Abstract
Bacillus subtilis tryC2, thyA, thyB, lysogenic for the phage DNA polymerase negative mutant SPO2 susL244, was induced under conditions preventing phage and bacterial DNA synthesis. The biological activity of DNA from induced cells and from uninduced controls was assayed by transformation and transfection, respectively. About 50% of the phage DNA biological activity in DNA extracted from induced cells was resistant to exposure to pH 11.8 TO 11.9. This DNA was operationally defined as alkali-resistant phage DNA. Transforming bacterial DNA from uninduced or induced cells and transfecting DNA from uninduced cells were more than 95% inactivated after exposure to high pH. The alkali-resistant phage DNA was characterized by sucrose gradient centrifugation, by centrifugation in cesium chloride-propidium iodide, and by electron microscopy. It was found to consist of a majority of covalently closed circular DNA molecules. Length measurements of a few relaxed circular molecules indicate a molecular weight of these similar to that previously found for mature SPO2DNA. Attempts to isolate similar covalently closed circular phage DNA from induced bacteria lysogenic for SPO2 phage with a functional DNA polymerase gene were unsuccessful. The gene order in mature and prophage SPO2 was determined by rescue of single and double markers from the respective type of DNA. The data obtained show that prophage DNA is (genetically) permuted relative to mature DNA. The phage attachment site is suggested to be located between genes I and J.
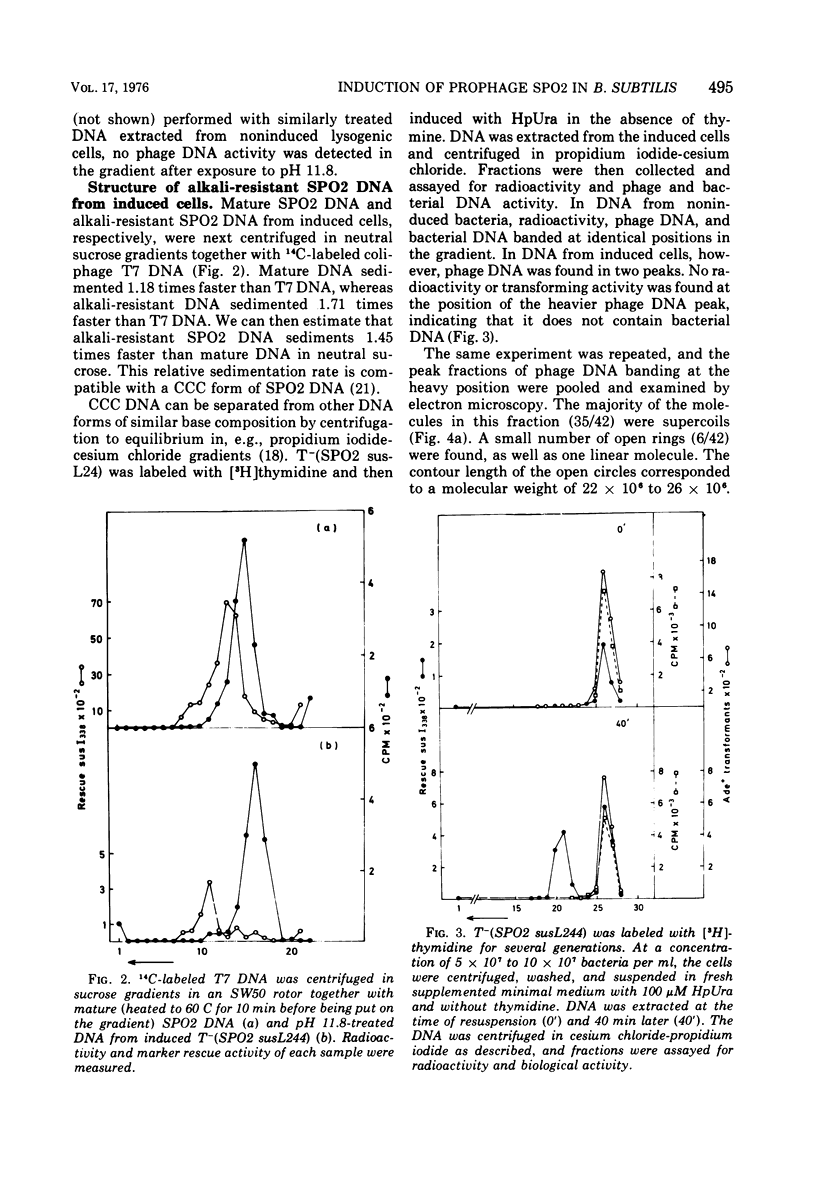
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentrout R. W., Rutberg L. Mapping of prophage and mature deoxyribonucleic acid from temperate Bacillus bacteriophage phi 105 by marker rescue. J Virol. 1970 Dec;6(6):760–767. doi: 10.1128/jvi.6.6.760-767.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentrout R. W., Skoog L., Rutberg L. Structure and biological activity of deoxyribonucleic acid from Bacillus bacteriophage phi 105: effects of Escherichia coli exonucleases. J Virol. 1971 Mar;7(3):359–371. doi: 10.1128/jvi.7.3.359-371.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Rutberg L. Induction of prophage SPO2 in Bacillus subtilis by 6-(para)-hydroxyphenylazouracil. J Virol. 1974 Dec;14(6):1470–1475. doi: 10.1128/jvi.14.6.1470-1475.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Rutberg L. Induction of prophage SPO2 in Bacillus subtilis: prophage excision in the absence of bacterial or bacteriophage DNA synthesis. J Virol. 1974 Dec;14(6):1476–1481. doi: 10.1128/jvi.14.6.1476-1481.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Venema G. Transformation in Bacillus subtilis. Fate of newly introduced transforming DNA. Mol Gen Genet. 1973;123(2):185–198. doi: 10.1007/BF00267334. [DOI] [PubMed] [Google Scholar]
- Boice L., Eiserling F. A., Romig W. R. Structure of bacillus subtilis phage SPO2 and its DNA: similarity of Bacillus subtilis phages SPO2, phi 1O5 and SPP1. Biochem Biophys Res Commun. 1969 Feb 21;34(4):398–403. doi: 10.1016/0006-291x(69)90395-7. [DOI] [PubMed] [Google Scholar]
