Abstract
Our recent study highlights the role of 2 glutathione transferases (GSTs) in the detoxification of the environmental pollutant, 2,4,6-trinitrotoluene (TNT) in Arabidopsis thaliana. TNT is toxic and highly resistant to biodegradation in the environment, raising both health and environmental concerns. Two GSTs, GST-U24 and GST-U25, are upregulated in response to TNT treatment, and expressed predominantly in the root tissues; the site of TNT location following uptake. Plants overexpressing GST-U24 and GST-U25 exhibited significantly enhanced ability to withstand and detoxify TNT, and remove TNT from contaminated soil. Analysis of the catalytic activities of these 2 enzymes revealed that they form 3 TNT-glutathionyl products. Of particular interest is 2-glutathionyl-4,6-dinitrotoluene as this represents a potentially favorable step toward subsequent degradation and mineralization of TNT. We demonstrate how GSTs fit into what is already known about pathways for TNT detoxification, and discuss the short and longer-term fate of TNT conjugates in planta.
Keywords: Arabidopsis thaliana, environmental pollutant, Glutathione transferases, TNT, xenobiotic detoxification, 2, 4, 6-trinitrotoluene
The environmental pollutant 2,4,6-trinitrotoluene (TNT) is of concern because it is toxic, classified as a carcinogen by the US Environmental Protection Agency, and is highly resistant to biodegradation in the environment.1 While pollution is limited mainly to military ranges and manufacturing sites, in the US alone, there are an estimated 16 million hectares of active and closed military sites contaminated with explosives,2 and the use of TNT in ordnance will continue for the foreseeable future.
Microorganisms have been isolated from explosives-contaminated soil and the biochemistry behind TNT-detoxification has been well characterized. This occurs predominantly via the transformation to hydroxylamino dinitrotoluenes (HADNTs) and amino dinitrotoluenes (ADNTs) by nitroreductases (reviewed in Rylott et al. 20111). However, despite the presence of TNT-detoxifying microbial populations in contaminated soil, TNT continues to be highly recalcitrant to degradation. Given the scale of TNT pollution in the environment, phytoremediation could offer an alternative means of cleaning-up contaminated sites. A problem with this approach is the phytotoxicity of TNT at the polluting levels found in the environment.3
The endogenous metabolism of TNT by plants has been characterized, with much research focusing on the model plant species Arabidopsis thaliana (Arabidopsis).1,4 As presented in Figure 1, TNT can be transformed in a similar pathway to that in microorganisms; to HADNTs, with a varying portion further reduced to ADNTs. In Arabidopsis, oxophytodienoate reductases are known to catalyze these steps.5 The additional functionality of HADNTs and ADNTs permits their subsequent conjugation to amino acids, organic acids and sugars.6,7 The conjugation of HADNT and ADNT isomers to glucose by Arabidopsis glucosyl transferases has been characterized,8 with research suggesting that these conjugates are subsequently sequestered within the cell walls.4 Previously, GSTs had been shown to be upregulated in response to TNT treatment, and their involvement in TNT detoxification hypothesized.8-12 Our study13 investigated GST-U24 and GST-U25, the 2 Arabidopsis GSTs most highly upregulated in response to TNT under our treatment conditions. We demonstrated that Arabidopsis plants over-expressing GST-U24 or GST-U25 removed more TNT from soil and were more resistant to TNT toxicity than wild type plants, due to the direct glutathionylation of TNT. However, the levels of GSH in leaves of wild-type plants grown in TNT-contaminated soil was 29 % less than those grown in uncontaminated soil; in the GST-U25 overexpressing lines, the decrease in GSH in the presence of TNT was 58 %. These results suggest that on TNT-contaminated sites, conjugation of GSH to TNT might deplete GSH pools, limiting TNT detoxification.
Figure 1.
