgG-based bispecific antibodies, various technologies have been developed. Carter and coworkers described the “knobs-into-holes” technology, where point mutations within the CH3 domain of the heavy chain are used to generate an asymmetric bispecific antibody molecule. This technology requires the co-expression of four different polypeptides within the same cell line used for production.
Scientists at Genmab introduced a technology (termed “Duobody™”) for the assembly of bispecific IgG products, based on the separate expression of two parental antibodies. Both antibodies, which carry a single matched point mutation in their CH3 domains, can be mixed together and then separated into HL half-molecules by reducing conditions in vitro. A subsequent re-assembly and purification step leads to the formation of IgG-based bispecific antibodies.
In double-variable-domains Immunoglobulins (DVD-IgGs™), the structure of an IgG is extended at the N-terminal extremities of heavy and light chains with additional VH and VL domains, respectively, thus creating a second antigen-binding specificity (86).
Bispecific antibody products can also be generated by appending antibody-like molecules (e.g., scFv fragments, globular domains of other proteins) at the extremities of heavy and light chains of an IgG molecule (87–89).
All bispecific formats described so far contain an Fc portion, which de facto renders the molecule multispecific. Indeed, the Fc moiety may interact with the neonatal FcRn receptor (thus contributing to a longer circulatory half-life in blood), with Fcγ receptors and with complement components (e.g., C1q). For certain pharmaceutical applications, however, it may be preferable to generate bispecific antibodies devoid of the Fc portion.
Examples of bispecific antibodies, consisting of antibody fragments, include products generated by chemical cross-linking of two Fab fragments (90), as well as recombinant proteins designed to incorporate two antigen-binding specificities without the need for chemical modification [Figure 2].
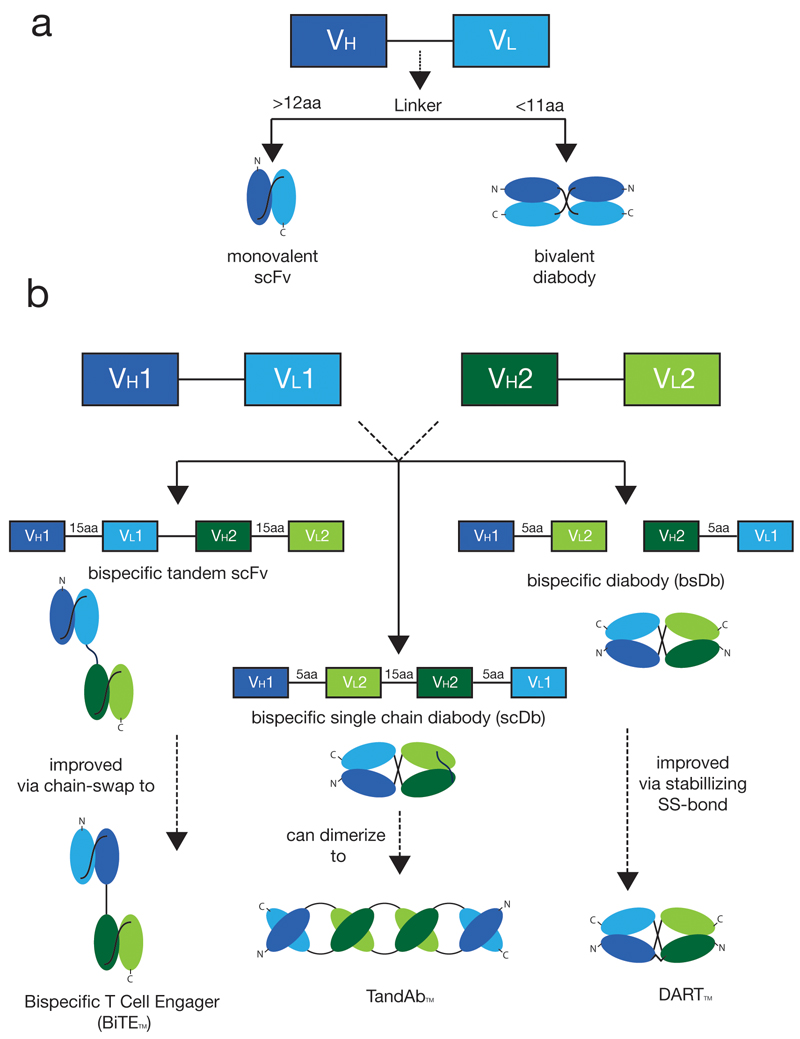
BiTEs™ are fusion proteins consisting of tandem repeats of two different scFv fragments. In most cases, short Gly-Ser-rich linkers are used and a domain order VLA-VHA-VHB-VLB is preferred (91), but other arrangements can be considered (92). The technology has been successfully used to generate clinical products (e.g., Blinatumomab), but could in principle lead to the undesired pairing of non-cognate VH and VL domains or to the formation of multimeric species (e.g., Tandabs™, see below) [Figure 3].
Fig. 3. Overview of commonly used scFv-based antibody fragments.
a) Shorter linkers force two scFv fragments to homo- or heterodimerize, resulting in a diabody fragment b) chain order and linker length variations lead to a great diversity of different bispecific antibody fragments
In 1993, Philipp Holliger and Sir Gregory Winter described a procedure to create bispecific antibodies (termed “diabodies”) by shortening the linker between VH and VL domain of scFv molecules. For linkers shorter than 11 aminoacids, the two domains cannot pair intramolecularly and are forced to homodimerize or heterodimerize [Figure 3]. A variation of this technology features the introduction of a cysteine residue at the end of each diabody subunit, thus leading to the formation of disulfide-stabilized diabodies, also known as “DARTs”™(93).