- Bron S., Venema G. Ultraviolet inactivation and excision-repair in Bacillus subtilis. II. Differential inactivation and differential repair of transforming markers. Mutat Res. 1972 May;15(1):11–22. doi: 10.1016/0027-5107(72)90087-5. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindahl G. Attachment of prophage P2: gene order at different host chromosomal sites. Virology. 1969 Dec;39(4):867–881. doi: 10.1016/0042-6822(69)90023-3. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope study of the structures of the Bacillus subtilis prophages, SPO2 and phi105. J Mol Biol. 1973 Apr 5;75(2):257–264. doi: 10.1016/0022-2836(73)90019-3. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Low R. L., Cozzarelli N. R. Inhibition of a DNA polymerase from Bacillus subtilis by hydroxyphenylazopyrimidines. Proc Natl Acad Sci U S A. 1973 Jan;70(1):103–107. doi: 10.1073/pnas.70.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingery R., Echols H. Mutants of bacteriophage lambda unable to integrate into the host chromosome. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1507–1514. doi: 10.1073/pnas.58.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havender W. R., Trautner T. A. Genetic and transfection studies with B. subtilis phage SP50. 3. Biological effects of DNA cleavage and the physical basis of the map. Mol Gen Genet. 1972;116(1):51–67. doi: 10.1007/BF00334260. [DOI] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. W., Eremenko-Volpe T., Greenwald L., Meadow W. L., Marmur J. Physical and genetic mapping of the SPO2 prophage on the chromosome of Bacillus subtilis 168. J Virol. 1969 Jun;3(6):627–628. doi: 10.1128/jvi.3.6.627-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A. D., Masuda T. Evidence for a prophage excision gene in lambda. J Mol Biol. 1970 Feb 14;47(3):557–564. doi: 10.1016/0022-2836(70)90322-0. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Young E. T., 2nd, Sinsheimer R. L. Purification and properties of intracellular lamba DNA rings. J Mol Biol. 1968 Apr 28;33(2):395–413. doi: 10.1016/0022-2836(68)90197-6. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. L. Transduction studies on the relation between prophage and host chromosome. J Mol Biol. 1965 Jul;12(3):892–912. doi: 10.1016/s0022-2836(65)80336-9. [DOI] [PubMed] [Google Scholar]
- Rupert C. S. Shapes of the U.V. inactivation curves for single and linked double markers in Haemophilus influenzae transforming DNA. Photochem Photobiol. 1968 May;7(5):437–449. doi: 10.1111/j.1751-1097.1968.tb07405.x. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Warner R. C. Alkali denaturation of covalently closed circular duplex deoxyribonucleic acid. J Biol Chem. 1970 May 25;245(10):2704–2708. [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W. Deoxyribonucleic acid polymerase activity in a deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis infected with temperature bacteriophage SPO2. J Virol. 1972 Oct;10(4):658–660. doi: 10.1128/jvi.10.4.658-660.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Yasunaka K., Tsukamoto H., Okubo S., Horiuchi T. Isolation and properties of suppressor-sensitive mutants of Bacillus subtilis bacteriophage SP02. J Virol. 1970 Jun;5(6):819–821. doi: 10.1128/jvi.5.6.819-821.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]





