Abstract
Aims
The purpose of this study is to describe the incidence and presenting features of patients with acute liver failure (ALF) due to ischemic hepatitis and the prognostic factors associated with short (three-week) and long-term outcomes.
Methods
Retrospective cohort analysis of adult patients enrolled in the Acute Liver Failure Study Group between 1998 and 2008 with ALF due to ischemic hepatitis. Predictors of adverse outcomes three weeks after presentation were identified by univariate and multivariate analysis.
Results
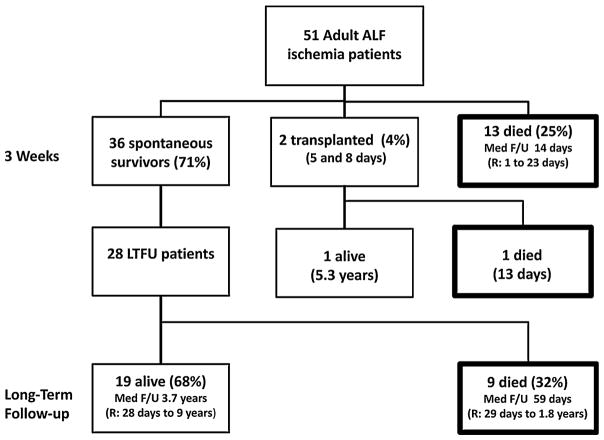
Ischemic hepatitis accounted for 51 (4.4%) of the 1147 ALF patients enrolled. Mean age was 50 years, 63% were female, and only 31% had known heart disease before presentation. However, a cardiopulmonary precipitant of hepatic ischemia was identified in 69%. Three-week spontaneous survival was 71%, two patients (4%) underwent liver transplantation, and the remaining 13 patients (25%) died of multi-organ failure. Adverse outcomes were more frequent in subjects with higher admission phosphate levels (HR 1.3, 95% CI 1.1–1.6, P = 0.008) and in subjects with grade 3/4 encephalopathy at presentation (HR: 8.4, 95% CI 1.1–66.5, P = 0.04). Nineteen of the 28 short-term survivors (68%) were still alive at a median follow-up of 3.7 years whereas nine (32%) others had died at a median follow-up of 2 months.
Conclusions
A higher admission serum phosphate level and more advanced encephalopathy are associated with a lower likelihood of short-term survival of hospitalized patients with ALF due to ischemic hepatitis. Long-term outcomes are largely determined by underlying cardiovascular morbidity and mortality.
Keywords: Liver transplantation, Hypoxic hepatitis, Aminotransferase levels, Coagulopathy, Encephalopathy
Introduction
Hypoxic liver injury or “ischemic hepatitis” is a common cause of marked serum aminotransferase elevation amongst hospitalized patients, particularly in intensive-care units [1–3]. Ischemic hepatitis most commonly arises because of arterial hypoxemia and/or insufficient hepatic perfusion resulting from passive liver congestion, circulatory collapse, and/or a low cardiac output state. Ischemic hepatitis is largely a clinical diagnosis that is characterized by:
cardiopulmonary or circulatory failure with or without associated hypotension;
massive and rapidly reversible increases in serum aminotransferase levels; and
exclusion of other causes of severe acute liver injury, for example acetaminophen overdose, viral hepatitis, and toxin-mediated liver damage [4, 5].
In rare patients who undergo liver biopsy, centrilobular necrosis of varying severity is found, indicative of reduced hepatic arterial oxygen delivery with resultant hepatocyte death [6]. Clinically, a transient coagulopathy characterized by hypoprothrombinemia because of reduction of clotting factor synthesis is typically seen but most patients have improvement or near normalization in their serum aminotransferase and international normalized ratio (INR) levels within 7–10 days of onset.
The frequency and circumstances wherein ischemic hepatitis leads to life-threatening acute liver failure (ALF) with associated coagulopathy and impaired mental status are not well-described. In addition, the natural history and associated prognostic factors in patients with ALF due to hepatic ischemia are largely unknown. The United States Acute Liver Failure Study Group (ALFSG) has been prospectively tracking causes and outcomes for adult patients with ALF enrolled in a multi-center prospective observational study since 1998 [7]. The purpose of this study is to characterize the risk factors, clinical presentation, and laboratory features associated with an adverse outcome in 51 consecutive adult ALF patients with ischemic hepatitis seen over a 10 year period. A secondary purpose was to characterize the long-term clinical outcomes in these patients after their acute illness.
Methods
US Acute Liver Failure Study Group
The ALFSG is a consortium of 23 referral centers with an interest and expertise in ALF that is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. An ongoing prospective observational study has set out to determine etiology, clinical features, and outcomes for adult patients with ALF. Enrollment criteria include the presence of coagulopathy with an INR ≥ 1.5 and any level of hepatic encephalopathy within 26 weeks of illness onset in a person with no underlying liver disease. The study population consisted of consecutive ALF patients with hepatic ischemia enrolled between January 1998 and October 2007.