The design of tandem scFv structures with linkers of suitable length has been used to generate higher order oligomeric structures, termed “Tandabs”™ (94) [Figure 2]. Although various types of molecular assemblies could be generated [Figure 3], the canonical Tandab™ format features the formation of two pairs of antigen-binding specificities.
Obviously, bispecific molecules could also be generated by the tandem arrangements of other antibody-like fragments, such as VHH domains of camelid antibodies (95) or other types of globular binding domains (96).
Antigen-binding specificities for bispecific antibody products
The majority of applications of bispecific antibodies have been, so far, in the Oncology field. In most cases, one antibody moiety was specific to tumor-associated antigens on the cell surface of cancer cells, while the second antigen-binding specificity was directed against a leukocyte antigen. In virtually all applications, the recruitment of T cells was mediated by the use of anti-CD3 binders, but other molecular targets (e.g., CD16 on NK cells) have also been proposed (97).
The cross-linking of a tumor cell with a T cell by means of a suitable bispecific antibody may lead to an MHC-independent retargeting of cytolytic activity. Indeed, the use of suitable bispecific antibodies and T cells in vitro may lead to a potent and selective killing of target tumor cells at extremely low concentration of biopharmaceutical agent. Little is known, however, about the tumor targeting performance of bispecific antibodies in vivo, as (to the best of our knowledge) there is only one published quantitative biodistribution study performed in an immunocompetent syngeneic setting (98). Most of the information on the tumor-targeting performance of bispecific antibody products derives from the observation of therapeutic activity in animal models or in patients.
In most cases, the same antibody products cannot be used in immunocompetent animal models and in patients, as the target antigens are not conserved. It is therefore common practice to implant human tumors in immunocompromised mice, followed by the administration of human hemapoietic stem cells (HSCs). However, therapy studies with fully-murine bispecific antibodies in immunocompetent settings have been described and have led to impressive anti-tumor activities, especially for the treatment of hematological malignancies (99).
Complete tumor regressions have been reported for the use of bispecific antibodies, directed against markers expressed on the surface of solid tumor cells. For those studies, human tumors were grafted into immunocompromised mice, which received an injection of human T cells prior to the administration of the bispecific antibody product. Tumor-associated antigens considered for bispecific antibody product development include prostate-specific membrane antigen (PSMA) (100), carcinoembryonic antigen (CEA) (101), epidermal growth factor receptor (EGFR) (102) and A33 (103).
Therapy with a bispecific antibody binding a tumor associated antigen (TAA) and CD3 could lead to problems due to unspecific binding of the molecules either to healthy cells expressing the target antigen or to Fc receptors (for molecules bearing a Fc moiety). Potentially, this could lead to off-target T cell activation due to unspecific CD3 crosslinking (104).
Toxicities associated with the use of bispecific antibodies were reported in earlier clinical studies (105, 106). For this reason, the administration of bispecific antibodies is often limited to very low doses, in most cases with serum levels below 1 ng/ml (107). Toxicology studies in Balb/c mice with a murine analogue of Blinatumomab revealed no difference in administration of either one daily IV bolus dosing or a twice daily subcutaneous injection (108). However, clinical differences were observed for different administration modalities, as outlined in the next section.
Selected examples
Blinatumomab (Blincyto™)
Blinatumomab is a bispecific antibody in the BiTE™ format, developed by Micromet (now Amgen). It targets the CD3 antigen on T cells and the CD19 antigen, expressed on B cells in a majority of B cell malignancies.
T cells are activated upon binding, which in turn induces release of inflammatory cytokines and their transient proliferation.
The product received accelerated approval from the U.S. Food and Drug Administration for the treatment of Philadelphia-negative (Ph-) relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL) in December 2014. In 2015, approval was also recommended for the E.U. by the Committee for Medicinal Products for Human Use (CHMP). At the moment, Blinatumomab is also being investigated in Phase II clinical studies for the treatment of relapsed/refractory diffuse large B-cell lymphoma and in Phase I for treatment with relapsed, indolent B-cell non-Hodgkin's Lymphoma (NHL).
Blinatumomab consists of two murine scFv antibody fragments fused together by a G4S-linker. The sequential arrangement of variable domains is VLA-VHA-(G4S)-VHB-VLB. It was shown that this bispecifc antibody construct is cytotoxically active at very low concentrations (109), making it much more potent than Rituximab, a chimeric anti-CD20 antibody (110) in in vitro comparative assays.
Blinatumomab was tested in humans for the first time in 2001. Three phase I dose escalation studies were started with short-term intravenous infusion. These administrations took place twice or three times a week, each being a short term 2 or 4 hour i.v. infusion. Due to neurologic adverse events, cytokine release syndrome and infections, all three phase I studies were terminated early. Three years later, in 2004, a new phase I study was started to evaluate the safety profile and the benefit/risk ratio of a continuous i.v. administration over a period of 4 or 8 weeks. Due to the ”sustained presence of blinatumomab in serum at highly predictable drug levels” (111), all further clinical studies were performed using continuous infusion. Continous i.v. infusions are administered via an implanted port and a portable pump system.
In a phase II clinical study, which represented the basis for regulatory approval, 81 out of 189 ALL patients reached the primary endpoint of complete response or hematological complete response (112).
Catumaxomab (Removab™)
Catumaxomab is a trifunctional bispecific antibody which targets CD3 and the antigen epithelial cell adhesion molecule (EpCam). The product was approved for malignant ascites in 2009. Phase II clinical studies for the treatment of gastrointestinal and breast cancer are ongoing. Regulatory approval was granted on the basis of a pivotal phase II/III study, in which treatment of patients with malignant ascites due to epithelial tumors resulted in a significant reduction of ascites signs and clinically relevant prolongation of puncture-free survival (113).
