Abstract
The importance of executive functioning for later life outcomes, along with its potential to be positively affected by intervention programs, motivates the need to find early markers of executive functioning. In this study, 18-month-olds performed three executive-function tasks—involving simple inhibition, working memory, and more complex inhibition—and a motion-capture task assessing prospective motor control during reaching. We demonstrated that prospective motor control, as measured by the peak velocity of the first movement unit, is related to infants’ performance on simple-inhibition and working memory tasks. The current study provides evidence that motor control and executive functioning are intertwined early in life, which suggests an embodied perspective on executive-functioning development. We argue that executive functions and prospective motor control develop from a common source and a single motive: to control action. This is the first demonstration that low-level movement planning is related to higher-order executive control early in life.
Keywords: prospective motor control, motor development, executive functions, reaching, infancy
In the current study, we assessed the link between prospective motor control and executive functions in infancy. We argue that they develop from a common source and a single motive: to control action. Executive functions are concerned with choosing, enacting, and sustaining goal-directed actions (Barkley, 2012); they include inhibition, working memory, and cognitive flexibility (Diamond, 2013). Executive functions affect quality of life and academic performance, and the positive effects of intervention programs (Diamond, 2013) have motivated the need to find early markers. However, little is known about the developmental origin of executive functions, which are generally assumed to develop from infancy in a hierarchical manner: Complex forms emerge from the development and integration of simpler forms (Garon, Bryson, & Smith, 2008). Posner and Rothbart (2000) noted that one early source of executive functions may be selective attention (Johansson, Marciszko, Gredebäck, Nyström, & Bohlin, 2015). Other researchers have demonstrated associations early in infancy between the development of executive functions, processing speed, and memory (Bell, 2012; Rose, Feldman, Jankowski, & Van Rossem, 2012). In addition to the cognitive foundations of executive functions, it is clear that the social context, particularly the child-parent relationship, affects the development of executive functions (Bernier, Carlson, Deschênes, & Matte-Gagné, 2012).
We take a different approach, arguing for an embodied perspective (Gentsch, Weber, Synofzik, Vosgerau, & Schütz-Bosbach, 2016; Marshall, 2016; Wilson, 2001) of the emergence of executive functions. More specifically, we propose that executive functions are grounded in an infant’s developing ability to control and plan motor actions (i.e., prospective motor control). A central aspect of executive functions is the regulation of actions and goals. From a motor-development perspective, these abilities are an integrated part of human life as early as they can be measured. Studies of fetal development have documented goal-directed action patterns as early as the 22nd week of gestation (Zoia et al., 2007). After birth, action planning develops quickly. Newborns direct their actions toward visible goals (van der Meer, van der Weel, & Lee, 1995), and most infants are able to prospectively control reaching by 5 months (von Hofsten, 2004). Complex action planning emerges a few months later, and infants can plan action sequences beginning at 10 months of age (Claxton, Keen, & McCarty, 2003). Thus, infants’ emerging prospective motor-control abilities possibly provide a foundation for executive control of actions (Diamond, 2013; Miyake et al., 2000; Thelen, Corbetta, & Spencer, 1996; Thelen, Schöner, Scheier, & Smith, 2001).
Three lines of evidence make plausible the tentative link between prospective motor control and executive functions. First, large overlaps exist in the neural structures, particularly the cerebellum and prefrontal cortex, that control action and executive functions (Barkley, 2012; Diamond, 2000). Second, Ridler et al. (2006) demonstrated correlations between the onset of walking and standing in infancy and the facility of working memory and categorization in 33- to 35-year-olds. Furthermore, early onset of walking in infancy and high executive function performance in adulthood were associated with increased gray-matter density in both the frontal lobes and the cerebellum (Ridler et al., 2006). Finally, executive functions and motor deficits share a large degree of comorbidity in a range of clinical diagnoses, including attention-deficit/hyperactivity disorder (Mariani & Barkley, 1997), autism spectrum disorder (Ekberg, Falck-Ytter, Bölte, Gredebäck, & EASE Team, 2016), and depression (Marvel & Paradiso, 2004).
If executive functions do emerge from developing prospective motor control, then this connection should be observable as it develops early in life, as soon as executive functions can be measured reliably. In the present study, 18-month-old infants participated in assessments of simple inhibition, working memory, and more complex inhibition (we refer to this task as the complex-inhibition task). The infants were also assessed with a task measuring prospective motor control (i.e., the ability to control and plan actions) in the smallest observable units of actions, known as movement units (von Hofsten, 1991). A movement unit comprises a few hundred milliseconds of the movement and includes one acceleration phase and one deceleration phase of a joint. The first movement unit reveals the initial motor plan before eventual adjustments are applied (Jeannerod, 1988) and can precisely index prospective motor control (von Hofsten, 1991, 1993). Velocity is a central parameter of goal-directed movements (Plamondon & Alimi, 1997; Thelen et al., 1996), and the peak velocity of the first movement unit can serve as a measurement of prospective motor control (Gottwald et al., 2016).
We propose that in 18-month-old infants, prospective motor control is associated with executive functions. Finding such an association would provide insights into executive-function development, demonstrate for the first time that low-level movement planning is related to higher-order executive functions, and lay the foundation for an embodied approach to executive functions. A higher peak velocity for the first movement unit should be associated with better performance on tasks assessing simple forms of executive functions, such as simple inhibition and working memory. We expected no correlation between prospective motor control and performance on the complex-inhibition task, given that the executive abilities needed for this task are assumed to not have developed sufficiently at 18 months of age (Garon et al., 2008). Furthermore, by controlling for general motor skills, we assessed whether individual differences in executive functions were specifically related to prospective motor control and not general maturity.