Data Collection
Local institutional review board approval was obtained at all of the participating sites and written informed consent was obtained from the patients’ next of kin, because all patients had impaired mental status at enrollment. Detailed patient demographics, medical history, clinical features, and laboratory values were collected at study enrollment. Serial laboratory and clinical data were prospectively recorded through three weeks of observation. At the end of three weeks, short-term outcomes were classified as spontaneous survival or adverse outcome (i.e., death or liver transplant, LT). The spontaneous survivors and LT recipients were further followed up at 12 and 24 months by the site investigator. The Social Security master death file was queried for all patients lost to follow-up. All data forms were sent to the data-coordinating center at the University of Texas Southwestern Medical Center at Dallas for review and entry into a central database. Annual site visits were conducted by the data coordinating center to verify source documents. A Certificate of Confidentiality was obtained from the National Institutes of Mental Health for the entire study.
Case Definition
Cases of ALF due to ischemic hepatitis were identified by the site investigators as subjects with:
a rapid rise and fall in serum ALT > 1,000 IU/ml;
in the proper clinical setting of decreased hepatic perfusion/arterial hypoxemia; and
after excluding other etiologies (i.e. acetaminophen, viral hepatitis etc.) by standard diagnostic studies and expert review.
The etiology of circulatory collapse was categorized into cardiopulmonary (pump or respiratory failure, myocardial infarction, arrhythmia), and extra-cardiac causes (sepsis, seizure, hepatic vascular occlusion, volume depletion, etc.).
Statistical Analysis
Results are expressed as percentages or mean ± SD unless otherwise stated. Comparison between group of patients with spontaneous survival and adverse outcomes was done using the two-tailed Student t test and chi-squared test where appropriate. P < 0.05 was considered statistically significant. For survival analysis, time of entry was defined as the date of study enrollment. The survival time was calculated from the date of enrollment to the date of death or censoring (i.e. three weeks from enrollment in the study). The univariate Cox proportional hazards model was used to estimate the effect of each factor, including demographic variables, co-morbidities, and presenting laboratory and clinical features. The variables with P < 0.10 on the univariate proportional hazards model were used in the Cox multivariate model to identify predictors of adverse outcome at three weeks. Backward conditional Cox regression modeling was used. All statistical analysis and graph formation was completed using SPSS version 15.0 (SPSS, Chicago, IL, USA).
Results
Adult ALF Patients with Hepatic Ischemia
The 51 patients with ALF due to ischemic hepatitis accounted for 4.4% of the 1,147 patients enrolled in the ALFSG over a 10-year period (Fig. 1). Patient demographics, medical co-morbidities, laboratory data, and clinical features on study day 1 are listed in Table 1. There was a modest female preponderance (63%) with a mean age of 50 years and Caucasians accounted for 82% of the patients. Clinical symptoms of fatigue, weakness, or abdominal pain had been present for a mean of eight days before enrollment. Known cardiovascular disease was present in only 31% of the patients and a smaller percentage had hypertension, diabetes, and/or chronic kidney disease.
Fig. 1.
Patients with ALF due to ischemic hepatitis. Severe hepatic ischemia accounted for 51 of the 1,147 (4.4%) ALF patients enrolled in the ALFSG between 1/98 and 10/07. At three weeks, there were 36 spontaneous survivors (71%), two patients had undergone liver transplantation (4%), and 13 had died of multi-organ failure (25%). Further follow-up of 28 initial survivors revealed that nine died of a variety of causes and the remaining 19 patients were alive at a median follow-up of 3.7 years
Table 1.