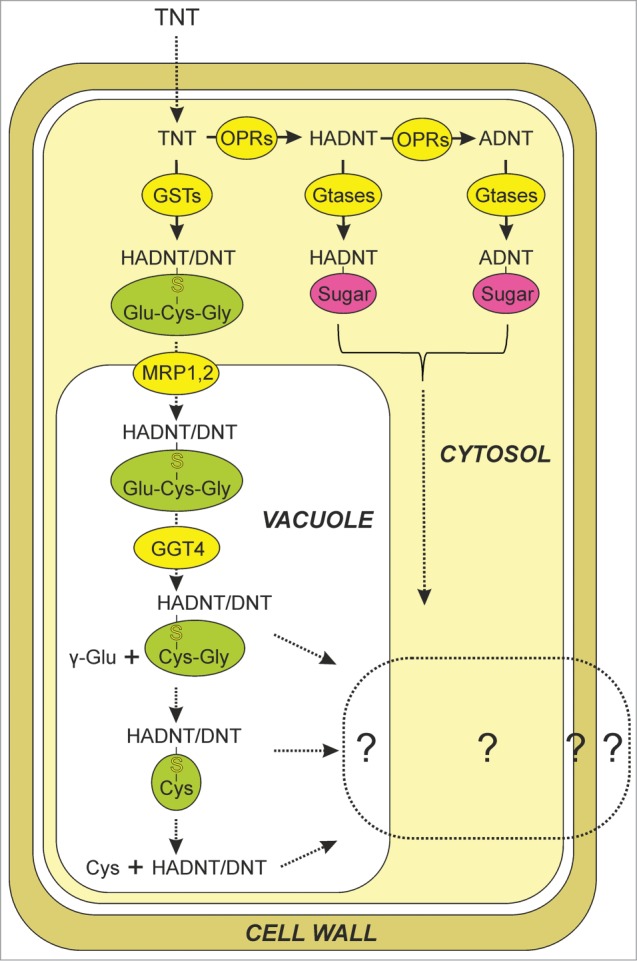
Schematic representation showing proposed detoxification pathway for 2,4,6 trinitrotoluene (TNT) in Arabidopsis roots. Mode of cellular TNT uptake and end fate of TNT-derived conjugates are still unknown. OPR, oxophytodienoate reductases; GSTs, glutathione transferases; GTases, glucosyl transferases; MRP1,2, multidrug resistance-associated protein; GGT, γ-glutamyl transpeptidase; HADNT, hydroxylamino dinitrotoluene; DNT, dinitrotoluene. Dotted lines represent putative pathways.
Characterization of recombinantly expressed, purified GST-U24 and GST-U25 showed that they produced 3 distinct products, identified using HPLC-MS and NMR analysis. GST-U24 produced predominantly conjugate 1 (C-glutathionylated 4-HADNT), whereas GST-U25 produced conjugates 1, 2 (C-glutathionylated 2-HADNT) and 3 (2-glutathionyl-4,6-dinitrotoluene).13 Conjugate 3 is of particular interest due to the denitration of TNT; the nitro groups offer stability to the ring through resonance.14 Biochemical pathways in bacteria that can mineralize the structurally-related compounds 2,4- and 2,6-dinitrotoluene (DNT) have been characterized.15,16
Following uptake, TNT and derivatives are located predominantly in the root tissues17 with conjugation to glutathione occurring in the cytosol. At pH 6.5 to 7.0, production of conjugate 3 by GST-U25 is preferred over the other 2 conjugates. That only conjugate 3 was detected in the root extracts from GST-U25 overexpressing plants fed TNT suggests that the root cytosol pH is favorable for conjugate 3 production13. Further studies are now required to investigate whether conjugate 3 is a step closer to the catabolism of TNT into intermediates of general metabolism, or possibly mineralization, of this organic pollutant. If conjugate 3 is more amenable to further breakdown, this could be used to genetically manipulate, or screen, for plants with enhanced production of conjugate 3. Both root pH and conjugate 3 producing activity have not yet been reported for phytoremediation-relevant species such as switchgrass and poplar, although studies to identify GSTs in poplar are underway.9-11
Studies on GSH-herbicide conjugates show that they are then rapidly sequestered to the vacuole by the ATP-binding cassette transporters, Multidrug Resistance-associated Proteins MRP1 and MRP2.18,19 Both MRP1 and MRP2 are up-regulated in response to TNT8 and it is likely that TNT-derived glutathione conjugates are transported to the vacuole. During glutathione catabolism in Arabidopsis, GGT4 in the vacuole, and predominantly GGT1 in the extracellular space, catalyze the production of cysteinylglycine (Cys-Gly) and γ-glutamate (γ-Glu).20-22 The Cys-Gly is hydrolysed in the cytosol by a dipeptidase, making these amino acids available for glutathione re-synthesis. The γ-Glu binds with amino acids to produce γ-Glu-alanine or other amino acids (γ-Glu-aa). The involvement of a cytosolic γ-glutamyl cyclotransferase (GGCT) has been confirmed,23 and more recently, the gene identified and enzyme characterized.24 The GGCT converts γ-Glu-alanine or any other γ-Glu-aa moiety (including γ-Glu-Cys) into pyroglutamate or 5-oxoproline, which is subsequently recycled into Glu by oxoprolinase.23,25
