Abstract
The incidence of osteochondral lesions, as well as osteoarthritis of the ankle joint following osteochondritis dissecans and trauma, has been reappraised in recent years. Consequently, an increasing number of surgical interventions using different cartilage repair techniques is performed in the ankle joint, which has resulted in a growing demand for repetitive and objective assessment of cartilage tissue and its repair. While morphological imaging does enable monitoring of macroscopic changes with increasing precision, it fails to provide information about the ultrastructural composition of cartilage. The significance of molecular changes in cartilage matrix composition, however, is increasingly recognized, as it is assumed that macroscopic cartilage degeneration is preceded by a loss in glycosaminoglycans and a disorganization of the collagen network. Recent advances in biochemical magnetic resonance imaging (MRI) have yielded sequences sensitive to these changes, thus providing invaluable insight into both early cartilage degeneration and maturation of repair tissue, on a molecular level. The aim of this review was to provide a comprehensive overview of these techniques, including water and collagen-sensitive T2/T2* mapping, as well as glycosaminoglycan-sensitive sequences such as delayed gadolinium-enhanced MRI of cartilage dGEMRIC, and sodium imaging, and describe their applications for the ankle joint.
Keywords: MRI, ankle, cartilage, T2 mapping, sodium imaging
Background
Osteoarthritis (OA) is a frequent source of disability and pain, affecting about 12% of the US population aged 25 to 74 years at one or more joints.1 While the knee and hip joint are predominantly affected by primary osteoarthritis, only 7% to 9%2,3 of ankle OA cases can be attributed to this pathogenesis. This discrepancy is explained by different joint anatomy and biomechanics, as well as differences in chondrocyte properties inherent to the articular cartilage of the respective joints.4 As a result, articular cartilage of the ankle joint is deemed to have a higher intrinsic regenerative potential than knee cartilage.5,6 At the same time, the ankle joint seems susceptible to osteochondritis dissecans and trauma, with successive development of posttraumatic osteoarthritis. Despite the higher intrinsic regenerative potential of ankle cartilage compared to that of knee cartilage, these lesions frequently require surgical treatment.7
There is a number of different cartilage repair techniques for treating osteochondral or chondral lesions of the ankle joint, which are aiming for defect filling and stabilization of adjacent cartilage regions. Depending on the patient’s age and defect size, microfracturing (MFX),8 mosaicplasty,9 (matrix-associated)10 autologous chondrocyte transplantation ((M)ACT),11 and newer techniques using bone marrow–derived cell transplantation12 may be performed.
The ability to assess and monitor the quality of cartilage (repair tissue) in vivo is pivotal for 2 reasons. Timely diagnosis of changes in cartilage composition indicative of early-onset OA would be a prerequisite for the initiation of OA therapy. Second, with the variety of treatment options available, meticulous examination of their outcomes is paramount particularly because it has been shown that the molecular composition of repair tissue (RT) affects long-term clinical outcome, with hyaline-like RT yielding better results than fibrocartilage-like RT.13 However, in most cases, the gold standard of histological assessment is not feasible due to its invasiveness and associated morbidity. Recent advances in magnetic resonance imaging (MRI) have resulted in the development of sequences that now meet the need for noninvasive cartilage assessment, also in the ankle joint.
Morphological Magnetic Resonance Imaging
In general, morphological MRI is a highly valuable modality for the assessment of cartilage lesions and degeneration. MRI of ankle cartilage, however, is particularly challenging due to the highly congruent articular surfaces,14 the curvature, and (compared to the knee) the relatively thin cartilage layers.15 This combination of curvature and thin cartilage layers, for which Millington et al.14 found values of 1.1 ± 0.18 mm for talar and 1.16 ± 0.14 mm for tibial cartilage, contribute to partial volume effects. Moving to higher field strengths appears to be very promising in addressing this issue, as the higher signal-to-noise-ratio can be traded for increased spatial resolution. As mentioned before, the development of innovative cartilage repair techniques generated a strong demand for an objective and reproducible assessment of the composition and quality of cartilage RT. The introduction of the magnetic resonance observation of cartilage repair tissue (MOCART) score16 marked a milestone by enabling semiquantitative assessment of cartilage RT. Its wide acceptance and use in the orthopedic community demonstrate its value. Initially designed for the knee joint, the MOCART score recently has been applied to the ankle joint.17,18 However, the MOCART score cannot fully account for the different nature of lesions in the ankle joint. Compared with the knee joint, osteochondral lesions comprise a higher percentage of all defects in the ankle. The etiology of these lesions is often osteochondritis dissecans, which inherently compromises the integrity of the subchondral lamina. Hence, different cartilage repair techniques cannot result in a different outcome with regard to this variable, and the assessment of its integrity—as mandated in the MOCART score—is of limited relevance. Another criterion of the MOCART score is the presence or absence of adhesions. Although adhesions play an important role in the knee joint, several studies revealed them to be insignificant in the ankle joint.18,19 However, subchondral cysts or bone marrow edema might play a more prominent role as a more frequent source of pain in the ankle joint. Therefore, it is currently under discussion whether an adaption of the MOCART score for the ankle joint might be of additional value.
Another morphological score, which has been recently established for the assessment of osteochondral allograft transplantation, is the Osteochondral Allograft MRI Scoring System (OCAMRISS).20 This scoring system features strong interobserver agreement and correlated well with clinical outcome.21 While initially developed for the knee joint, it might also have the potential to monitor osteochondral transplants in the ankle joint.
Although morphological MRI does allow for the evaluation of macroscopic structural changes of cartilage and in conjunction with the MOCART score that of cartilage RT, its biochemical composition remains elusive.