Method
Participants
The final sample included 53 infants (22 girls, 31 boys) aged 18 months (mean age = 542 days, SD = 9, age range = 529–561 days). An additional 17 infants were tested but excluded from analysis because of incomplete task performance (n = 11), technical error (n = 4), or low-quality motion-tracking data (n = 2).1 To be included in the sample, the infants had to complete all experimental tasks. Across the various tasks, completion rate ranged from 84% to 96% of all participants. Informed consent was obtained from both parents of all participants included in the study. Participants were recruited from the lab’s database of parents who had expressed interest in participating in research studies with their child. The parents received a gift voucher worth 100 Swedish kronor (approximately €10) for their participation. All procedures involving human participants were performed in accordance with the ethical standards of the regional ethics committee and the 1964 Declaration of Helsinki (World Medical Association, 2013) and its later amendments or comparable ethical standards.
Design
In this study, we combined measures of three components of executive function (simple inhibition, working memory, and more complex inhibition) and a measure of prospective motor control. The tasks were performed in the following order: complex inhibition, simple inhibition, prospective motor control, and working memory. In addition, general motor skills were assessed by using 40 items on gross motor skills and 36 items on fine motor skills from the Vineland Scales of Adaptive Behavior (Sparrow, Cicchetti, & Balla, 2005).
Materials and procedure
Before visiting the lab, the parents rated their infant’s gross and fine motor skills (i.e., their general motor skills) by filling out the 76 items from the Vineland Scales of Adaptive Behavior (Sparrow et al., 2005). After filling out a consent form, the caregivers sat at a table with their infants on their laps, facing the experimenter. The infants then performed the tasks. The parents were instructed to stay neutral and not to interfere with their infant’s behavior. Including breaks and instructions, the whole procedure took approximately 30 min.
Complex-inhibition task
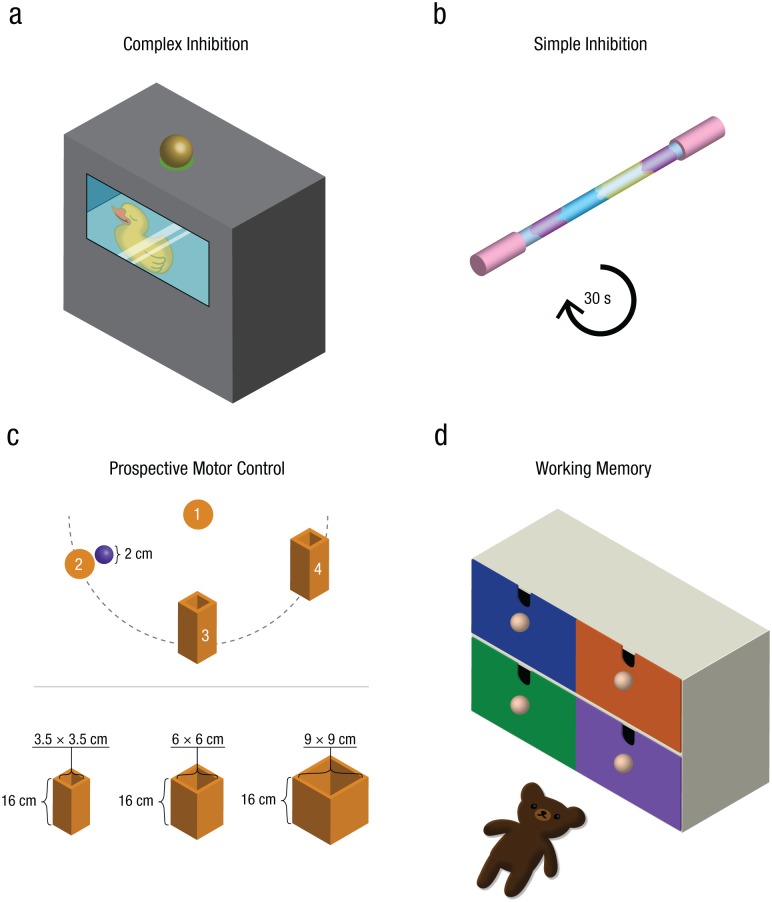
This task was a modified version of the tricky-box task used by Garon, Smith, and Bryson (2014), measuring complex inhibition. For this task, which took approximately 6 min, a custom-built black wooden box (22 × 22 × 12.5 cm) was used (Fig. 1a). It had one wooden knob (4.5 cm in diameter) on top and a Plexiglas window (15 × 8.5 cm) on the front. A shelf inside the box could be seen through the window, and on the shelf sat a toy duck. An electric switch that controlled the opening of the window was attached to the back of the box. The infant had to inhibit one action (i.e., reaching directly toward the duck behind the window) and perform another action first (i.e., reaching for and pulling the knob that opened the window) to retrieve the duck. Reaching directly for the knob to open the window indicated better complex inhibition ability, whereas reaching for the window indicated poorer complex inhibition ability.
Fig. 1.