Day 1 clinical and laboratory features of patients with ALF due to ischemic hepatitis
| Total (n = 51) | Spontaneous survivors (n = 36) | Died/transplanted (n = 15) | P | |
|---|---|---|---|---|
| Age (years) | 50 ± 16 | 51 ± 17 | 46 ± 15 | 0.29 |
| Female | 63% | 61% | 67% | 0.71 |
| Race/ethnicity | ||||
| Caucasian | 82% | 86% | 73% | 0.28 |
| African American | 10% | 11% | 7% | 0.63 |
| Asian | 2% | 3% | – | – |
| Other | 6% | – | 20% | – |
| Symptom onset to enrollment (days) | 8 ± 7 | 9 ± 8 | 6 ± 5 | 0.18 |
| Hospital admission to enrollment (days) | 5 ± 6 | 4 ± 3 | 7 ± 9 | 0.08 |
| Medical history | ||||
| Heart disease | 31% | 33% | 27% | 0.64 |
| Hypertension | 25% | 28% | 20% | 0.56 |
| Diabetes | 14% | 17% | 7% | 0.34 |
| Renal disease | 10% | 11% | 7% | 0.63 |
| Day 1 labs | ||||
| Hemoglobin (g/dl) | 10.6 ± 1.6 | 10.7 ± 1.5 | 10.5 ± 1.9 | 0.69 |
| WBC (103/ml) | 15.5 ± 12.2 | 13.6 ± 6 | 20.1 ± 20.3 | 0.08 |
| Platelet count (103/ml) | 96.1 ± 57.6 | 105.8 ± 59 | 73 ± 48.1 | 0.06 |
| INR | 2.8 ± 1.9 | 2.8 ± 2.2 | 2.7 ± 1.1 | 0.91 |
| ALT (IU/l) | 3,014 ± 2.325 | 2,543 ± 1,538 | 4,146 ± 3,440 | 0.10 |
| AST (IU/l) | 3,681 ± 3,825 | 3,326 ± 3,877 | 4,511 ± 3,695 | 0.32 |
| Alkaline phosphatase (IU/l) | 180 ± 134 | 142 ± 78 | 263 ± 188 | 0.03 |
| Total bilirubin (mg/dl) | 6 ± 7 | 5 ± 4 | 10.6 ± 10.2 | 0.05 |
| Creatinine (mg/dl) | 2.9 ± 1.5 | 2.7 ± 1.4 | 3.3 ± 1.6 | 0.15 |
| Phosphate (mg/dl) | 4.8 ± 2.8 | 4 ± 1.7 | 6.6 ± 3.8 | 0.03 |
| HCO3 (mmol/l) | 21.5 ± 5.6 | 22.5 ± 5.1 | 19 ± 6 | 0.06 |
| Arterial pH | 7.38 ± 0.10 | 7.39 ± 0.08 | 7.38 ± 0.13 | 0.67 |
| Day 1 clinical status | ||||
| Mean arterial pressure (mm Hg) | 87 ± 16 | 88 ± 14 | 83 ± 21 | 0.44 |
| Pulse (BPM) | 101 ± 19 | 100 ± 19 | 103 ± 20 | 0.63 |
| Grade 3–4 encephalopathy | 55% | 39% | 93% | 0.0004 |
| Cardiac arrhythmia | 33% | 28% | 47% | 0.19 |
| On pressors | 33% | 33% | 33% | 1.00 |
| On dialysis | 25% | 25% | 27% | 0.90 |
| Intubated | 71% | 64% | 87% | 0.10 |
As expected, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and INR levels were markedly elevated at study admission consistent with a diagnosis of severe hepatic ischemia. All but one patient had a peak serum ALT greater than 1,000 IU/ml and rapid normalization of serum aminotransferase levels were noted in 90% within seven days of follow-up. The mean platelet count was substantially reduced at enrollment to 96 × 103/ml and 54% of subjects had a total bilirubin of >3 mg/dl. Mean serum creatinine at enrollment was 2.9 mg/dl and 25% of the patients were on dialysis. More than half of the patients were reported to have advanced encephalopathy (i.e. grade 3 or 4) and over 70% were intubated on day 1.
Twenty-eight patients (55%) had experienced an episode of documented hypotension. Cardiopulmonary decompensation including congestive heart failure (CHF), cardiopulmonary arrest, acute myocardial infarction (MI), and cardiac arrhythmias were the most commonly identified precipitants of ischemic hepatitis (69%). The remainder of the patients had hepatic ischemia due to a multitude of non-cardiac etiologies including severe sepsis (10%), seizures (8%), vascular occlusion of the porta hepatis (4%), severe intravascular volume depletion (2%), and other etiologies (7%). Echocardiogram reports were available for twenty-six patients with all but five demonstrating severely depressed left ventricular systolic function with an ejection fraction <20%. Three patients had severe pulmonary hypertension with right heart failure and another patient had severe aortic stenosis. Liver pathology from biopsy (8), explant (1), or autopsy (3) specimens confirmed ischemic hepatitis with zone 3 centrilobular necrosis of varying severity in twelve patients.
Three-Week Outcomes
At the end of the study, 36 patients (71%) had recovered with supportive care and 13 others had died (25%). In addition, two patients had undergone liver transplantation (4%) (Fig. 1). The first transplanted patient was a 47-year-old Hispanic female who developed severe hepatic ischemia after inadvertent clamping of her hepatic artery and portal vein during a laparoscopic cholecystectomy. She underwent transplantation on study day 2 and is doing well at 5.3 years post-transplant. The second liver transplant recipient was a 21-year-old Hispanic female with a body mass index of 53 kg/m2 who experienced inadvertent clamping of her porta hepatis during an attempted laparoscopic gastric bypass surgery. Her first liver transplant was complicated by primary non-function and she died of fungal sepsis five days after retransplantation.
The mean subject age and gender distributions were not significantly different between the spontaneous survivors and those with adverse outcomes (Table 1). In addition, the mean time from symptom onset to initial hospitalization and the frequency of various medical co-morbidities were similar in the two patient groups.