Studies using monobromobimane (mBB), which is conjugated to glutathione and sequestered into the vacuole, revealed that in Arabidopsis root vacuoles, the N-terminal degradation products, Cys-Gly-mBB and γ-Glu-mBB, are produced by GGT4.20,22 Studies on ggt4 mutants demonstrated that the gene is responsible for the majority of GSH-mBB-derived conjugates, and that C-terminal degradation is not significant.22 Some γ-Glu-Cys conjugates have been isolated from Silene cucubalus cell suspension cultures heterologously expressing an Arabidopsis cytosolic phytochelatin synthase and fed mBB. However, while the formation of γ-Glu-Cys conjugates indicates that C-terminal degradation of glutathione conjugates is possible in the Arabidopsis cytosol,26 studies in Arabidopsis indicated that vacuolar sequestration out-competes cytosolic phytochelatin synthase-catalyzed, C-terminal carboxypeptidase activity.21 A barley vacuolar carboxypeptidase that cleaves alachlor glutathione conjugates C-terminally to produce γ-Glu-Cys conjugates has been reported,27 demonstrating that there are species differences.
Along with the studies using mBB, ggt4 was found to be more susceptible than wild-type plants to the herbicides 2,4-dichlorophenoxyacetic acid and metolachlor22. While the fate of TNT-derived glutathione conjugates in plants is not known, the above studies suggest that these would be further processed via the N-terminal, GGT4 catalyzed, pathway in Arabidopsis roots (Fig. 1). The glutathione conjugation of 2,4-dichlorophenoxyacetic acid and metolachlor have been shown to be sufficient to alleviate their phytotoxicity,22 and whether these herbicides, or TNT are catabolized beyond Cys-conjugates is unclear. So far, it has not been proven whether there is a nitrogen-related advantage in salvaging Glu and Gly components from glutathione conjugates.22 Further studies could test whether limiting nitrogen or sulfur affect TNT-conjugating GST activity and phytotoxicity in Arabidopsis and more phytoremediation-relevant species. Both nitrogen and sulfur are elements that are likely to be limiting in field environments such as military training ranges.
In plant species suitable for phytoremediation of TNT, such as poplar and perennial grasses, the cytosolic contents are lost during the conversion to woody biomass. While evidence suggests that glycosylated TNT derivatives are bound up in plant macromolecules such as lignin,4 the longer-term fate of TNT-derived glutathione conjugates following emptying of the cell contents and conversion to woody xylem is unknown. Studies are needed to establish the longer-term bioavailability and toxicity of transformed, conjugated xenobiotics and, as with all persistent aromatic hydrocarbon pollutants, true remediation requires their catabolism into intermediates of general metabolism, or mineralization.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the Strategic Environmental Research and Development Program of the US Department of Defense. H. Sparrow and E.J. Johnston acknowledge funding from the BBSRC and K. Tzafestas acknowledges funding from a Burgess studentship.