Catumaxomab is a hybrid between a mouse IgG2a and a rat IgG2a and is produced in mouse-rat quadromas (85). As a consequence, the molecule is also able to bind human Fc gamma receptors I and III. It has been proposed that the multifunctionality of Catumaxomab facilitates the interaction of the antibody with tumor cells, the recruitment of T cells by interaction with the epsilon domain of CD3, as well as the activation of NK cells, dendritic cells, monocytes and macrophages (114), creating complex immunological synapses.
In a phase I /II study the maximum tolerated dose was defined at 10, 20, 50, 200 and 200 µg for the first five doses. Side effects included fever, nausea and vomiting in the majority of patients. Treatment prevented the accumulation of ascites and eliminated tumor cells (115).
Other products in clinical development
Other bispecific antibodies in BiTE™ format which are currently being studied in the clinic include MT111 (targeting CD3 and CEA, for the treatment of advanced gastric cancer and colon adenocarcinoma), MT112 (targeting CD3 and PSMA, for the treatment of prostate cancer) and MT110 (targeting CD3 and EpCam, for the treatment of colorectal cancer, lung cancer and gastrointestinal cancer).
Clinical-stage products in DART™ format include MGD006 (CD123 x CD3) (116), MGD007 (gpA33 x CD3) (103) and MGD011 (CD19 x CD3) (117). MGD006, targeting CD123 (the IL3 receptor alpha chain) is being investigated in a phase I dose-escalation study in patients with refractory acute myeloid leukemia (AML). MGD007 is currently being studied in a phase I dose finding study in two cohorts of patients: patients with K-Ras wildtype metastatic colorectal cancer and patients with K-Ras mutant colorectal cancer. For the treatment of hematological B cell malignancies, MGD011 is being developed as a Fc fusion to improve the half-life of the molecule, allowing for a more convenient dosing than CD19xCD3 products of competitors.
Analogies and differences between the two approaches
In principle, certain immunocytokine products could mimic the action of bispecific antibodies. The cytokine moiety can engage in a binding interaction with its cognate receptor on the surface of T-cells, thus creating an immunological synapse with the tumor cell. It remains to be investigated to which extent this mechanism happens in vivo and whether it contributes to selective tumor cell killing.
There are fundamental mechanistic differences between the anti-tumor activities of immunocytokines and bispecific antibodies. The latter class of molecules crucially depends on the formation of a “bridge” between tumor cells and leukocytes (in most cases, T cells). Experimental evidence suggests that it may be difficult for antibody products to diffuse into solid tumor masses and reach all neoplastic cells (118). However, bispecific antibodies are extremely efficient in mediating targeted cell killing even at low concentrations, provided that accessory lymphocytes are available at the site of action.
Immunocytokines can be directed against tumor cell antigens or against targets found in the tumor extracellular matrix. In both cases, an influx into the tumor mass and a potent activation of various types of leukocytes has been reported, both in animal models and in cancer patients. The exploitation of multiple cell types (e.g., T cells and NK cells) to fight malignancies represents an attractive feature of immunocytokines. Importantly, these products typically do not display myelotoxicity, making them ideally suited for combination with certain cytotoxic drugs (23, 28, 59).
It is not known, at this moment in time, whether bispecific antibodies and immunocytokines may display an additive or synergistic activity, when used in combination.
Discussion and Outlook
Both immunocytokines and bispecific antibodies have exhibited impressive anti-cancer activity in preclinical cancer models. Two bispecific antibodies have gained marketing approval, while at present no immunocytokine product has been introduced in the market.
The tumor homing performance of immunocytokines has extensively been analyzed in animal models using quantitative biodistribution studies with radiolabeled protein preparation. A similar analysis has not been performed for bispecific antibodies. The choice of individual formats for bispecific antibodies (and, to a certain extent, for immunocytokines) continues to be guided by preferences in product manufacturing and by an empirical testing of biological activities. Nuclear Medicine studies with radiolabeled products would be invaluable, in order to better assess the tumor homing properties of these biopharmaceuticals in cancer patients.
Success with bispecific antibody products will crucially rely on the availability of good-quality tumor-associated antigens. Down-regulation of these targets on the surface of tumor cells could represent an easy avenue to generate resistance to treatment. Validated targets are available for hematological malignancies. On-going clinical investigations will reveal the potential of bispecific antibodies for the treatment of disseminated solid tumors.
Immunocytokines typically do not cure cancer when used as single agents (even notable exceptions have been observed, both in mouse models and in individual patients). However, these biopharmaceuticals have proven to be versatile agents, capable of boosting the activity of other classes of therapeutic drugs. Not all anti-cancer agents can be potentiated by immunocytokines. For this reason, a judicious evaluation of the best combination strategies (including the choice of the best targets and cytokine payloads) will continue to need experimental studies. Dosing and treatment schedules have a strong impact on therapeutic outcome (59).
As cancer cures with immunocytokine-based regimens are increasingly being observed in mouse models, it becomes possible to perform mechanistic studies, aimed at the identification of tumor-rejection antigens and of the cellular contribution to the insurgence of protective immunity. Innovative techniques, such as mass spectrometry-based HLA peptidome analysis (119) and multiplex tetramer analysis (120), should facilitate mechanistic investigations.