On study day 1, serum alkaline phosphatase and phosphate levels were significantly higher for patients with adverse outcomes than for spontaneous survivors. In addition, other laboratory data suggestive of more severe illness, for example total bilirubin, creatinine, and bicarbonate levels, were also worse in this group but these trends did not reach statistical significance. Only 39% of spontaneous survivors had grade 3 or 4 hepatic encephalopathy on day 1 compared with 93% of patients who died or required liver transplant (P = 0.0004). Spontaneous survivors were also less likely to have cardiac arrhythmias or require mechanical ventilation and hemodialysis at presentation than patients with adverse outcomes.
Over the course of the study, non-survivors had significantly higher peak ALT levels (6,722 vs. 3,722 IU/l, P = 0.02) and were more likely to be intubated (98% vs. 53%, P = 0.01) than spontaneous survivors (Table 2). Although the mean peak INR, AST, total bilirubin, and creatinine, and the frequency of other clinical complications were higher in patients with adverse outcomes, none of these trends was statistically significant. During their hospital course, five (10%) subjects had an intracranial pressure monitor placed, including one patient who underwent liver transplantation, one subject with refractory seizures, one cardiac arrest patient with cerebral edema on head CT, one subject with a brain tumor and ventricular arrhythmia, and another with a drug overdose. The methadone overdose patient experienced uncal herniation on head CT but survived and is doing well at 2.5 years of follow-up. In addition, nine (18%) patients were treated with N-acetylcysteine and eight (89%) of these patients were spontaneous survivors despite grade 4 encephalopathy at presentation in five of them.
Table 2.
Peak laboratory values and complications through week 3
| Total (n = 51) | Spontaneous survivors (n = 36) | Death/transplant (n = 15) | P | |
|---|---|---|---|---|
| INR | 4.1 ± 2.9 | 4 ± 3.1 | 4.3 ± 2.7 | 0.71 |
| ALT (IU/l) | 4,640 ± 3,258 | 3,772 ± 2,494 | 6,722 ± 3,972 | 0.02 |
| AST (IU/l) | 7,127 ± 6,917 | 6,147 ± 6,509 | 9,478 ± 7,519 | 0.12 |
| Total bilirubin (mg/dl) | 9.5 ± 9.2 | 7.9 ± 7.4 | 13.3 ± 11.8 | 0.11 |
| Creatinine (mg/dl) | 3.9 ± 2.2 | 3.7 ± 2.4 | 4.2 ± 2 | 0.45 |
| Arterial pH (nadir) | 7.32 ± 0.13 | 7.33 ± 0.10 | 7.29 ± 0.16 | 0.20 |
| Cardiac arrhythmia | 55% | 50% | 33% | 0.27 |
| On pressors | 43% | 36% | 60% | 0.12 |
| On dialysis | 35% | 33% | 40% | 0.65 |
| Intubated | 72% | 64% | 93% | 0.03 |
| N-acetylcysteine | 18% | 22% | 6% | 0.092 |
Predictors of Three-Week Survival
The day 1, levels of serum ALT, alkaline phosphatase, and phosphate, and the presence of grade 3 or 4 encephalopathy were significantly associated with adverse outcomes on the univariate Cox proportional hazards model. In multivariate Cox regression modeling, only advanced encephalopathy and high levels of serum phosphate on study day 1 were independent and significant predictors of adverse outcomes at three weeks. The hazard ratio for an adverse outcome with grade 3 or 4 encephalopathy was 8.4 (95% CI 1.1–66.5, P = 0.04) and the hazard ratio for each increase in 1.0 mg/dl of serum phosphate was 1.3 (95% CI 1.1–1.6, P = 0.008).
Long-Term Outcomes for the Initial Three-Week Survivors
Long-term outcomes could be tracked in 28 of the 36 (78%) three week spontaneous survivors (Fig. 1). Nineteen of these patients (67%) were still alive at last follow up whereas nine patients (37%) had died. Causes of death of these nine patients included CHF (2), disseminated Aspergillus (1), cerebral vascular accident (1), multi-organ failure (1), and unknown (4). The median time from study enrollment to death was 57 days (range 29 days–1.8 years). Therefore, of the 51 patients with ALF due to ischemic hepatitis, 14 (27%) died during their initial hospitalization and nine (17%) additional patients died of other causes with two-year actuarial survival of 45%. Of the 19 patients still alive at the end of follow-up, more detailed information was available on 13 (68%). All of the long-term survivors had normalization of their serum AST, ALT, alkaline phosphatase, and bilirubin levels. Over half reported continued heart failure symptoms with one patient actually undergoing successful heart transplantation seven months after his initial presentation (Fig. 2). Persistent seizures were reported in three patients and one patient remained on hemodialysis. Only 23% of the long-term survivors experienced no further medical complications since their initial ALF hospitalization.
Fig. 2.