Materials and setup for the four tasks. In the complex-inhibition task (a), the infants had to open the window of a box via a knob in order to retrieve a duck that was inside. In the simple-inhibition task (b), the infants were told not to touch a glittering wand. After 30 s, they were told they could touch it. In the prospective-motor-control task (c), the infants placed a hand in a marked area (1), reached for an object (2), and placed the object in a box that was positioned either at a short distance (3) or a long distance (4) from the object. Each participant placed an object in a small box, a medium box, and a big box. In the working memory task (d), one of three toys (in this example, a teddy bear) was hidden in one of the drawers of a chest, and the infants then searched for it in the four drawers. There was a time delay of 5 s before search began.
First, the experimenter presented the black box and demonstrated how to open the window by pulling the knob on top of the box. However, the opening was actually controlled by the switch on the back of the box. In the warm-up phase, the experimenter pushed the box to the infant and said, “Now you can try! Can you open the window?” If the infant did not act, the experimenter again asked the infant to open the box. If the infant reached for the window, the experimenter reminded the infant about the knob by pointing to it and saying, “You have to pull here!” When the infant pulled the knob, the experimenter used the switch to open the box; the infant could not see the experimenter’s actions. This process was repeated until the infant opened the box two times to ensure that he or she understood the mechanism and could perform it. After this, an attractive toy (color-changing plastic duck) was presented and given to the infant for 10 to 15 s. In the subsequent four test trials, the toy was placed behind the window on the shelf inside the box. Then the experimenter pushed the box toward the infant and said, “Now you can take the duck.” The parent was instructed to gently hold the infant’s arms until the box came to a stop in front of the infant to prevent early reaches while the box was still moving. If the infant reached only for the window, the experimenter waited for 10 s and then pointed to the knob and said, “You have to pull here!” If the infant did not pull the knob, the experimenter opened the window by pulling the knob and took out the toy and gave it to the infant. After getting the toy, the infant was allowed to play with it for 5 to 10 s.
Simple-inhibition task
The task established by Friedman, Miyake, Robinson, and Hewitt (2011) was used to measure simple inhibition. Specifically, we measured the infants’ ability to inhibit reaching for an attractive toy (a colorful glittering wand, 31 cm long and 2 cm in diameter) for 30 s (Fig. 1b). The experimenter made eye contact with the infant, presented the wand by holding it in front of her, and then placed it on the table within the infant’s reach. Simultaneously, the experimenter shook her head and said, “Now, [infant’s name], you are not allowed to touch this.” Then she looked away with a neutral facial expression. After 30 s had passed (earlier if the infant touched the toy), the experimenter encouraged the infant to play with the toy by saying, “It’s okay, you can touch it now.”
Prospective-motor-control task
The task was adopted from Claxton et al. (2003) and Rosander and von Hofsten (2011) and has been used previously to study prospective motor control in 14-month-old infants (Gottwald et al., 2016). Prospective motor control was measured as the peak velocity of a reaching action’s first movement unit; high movement velocity in valid trials (for details, see Data Coding and Analysis) indicated high prospective-motor-control abilities. The infant had to reach for an object (a plush toy plum, 2 cm in diameter) and place it in a wooden box (Fig. 1c). Large, medium, and small boxes that were used measured 16 cm tall, with inner measures of 9 × 9 cm, 6 × 6 cm, or 3.5 × 3.5 cm, respectively. First, the experimenter showed the object and one of the boxes to the caregiver and the infant. Next, she placed the object and box at defined positions on the table and said, “Look, the plum! Can you place it into the box?” The object and boxes were placed in a half-circle around the infant. The caregiver subsequently reached for the object and joyfully placed it into the box. This was done twice to show the infant the expected action.
In the following test trials, the experimenter presented the infant with the object and one of the boxes. She then placed both the object and the box on the table. The distance between the object and the box was either 12 cm or 37 cm. The positions and sizes of the boxes were counterbalanced across trials. Infants were instructed to place their right hands on the starting area marked by a 5-cm colored circle. If the infants did not follow the instructions, the caregivers were instructed to assist by holding the infant’s arm gently from behind so that the infant’s hand was touching the starting area. They released the arm when the experimenter indicated a new trial by saying “again.” The infant was verbally encouraged to place the object in the boxes, and both the experimenter and the caregiver praised the infant after he or she performed the action. Eighteen trials were performed in a counterbalanced order in blocks of three identical trials (e.g., three trials of medium boxes at the longer distance followed by three trials of small boxes at the shorter distance) and continued until the infant lost interest in the task. The task took approximately 10 min to perform.
Working memory task
Working memory was measured using a classic hide-and-seek task (Garon et al., 2008) with a time delay before searching. The task took approximately 6 min. A small chest of four drawers (21 × 28.5 × 18.5 cm) was used. The drawers were painted different colors (Fig. 1d). A cloth was attached to the chest to hide the drawers during the delay before searching. In the warm-up phase, the infant first selected one of three toys that was to be hidden in one of the four drawers. After two warm-up trials in which the toy was hidden, and the infants searched for it without time delay, four test trials were performed. On each trial, the experimenter showed the toy to the infant, holding it above one drawer and slowly hiding it inside while saying, “Now I am hiding it here.” Then she covered the chest with the cloth, looked away with a neutral facial expression, and waited for 5 s. The parent was instructed to hold the infant’s arms during the delay to prevent the infant from pointing to the drawers. Next, the experimenter pushed the chest to the infant, saying, “Now you can search!” The parents were instructed to release the infant’s arms and to not interfere with the infant’s behavior. The infant could search for the toy a maximum four times before the experimenter started a new trial. When the infant found the toy the experimenter said, “There it was!” When the infant did not find the toy, she said, “Where is it?” to motivate more searching. The toy was hidden in a new location in each of the four test trials, with the same order used for all participants.