Biochemical Magnetic Resonance Imaging
The underlying idea of biochemical MRI is to enable the quantification of molecular biomarkers in vivo and to use them as hallmarks for disease diagnosis and progression. The assessment of 2 key constituents of cartilage—collagen and glycosaminoglycans (GAGs)—is of particular interest in this regard, as they are integral to proper cartilage homeostasis and function. Notably, the early stages of cartilage degeneration are assumed to accompany a decrease in GAG content and disorganization, as well as loss of collagen fibers.6,22 Special emphasis must be placed on the assessment of GAG content, as it has also been shown to significantly correlate with biomechanical cartilage properties, predominantly compressive stiffness.23 In the evaluation of RT after cartilage repair, the quantification of these 2 matrix components is also of key importance, as the main difference between desired “hyaline-like” cartilage and unwanted “fibrocartilage-like” RT manifests as differences in collagen orientation and GAG content.24
For the assessment of water content and of the collagen network—content as well as fiber orientation—T2-mapping and T2*-mapping have been successfully introduced for the ankle joint. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and sodium imaging25 have been employed for the evaluation of the GAG content in the ankle joint. Two additional techniques (T1rho and gagCEST [short for glycosaminoglycan chemical exchange saturation transfer]) have been proposed for the evaluation of GAG content; however, neither of those has been applied to the ankle joint as yet. There are several articles that have reported promising initial data on gagCEST, but its performance in thin cartilage structures, such as those of the ankle joint, still awaits investigation.
T2-Mapping
T2-weighted images are commonly known from routine morphological imaging. They are optimized to provide maximal contrast between the structures of interest. This optimization is performed by carefully setting the echo time in such a way as to maximize the difference in signal intensities between 2 structures of interest. To yield optimal contrast; however, a prior estimate of T2 values of the structures of interest is useful.
T2-mapping relies on the measurement of T2-weighted images as well, but takes a different approach, to provide more specific information. From a T2-map, a time constant of the signal decay (T2 relaxation time) of a given structure may be quantified. To achieve this, a series of individual T2-weighted images with increasing echo times can be acquired, for example, in a standard multi-echo spin-echo (MESE) sequence. T2 values in cartilage are influenced by the amount and degree of the interaction between water molecules and biomacromolecules, particularly collagen fibers. The adequate selection of the number of echoes, as well as echo times, is crucial. Normally, the shortest possible echo time is sought for the first echo, while the longest echo time should not exceed a certain cutoff, after which predominantly noise would be collected, which would artificially prolong T2 values. Subsequently, a fitting procedure is applied, which calculates the time constant of exponential signal decay for each voxel independently. Altogether, these voxels comprise the resulting T2-map. The first echo however, is excluded from this procedure, as it is not stimulated by previous echoes.
The aforementioned interaction between free water and collagen fibrils also gives rise to a complication in the T2-mapping approach, the magic angle effect. T2 relaxation times increase when collagen fibrils in cartilage are at an angle of about 55° relative to the direction of the B0 field.26,27 This effect must be considered during the evaluation of T2-maps.
Healthy hyaline cartilage is characterized by a zonal anatomy, resulting from a distinct organization of collagen fibrils. T2-mapping reflects this zonal compositional variation, as well as a moderate zonal difference in water content,22 by an increase of T2 values from the deep layers to the more superficial layers.28 In fibrocartilage, however, this pattern is lost. Moreover, White at al.24 were able to demonstrate, through histological validation in equine subjects, that zonal evaluation of quantitative T2-mapping can be used to differentiate between hyaline-like and fibrocartilage-like RT after cartilage repair. In addition, based on their findings, they conjectured that MFX yielded inferior RT quality compared to MACT. Consequently, T2-mapping has been successfully employed for the evaluation of RT quality, after different repair techniques in human subjects in vivo by Welsch et al.29 Similar to the study by White et al.,24 RT after MFX lacked a zonal increase in T2 values. RT after MACT, however, showed significant zonal variation, indicating a distinct organization of the collagen network as a surrogate for hyaline-like cartilage. After the first T2-mapping studies, which focused on larger joints, T2-mapping was soon extended to the ankle joint.
As already discussed for morphological imaging, the anatomical properties of the talocrural cartilage are particularly challenging. Nonetheless, Welsch et al.30 demonstrated promising results in a feasibility study on healthy volunteers at 3 T. Using a dedicated ankle coil and an MESE T2 approach, excellent image quality and reproducibility were achieved within a clinically tolerable measurement time of 9:49 minutes. Subsequently, many studies employed T2-mapping for the noninvasive evaluation of alternative cartilage repair techniques. Giannini et al.31 were the first to investigate autologous chondrocyte implantation (ACI) in the ankle joint in a case series of six patients after a 10-year follow-up. The patients experienced excellent clinical outcomes, as reflected by an increase in the American Orthopedic Foot and Ankle (AOFAS) score from 37.9 ± 17.9 points preoperatively to 92.7 ± 9.9 points at the 10-year follow-up. Furthermore, T2-mapping demonstrated similar values for RT (46 ms; range, 34-50 ms) and for healthy reference cartilage (40 ms; range, 30-50 ms). This corresponds well to their findings of second-look arthroscopies and biopsies, which were performed 15 months after surgery, during hardware removal, and demonstrated hyaline-like cartilage repair tissue.
Successive larger studies32,33 on (M)ACT in the ankle joint corroborated these initial findings ( Fig. 1 ).32 Domayer et al.,34 Tao et al.,35 as well as Becher et al.,36 investigated RT after MFX and, surprisingly, unanimously reported no significant difference in global T2 between RT and adjacent healthy reference cartilage—in contradiction to, what has previously been observed in the knee joint.