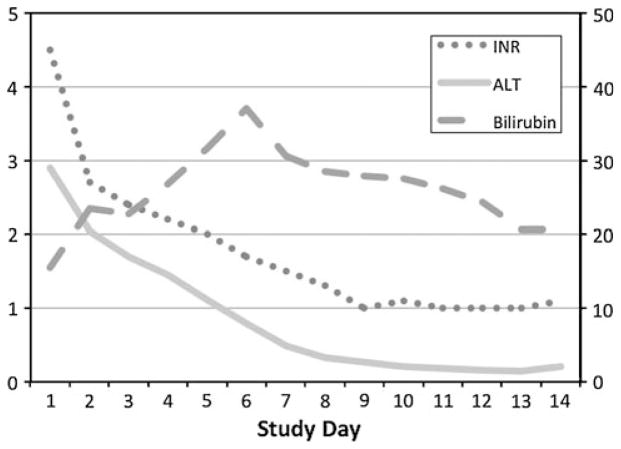
A patient with ALF due to hepatic ischemia from an unrecognized cardiomyopathy. A 24-year-old previously healthy Caucasian male presented with a two-week history of unexplained nausea, vomiting, abdominal pain, and jaundice. At admission, his serum AST was 2,962 IU/l, ALT 2,902 IU/l, total bilirubin 15 mg/dl, and INR 4.5. In addition, he had acute kidney injury with a serum creatinine of 4.4 mg/dl and a phosphate level of 6.6 mg/dl. Evaluation for hepatitis A, B, and C, autoimmune hepatitis, and toxic liver injury was negative and a liver ultrasound demonstrated no biliary tract disease. At enrollment, he had grade 2 encephalopathy and was started on renal replacement therapy on hospital day 2. A surface echocardiogram revealed severe biventricular heart failure with an ejection fraction of <10%. Because of hypotension refractory to inotropes, an aortic balloon pump was placed on hospital day #4 followed by a left ventricular assist device on day #5 and his liver and kidney function gradually improved over the following month. At discharge on hospital day #68, his ALT was 74 IU/l and bilirubin 2.1 mg/dl. Eventually he underwent heart transplantation and his explant revealed evidence of a diffuse idiopathic cardiomyopathy. The patient is currently alive and well nine years after presentation with normal cardiac and hepatic function. (Serum ALT reported in units of 1,000 IU/l)
Discussion
The results of our prospective study demonstrate that although hepatic ischemia is commonly encountered in an ICU, it is an uncommon cause of ALF amongst adult Americans, accounting for only 4.4% of consecutive ALFSG cases. Overall, ALF due to ischemic hepatitis was associated with a high likelihood of recovery during short-term follow-up despite the presence of multi-organ failure in many patients at enrollment (Table 1). The relatively small number of cases (51) over a 10-year period in this multicenter study suggests that either hepatic ischemia rarely leads to ALF or that these patients may have been less commonly referred for enrollment. Because ischemic hepatitis is diagnosed in up to 0.16% of all hospitalized patients and nearly 1% of all ICU patients, it is likely that many patients were not referred because of the generally favorable likelihood of recovery with supportive care [4, 5]. However, some patients without known heart disease may continue to be referred to liver-transplant centers for presumed “idiopathic” ALF that is actually due to hepatic ischemia [5, 6]. In a recent series of 14 ischemic hepatitis patients that presented with nausea, vomiting, and abdominal pain, six had jaundice and elevated serum alkaline phosphatase levels, as we noted in several of our patients (Fig. 2). However, eight of these patients had absolutely no cardiopulmonary symptoms before presentation [5].
As with other more common causes of ALF in the United States, a female preponderance (63%) was noted in our series of ischemic hepatitis patients, for reasons that are unclear [8]. This observation is of particular interest, because previous reports have suggested that ischemic hepatitis is more common in men [7]. However, the racial distribution and other demographic features of patients were generally similar to that of the general US population and the overall ALFSG cohort [8]. The mean age of 50 in the ischemia ALF patients is older than the mean age of 43 in the overall cohort of non-acetaminophen ALF patients enrolled during the same time period. However, the ALF ischemic hepatitis patients are still younger than the mean age of 70 reported in large series of consecutive ischemic hepatitis patients [4, 7]. Surprisingly few of our patients had underlying hypertension or diabetes and the frequency of these co-morbidities was similar to that for the overall ALFSG cohort [9]. Most of the ischemic hepatitis patients had been hospitalized for several days before study entry and reported non-specific symptoms of abdominal pain and fatigue that started a mean of eight days before enrollment. The high incidence of thrombocytopenia (i.e. admission platelet count < 150 k/ml) in our series of 84% is similar to that reported for other series of ischemic hepatitis patients [5]. The thrombocytopenia is likely to be multi-factorial in nature with contributions from impaired hepatic thrombopoietin production, transient portal hypertension from the ALF, medications, and intravascular coagulation [10]. Higher levels of both serum AST and ALT at presentation were associated with poorer prognosis, presumably reflecting more severe and extensive liver necrosis. In support of this, the admission and peak total bilirubin levels tended to be higher in the non-survivors but these trends did not reach statistical significance, presumably because of the limited sample size (Table 1). Our data are consistent with those from another recent study of 117 ischemic hepatitis ICU patients, among whom those with higher peak serum aminotransferase and INR levels also had poorer outcomes [11]. Overall, patients with adverse outcomes seemed to be sicker at enrollment, on the basis of laboratory data, but the frequency of respiratory, renal, and cardiac failure was remarkably similar. By contrast, the presence of more advanced encephalopathy proved to be a distinguishing clinical feature in both univariate and multivariate analysis for adverse outcomes in our patient population. This finding is in keeping with previous studies demonstrating poor spontaneous survival of patients with non-acetaminophen ALF who present with or progress to grade 3 or 4 encephalopathy [12–14]. In the current series, it was difficult to determine whether the advanced encephalopathy was solely due to cerebral edema, because only five of our patients had intracranial pressure monitors placed and detailed data regarding the pressure measurements were not recorded.