Data recording
The entire session was filmed by video cameras from above (a bird’s-eye view) and from the side. Kinematic data were recorded by a motion-tracking device (Qualisys Motion Capture Systems, Gothenburg, Sweden) at a sampling rate of 240 Hz. An eight-camera motion-capture system was used to identify and track the motion of reflective markers (0.6 cm in diameter); each infant had two markers, one on each hand.
Data coding and analysis
Complex-inhibition task
This task measured the infants’ ability to inhibit reaching for a window showing a toy in a box rather than reaching for the knob on the top of the box to open the window. The warm-up trials were coded for whether the infants opened or did not open the box via the knob and whether or not the infants needed to be reminded of the knob by the experimenter. The coding revealed that, in at least one of the two warm-up trials, 85% of the final sample opened the box via the knob without a reminder, 13% opened the box with a reminder, and 2% (i.e., 1 participant) did not open the box. The infants received a score of 2 points if they reached directly for the knob, 1 point if they reached for the window first and then self-corrected and went for the knob, and 0 points if they reached for the window and then reached for the knob only after being reminded by the experimenter or if they did not reach for the knob at all. The mean score over all test trials was calculated for every participant, ensuring that the variable used was not binary. Higher values indicated higher inhibition (the maximum was 2 points). A second rater double-coded 20 videos, and interrater reliability was high (intraclass correlation coefficient, or ICC = .98). A valid trial consisted of a successful action sequence that ended with the retrieval of the object. Participants had to have at least one valid trial out of four to be included in the analysis. Four infants were excluded because they lacked any valid test trials, 1 infant was excluded because we made a technical error, and 1 infant was excluded because he did not participate in the task. Thus, 65 participants (93%) contributed valid data.
Simple-inhibition task
This task measured the infants’ ability to inhibit reaching for an attractive toy. Videos were coded for the time when the experimenter let go of the wand and, if applicable, the time when the infant touched the wand. A second rater double-coded 20 videos, and interrater reliability was high (ICC = .99). The latency between the two events (i.e., the infant’s waiting time) was calculated in seconds, which resulted in interval-scaled values. If the infant did not touch the wand, the maximal value of 30 s was assigned. High inhibition was indicated by a high value (the maximum was 30 points). A valid trial included no parental interference and ended when the infant touched the toy or 30 s had passed. One infant had to be excluded because the parent interfered with the procedure, and 2 infants were excluded because of a technical failure. Thus, 67 infants (96%) contributed usable data.
Prospective-motor-control task
This reach-to-place task measured the infants’ prospective motor control via the peak velocity of the first movement unit during the initial reach toward the to-be-placed object. Videos were coded for the beginning and end of the reaching actions using Qualisys Track Manager (Version 2.12). The last frame before the start of the movement of the right hand and the first contact between the hand and object were defined as the start and end points of the movement, respectively. The kinematic data for the right hand were analyzed. Left-handed or both-handed reaches were seldom performed (19 infants in the final sample performed 42 non-right-handed reaches, an average of less than one trial per infant in the whole sample of 53 infants), and the experimental design requested right-hand actions. Reaching movements starting from the marked area, aiming directly for the object, and followed by direct placement movements were counted as valid trials.
Eleven infants had to be excluded: 3 because they did not participate in the task, 4 because we made a technical error, 2 because of low-quality motion-tracking data, and 2 because they contributed fewer than three valid trials. Thus, 59 infants (84%) contributed valid data and were included in the final sample; these infants performed 25 trials on average. (Inspection of the data from the total sample revealed that infants performed 24 trials on average.) In the final sample, 50% (13 trials on average) of all trials performed were considered valid, whereas 48% of the trials performed by all participants were valid. A second rater double-coded 20 of the 70 videos to judge trial validity, and interrater reliability was high (ICC = .97).
A movement unit is defined by the movement’s velocity profile and contains one acceleration phase and one deceleration phase. Velocity reaches its peak at the end of the acceleration phase (Gottwald & Gredebäck, 2015; von Hofsten, 1991). The peak velocity of the first movement unit was extracted from the kinematic data. The position data were polynomially interpolated using Qualisys Track Manager (the criterion for interpolation was a maximal data gap of 30 frames) before being exported to TimeStudio (Version 3.03; http://timestudioproject.com; Nyström, Falck-Ytter, & Gredebäck, 2016; the exact analysis used in this study can be downloaded using uwid ts-9d7-086 from within the TimeStudio program), a MATLAB-based open-access analysis tool.
As in previous research (Grönqvist, Strand Brodd, & von Hofsten, 2011), the data were filtered separately for x-, y-, and z-coordinates by a three-sample-median filter to remove outliers, and a Butterworth low-pass filter at 10 Hz was applied to position data. Subsequently, three-dimensional velocity was calculated and again smoothed using the Butterworth low-pass filter at 10 Hz. Movement units were semiautomatically detected using the criteria specified by Gottwald et al. (2016): a minimal movement-unit peak distance of one sample and a movement-unit merge threshold of eight samples. After visual inspection, a few trials were excluded if the first movement unit was incomplete (5% of performed trials, 9% of valid trials) or if the full trial was noisy (1% of performed trials, 2% of valid trials). The peak velocity of the first movement unit was determined, and average peak velocities were calculated for every participant. High velocities in valid trials indicated high prospective motor control abilities.