References
- 1. Rylott EL, Lorenz A, Bruce NC. Biodegradation and biotransformation of explosives. Curr Opin Biotechnol 2011; 22:434-40. PMID:21094036; http://dx.doi.org/ 10.1016/j.copbio.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 2. United States General Accounting Office Department of defense operational ranges, more reliable cleanup cost estimates and a proactive approach to identifying contamination are needed. Report to Congressional Requesters 2004; http://www.gao.gov/new.items/d04601.pdf [Google Scholar]
- 3. Hannink NK, Rosser SJ, Bruce NC. Phytoremediation of explosives. Crit Rev Plant Sci 2002; 21:511-38. http://dx.doi.org/ 10.1080/0735-260291044340 [DOI] [Google Scholar]
- 4. Rylott E, Bruce N. Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends Biotechnol 2009; 27:73-81. PMID:19110329; http://dx.doi.org/ 10.1016/j.tibtech.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 5. Beynon ER, Symons ZC, Jackson RG, Lorenz A, Rylott EL, Bruce NC. The role of oxophytodienoate reductases in the detoxification of the explosive 2,4,6-trinitrotoluene by Arabidopsis. Plant Physiol 2009; 151:253-61. PMID:19605548; http://dx.doi.org/ 10.1104/pp.109.141598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhadra R, Wayment DG, Williams RK, Barman SN, Stone MB, Hughes JB, Shanks JV. Studies on plant-mediated fate of the explosives RDX and HMX. Chemosphere 2001; 44:1259-64. PMID:11513416; http://dx.doi.org/ 10.1016/S0045-6535(00)00272-1 [DOI] [PubMed] [Google Scholar]
- 7. Bhadra R, Wayment DG, Hughes JB, Shanks JV. Confirmation of conjugation processes during TNT metabolism by axenic plant roots. Environ Sci Technol 1999; 33:446-52. http://dx.doi.org/ 10.1021/es980635m [DOI] [Google Scholar]
- 8. Gandia-Herrero F, Lorenz A, Larson T, Graham IA, Bowles DJ, Rylott EL, Bruce NC. Detoxification of the explosive 2,4,6-trinitrotoluene in Arabidopsis: discovery of bifunctional O- and C-glucosyltransferases. Plant J 2008; 56:963-74. PMID:18702669; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03653.x [DOI] [PubMed] [Google Scholar]
- 9. Tanaka S, Brentner LB, Merchie KM, Schnoor JL, Yoon JM, Van Aken B. Analysis of gene expression in poplar trees (Populus deltoides x nigra, DN34) exposed to the toxic explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Int J Phytoremed 2007; 9:15-30. PMID:18246712; http://dx.doi.org/ 10.1080/15226510601139375 [DOI] [PubMed] [Google Scholar]
- 10. Brentner LB, Mukherji ST, Merchie KM, Yoon JM, Schnoor JL, Aken BV. Expression of glutathione S-transferases in poplar trees (Populus trichocarpa) exposed to 2,4,6-trinitrotoluene (TNT). Chemosphere 2008; 73:657-62. PMID:18774158; http://dx.doi.org/ 10.1016/j.chemosphere.2008.07.059 [DOI] [PubMed] [Google Scholar]
- 11. Ekman DR, Lorenz WW, Przybyla AE, Wolfe NL, Dean JF. SAGE analysis of transcriptome responses in Arabidopsis roots exposed to 2,4,6-trinitrotoluene. Plant Physiol 2003; 133:1397-406. PMID:14551330; http://dx.doi.org/ 10.1104/pp.103.028019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mezzari MP, Walters K, Jelinkova M, Shih MC, Just CL, Schnoor JL. Gene expression and microscopic analysis of Arabidopsis exposed to chloroacetanilide herbicides and explosive compounds. A phytoremediation approach. Plant Physiol 2005; 138:858-69. PMID:15923336; http://dx.doi.org/ 10.1104/pp.104.056168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gunning V, Tzafestas K, Sparrow H, Johnston EJ, Brentnall AS, Potts JR, Rylott EL, Bruce NC. Arabidopsis glutathione transferases U24 and U25 exhibit a range of detoxification activities with the environmental pollutant and explosive, 2,4,6-trinitrotoluene. Plant Physiol 2014; 165:854-65. PMID:24733884; http://dx.doi.org/ 10.1104/pp.114.237180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qasim M, Gorb L, Magers D, Honea P, Leszczynski J, Moore B, Taylor L, Middleton M. Structure and reactivity of TNT and related species: application of spectroscopic approaches and quantum-chemical approximations toward understanding transformation mechanisms. J Hazard Mater 2009; 167:154-63. PMID:19200649; http://dx.doi.org/ 10.1016/j.jhazmat.2008.12.105 [DOI] [PubMed] [Google Scholar]