Our prospective study demonstrated that underlying cardiopulmonary disease was the most common precipitant of ischemic hepatitis, accounting for 69% of cases. However, the etiology of ischemia did not seem to be associated with short-term clinical outcomes. Surprisingly, two cases of iatrogenic ischemia because of the inadvertent clamping of the porta hepatis during laparoscopic abdominal surgery were reported, both of whom required liver transplantation. Fortunately, this cause of hepatic ischemia seems to be exceedingly rare. Although eight of the nine patients treated with N-acetylcysteine were spontaneous survivors, it remains unclear whether this treatment may be of benefit to other patients, because hepatic ischemia patients were excluded from the randomized controlled trial of NAC in non-acetaminophen adult ALF patients [13].
In multivariate analysis, elevated serum phosphate levels at presentation were associated with a greater likelihood of adverse outcomes. This finding is consistent with several previous studies of patients with ALF of different etiology that also revealed poorer outcomes for patients with hyperphosphatemia [15]. Although the mechanism remains unclear, it is speculated that impaired hepatic regeneration may affect patients with severe acute liver injury who do not rapidly consume phosphate. Alternatively, hyperphosphatemia could simply be a surrogate marker of concomitant renal failure which is also a predictor of poor outcomes in non-acetaminophen ALF [12]. Unfortunately, we did not have serial serum phosphate levels to determine whether persistently elevated phosphate levels may be a better predictor than admission levels. In addition, in future studies it may prove worthwhile to test for other markers of liver regeneration, including phosphatonins [16].
A unique aspect of our study was determining long-term outcomes for the ALF patients with hepatic ischemia. A number of small retrospective studies have suggested that spontaneous survivors of ALF may have residual neurological and hepatic abnormalities during follow-up, but the number of patients followed and the methods used have been variable [17, 18]. To improve our understanding in this area, the ALFSG has begun to track enrolled patients one and two years after presentation [19]. In addition to determining long-term clinical outcomes, we were also interested in finding evidence of residual hepatic disease as suggested by others in patients with severe idiosyncratic drug-induced liver injury [20]. With long-term follow-up available in 28 of the 36 spontaneous survivors, we identified a moderate proportion of patients (32%) who died primarily from underlying cardiovascular disease and none of the long-term survivors seemed to have evidence of persistent liver dysfunction. These data suggest that additional studies of long-term outcomes in patients with ALF due to other etiologies are warranted [21].
Limitations of our study include the small overall number of patients that were available for analysis. However, this series is still the largest cohort of ALF patients with ischemic hepatitis reported to date. Another potential limitation of our study was the means by which a diagnosis was established. By definition, patients had encephalopathy at enrollment but it was difficult to discern the contribution of sedation, hypoxemia, and cerebral edema to the altered mental status. In accordance with the study protocol, hepatic ischemia ALF patients had to have a rapid rise and resolution in serum aminotransferase levels with serum ALT >1,000 IU/ml in a proper clinical context after exclusion of more common causes of liver injury. Although echocardiograms were not mandated for this observational study, review of the available reports confirmed clinically significant structural heart disease in 25 patients. As in our study, previous case series of ischemic hepatitis patients have been unable to document an acute hypotensive episode or arrhythmia in up to 50% of subjects [2]. Our group has previously looked at the diagnostic and prognostic significance of serum troponin levels in adult ALF patients [22]. However, the frequent elevations of serum troponin levels in ALF patients independent of the etiology of ALF, and their non-specific increase with renal failure make clarifying a diagnosis of ischemic hepatitis because of an acute cardiac event quite challenging [23].