Working memory task
This task measured the infants’ working memory using a hide-and-seek task with a time delay before searching. The warm-up trials were coded for opening and nonopening of the drawers, which revealed that all participating infants opened drawers in both warm-up trials. The test trials were coded for successful searches: The infants received scores of 4 points, 3 points, 2 points, or 1 point according to whether they were successful on the first, second, third, or fourth try, respectively. Infants who did not succeed after four attempts were given no points. The mean score over all test trials was calculated for every participant. Higher values indicate greater working memory performance (the maximum was 4 points). Participants had to have at least one valid trial out of four to be included in the analysis. Nonvalid trials were those in which the infants did not search for the toy. A second rater double-coded 20 videos, and interrater reliability was high (ICC = .92). Three infants were excluded because we made a technical error, 3 infants were excluded because they lacked any valid test trials, and 1 infant did not participate in the task. Thus, 63 participants (90%) provided valid data.
Statistical analysis
One outlier in the kinematic data (3 SD from the mean) was removed. Skewness and kurtosis were within acceptable ranges (skewness = −0.496 to 1.432; kurtosis = −1.017 to 1.859; see Kline, 2005). Bivariate correlations (Pearson’s point-biserial correlations, respectively) were calculated to investigate the relationships between all variables. We used t tests to check for gender differences in prospective motor control and executive-function performance. Afterward, we ran a hierarchical regression on peak velocity of the first movement unit during reaching with the variables fine motor skills, gross motor skills, age, and gender (Step 1), and then we added simple inhibition, working memory, and complex inhibition to the regression (Step 2). In Step 1, we investigated merely the contributions of the control variables, whereas in Step 2, we took into account all measures of executive functions. All reported statistical tests were two-tailed.
Results
Complex inhibition
On average, participants in the sample contributed 3.81 valid trials out of 4 trials (SD = 0.81). The average score was 1.28 out of 2 possible points (range = 0–2 points, SD = 0.68). We found no gender differences in task performance, t(51) = −0.21, p > .250.
Simple inhibition
The average waiting time was 7 s out of the maximum 30 s (range = 0–30 s, SD = 11.64). Nine participants in the sample waited the maximum 30 s before touching the attractive toy, whereas 17 touched it immediately. We found no gender differences in task performance, t(51) = −0.98, p > .250.
Prospective motor control
The average peak velocity of the first movement unit during reaching was 562.12 mm per second (range = 363.42–815.14 mm/s, SD = 87.25). We found no gender differences in task performance, t(51) = −0.66, p > .250.
Working memory
Participants in the sample completed an average of 3.77 valid trials out of 4 trials (SD = 0.56). The average score was 2.79 out of 4 possible points (range = 0.75–4 points, SD = 0.72). We found a significant gender difference in task performance: Girls (M = 3.03, SD = 0.64, n = 22) scored higher on working memory than boys did (M = 2.63, SD = 0.74, n = 31), t(51) = −2.06, p = .045.
Vineland gross and fine motor skills
The data were complete for all participants. On average, participants scored 1.28 out of the maximum 2 points on gross motor skills (range = 0.27−1.84 points, SD = 0.21) and 0.63 out of the maximum 2 points on fine motor skills (range = 0.43−0.91 points, SD = 0.11).
Associations between prospective motor control and executive functions
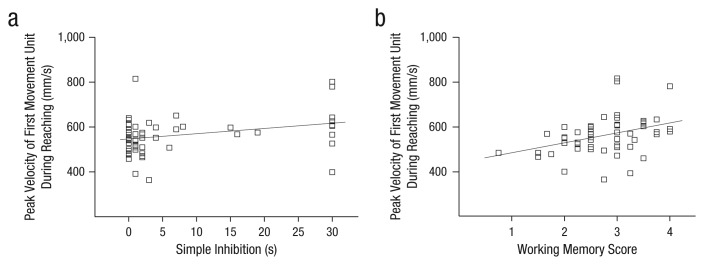
Prospective motor control correlated significantly with simple inhibition, r = .31, p = .026, and working memory, r = .39, p = .004 (Fig. 2), but not with complex inhibition, age, gender, fine motor skills, or gross motor skills. Fine motor skills and gross motor skills were significantly interrelated, r = .42, p = .002, but they were not related to any of the other variables. No significant correlations with participant age were found (Table 1).
Fig. 2.
Scatterplots (with best-fitting regression lines) showing the relationship between the peak velocity of the first movement unit during reaching and performance on the (a) simple-inhibition and (b) working memory tasks.
Table 1.
Correlations Among All Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Prospective motor control | — | ||||||
| 2. Simple inhibition | .31* | — | |||||
| 3. Working memory | .39** | .13 | — | ||||
| 4. Complex inhibition | .08 | –.12 | .02 | — | |||
| 5. Fine motor skills | –.06 | –.04 | –.05 | .14 | — | ||
| 6. Gross motor skills | .17 | .12 | .11 | .09 | .42** | — | |
| 7. Age | –.06 | .19 | .07 | .15 | .01 | –.17 | — |
| 8. Gender | –.08 | –.14 | –.28* | .03 | –.24 | –.19 | –.16 |
Note: The coefficients for gender are point biserial correlations; all others are Pearson’s correlations.
p < .05. **p < .01.