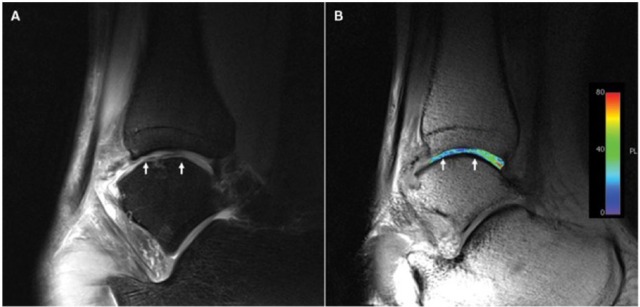
Figure 1.
T2-mapping of a patient after matrix associated chondrocyte transplantation (MACT) of the talus at 3 T: morphological proton density-weighted image (A) and a corresponding T2-map (B), both in sagittal orientation. The T2-map displays homogenously distributed T2 values (B), with a small decrease in the repair tissue. As demonstrated, the resolution is insufficient for zonal evaluation at 3 T.32
However, all these studies were performed at 3 T and had to go without the assessment of zonal variation of T2 values, due to insufficient spatial resolution. As a remedy, Domayer et al.37 moved to an ultra-high field (7 T) system. By trading the gain in signal-to-noise-ratio for increased spatial resolution, zonal cartilage assessment in the ankle joint became possible for the first time ( Fig. 2 ).37 Ten asymptomatic volunteer cases with an AOFAS score of 100, along with ten cases after MACT and MFX, were included in the study. The mean follow-up after MACT was 65.4 months, compared to 113.8 months after MFX. The morphological outcome, as well as the AOFAS score, showed no significant differences at follow-up. As expected, a significant zonal increase in T2 values was observed in the healthy volunteers from the deep to the superficial cartilage layers (21.1 ± 3.1 vs. 39.3 ± 5.9 ms, P < 0.001). Both techniques yielded RT with a significant zonal variation of T2 values (MFX, P = 0.009; MACT, P = 0.003). In addition, there was no difference between RT after MFX and MACT with regard to superficial (P = 0.796) or deep (P = 0.507) T2 values. When comparing the respective RTs with healthy reference cartilage, however, both techniques exhibited significantly increased T2 values in the deep layer (MFX, P = 0.004; MACT, P = 0.001), which resulted in significant differences in global T2 values (MFX, P = 0.025; MACT, P = 0.015). These results reinforce the findings of previous studies on MFX in the ankle joint by directly comparing it with MACT for the first time, and indicate that both techniques yield RTs of similar structure in the ankle joint.
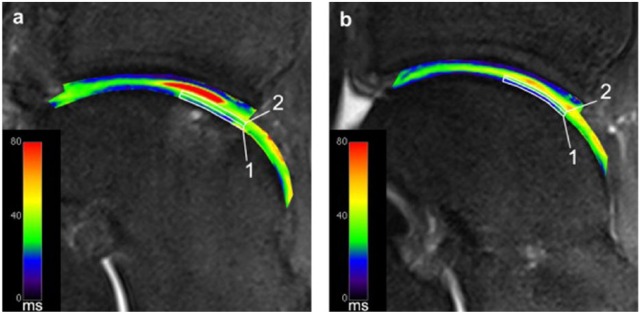
Figure 2.
T2-mapping of a patient after matrix-associated chondrocyte transplantation (MACT) of the talus at 7 T. Sufficient spatial resolution to allow for zonal cartilage evaluation is demonstrated. Image (a) displays region of interest (ROI) analysis of repair tissue, and image (b) shows ROI analysis of healthy reference cartilage in the same patient.37
Moving to 7 T provided sufficient spatial resolution to allow for zonal cartilage evaluation. However, with increasing field strengths, the specific absorption rate (SAR) limits image acquisition times for standard MESE sequences. As an unfavorable countermeasure, the number of acquired slices must be reduced in order to achieve a clinically tolerable scan time.
Alternatively, a recently developed T2-mapping sequence, 3-dimensional (3D) triple-echo steady-state (3D-TESS)38 may be used. 3D-TESS uses a different approach than standard MESE sequences. Three images with the same echo time, but different relaxation times, are acquired, causing different T1- and T2-weighting.38 These images are then used to calculate the T2 map by iterations. In that way, 3D-TESS may also be used to calculate a T1-map. However, it is fundamental to the principle of 3D-TESS that the relaxation times used are significantly shorter than the T2 of the investigated tissue. In the knee, 3D-TESS demonstrated a similar performance with respect to a standard MESE sequence, while providing significant advantages, such as shortening of acquisition time and insensitivity to B0 and B1 changes.39 Future studies are needed to evaluate the potential of this new technique in the ankle joint.