Acknowledgements
The authors are grateful to Eidgenössische Technische Hochschule (ETH) Zürich, to the European Research Council, to the Swiss National Science Foundation and to the Bovena Stiftung for fincancial contribution.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytoxicity
- AML
acute myeloid leukemia
- CEA
carcinoembryonic antigen
- EDA
extradomain A of fibronectin
- EDB
extradomain A of fibronectin
- EGFR
epidermal growth factor receptor
- GD2
disialoganglioside
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IFNa
interferon alpha
- IFNg
interferon gamma
- IgG
immunoglobulin G
- IL[1-21]
interleukin [1-21]
- MTD
maximum tolerated dose
- NK
natural killer
- PSMA
prostate-specific membrane antigen
- scFV
single-chain variable fragment
- SCID
severe combined immunodeficiency
- TGF-β
transforming growth factor beta
- TNF
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
References
- 1.Krall N, Scheuermann J, Neri D. Small targeted cytotoxics: current state and promises from DNA-encoded chemical libraries. Angew Chem Int Ed Engl. 2013;52:1384–1402. doi: 10.1002/anie.201204631. [DOI] [PubMed] [Google Scholar]
- 2.van der Veldt AA, et al. Toward prediction of efficacy of chemotherapy: a proof of concept study in lung cancer patients using [(1)(1)C]docetaxel and positron emission tomography. Clin Cancer Res. 2013;19:4163–4173. doi: 10.1158/1078-0432.CCR-12-3779. [DOI] [PubMed] [Google Scholar]
- 3.van der Veldt AA, et al. Absolute quantification of [(11)C]docetaxel kinetics in lung cancer patients using positron emission tomography. Clin Cancer Res. 2011;17:4814–4824. doi: 10.1158/1078-0432.CCR-10-2933. [DOI] [PubMed] [Google Scholar]
- 4.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9:767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 5.Walsh G. Biopharmaceutical benchmarks 2014. Nat Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 6.Hess C, Venetz D, Neri D. Emerging classes of armed antibody therapeutics against cancer. Medchemcomm. 2014;5:408–431. [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 9.Tayal V, Kalra BS. Cytokines and anti-cytokines as therapeutics--an update. Eur J Pharmacol. 2008;579:1–12. doi: 10.1016/j.ejphar.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Melero I, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 11.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13:525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 12.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy K, Travers P, Walport M, Janeway C. Janeway's immunobiology. 8th ed. New York: Garland Science; 2012. [Google Scholar]
- 14.Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(Suppl 4):A40–45. doi: 10.1016/s0264-410x(02)00386-9. [DOI] [PubMed] [Google Scholar]
- 15.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 16.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YL, Chen SH, Wang JY, Yang BC. Fas ligand on tumor cells mediates inactivation of neutrophils. J Immunol. 2003;171:1183–1191. doi: 10.4049/jimmunol.171.3.1183. [DOI] [PubMed] [Google Scholar]
- 18.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241:104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 20.Becker JC, Pancook JD, Gillies SD, Furukawa K, Reisfeld RA. T cell-mediated eradication of murine metastatic melanoma induced by targeted interleukin 2 therapy. J Exp Med. 1996;183:2361–2366. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villa A, et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122:2405–2413. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 22.Pini A, et al. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem. 1998;273:21769–21776. doi: 10.1074/jbc.273.34.21769. [DOI] [PubMed] [Google Scholar]
- 23.Gutbrodt KL, et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5:201–118. doi: 10.1126/scitranslmed.3006221. [DOI] [PubMed] [Google Scholar]
- 24.Hemmerle T, Doll F, Neri D. Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis. Proc Natl Acad Sci U S A. 2014;111:12008–12012. doi: 10.1073/pnas.1402783111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess C, Neri D. Tumor-targeting properties of novel immunocytokines based on murine IL1beta and IL6. Protein Eng Des Sel. 2014;27:207–213. doi: 10.1093/protein/gzu013. [DOI] [PubMed] [Google Scholar]
- 26.Doll F, Schwager K, Hemmerle T, Neri D. Murine analogues of etanercept and of F8-IL10 inhibit the progression of collagen-induced arthritis in the mouse. Arthritis Res Ther. 2013;15:R138. doi: 10.1186/ar4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey K, Zivanovic A, Schwager K, Neri D. Antibody-based targeting of interferon-alpha to the tumor neovasculature: a critical evaluation. Integr Biol (Camb) 2011;3:468–478. doi: 10.1039/c0ib00099j. [DOI] [PubMed] [Google Scholar]
- 28.Hemmerle T, Probst P, Giovannoni L, Green AJ, Meyer T, Neri D. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109:1206–1213. doi: 10.1038/bjc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gafner V, Trachsel E, Neri D. An engineered antibody-interleukin-12 fusion protein with enhanced tumor vascular targeting properties. Int J Cancer. 2006;119:2205–2212. doi: 10.1002/ijc.22101. [DOI] [PubMed] [Google Scholar]
- 30.Hemmerle T, Neri D. The dose-dependent tumor targeting of antibody-IFNgamma fusion proteins reveals an unexpected receptor-trapping mechanism in vivo. Cancer Immunol Res. 2014;2:559–567. doi: 10.1158/2326-6066.CIR-13-0182. [DOI] [PubMed] [Google Scholar]
- 31.Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67:4940–4948. doi: 10.1158/0008-5472.CAN-07-0283. [DOI] [PubMed] [Google Scholar]
- 32.Halin C, Niesner U, Villani ME, Zardi L, Neri D. Tumor-targeting properties of antibody-vascular endothelial growth factor fusion proteins. Int J Cancer. 2002;102:109–116. doi: 10.1002/ijc.10674. [DOI] [PubMed] [Google Scholar]