Hierarchical regression analysis
Step 1, in which the predictors were fine motor skills, gross motor skills, age, and gender, did not produce significant results, F(4, 48) = 0.70, p > .250. This suggests that none of the control variables accounted for the relationship between executive functions and prospective motor control (Table 2). Step 2, in which we added scores for simple inhibition, working memory, and complex inhibition as predictors, did produce significant results, F(7, 45) = 2.29, p = .044. This analysis explained 26% of the variance in the peak velocity of the first movement unit. Simple inhibition, β = 0.29, p = .037, and working memory, β = 0.35, p = .013, made significant independent contributions. Complex inhibition did not contribute significantly.2
Table 2.
Results From the Hierarchical Regression Analysis Predicting the Velocity of the First Movement Unit During Reaching
| Step and predictor | b | SE (b) | β |
|---|---|---|---|
| Step 1: R2 = .06, F(4, 48) = 0.70 | |||
| Fine motor skills | –138.39 | 126.98 | –0.17 |
| Gross motor skills | 89.56 | 64.87 | 0.22 |
| Age (days) | –0.30 | 1.48 | –0.03 |
| Gender | –14.21 | 25.87 | 0.08 |
| Step 2: R2 = .26, F(7, 45) = 2.29* | |||
| Fine motor skills | –66.32 | 118.99 | –0.08 |
| Gross motor skills | 41.20 | 60.90 | 0.10 |
| Age (days) | –1.35 | 1.42 | –0.13 |
| Gender | 6.25 | 24.74 | 0.04 |
| Simple inhibition | 2.23 | 1.06 | 0.29* |
| Working memory | 42.81 | 16.49 | 0.35* |
| Complex inhibition | 16.76 | 17.27 | 0.13 |
Note: The Vineland Scales of Adaptive Behavior (Sparrow, Cicchetti, & Balla, 2005) were used to assess fine and gross motor skills.
p < .05.
Discussion
Our results demonstrated that executive functions are related to prospective motor control. As we predicted, simple inhibition and working memory were positively related to prospective motor control, whereas the complex inhibition and control variables were not.
We propose an embodied perspective of the development of executive functions. According to this account, people’s ability to control and plan their actions (in an executive sense) begins in infancy with their need and ability to prospectively control their motor actions. Whereas the motor system is engaged in achieving low-level goals, such as reaching for a ball while reducing error in performance and adjusting to the environment (Wolpert, Diedrichsen, & Flanagan, 2011), executive functions are related to goals on a higher cognitive level that do not directly deal with the movements of individual joints, but rather with the long-term benefits of the system as a whole (Barkley, 2012). The embodied account suggests that the development of executive functions is grounded in prospective motor control in infancy. We assume that early executive functions proliferate during childhood into their own separate domain of higher order action control, which is consistent with the suggestion that cognitive processes differentiate over development (Karmiloff-Smith, 2015). This assumption is supported by the findings of the current study. We demonstrated that the peak velocity of the first movement unit during reaching is related to performance on simple-inhibition and working memory tasks. In other words, the ability to plan reaching actions (as measured on the smallest possible scale, that of movement units) is related to higher-order control (as indexed by standardized behavioral tasks used to assess executive functions). Thus, our results offer the first demonstration that low-level movement planning is related to higher-order executive control early in life.
Our suggestion of an embodied account of early executive-function development is supported by the theoretical work by Thelen et al. (2001), in which they proposed an embodied account of executive-function development using the A-not-B task. According to the dynamic-systems approach, cognitive development can be understood as continuous interactions among the brain, the body, and the ever-changing environment (Thelen et al., 1996, 2001), a notion that is highly consistent with the approach taken in the current study.
One potentially limiting factor is that our study does not include longitudinal data; from this study alone, we cannot conclude whether prospective motor control affects executive functions or vice versa. However, there are two reasons why we argue for a pathway leading from motor control to cognitive control. First, prospective dimensions of motor control develop during the first few months of life (van der Meer et al., 1995; von Hofsten, 2004; Zoia et al., 2007), whereas executive functions generally have been described as emerging later in infancy (Barkley, 2012; Diamond, 2000). Second, Ridler et al. (2006) demonstrated correlations between the onset of standing and walking in infancy and executive functions in adulthood. The long-term predictive value of early motor proficiencies is in accord with the notion that executive functions develop from motor-control processes. It is not clear from the work of Ridler et al. (2006) how the onset of standing and walking, which was used to assess motor development in their study, relates to later executive functions.
These motor milestones are influenced by several factors and have large consequences for the experiences that infants gain from interacting with the world. The onset of standing and walking may be related to individual differences in general maturation, psychosocial context, or personality. In addition, walking enables the infant to perceive the world differently than when crawling. Walkers see more, move more, and have their hands free for object exploration and social interaction (Adolph & Tamis-LeMonda, 2014). All of these factors may also be associated with executive functions without a direct link between motor development and later executive functions.
Taken together, the current results and the prior work by Ridler et al. (2006) and Thelen et al. (1996, 2001) suggest that prospective motor control is an important component in the development of executive functions. We suggest that prospective motor control we report and the predictive relationship between executive functions and motor milestones reported by Ridler et al. reflect the same embodied developmental process. Controlling reaching, standing, and walking requires prospective motor control. Infants who develop these abilities earlier develop better executive-function skills, and these developmental differences remain throughout life.