T2*-Mapping
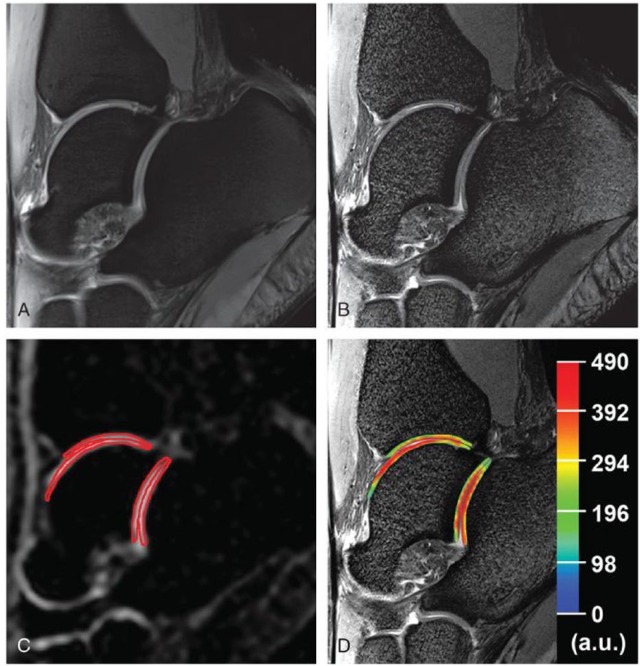
Another alternative to address the problem of SAR limits at ultra-high fields is the use of a different imaging approach, namely T2*-mapping. Upon its introduction, T2* relaxation time measurements have been proposed as an alternative biomarker for the biochemical assessment of articular cartilage, in addition to T2 relaxation time measurements. As opposed to quantitative T2-mapping, T2*-mapping is obtained with a multigradient-recalled echo (GRE) sequence. This highlights its key advantages over T2-mapping based on an MESE sequence: shorter acquisition times, lower SAR as well as the ability to obtain 3D measurements. The different sequence design also explains the main differences between the 2 approaches. T2* comprises information about the field inhomogeneity on a molecular level and also on a macroscopic (pixel size) level. The relative contribution of both effects is hard to assess and may vary. Generally speaking, the shorter the T2 values of a given structure, the smaller the contribution at the macroscopic level. Hence, T2-mapping and T2*-mapping will yield similar relaxation times for tissues with short T2 relaxation times, such as cartilage, as demonstrated by Welsch et al.40 in the evaluation of RT at 7 T. A study that used T2-mapping ( Fig. 3 )19 and T2*-mapping ( Fig. 4 )19 for the evaluation of MACT in the ankle joint came to a similar conclusion.19 It is worth noting that both studies reported consistently lower relaxation times for T2*-mapping, which is an expected feature of the applied GRE sequence in T2*-mapping.
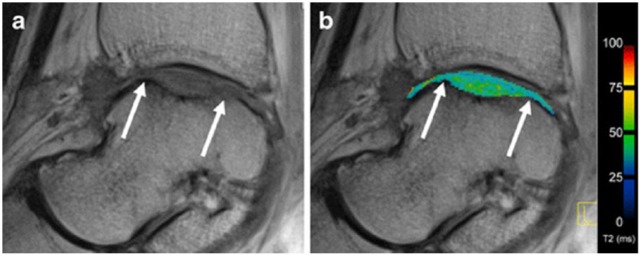
Figure 3.
T2-mapping depicting the ankle joint of a patient after matrix-associated autologous chondrocyte transplantation (MACT) in sagittal orientation: on the left displayed in grayscale using the shortest echo time (a); on the right depicted as an overlay of the pseudo-colored map over the grayscale image (b). White arrows indicate the borders of the repair tissue.19
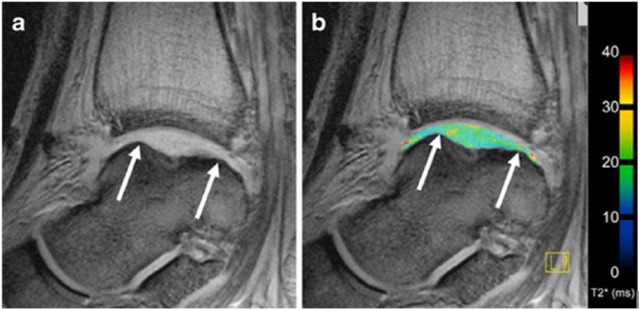
Figure 4.
Sagittal T2*-mapping after matrix-associated autologous chondrocyte transplantation (MACT) in the talus of the same patient as in Figure 3 . Likewise, a grayscale image (a) and an image with overlaid T2* values (b) are displayed. White arrows indicate the borders of the repair tissue.19
Krause et al.41 investigated the ability of T2*-mapping as a marker of early osteoarthrosis in pes cavovarus, a foot deformity, which alters biomechanics and consequently increases the risk of cartilage degeneration in the ankle joint. Whereas T2* values in the talocrural joint of symptomatic cavovarus patients differed significantly from asymptomatic cavovarus patients, as well as from healthy volunteers, no difference between asymptomatic cavovarus patients and healthy volunteers could be observed.
Subsequently, T2*-mapping was applied to investigate the effect of ultra-long-distance running on ankle cartilage by repeated examination, in a mobile 1.5 T MRI, of participants of the TransEurope FootRace (TEFR).42 While morphological imaging indicated stable cartilage conditions over the entire race, an analysis of T2* values demonstrated an initial increase (25.6% on average) during the first 2000 km. Surprisingly, this was followed by a decrease in T2* relaxation times of 6% to 10% for runners who continued after 2500 km, indicating the ability of ankle cartilage to partially adapt to an ongoing ultramarathon burden.