- 33.Venetz D, Hess C, Lin CW, Aebi M, Neri D. Glycosylation profiles determine extravasation and disease-targeting properties of armed antibodies. Proc Natl Acad Sci U S A. 2015;112:2000–2005. doi: 10.1073/pnas.1416694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmerle T, Wulhfard S, Neri D. A critical evaluation of the tumor-targeting properties of bispecific antibodies based on quantitative biodistribution data. Protein Eng Des Sel. 2012;25:851–854. doi: 10.1093/protein/gzs061. [DOI] [PubMed] [Google Scholar]
- 35.Carnemolla B, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99:1659–1665. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 36.Halin C, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20:264–269. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 37.Borsi L, et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood. 2003;102:4384–4392. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- 38.Halin C, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63:3202–3210. [PubMed] [Google Scholar]
- 39.Hornick JL, Khawli LA, Hu P, Sharifi J, Khanna C, Epstein AL. Pretreatment with a monoclonal antibody/interleukin-2 fusion protein directed against DNA enhances the delivery of therapeutic molecules to solid tumors. Clin Cancer Res. 1999;5:51–60. [PubMed] [Google Scholar]
- 40.Zhu EF, et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 2015;27:489–501. doi: 10.1016/j.ccell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134:467–477. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- 42.Tzeng A, Kwan BH, Opel CF, Navaratna T, Wittrup KD. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc Natl Acad Sci U S A. 2015;112:3320–3325. doi: 10.1073/pnas.1416159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo EM, et al. Anti-CD138-targeted interferon is a potent therapeutic against multiple myeloma. J Interferon Cytokine Res. 2015;35:281–291. doi: 10.1089/jir.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillies SD, Lan Y, Brunkhorst B, Wong WK, Li Y, Lo KM. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51:449–460. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang R, et al. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res. 1997;57:4948–4955. [PubMed] [Google Scholar]
- 46.Mizokami MM, Hu P, Khawli LA, Li J, Epstein AL. Chimeric TNT-3 antibody/murine interferon-gamma fusion protein for the immunotherapy of solid malignancies. Hybrid Hybridomics. 2003;22:197–207. doi: 10.1089/153685903322328929. [DOI] [PubMed] [Google Scholar]
- 47.Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115:2864–2871. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niethammer AG, et al. Targeted interleukin 2 therapy enhances protective immunity induced by an autologous oral DNA vaccine against murine melanoma. Cancer Res. 2001;61:6178–6184. [PubMed] [Google Scholar]
- 49.Sondel PM, Gillies SD. Current and Potential Uses of Immunocytokines as Cancer Immunotherapy. Antibodies (Basel) 2012;1:149–171. doi: 10.3390/antib1020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol. 2013;5:29–45. doi: 10.2147/CPAA.S49231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young PA, Morrison SL, Timmerman JM. Antibody-cytokine fusion proteins for treatment of cancer: engineering cytokines for improved efficacy and safety. Semin Oncol. 2014;41:623–636. doi: 10.1053/j.seminoncol.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillies SD, et al. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin Cancer Res. 2011;17:3673–3685. doi: 10.1158/1078-0432.CCR-10-2921. [DOI] [PubMed] [Google Scholar]
- 53.Lode HN, et al. Melanoma immunotherapy by targeted IL-2 depends on CD4(+) T-cell help mediated by CD40/CD40L interaction. J Clin Invest. 2000;105:1623–1630. doi: 10.1172/JCI9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rekers NH, et al. Combination of radiotherapy with the immunocytokine L19-IL2: Additive effect in a NK cell dependent tumour model. Radiother Oncol. 2015 doi: 10.1016/j.radonc.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 55.Zegers CM, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res. 2015;21:1151–1160. doi: 10.1158/1078-0432.CCR-14-2676. [DOI] [PubMed] [Google Scholar]
- 56.Rekers NH, Zegers CM, Germeraad WT, Dubois L, Lambin P. Long-lasting antitumor effects provided by radiotherapy combined with the immunocytokine L19-IL2. Oncoimmunology. 2015;4:e1021541. doi: 10.1080/2162402X.2015.1021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Heuvel MM, et al. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med. 2015;13:32. doi: 10.1186/s12967-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borsi L, et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer. 2002;102:75–85. doi: 10.1002/ijc.10662. [DOI] [PubMed] [Google Scholar]
- 59.Moschetta M, et al. Paclitaxel enhances therapeutic efficacy of the F8-IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012;72:1814–1824. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- 60.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133:751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 61.Schliemann C, et al. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood. 2009;113:2275–2283. doi: 10.1182/blood-2008-05-160747. [DOI] [PubMed] [Google Scholar]
- 62.Balza E, et al. Therapy-induced antitumor vaccination in neuroblastomas by the combined targeting of IL-2 and TNFalpha. Int J Cancer. 2010;127:101–110. doi: 10.1002/ijc.25018. [DOI] [PubMed] [Google Scholar]
- 63.Spitaleri G, et al. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol. 2013;139:447–455. doi: 10.1007/s00432-012-1327-7. [DOI] [PubMed] [Google Scholar]
- 64.Johannsen M, et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46:2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 65.Eigentler TK, et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17:7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 66.Weide B, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2:668–678. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 67.Danielli R, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64:999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brack SS, Silacci M, Birchler M, Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res. 2006;12:3200–3208. doi: 10.1158/1078-0432.CCR-05-2804. [DOI] [PubMed] [Google Scholar]