In summary, 4.4% of the US adults enrolled in the ALFSG registry had ischemic hepatitis as their final diagnosis. The demographic features of the ALF patients with hepatic ischemia were similar to those of other subgroups of patients with non-acetaminophen ALF except for the higher mean patient age. Heart disease was known in only 31% of the patients at presentation but a cardiopulmonary precipitant of the hepatic ischemia was identified in 69% of the patients during their hospital course. Because some of these patients had no cardiopulmonary symptoms at presentation with a clinical picture of idiopathic hepatitis and prominent fatigue, epigastric pain, and jaundice, it is important to obtain an echocardiogram early, to help identify and treat underlying structural heart disease (Fig. 2). The short-term prognosis for ALF patients with hepatic ischemia was generally favorable with a 70% actuarial three -week survival. Lower serum phosphate levels at enrollment were associated with a greater likelihood of spontaneous survival. In addition, patients with more advanced encephalopathy at enrollment (i.e. grade 3 or 4) had a significantly poorer outcome. For 28 spontaneous survivors, long-term outcomes were ongoing cardiovascular morbidity and mortality with preserved hepatic function.
Acknowledgments
We gratefully acknowledge the support provided by the members of The Acute Liver Failure Study Group 1998–2008. This study was funded by NIH grant DK U-01 58369 for the Acute Liver Failure Study Group provided by the National Institute of Diabetes, Digestive, and Kidney Disease. Additional funding was provided by the Tips Fund of the Northwestern Medical Foundation and the Jeanne Roberts and Rollin and Mary Ella King Funds of the Southwestern Medical Foundation, and T-32 DK007745-12 to DD.
Abbreviations
- ALF
Acute liver failure
- ALFSG
Acute Liver failure study Group
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CHF
Congestive heart failure
- INR
International normalized ratio
- MI
Myocardial infarction
Appendix
Members and institutions participating in the Acute Liver Failure Study Group 1998–2006: W. M. Lee, MD (Principal Investigator), George A. Ostapowicz, MD, Frank V. Schiødt, MD, Julie Polson, MD, University of Texas Southwestern, Dallas, TX; Anne M. Larson, MD, University of Washington, Seattle, WA; Timothy Davern, MD, University of California, San Francisco, CA; Michael Schilsky, MD, Mount Sinai School of Medicine, NY, NY; Timothy McCashland, MD, University of Nebraska, Omaha, NE; J. Eileen Hay, MBBS, Mayo Clinic, Rochester, MN; Natalie Murray, MD, Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, MD, University of Pittsburgh, Pittsburgh, PA; Andres Blei, MD, Northwestern University, Chicago, IL; Atif Zaman, MD, University of Oregon, Portland, OR; Steven H.B. Han, MD, University of California, Los Angeles, CA; Robert Fontana, MD, University of Michigan, Ann Arbor, MI; Brendan McGuire, MD, University of Alabama, Birmingham, AL; Ray Chung, MD, Massachusetts General Hospital, Boston, MA; Alastair Smith, MB, ChB, Duke University Medical Center, Durham, NC; Robert Brown, MD, Cornell/Columbia University, NY, NY; Jeffrey Crippin, MD, Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, MBBS, Medical University of South Carolina, Charleston, SC; Santiago Munoz, MD, Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, MD, University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, MD, Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, MD, University of California Davis, Sacramento, CA; Raj Satyanarayana, MD, Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, MD, University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, Nahid Attar, and Corron Sanders, PhD and the Statistics and Data Management Group included, Joan Reisch, PhD, Linda Hynan, PhD, Janet P. Smith, Joe W. Webster, and Mechelle Murray. We further acknowledge all the coordinators from the study sites, and the patients and their families who participated in this study.
Footnotes
This study was conducted for the Acute Liver Failure Study Group. Please refer to the “Appendix” for members of the Acute Liver Failure Study Group.
Contributor Information
Ryan M. Taylor, Email: rtaylor2@kumc.edu, Department of Internal Medicine, University of Kansas, Kansas City, KS, USA
Shannan Tujios, Email: Shannan.Tujios@UTSouthwestern.edu, Department of Internal Medicine, University of Michigan Medical School, University of Michigan Medical Center, 3912 Taubman Center, Ann Arbor, MI 48109-0362, USA.
Kartik Jinjuvadia, Email: kartik.jinjuvadia@gmail.com, Department of Internal Medicine, University of Michigan Medical School, University of Michigan Medical Center, 3912 Taubman Center, Ann Arbor, MI 48109-0362, USA.
Timothy Davern, Email: davernt@sutterhealth.org, California Pacific Medical Center, San Francisco, CA, USA.
Obaid S. Shaikh, Email: obiad@pitt.edu, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
Steve Han, Email: steven.han@ucla.edu, University of California Los Angeles, Los Angeles, CA, USA.