These studies already demonstrated the wide applicability of T2*-mapping, including its distinct advantages over T2-mapping. As mentioned before, in T2-mapping, zonal variation analysis was established as a valuable evaluation method that enabled differentiation between healthy cartilage and cartilage with incipient matrix disorganization. At 7 T, zonal variation analysis could even be successfully used in the ankle joint. In GRE-based T2*-mapping, however, the interface between cartilage and cortical bone may yield very low T2* values due to macroscopic susceptibility effects.40 This can cause the illusion of zonal variation even in defect regions, which would lack zonal differences on T2-maps. Therefore, zonal variation analysis should not be performed in T2* images. Instead, evaluation should be limited to the assessment of the global cartilage layer. In addition, the indication for T2*-mapping in the postoperative assessment of patients should be approached cautiously, as susceptibility artifacts from metalwork may significantly impair the quality of the T2* measurements.
dGEMRIC
The dGEMRIC technique relies on the equilibration of gadolinium diethylenetriamine penta-acetic acid (Gd-DTPA−) throughout the cartilage. In the majority of cases this is achieved by intravenous administration of this negatively charged contrast agent prior to MRI. After administration, Gd-DTPA2− diffuses into cartilage and distributes in an inversely proportional manner to the negative charge density, which is dominated by the negatively charged GAGs present in cartilage.43 In healthy cartilage the negative charge of the GAG molecules counteracts the diffusion of the negatively charged contrast agent molecules. With a focal loss of GAG, more contrast agent molecules can accumulate, thus reducing the T1 relaxation times in a concentration dependent manner. This allows for the specific evaluation and quantification of GAG content, as demonstrated in several studies.44,45 To ensure sufficient penetration of articular cartilage, a certain time-interval must be maintained between the administration of contrast agent and MRI. Additionally the patient should repetitively load and unload the joint in the meantime. This interval, which is responsible for the attribute “delayed” in dGEMRIC has to be adapted for different joints, in the ankle joint being optimal around 45 minutes.46 The evaluation of GAG content may be based on the dGEMRIC index (T1(Gd)), which is the mean T1 in a confined region of interest (ROI) at a certain time point after contrast media administration.47 For studies designed to evaluate cartilage RT, the relative delta relaxation rate (rΔR1) might be useful, as published by Watanabe et al.48 The delta relaxation rate (ΔR1) is defined as the difference between postcontrast relaxation rate (R1post = 1/T1post) and precontrast relaxation rate (R1pre = 1/T1pre). The aforementioned relative delta relaxation rate is the ΔR1 of RT divided by the ΔR1 of healthy reference cartilage, and has been shown to correlate well with biochemically assessed GAG content in RT.48 While healthy cartilage is expected to yield an rΔR1 of approximately 1, higher values would go along with a decrease in GAG content of the RT. However, many confounding variables, such as cartilage thickness, contrast dose, T1-specific imaging protocols as well as the time interval between contrast media administration and imaging, influence T1(Gd). Therefore, exact standardization of the study protocol is a fundamental prerequisite to yield exact and reproducible measurements.47,49
Several studies that have investigated cartilage GAG content based on dGEMRIC have been performed in the knee and hip. The ankle joint, however, is particularly challenging due to its thin cartilage layer. Domayer et al.46 addressed this issue with the use of a multichannel (phased array) flexible multipurpose coil, which was able to provide sufficient signal-to-noise-ratio (SNR) and resolution for the separate evaluation of the talar and tibial cartilage layers at 3 T. A volume interpolated breath-hold examination (VIBE) sequence was applied. By performing 2 consecutive acquisitions with different flip angles, this sequence enables the calculation of quantitative T1-mapping images. The optimal time interval between the administration of contrast media and the acquisition of postcontrast T1 images was assessed in a volunteer study, which indicated that thorough contrast agent diffusion is reached after 45 minutes. Subsequently, 10 patients after MACT of the talar dome, with a mean follow-up period of 51 months, were examined with this optimized protocol and pre- and postcontrast images were obtained ( Fig. 5 ).46 The ROI analysis demonstrated a considerable interindividual variation in postcontrast relaxation rates for both, reference cartilage and RT. This variability, however, was ameliorated when calculating the ΔR1 and rΔR1. Regarding ΔR1, no significant difference was found between RT and reference cartilage, although this finding was limited by the relatively small sample size. Average rΔR1 was 1.34, and therefore significantly closer to healthy reference cartilage than the values reported for RT after MACT in the knee, where values of 2.35 and 2.40 were reported.50,51 These findings once again puts emphasize assumption that ankle cartilage has increased regenerative potential.5,6
Figure 5.
Delayed gadolinium-enhanced magnetic resonance imaging of the talus after matrix-associated autologous chondrocyte implantation (MACI) at 3 T. White arrows indicate repair tissue borders. Image (a) displays precontrast T1-maps. Image (b) displays postcontrast T1-maps.46
Recent studies were able to demonstrate the potential of dGEMRIC not only in larger joints but also in the ankle joint. However, the necessity of contrast media administration, as well as the obligatory time interval before quantitative T1-mapping, may constitute hindrances for widespread clinical application.
Sodium Imaging
Sodium imaging allows for the direct measurement of sodium concentrations in a volume of interest. Its use for the assessment of the GAG content of articular cartilage is based on the premise that the negatively charged sites on GAGs are equalized by positively charged sodium ions. By including phantoms with known sodium concentrations in in vivo measurements, sodium-corrected signal intensities (cSI) or even absolute sodium concentrations can be calculated in native cartilage, as well as cartilage RT. Under consideration of this directly proportional relationship between the concentration of sodium ions and GAGs, cartilage GAG content can be deduced from the sodium values. Its high sensitivity and straightforward approach render sodium imaging a very promising modality for the assessment of GAG content.
However, sodium imaging is a challenging method. It requires special multinuclear hardware and has to deal with sodium concentrations considerably lower than in proton-based imaging. In addition, the physical properties of the sodium nuclei make the situation even more complicated, as their gyromagnetic ratio is only one-fourth that of protons. Four times higher gradient strengths are necessary for sodium to compensate for this. Altogether, the sensitivity of sodium in cartilage is about 4000 times lower compared to that of protons. This results in an inherently low SNR, limiting spatial resolution of sodium images acquired at 3 T. The remedy for this issue is the use of higher field strengths, which, in contrast to proton based MR imaging brings no disadvantages, but offers a substantial increase in SNR. This increase in SNR per unit of time, provided by 7-T systems, can be invested into sodium images with higher spatial resolution.