- 69.Marlind J, et al. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res. 2008;14:6515–6524. doi: 10.1158/1078-0432.CCR-07-5041. [DOI] [PubMed] [Google Scholar]
- 70.Pedretti M, et al. Combination of temozolomide with immunocytokine F16-IL2 for the treatment of glioblastoma. Br J Cancer. 2010;103:827–836. doi: 10.1038/sj.bjc.6605832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catania C, et al. The tumor-targeting immunocytokine F16-IL2 in combination with doxorubicin: dose escalation in patients with advanced solid tumors and expansion into patients with metastatic breast cancer. Cell Adh Migr. 2015;9:14–21. doi: 10.4161/19336918.2014.983785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schliemann C, et al. Targeting interleukin-2 to the bone marrow stroma for therapy of acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Cancer Immunol Res. 2015;3:547–556. doi: 10.1158/2326-6066.CIR-14-0179. [DOI] [PubMed] [Google Scholar]
- 73.Gillies SD, Lo KM, Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods. 1989;125:191–202. doi: 10.1016/0022-1759(89)90093-8. [DOI] [PubMed] [Google Scholar]
- 74.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 75.Becker JC, Varki N, Gillies SD, Furukawa K, Reisfeld RA. Long-lived and transferable tumor immunity in mice after targeted interleukin-2 therapy. J Clin Invest. 1996;98:2801–2804. doi: 10.1172/JCI119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osenga KL, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King DM, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shusterman S, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribas A, et al. Phase I/II open-label study of the biologic effects of the interleukin-2 immunocytokine EMD 273063 (hu14.18-IL2) in patients with metastatic malignant melanoma. J Transl Med. 2009;7:68. doi: 10.1186/1479-5876-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Epstein AL, Chen FM, Taylor CR. A novel method for the detection of necrotic lesions in human cancers. Cancer Res. 1988;48:5842–5848. [PubMed] [Google Scholar]
- 81.Li J, Hu P, Khawli LA, Yun A, Epstein AL. chTNT-3/hu IL-12 fusion protein for the immunotherapy of experimental solid tumors. Hybrid Hybridomics. 2004;23:1–10. doi: 10.1089/153685904322771962. [DOI] [PubMed] [Google Scholar]
- 82.Kim JW, et al. First-in-human phase I trial of NHS-IL12 in advanced solid tumors. Journal of Clinical Oncology. 2012:30. [Google Scholar]
- 83.Lo KM, et al. huBC1-IL12, an immunocytokine which targets EDB-containing oncofetal fibronectin in tumors and tumor vasculature, shows potent anti-tumor activity in human tumor models. Cancer Immunol Immunother. 2007;56:447–457. doi: 10.1007/s00262-006-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rudman SM, et al. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011;17:1998–2005. doi: 10.1158/1078-0432.CCR-10-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol. 1995;155:219–225. [PubMed] [Google Scholar]
- 86.Wu C, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25:1290–1297. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 87.Brack S, et al. A bispecific HER2-targeting FynomAb with superior antitumor activity and novel mode of action. Mol Cancer Ther. 2014;13:2030–2039. doi: 10.1158/1535-7163.MCT-14-0046-T. [DOI] [PubMed] [Google Scholar]
- 88.Silacci M, et al. Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. MAbs. 2015;0 doi: 10.1080/19420862.2015.1093266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jendreyko N, et al. Intradiabodies, bispecific, tetravalent antibodies for the simultaneous functional knockout of two cell surface receptors. J Biol Chem. 2003;278:47812–47819. doi: 10.1074/jbc.M307002200. [DOI] [PubMed] [Google Scholar]
- 90.Borchmann P, et al. Phase 1 trial of the novel bispecific molecule H22xKi-4 in patients with refractory Hodgkin lymphoma. Blood. 2002;100:3101–3107. doi: 10.1182/blood-2001-12-0295. [DOI] [PubMed] [Google Scholar]
- 91.Nagorsen D, Baeuerle PA. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp Cell Res. 2011;317:1255–1260. doi: 10.1016/j.yexcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 92.Neri D, Momo M, Prospero T, Winter G. High-affinity antigen binding by chelating recombinant antibodies (CRAbs) J Mol Biol. 1995;246:367–373. doi: 10.1006/jmbi.1994.0091. [DOI] [PubMed] [Google Scholar]
- 93.Johnson S, et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol. 2010;399:436–449. doi: 10.1016/j.jmb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Kipriyanov SM, et al. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol. 1999;293:41–56. doi: 10.1006/jmbi.1999.3156. [DOI] [PubMed] [Google Scholar]
- 95.Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007;77:13–22. doi: 10.1007/s00253-007-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 97.Reusch U, et al. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs. 2014;6:728–739. doi: 10.4161/mabs.28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.List T, Neri D. Biodistribution studies with tumor-targeting bispecific antibodies reveal selective accumulation at the tumor site. MAbs. 2012;4:775–783. doi: 10.4161/mabs.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Demanet C, Brissinck J, De Jonge J, Thielemans K. In vivo studies using bispecific antibodies (anti-CD3 x anti-idiotype) and CD28-induced costimulation in the BCL1 lymphoma. J Hematother. 1995;4:363–368. doi: 10.1089/scd.1.1995.4.363. [DOI] [PubMed] [Google Scholar]