When focusing on concurrent relationships in a developmental sample, it is possible that other factors, such as general maturity, are the real source of covariation between variables of interest. In the current study, this is not the most parsimonious explanation given that motor control is related to two forms of executive control (simple inhibition and working memory) but not complex inhibition or general motor skills as measured by the Vineland scales. The latter assess motor functionality and are often used to control for differences in general maturation. The lack of relationship with complex inhibition is in line with the framework proposed by Garon et al. (2008) and further indicates that complex inhibition is not fully developed at 18 months. The average performance on this task was rather low, which also provides evidence for this assumption.
Given that all tasks in the current experiment involved manual behavior, it is important to consider the possibility that the observed individual differences in results of executive-function tasks did not capture meaningful variance in subcomponents of executive functions. Instead, they may have captured variability in low-level motor control, which creates a situation in which several assessments of prospective motor control correlate with each other. This is not likely in the current study because of the level of cognitive performance required to pass each executive-function task. The working memory task involved reaching toward a goal, but the infants were scored on the basis of whether the goal location contained a previously demonstrated reward. The task indexes memory of prior events instead of infants’ proficiency in prospectively controlled reaching. In the simple-inhibition task, the infants were told not to touch an interesting toy, and good performance involved inhibiting a behavioral response—the opposite of what was required in the prospective-motor-control task.
Future research should assess the link between prospective motor control and executive functions longitudinally to investigate the direction of the observed effects, starting at 4 to 6 months and continuing until executive functions can be reliably assessed at 18 months. Active training studies provide further research opportunities: By having several testing occasions, effects of the specific prospective-motor-control training on executive-function abilities could be compared with effects of general motor training or no training.
In summary, we demonstrated an association between prospective motor control and executive functions in infancy. These findings are consistent with the suggestion that executive functions emerge from prospective motor control in everyday actions, such as reaching or walking. In other words, the emergence of executive functions should be seen as being grounded in the development of motor control.
Acknowledgments
We thank Matilda Göranzon and Elin Schröder for help with data collection, other members of the Uppsala Child and Baby Lab for comments on the manuscript, Mattias Stridbeck for illustrations, and the families for participating in the study.
Some infants were excluded for more than one reason or more than one task; they are counted only once in this total but are counted more than once in the task-specific exclusion information in the Data Coding and Analysis section.
As noted in the Statistical Analysis section, the values for kurtosis and skewness did not indicate violation of normal distribution. However, the distribution of the simple inhibition score in Figure 2 may lead to questions about normality. Therefore, we log-transformed the data (log2 (simple inhibition + 1)). Additional correlation and regression analyses with the transformed data demonstrated similar effects; there was a significant correlation between prospective motor control and both simple inhibition, r2 = .26, p = .029, and between prospective motor control and working memory, r2 = .39, p = .002. Step 2 of the regression analysis with the target variables explained 25% of the variation, F(7, 45) = 2.11, p = .062, along with the contributions of simple inhibition, β = 0.26, p = .063, and working memory, β = 0.37, p = .010.
Footnotes
Action Editor: Marc J. Buehner served as action editor for this article.
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This project was funded by two grants from the Marie Skłodowska-Curie Initial Training Network (“Action research: Improving understanding and methodologies in early development” Grant 289404 and “Brainview” Grant 642996), by a grant from the Developmental Social Cognition and Action Understanding Project (CACTUS) of the European Research Council (No. 312292), and by a Wallenberg Academy Fellowship (No. 2012.012).
References
- Adolph K. E., Tamis-LeMonda C. S. (2014). The costs and benefits of development: The transition from crawling to walking. Child Development Perspectives, 8, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. A. (2012). Executive functions: What they are, how they work, and why they evolved. New York, NY: Guilford Press. [Google Scholar]
- Bell M. A. (2012). A psychobiological perspective on working memory performance at 8 months of age. Child Development, 83, 251–265. [DOI] [PubMed] [Google Scholar]
- Bernier A., Carlson S. M., Deschênes M., Matte-Gagné C. (2012). Social factors in the development of early executive functioning: A closer look at the caregiving environment. Developmental Science, 15, 12–24. [DOI] [PubMed] [Google Scholar]
- Claxton L. J., Keen R., McCarty M. E. (2003). Evidence of motor planning in infant reaching behavior. Psychological Science, 14, 354–356. [DOI] [PubMed] [Google Scholar]
- Diamond A. (2000). Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development, 71, 44–56. [DOI] [PubMed] [Google Scholar]
- Diamond A. (2013). Executive functions. Annual Review of Clinical Psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekberg T. L., Falck-Ytter T., Bölte S., Gredebäck G., & EASE Team. (2016). Reduced prospective motor control in 10-month-olds at risk for autism spectrum disorder. Clinical Psychological Science, 4, 129–135. [Google Scholar]