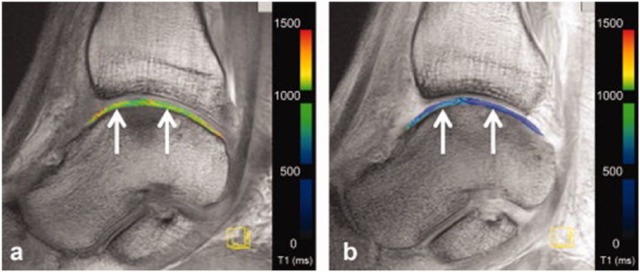
After several studies showing the possibility of sodium imaging for the evaluation of knee cartilage, including a demonstration of its correlation with dGEMRIC,52-54 the first study on sodium imaging in the ankle joint was recently performed.55 The authors successfully validated their protocol in ankle joint cadaver specimens ex vivo, correlating the corrected signal intensities with the histochemically evaluated GAG content. A strong correlation was observed, demonstrating that even the thin cartilage layer of the ankle joint is amenable to sodium imaging at 7 T, with high sensitivity to changes in GAG content. Subsequently, baseline values were established in healthy volunteers ( Fig. 6 )55 and compared with those of RT after MFX and MACT of the talar dome. RT after both, MFX and MACT, exhibited significantly lower cSI and therefore GAG content than healthy reference cartilage. In line with the findings regarding the collagen network by an aforementioned T2 mapping study,37 no significant difference between MFX and MACT was observed with respect to the GAG content. This study demonstrated that the value of sodium imaging in cartilage assessment also applies to the ankle joint. Because of its high sensitivity, it has the ability to serve as a reference modality for novel GAG specific sequences in the future.
Figure 6.
Sagittal proton density–weighted 2-dimensional turbo spin echo (TSE) images (A and B) and corresponding sodium images (C and D) display the ankle joint of a healthy volunteer acquired at 7 T. Image B has additional fat suppression and slightly higher resolution than image A. Image C was acquired with a sagittal sodium 3-dimensional gradient echo (GRE) sequence now displayed with additional red regions of interest (ROIs) for quantitative evaluation purposes. Image D displays a color-coded corrected signal intensity map of sodium superimposed on the corresponding morphological image B.55
Conclusion
The role of biochemical MRI in the assessment of cartilage quality cannot be emphasized enough. There are multiple different imaging approaches at disposal, which differ in target parameters, as well as in specific advantages and disadvantages ( Table 1 ).56 All of these approaches benefit from the use of high field (3 T) and ultra-high field (7 T) MRI, particularly from a gain in SNR and spectral resolution. Increased SNR might be traded for either higher spatial resolution while preserving measurement time or it might be traded for higher temporal resolution while preserving spatial resolution. With improvements in software and hardware (stronger gradients, radiofrequency coils with better B1-homogeneity, efficient fat suppression), most of the former limitations of 7 T, such as field inhomogeneities, increased sensitivity to chemical shift artifacts, as well as to susceptibility artifacts have been addressed and ameliorated. If adequately optimized, most sequences are expected to yield images of higher quality at 7 T in comparison with 3T. The inherent drawbacks of 7 T include SAR limits and increased inhomogeneity of the static magnetic field, as well as the B1 field. Overall, however, there is a substantial preponderance of the benefits of 7-T MRI. Therefore, with increasing availability the introduction of 7-T systems to the clinical routine can be expected in the near future. Nevertheless, with the exception of sodium imaging, all of the discussed sequences have already been successfully applied in the ankle joint at lower field strengths as well. In the ankle joint, biochemical MRI is currently focused on the postoperative evaluation of talar dome lesions and the evaluation of repair tissue after different cartilage surgeries in a research setting. However, as with 7-T systems, the translation of these innovative approaches to a clinical setting is expected.
Table 1.
Overview Over Biochemical Magnetic Resonance Imaging Techniques for the Assessment of Articular Cartilage of the Ankle Joint56.
| MRI | Ankle | Cartilage | T2-Mapping |
|---|---|---|---|
| T2-mapping | Free water, collagen fiber content and network | Robust technique, various studies; available in clinical routine | Magic angle effect, limited sensitivity to early onset osteoarthritis; weight bearing related daily variations Zonal evaluation in the ankle joint limited to ultra-high fields |
| T2*-mapping | Free water, collagen fiber content and network | Similar information as T2-mapping, shorter measurement times, 3-dimensional acquisition; available in clinical routine; lower SAR than T2-mapping | Magic angle effect, sensitive to susceptibility artifacts, limited sensitivity to early-onset osteoarthritis; weightbearing-related daily variations, zonal evaluation has not been performed in the ankle joint |
| dGEMRIC | GAG content | Short measurement times, high specificity and sensitivity | Dependent on administration of contrast media; obligatory time interval between Gd-DTPA-administration and imaging; distribution of contrast media is subject to cartilage thickness and other patient-specific factors |
| Sodium imaging | GAG content | High specificity and sensitivity; reference technique for evaluation of GAG content | Limited to ultra-high fields, need for special hardware, technically demanding, long measurement times, low spatial resolution, currently a research technique |
dGEMRIC = delayed gadolinium-enhanced MRI of cartilage; GAG = glycosaminoglycan; Gd-DTPA = gadolinium diethylenetriamine penta-acetic acid; MRI = magnetic resonance imaging; SAR = specific absorption rate.