- 100.Friedrich M, et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther. 2012;11:2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- 101.Peng L, et al. The CEA/CD3-bispecific antibody MEDI-565 (MT111) binds a nonlinear epitope in the full-length but not a short splice variant of CEA. PLoS One. 2012;7:e36412. doi: 10.1371/journal.pone.0036412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lutterbuese R, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci U S A. 2010;107:12605–12610. doi: 10.1073/pnas.1000976107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moore PA, et al. Development of MGD007, a gpA33 x CD3 bispecific DART for T-cell immunotherapy of metastatic colorectal cancer. Cancer Research. 2014;74 doi: 10.1158/1535-7163.MCT-17-1086. [DOI] [PubMed] [Google Scholar]
- 104.Durben M, et al. Characterization of a bispecific FLT3 X CD3 antibody in an improved, recombinant format for the treatment of leukemia. Mol Ther. 2015;23:648–655. doi: 10.1038/mt.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kroesen BJ, et al. Phase I study of intravenously applied bispecific antibody in renal cell cancer patients receiving subcutaneous interleukin 2. Br J Cancer. 1994;70:652–661. doi: 10.1038/bjc.1994.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tibben JG, Boerman OC, Massuger LF, Schijf CP, Claessens RA, Corstens FH. Pharmacokinetics, biodistribution and biological effects of intravenously administered bispecific monoclonal antibody OC/TR F(ab')2 in ovarian carcinoma patients. Int J Cancer. 1996;66:477–483. doi: 10.1002/(SICI)1097-0215(19960516)66:4<477::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 107.Klinger M, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–6233. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]
- 108.Amgen. Blinatumomab (AMG103) Background information for the pediatric subcommittee of the oncologic drugs advisory committee meeting; 2012. [Google Scholar]
- 109.Loffler A, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 110.Bargou R, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 111.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 112.Topp MS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 113.Parsons S, et al. Intraperitoneal treatment of malignant ascites due to epithelial tumors with catumaxomab: A phase II/III study. Journal of Clinical Oncology. 2008:26. [Google Scholar]
- 114.Zeidler R, et al. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer. 2000;83:261–266. doi: 10.1054/bjoc.2000.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burges A, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13:3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 116.Moore P, et al. Preclinical activity and safety of MGD006, a CD123xCD3 Bispecific DART (R) molecule for the treatment of hematological malignancies. European Journal of Cancer. 2014;50:48–48. [Google Scholar]
- 117.Liu LQ, et al. MGD011, Humanized CD19 x CD3 DART (R) Protein with Enhanced Pharmacokinetic Properties, Demonstrates Potent T-Cell Mediated Anti-Tumor Activity in Preclinical Models and Durable B-Cell Depletion in Cynomolgus Monkeys Following Once-a-Week Dosing. Blood. 2014:124. [Google Scholar]
- 118.Dennis MS, et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67:254–261. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- 119.Bassani-Sternberg M, Barnea E, Beer I, Avivi I, Katz T, Admon A. Soluble plasma HLA peptidome as a potential source for cancer biomarkers. Proc Natl Acad Sci U S A. 2010;107:18769–18776. doi: 10.1073/pnas.1008501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hadrup SR, Schumacher TN. MHC-based detection of antigen-specific CD8+ T cell responses. Cancer Immunol Immunother. 2010;59:1425–1433. doi: 10.1007/s00262-010-0824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dela Cruz JS, Trinh KR, Morrison SL, Penichet ML. Recombinant anti-human HER2/neu IgG3-(GM-CSF) fusion protein retains antigen specificity and cytokine function and demonstrates antitumor activity. J Immunol. 2000;165:5112–5121. doi: 10.4049/jimmunol.165.9.5112. [DOI] [PubMed] [Google Scholar]
- 122.Hornick JL, Khawli LA, Hu P, Lynch M, Anderson PM, Epstein AL. Chimeric CLL-1 antibody fusion proteins containing granulocyte-macrophage colony-stimulating factor or interleukin-2 with specificity for B-cell malignancies exhibit enhanced effector functions while retaining tumor targeting properties. Blood. 1997;89:4437–4447. [PubMed] [Google Scholar]
- 123.Bauer S, et al. Targeted bioactivity of membrane-anchored TNF by an antibody-derived TNF fusion protein. J Immunol. 2004;172:3930–3939. doi: 10.4049/jimmunol.172.6.3930. [DOI] [PubMed] [Google Scholar]
- 124.Bauer S, et al. Targeted therapy of renal cell carcinoma: synergistic activity of cG250-TNF and IFNg. Int J Cancer. 2009;125:115–123. doi: 10.1002/ijc.24359. [DOI] [PubMed] [Google Scholar]
- 125.Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. CD20-targeted tetrameric interferon-alpha, a novel and potent immunocytokine for the therapy of B-cell lymphomas. Blood. 2009;114:3864–3871. doi: 10.1182/blood-2009-06-228890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cooke SP, Pedley RB, Boden R, Begent RH, Chester KA. In vivo tumor delivery of a recombinant single chain Fv::tumor necrosis factor-alpha fusion [correction of factor: a fusion] protein. Bioconjug Chem. 2002;13:7–15. doi: 10.1021/bc000178a. [DOI] [PubMed] [Google Scholar]
- 127.Huang TH, Chintalacharuvu KR, Morrison SL. Targeting IFN-alpha to B cell lymphoma by a tumor-specific antibody elicits potent antitumor activities. J Immunol. 2007;179:6881–6888. doi: 10.4049/jimmunol.179.10.6881. [DOI] [PubMed] [Google Scholar]
- 128.Liu Y, Cheung LH, Marks JW, Rosenblum MG. Recombinant single-chain antibody fusion construct targeting human melanoma cells and containing tumor necrosis factor. Int J Cancer. 2004;108:549–557. doi: 10.1002/ijc.11524. [DOI] [PubMed] [Google Scholar]
- 129.Rossi EA, Rossi DL, Cardillo TM, Stein R, Goldenberg DM, Chang CH. Preclinical studies on targeted delivery of multiple IFNalpha2b to HLA-DR in diverse hematologic cancers. Blood. 2011;118:1877–1884. doi: 10.1182/blood-2011-03-343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scherf U, Benhar I, Webber KO, Pastan I, Brinkmann U. Cytotoxic and antitumor activity of a recombinant tumor necrosis factor-B1(Fv) fusion protein on LeY antigen-expressing human cancer cells. Clin Cancer Res. 1996;2:1523–1531. [PubMed] [Google Scholar]