- Friedman N. P., Miyake A., Robinson J. L., Hewitt J. K. (2011). Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioural genetic analysis. Developmental Psychology, 47, 1410–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N., Bryson S. E., Smith I. M. (2008). Executive function in preschoolers: A review using an integrative framework. Psychology Bulletin, 134, 31–60. [DOI] [PubMed] [Google Scholar]
- Garon N., Smith I. M., Bryson S. E. (2014). A novel executive function battery for preschoolers: Sensitivity to age differences. Child Neuropsychology, 20, 713–736. [DOI] [PubMed] [Google Scholar]
- Gentsch A., Weber A., Synofzik M., Vosgerau G., Schütz-Bosbach S. (2016). Towards a common framework of grounded action cognition: Relating motor control, perception and cognition. Cognition, 146, 81–89. [DOI] [PubMed] [Google Scholar]
- Gottwald J. M., de Bortoli Vizioli A., Lindskog M., Nyström P., Ekberg T. L., von Hofsten C., Gredebäck G. (2016, May). What’s next? Infants prospectively control their reaching movements based on the difficulty of their subsequent action. Paper presented at the 20th International Conference on Infant Studies, New Orleans, LA. [Google Scholar]
- Gottwald J. M., Gredebäck G. (2015). Infants’ prospective control during object manipulation in an uncertain environment. Experimental Brain Research, 233, 2383–2390. [DOI] [PubMed] [Google Scholar]
- Grönqvist H., Strand Brodd K., von Hofsten C. (2011). Reaching strategies of very preterm infants at 8 months corrected age. Experimental Brain Research, 209, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. (1988). The neural and behavioural organization of goal-directed movements. New York, NY: Clarendon Press/Oxford University Press. [Google Scholar]
- Johansson M., Marciszko C., Gredebäck G., Nyström P., Bohlin G. (2015). Sustained attention in infancy as a longitudinal predictor of self-regulatory functions. Infant Behavior & Development, 41, 1–11. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. (2015). An alternative to domain-general or domain-specific frameworks for theorizing about human evolution and ontogenesis. AIMS Neuroscience, 2, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. (2005). Principles and practice of structural equation modeling (2nd ed.). New York: Guilford Press. [Google Scholar]
- Mariani M. A., Barkley R. A. (1997). Neuropsychological and academic functioning in preschool boys with attention deficit hyperactivity disorder. Developmental Neuropsychology, 13, 111–129. [Google Scholar]
- Marshall P. J. (2016). Embodiment and human development. Child Development Perspectives. Advance online publication. doi: 10.1111/cdep.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel C. L., Paradiso S. (2004). Cognitive and neurological impairment in mood disorders. Psychiatric Clinics of North America, 27, 19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., Wager T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Nyström P., Falck-Ytter T., Gredebäck G. (2016). The TimeStudio Project: An open source scientific workflow system for the behavioral and brain sciences. Behavior Research Methods, 48, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamondon R., Alimi A. M. (1997). Speed/accuracy trade-offs in target-directed movements. Behavioral and Brain Sciences, 20, 279–349. doi: 10.1017/S0140525X97001441 [DOI] [PubMed] [Google Scholar]
- Posner M., Rothbart M. (2000). Developing mechanisms of self-regulation in early life. Development and Psychopathology, 12, 427–441. [DOI] [PubMed] [Google Scholar]
- Ridler K., Veijola J. M., Tanskanen P., Miettunen J., Chitnis X., Suckling J., . . . Bullmore E. T. (2006). Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proceedings of the National Academy of Sciences, USA, 103, 15651–15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosander K., von Hofsten C. (2011). Predictive gaze shifts elicited during observed and performed actions in 10-month-old infants and adults. Neuropsychologia, 49, 2911–2917. [DOI] [PubMed] [Google Scholar]
- Rose S. A., Feldman J. F., Jankowski J. J., Van Rossem R. (2012). Information processing from infancy to 11 years: Continuities and prediction of IQ. Intelligence, 40, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S. S., Cicchetti D. V., Balla D. A. (2005). Vineland Adaptive Behavior Scales (2nd ed.). London, England: Pearson. [Google Scholar]
- Thelen E., Corbetta D., Spencer J. P. (1996). Development of reaching during the first year: Role of movement speed. Journal of Experimental Psychology: Human Perception and Performance, 22, 1059–1076. doi: 10.1037/0096-1523.22.5.1059 [DOI] [PubMed] [Google Scholar]
- Thelen E., Schöner G., Scheier C., Smith L. B. (2001). The dynamics of embodiment: A field theory of infant perseverative reaching [Target article and commentaries]. Behavioral and Brain Sciences, 24, 1–86. doi: 10.1017/S0140525X01003910 [DOI] [PubMed] [Google Scholar]
- van der Meer A. L., van der Weel F. R., Lee D. N. (1995). The functional significance of arm movements in neonates. Science, 267, 693–695. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. (1991). Structuring of early reaching movements: A longitudinal study. Journal of Motor Behavior, 23, 280–292. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. (1993). Prospective control: A basic aspect of action development. Human Development, 36, 253–270. [Google Scholar]
- von Hofsten C. (2004). An action perspective on motor development. Trends in Cognitive Sciences, 8, 266–272. [DOI] [PubMed] [Google Scholar]
- Wilson M. (2001). The case for sensorimotor coding in working memory. Psychonomic Bulletin & Review, 8, 44–57. [DOI] [PubMed] [Google Scholar]
- Wolpert D. M., Diedrichsen J., Flanagan J. R. (2011). Principles of sensorimotor learning. Nature Reviews Neuroscience, 12, 739–751. [DOI] [PubMed] [Google Scholar]
- World Medical Association. (2013). WMA Declaration of Helsinki - Ethical principles for medical research involving human subjects. Retrieved from http://www.wma.net/en/30publications/10policies/b3/ [DOI] [PubMed]
- Zoia S., Blason L., D’Ottavio G., Bulgheroni M., Pezzetta E., Scabar A., Castiello U. (2007). Evidence of early development of action planning in the human foetus: A kinematic study. Experimental Brain Research, 176, 217–226. [DOI] [PubMed] [Google Scholar]