Footnotes
Acknowledgments and Funding: Funding of this project was provided by the Vienna Science and Technology Fund, Project WWTF-LS11-018.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. Am J Public Health. 1984;74:574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-6. [PMC free article] [PubMed] [Google Scholar]
- 4. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-74. [DOI] [PubMed] [Google Scholar]
- 5. Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13:93-103. [DOI] [PubMed] [Google Scholar]
- 6. Aurich M, Mwale F, Reiner A, Mollenhauer JA, Anders JO, Fuhrmann RA, et al. Collagen and proteoglycan turnover in focally damaged human ankle cartilage: evidence for a generalized response and active matrix remodeling across the entire joint surface. Arthritis Rheum. 2006;54:244-52. [DOI] [PubMed] [Google Scholar]
- 7. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63. [DOI] [PubMed] [Google Scholar]
- 8. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 9. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(Suppl 2):25-32. [DOI] [PubMed] [Google Scholar]
- 10. Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3-8. [DOI] [PubMed] [Google Scholar]
- 11. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 12. Buda R, Vannini F, Cavallo M, Baldassarri M, Natali S, Castagnini F, et al. One-step bone marrow-derived cell transplantation in talarosteochondral lesions: mid-term results. Joints. 2013;1:102-7. [PMC free article] [PubMed] [Google Scholar]
- 13. Henderson I, Lavigne P, Valenzuela H, Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253-61. [DOI] [PubMed] [Google Scholar]
- 14. Millington SA, Li B, Tang J, Trattnig S, Crandall JR, Hurwitz SR, et al. Quantitative and topographical evaluation of ankle articular cartilage using high resolution MRI. J Orthop Res. 2007;25:143-51. [DOI] [PubMed] [Google Scholar]
- 15. Trattnig S, Breitenseher MJ, Huber M, Zettl R, Rottmann B, Haller J, et al. Determination of cartilage thickness in the ankle joint. An MRT (1.5)-anatomical comparative study. Rofo. 1997;166:303-6. [DOI] [PubMed] [Google Scholar]
- 16. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16-23. [DOI] [PubMed] [Google Scholar]
- 17. Apprich S, Trattnig S, Welsch GH, Noebauer-Huhmann IM, Sokolowski M, Hirschfeld C, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthritis Cartilage. 2012;20:703-11. [DOI] [PubMed] [Google Scholar]
- 18. Aurich M, Bedi HS, Smith PJ, Rolauffs B, Muckley T, Clayton J, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation early clinical and magnetic resonance imaging results. Am J Sport Med. 2011;39:311-9. [DOI] [PubMed] [Google Scholar]
- 19. Quirbach S, Trattnig S, Marlovits S, Zimmermann V, Domayer S, Dorotka R, et al. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009;38:751-60. [DOI] [PubMed] [Google Scholar]
- 20. Chang EY, Pallante-Kichura AL, Bae WC, Du J, Statum S, Wolfson T, et al. Development of a comprehensive Osteochondral Allograft MRI Scoring System (OCAMRISS) with histopathologic, micro-computed tomography, and biomechanical validation. Cartilage. 2014;5:16-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meric G, Gracitelli GC, McCauley JC, Pulido PA, Chang EY, Chung CB, et al. Osteochondral Allograft MRI Scoring System (OCAMRISS) in the knee: interobserver agreement and clinical application. Cartilage. 2015;6:142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36:121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kempson GE, Muir H, Swanson SA, Freeman MA. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970;215:70-7. [DOI] [PubMed] [Google Scholar]
- 24. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407-14. [DOI] [PubMed] [Google Scholar]
- 25. Wheaton AJ, Borthakur A, Shapiro EM, Regatte RR, Akella SV, Kneeland JB, et al. Proteoglycan loss in human knee cartilage: quantitation with sodium MR imaging—feasibility study. Radiology. 2004;231:900-5. [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Regatte RR. Investigation of regional influence of magic-angle effect on T2 in human articular cartilage with osteoarthritis at 3 T. Acad Radiol. 2015;22:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia Y, Moody JB, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med. 2002;48:460-9. [DOI] [PubMed] [Google Scholar]
- 28. Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50-5. [DOI] [PubMed] [Google Scholar]
- 29. Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology. 2008;247:154-61. [DOI] [PubMed] [Google Scholar]
- 30. Welsch GH, Mamisch TC, Weber M, Horger W, Bohndorf K, Trattnig S. High-resolution morphological and biochemical imaging of articular cartilage of the ankle joint at 3.0 T using a new dedicated phased array coil: in vivo reproducibility study. Skeletal Radiol. 2008;37:519-26. [DOI] [PubMed] [Google Scholar]
- 31. Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37(Suppl 1):112S-8S. [DOI] [PubMed] [Google Scholar]
- 32. Nehrer S, Domayer SE, Hirschfeld C, Stelzeneder D, Trattnig S, Dorotka R. Matrix-associated and autologous chondrocyte transplantation in the ankle: clinical and MRI follow-up after 2 to 11 years. Cartilage. 2011;2:81-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Battaglia M, Vannini F, Buda R, Cavallo M, Ruffilli A, Monti C, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19:1376-84. [DOI] [PubMed] [Google Scholar]