- 131.Christ O, Seiter S, Matzku S, Burger C, Zoller M. Efficacy of local versus systemic application of antibody-cytokine fusion proteins in tumor therapy. Clin Cancer Res. 2001;7:985–998. [PubMed] [Google Scholar]
- 132.Sharifi J, Khawli LA, Hu P, Li J, Epstein AL. Generation of human interferon gamma and tumor Necrosis factor alpha chimeric TNT-3 fusion proteins. Hybrid Hybridomics. 2002;21:421–432. doi: 10.1089/153685902321043954. [DOI] [PubMed] [Google Scholar]
- 133.Ebbinghaus C, et al. Engineered vascular-targeting antibody-interferon-gamma fusion protein for cancer therapy. Int J Cancer. 2005;116:304–313. doi: 10.1002/ijc.20952. [DOI] [PubMed] [Google Scholar]
- 134.Rosenblum MG, Cheung L, Mujoo K, Murray JL. An antimelanoma immunotoxin containing recombinant human tumor necrosis factor: tissue disposition, pharmacokinetic, and therapeutic studies in xenograft models. Cancer Immunol Immunother. 1995;40:322–328. doi: 10.1007/BF01519633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang X, Ye D, Thorpe PE. Enhancing the potency of a whole-cell breast cancer vaccine in mice with an antibody-IL-2 immunocytokine that targets exposed phosphatidylserine. Vaccine. 2011;29:4785–4793. doi: 10.1016/j.vaccine.2011.04.082. [DOI] [PubMed] [Google Scholar]
- 136.Hess C, Neri D. Evaluation of antibody-chemokine fusion proteins for tumor-targeting applications. Exp Biol Med (Maywood) 2014;239:842–852. doi: 10.1177/1535370214536667. [DOI] [PubMed] [Google Scholar]
- 137.Xu X, et al. Targeting and therapy of carcinoembryonic antigen-expressing tumors in transgenic mice with an antibody-interleukin 2 fusion protein. Cancer Res. 2000;60:4475–4484. [PubMed] [Google Scholar]
- 138.Gillies SD, et al. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood. 2005;105:3972–3978. doi: 10.1182/blood-2004-09-3533. [DOI] [PubMed] [Google Scholar]
- 139.Penichet ML, Dela Cruz JS, Shin SU, Morrison SL. A recombinant IgG3-(IL-2) fusion protein for the treatment of human HER2/neu expressing tumors. Hum Antibodies. 2001;10:43–49. [PubMed] [Google Scholar]
- 140.Klein C. Novel CEA-targeted IL2 variant immunocytokine for immunotherapy of cancer. J Immunother Cancer. 2014:2. [Google Scholar]
- 141.Frey K, Schliemann C, Schwager K, Giavazzi R, Johannsen M, Neri D. The immunocytokine F8-IL2 improves the therapeutic performance of sunitinib in a mouse model of renal cell carcinoma. J Urol. 2010;184:2540–2548. doi: 10.1016/j.juro.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 142.Matsumoto H, et al. Targeting of interleukin-2 to human MK-1-expressing carcinoma by fusion with a single-chain Fv of anti-MK-1 antibody. Anticancer Res. 2002;22:2001–2007. [PubMed] [Google Scholar]
- 143.Hemmerle T, Hess C, Venetz D, Neri D. Tumor targeting properties of antibody fusion proteins based on different members of the murine tumor necrosis superfamily. J Biotechnol. 2014;172:73–76. doi: 10.1016/j.jbiotec.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 144.Melani C, et al. Targeting of interleukin 2 to human ovarian carcinoma by fusion with a single-chain Fv of antifolate receptor antibody. Cancer Res. 1998;58:4146–4154. [PubMed] [Google Scholar]
- 145.Pasche N, Woytschak J, Wulhfard S, Villa A, Frey K, Neri D. Cloning and characterization of novel tumor-targeting immunocytokines based on murine IL7. J Biotechnol. 2011;154:84–92. doi: 10.1016/j.jbiotec.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 146.Schwager K, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther. 2009;11:R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trachsel E, Bootz F, Silacci M, Kaspar M, Kosmehl H, Neri D. Antibody-mediated delivery of IL-10 inhibits the progression of established collagen-induced arthritis. Arthritis Res Ther. 2007;9:R9. doi: 10.1186/ar2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sommavilla R, et al. Expression, engineering and characterization of the tumor-targeting heterodimeric immunocytokine F8-IL12. Protein Eng Des Sel. 2010;23:653–661. doi: 10.1093/protein/gzq038. [DOI] [PubMed] [Google Scholar]
- 149.Nilsson F, Kosmehl H, Zardi L, Neri D. Targeted delivery of tissue factor to the ED-B domain of fibronectin, a marker of angiogenesis, mediates the infarction of solid tumors in mice. Cancer Res. 2001;61:711–716. [PubMed] [Google Scholar]
- 150.Kim H, Gao W, Ho M. Novel immunocytokine IL12-SS1 (Fv) inhibits mesothelioma tumor growth in nude mice. PLoS One. 2013;8:e81919. doi: 10.1371/journal.pone.0081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gillies SD, et al. Antibody-IL-12 fusion proteins are effective in SCID mouse models of prostate and colon carcinoma metastases. J Immunol. 1998;160:6195–6203. [PubMed] [Google Scholar]
- 152.Peng LS, Penichet ML, Morrison SL. A single-chain IL-12 IgG3 antibody fusion protein retains antibody specificity and IL-12 bioactivity and demonstrates antitumor activity. J Immunol. 1999;163:250–258. [PubMed] [Google Scholar]
- 153.Hess C, Neri D. The antibody-mediated targeted delivery of interleukin-13 to syngeneic murine tumors mediates a potent anticancer activity. Cancer Immunol Immunother. 2015;64:635–644. doi: 10.1007/s00262-015-1666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vincent M, et al. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int J Cancer. 2013;133:757–765. doi: 10.1002/ijc.28059. [DOI] [PubMed] [Google Scholar]
- 155.Pasche N, Frey K, Neri D. The targeted delivery of IL17 to the mouse tumor neo-vasculature enhances angiogenesis but does not reduce tumor growth rate. Angiogenesis. 2012;15:165–169. doi: 10.1007/s10456-011-9239-8. [DOI] [PubMed] [Google Scholar]
- 156.Bhatt SZ D, Jiang X, Shin S, Timmermann JM, Rosenblatt JD, Lossos IS. Targeting B-Cell Malignancies With Anti-CD20-Interleukin-21 Fusokine. Blood. 2013:122. doi: 10.1182/blood-2016-09-738211. [DOI] [PubMed] [Google Scholar]