- 15. Nishino SF, Paoli GC, Spain JC. Aerobic degradation of dinitrotoluenes and pathway for bacterial degradation of 2,6-dinitrotoluene. Appl Environ Microbiol 2000; 66:2139-47. PMID:10788393; http://dx.doi.org/ 10.1128/AEM.66.5.2139-2147.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spanggord RJ, Spain JC, Nishino SF, Mortelmans KE. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol 1991; 57:3200-5. PMID:1781682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brentner L, Mukherji S, Walsh S, Schnoor J. Localization of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and 2,4,6-trinitrotoluene (TNT) in poplar and switchgrass plants using phosphor imager autoradiography. Environmental Pollution 2010:470-5. PMID:19782446; http://dx.doi.org/ 10.1016/j.envpol.2009.08.022 [DOI] [PubMed] [Google Scholar]
- 18. Cummins I, Dixon D, Freitag-Pohl S, Skipsey M, Edwards R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Met Rev 2011; 43:266-80. PMID:21425939; http://dx.doi.org/ 10.3109/03602532.2011.552910 [DOI] [PubMed] [Google Scholar]
- 19. Klein M, Burla B, Martinoia E. The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett 2006; 580:1112-22. PMID:16375897; http://dx.doi.org/ 10.1016/j.febslet.2005.11.056 [DOI] [PubMed] [Google Scholar]
- 20. Grzam A, Martin MN, Hell R, Meyer AJ. γ-Glutamyl transpeptidase GGT4 initiates vacuolar degradation of glutathione S-conjugates in Arabidopsis. FEBS Lett 2007; 581:3131-8. PMID:17561001; http://dx.doi.org/ 10.1016/j.febslet.2007.05.071 [DOI] [PubMed] [Google Scholar]
- 21. Grzam A, Tennstedt P, Clemens S, Hell R, Meyer AJ. Vacuolar sequestration of glutathione S-conjugates outcompetes a possible degradation of the glutathione moiety by phytochelatin synthase. FEBS Lett 2006; 580:6384-90. PMID:17097087; http://dx.doi.org/ 10.1016/j.febslet.2006.10.050 [DOI] [PubMed] [Google Scholar]
- 22. Ohkama-Ohtsu N, Zhao P, Xiang C, Oliver D. Glutathione conjugates in the vacuole are degraded by γ-glutamyl transpeptidase GGT3 in Arabidopsis. Plant J 2007; 49:878-88. PMID:17316176; http://dx.doi.org/ 10.1111/j.1365-313X.2006.03005.x [DOI] [PubMed] [Google Scholar]
- 23. Ohkama-Ohtsu N, Oikawa A, Zhao P, Xiang C, Saito K, Oliver D. A γ-glutamyl transpeptidase-independent pathway of glutathione catabolism to glutamate via 5-oxoproline in Arabidopsis. Plant Physiology 2008; 148:1603-13. PMID:18768907; http://dx.doi.org/ 10.1104/pp.108.125716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paulose B, Chhikara S, Coomey J, Jung HI, Vatamaniuk O, Dhankher OP. A γ-glutamyl cyclotransferase protects Arabidopsis plants from heavy metal toxicity by recycling glutamate to maintain glutathione homeostasis. Plant Cell 2013; 25:4580-95. PMID:24214398; http://dx.doi.org/ 10.1105/tpc.113.111815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazelis M, Creveling RK. Five-oxoprolinase (l-pyroglutamate hydrolase) in higher plants: partial purification and characterization of the wheat germ enzyme. Plant Physiol 1978; 62:798-801. PMID:16660609; http://dx.doi.org/ 10.1104/pp.62.5.798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beck A, Lendzian K, Oven M, Christmann A, Grill E. Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates. Phytochemistry 2003; 62:423-31. PMID:12620355; http://dx.doi.org/ 10.1016/S0031-9422(02)00565-4 [DOI] [PubMed] [Google Scholar]
- 27. Wolf AE, Dietz KJ, Schroder P. Degradation of glutathione S-conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett 1996; 384:31-4. PMID:8797797; http://dx.doi.org/ 10.1016/0014-5793(96)00272-4 [DOI] [PubMed] [Google Scholar]