- 34. Domayer SE, Welsch GH, Stelzeneder D, Hirschfeld C, Quirbach S, Nehrer S, et al. Microfracture in the ankle: clinical results and MRI with T2-mapping at 3.0 T after 1 to 8 years. Cartilage. 2011;2:73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tao H, Shang X, Lu R, Li H, Hua Y, Feng X, et al. Quantitative magnetic resonance imaging (MRI) evaluation of cartilage repair after microfracture (MF) treatment for adult unstable osteochondritis dissecans (OCD) in the ankle: correlations with clinical outcome. Eur Radiol. 2014;24:1758-67. [DOI] [PubMed] [Google Scholar]
- 36. Becher C, Zuhlke D, Plaas C, Ewig M, Calliess T, Stukenborg-Colsman C, et al. T2-mapping at 3 T after microfracture in the treatment of osteochondral defects of the talus at an average follow-up of 8 years. Knee Surg Sports Traumatol Arthrosc. 2015;23:2406-12. [DOI] [PubMed] [Google Scholar]
- 37. Domayer SE, Apprich S, Stelzeneder D, Hirschfeld C, Sokolowski M, Kronnerwetter C, et al. Cartilage repair of the ankle: first results of T2 mapping at 7.0 T after microfracture and matrix associated autologous cartilage transplantation. Osteoarthritis Cartilage. 2012;20:829-36. [DOI] [PubMed] [Google Scholar]
- 38. Heule R, Ganter C, Bieri O. Triple echo steady-state (TESS) relaxometry. Magn Reson Med. 2014;71:230-7. [DOI] [PubMed] [Google Scholar]
- 39. Juras V, Bohndorf K, Heule R, Kronnerwetter C, Szomolanyi P, Hager B, et al. A comparison of multi-echo spin-echo and triple-echo steady-state T2 mapping for in vivo evaluation of articular cartilage. Eur Radiol. Epub 2015, September 3. doi: 10.1007/s00330-015-3979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Welsch GH, Mamisch TC, Hughes T, Zilkens C, Quirbach S, Scheffler K, et al. In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol. 2008;43:619-26. [DOI] [PubMed] [Google Scholar]
- 41. Krause FG, Klammer G, Benneker LM, Werlen S, Mamisch TC, Weber M. Biochemical T2* MR quantification of ankle arthrosis in pes cavovarus. J Orthop Res. 2010;28:1562-8. [DOI] [PubMed] [Google Scholar]
- 42. Schutz UH, Ellermann J, Schoss D, Wiedelbach H, Beer M, Billich C. Biochemical cartilage alteration and unexpected signal recovery in T2* mapping observed in ankle joints with mobile MRI during a transcontinental multistage footrace over 4486 km.Osteoarthritis Cartilage. 2014;22:1840-50. [DOI] [PubMed] [Google Scholar]
- 43. Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969;177:492-500. [DOI] [PubMed] [Google Scholar]
- 44. Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205:551-8. [DOI] [PubMed] [Google Scholar]
- 45. Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857-65. [DOI] [PubMed] [Google Scholar]
- 46. Domayer SE, Trattnig S, Stelzeneder D, Hirschfeld C, Quirbach S, Dorotka R, et al. Delayed gadolinium-enhanced MRI of cartilage in the ankle at 3 T: feasibility and preliminary results after matrix-associated autologous chondrocyte implantation. J Magn Reson Imaging. 2010;31:732-9. [DOI] [PubMed] [Google Scholar]
- 47. Tiderius CJ, Jessel R, Kim YJ, Burstein D. Hip dGEMRIC in asymptomatic volunteers and patients with early osteoarthritis: the influence of timing after contrast injection. Magn Reson Med. 2007;57:803-5. [DOI] [PubMed] [Google Scholar]
- 48. Watanabe A, Wada Y, Obata T, Ueda T, Tamura M, Ikehira H, et al. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239:201-8. [DOI] [PubMed] [Google Scholar]
- 49. Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA) (2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36-41. [DOI] [PubMed] [Google Scholar]
- 50. Trattnig S, Marlovits S, Gebetsroither S, Szomolanyi P, Welsch GH, Salomonowitz E, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0T: preliminary results. J Magn Reson Imaging. 2007;26:974-82. [DOI] [PubMed] [Google Scholar]
- 51. Trattnig S, Mamisch TC, Pinker K, Domayer S, Szomolanyi P, Marlovits S, et al. Differentiating normal hyaline cartilage from post-surgical repair tissue using fast gradient echo imaging in delayed gadolinium-enhanced MRI (dGEMRIC) at 3 Tesla. Eur Radiol. 2008;18:1251-9. [DOI] [PubMed] [Google Scholar]
- 52. Widhalm H, Brix M, Apprich S, Welsch G, Zbyn S, Vekszler G, et al. 7 Tesla sodium (23Na) imaging for the assessment of patellar cartilage damage after patella-dislocation: preliminary results. Osteoarthritis Cartilage. 2013;21(Suppl):S193. [Google Scholar]
- 53. Zbyn S, Stelzeneder D, Welsch GH, Negrin LL, Juras V, Mayerhoefer ME, et al. Evaluation of native hyaline cartilage and repair tissue after two cartilage repair surgery techniques with 23Na MR imaging at 7 T: initial experience. Osteoarthritis Cartilage. 2012;20:837-45. [DOI] [PubMed] [Google Scholar]
- 54. Trattnig S, Welsch GH, Juras V, Szomolanyi P, Mayerhoefer ME, Stelzeneder D, et al. 23Na MR imaging at 7 T after knee matrix-associated autologous chondrocyte transplantation preliminary results. Radiology. 2010;257:175-84. [DOI] [PubMed] [Google Scholar]
- 55. Zbyn S, Brix MO, Juras V, Domayer SE, Walzer SM, Mlynarik V, et al. Sodium magnetic resonance imaging of ankle joint in cadaver specimens, volunteers, and patients after different cartilage repair techniques at 7 T: initial results. Invest Radiol. 2015;50:246-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hesper T, Hosalkar HS, Bittersohl D, Welsch GH, Krauspe R, Zilkens C, et al. T2* mapping for articular cartilage assessment: principles, current applications, and future prospects. Skeletal Radiol. 2014;43:1429-45. [DOI] [PubMed] [Google Scholar]