Raymond T. Chung, Email: rtchung@partners.org, Massachusetts General Hospital, Boston, MA, USA
William M. Lee, Email: william.lee@utsouthwestern.edu, University of Texas Southwestern Medical Center, Dallas, TX, USA
Robert J. Fontana, Email: rfontana@med.umich.edu, Department of Internal Medicine, University of Michigan Medical School, University of Michigan Medical Center, 3912 Taubman Center, Ann Arbor, MI 48109-0362, USA
References
- 1.Hickman PE, Potter JM. Mortality associated with ischemic hepatitis. Aust NZ J Med. 1990;20:32–34. doi: 10.1111/j.1445-5994.1990.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 2.Henrion J, Schapira M, Luwaert R, et al. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine. 2003;82:392–406. doi: 10.1097/01.md.0000101573.54295.bd. [DOI] [PubMed] [Google Scholar]
- 3.Henrion J, Descamps O, Luwaert R, et al. Hypoxic hepatitis in patients with cardiac failure: incidence in a coronary care unit and measurement of hepatic blood flow. J Hepatol. 1994;21:696–703. doi: 10.1016/s0168-8278(94)80226-2. [DOI] [PubMed] [Google Scholar]
- 4.Ebert EC. Hypoxic liver injury. Mayo Clin Proc. 2006;81:1232–1236. doi: 10.4065/81.9.1232. [DOI] [PubMed] [Google Scholar]
- 5.Denis C, deKerguennec C, Bernuau J, et al. Acute hypoxic hepatitis (liver shock): still a frequently overlooked cardiological diagnosis. Eur J Heart Fail. 2004;6:561–565. doi: 10.1016/j.ejheart.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman BJ, Pate MB, Marsh WH, et al. Cardiomyopathy unrecognized as a cause of hepatic failure. J Clin Gastroenterol. 1990;12:306–309. doi: 10.1097/00004836-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Fuhrmann V, Jager B, Zubkova A, et al. Hypoxic hepatitis—epidemiology, pathophysiology, and clinical management. Wien Klin Wochenschr. 2010;122:129–139. doi: 10.1007/s00508-010-1357-6. [DOI] [PubMed] [Google Scholar]
- 8.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Forde KA, Reddy KR, Troxel AB, et al. Racial and ethnic differences in presentation, etiology, and outcomes of acute liver failure in the United States. Clin Gastroenterol Hepatol. 2009;7:1121–1126. doi: 10.1016/j.cgh.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiodt FV, Balko J, Schilsky M, et al. Thrombopoietin in acute liver failure. Hepatology. 2003;37:558–561. doi: 10.1053/jhep.2003.50113. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrmann V, Kneidinger N, Herkner H, et al. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009;35:1397–1405. doi: 10.1007/s00134-009-1508-2. [DOI] [PubMed] [Google Scholar]
- 12.Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755–764. doi: 10.1016/s0016-5085(03)01051-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-Acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Grady JG, Alexander GH, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 15.Chung PY, Sitrin MD, Te HS. Serum phosphorus levels predict clinical outcome in fulminant hepatic failure. Liver Transplant. 2003;9:248–253. doi: 10.1053/jlts.2003.50053. [DOI] [PubMed] [Google Scholar]
- 16.Kuroo M. Overview of the FGF23-Klotho axis. Pediatric Nephrol. 2010;25:583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 17.Jackson EW, Zacks S, Zinn S, et al. Delayed neuropsychological dysfunction after liver transplantation for acute liver failure: a matched, case controlled study. Liver Transplant. 2002;8:932–936. doi: 10.1053/jlts.2002.35550. [DOI] [PubMed] [Google Scholar]
- 18.Youssef WI, Mullen KD. Liver transplantation in advanced liver failure: neurologic outcome in acute versus chronic liver disease. Liver Transplant. 2002;8:937–938. doi: 10.1053/jlts.2002.35925. [DOI] [PubMed] [Google Scholar]
- 19.Fontana RJ, Rossaro L, Hynan L, et al. Long-term survival is significantly higher in liver transplant recipients compared to spontaneous survivors with acute liver failure (Abstract) Hepatology. 2009;50 Abstract #246. [Google Scholar]
- 20.Aithal PG, Day CP. The natural history of histologically proved drug induced liver disease. Gut. 1999;44:731–735. doi: 10.1136/gut.44.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jepsen P, Schmidt LE, Larsen FS, et al. Long-term prognosis for transplant-free survivors of paracetamol induced acute liver failure. Aliment Pharmacol Ther. 2010;32:894–900. doi: 10.1111/j.1365-2036.2010.04419.x. [DOI] [PubMed] [Google Scholar]
- 22.Parekh NK, Hynan LS, De Lemos J, et al. Elevated troponin 1 levels in acute liver failure: Is myocardial injury an integral part of acute liver failure? Hepatology. 2007;45:1489–1495. doi: 10.1002/hep.21640. [DOI] [PubMed] [Google Scholar]
- 23.Freda BJ, Tang HW, Van Lente F, et al. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Col Cardiol. 2002;40:2065–2071. doi: 10.1016/s0735-1097(02)02608-6. [DOI] [PubMed] [Google Scholar]