Abstract
Objective
To report on our institution’s early results from juvenile particulate cartilage allograft transplantation of the talus.
Design
Because of the relative rarity of the procedure at the talus, it was decided to provide a comprehensive understanding of the currently available evidence via a 2-part study with (1) a systematic review of the literature and (2) a retrospective single-center cohort study of the authors’ patients, their demographics, and their early outcomes.
Results
(1) Four studies were included with 33 ankles with a weighted mean follow-up of 14.3 months. Only 1 ankle (3.3%) was converted to a revision open osteochondral allograft with medial malleolar osteotomy at 16 months postoperative; 6 (18.2%) required nonrevision type reoperations at an average of 15 months postoperative. (2) Six patients with mean age 35.7 ± 14.4 years were evaluated from the authors’ institution at mean 13.04 ± 8.35 months’ follow-up. All reported subjective improvements in pain and motion, and functional improvements, although postoperative magnetic resonance imaging in 3 patients at time points between 3 months and 2 years postoperative demonstrate persistent subchondral edema and nonuniform chondral surface in the talus. There were no intraoperative or postoperative complications, and there have been no reoperations.
Conclusion
Preliminary data suggest that treatment of large, traumatic or atraumatic, symptomatic osteochondral talar defects with particulated juvenile cartilage transplantation may improve patient subjective complaints of pain and function. Systematic review of the available literature highlights the need for future prospective, larger cohort studies of its use on the talus but suggests similar potential for the technology.
Keywords: talus, osteochondral, particulated, juvenile, cartilage
Introduction
Osteochondral lesions of the talus (OLT) are by definition a defect of the articular cartilage and the adjacent subchondral bone.1 They are often symptomatic, with deep ankle pain, limited range of motion, and swelling with the consequence of limitations in activities of daily living or sports participation.2 The etiology of these lesions is typically traumatic, either through a single episode such as an ankle sprain or fracture, or with multiple events as in recurrent ankle instability.1,3 While some lesions may be amenable to nonoperative management, most lesions—particularly those of larger size—require operative intervention, due to the inherent inability of native avascular cartilage to heal.4 Often the first tier of operative intervention is with marrow stimulation techniques such as microfracture or transarticular drilling, to enhance bleeding through the penetration of the subchondral bone and the subsequent flooding with cells forming fibrocartilage.5 Other advanced procedures for recalcitrant lesions include osteochondral allograft or autograft transplantation (OAT), autologous chondrocyte implantation (ACI), or matrix-induced ACI (MACI) have been developed, but each has its limitations of donor site morbidity, poor interface integration, the need for multiple procedures, or histological differences from native cartilage, which limit their efficacy.6
Recent technological advances in the field of cartilage restoration methods has led to the utilization of fresh particulated juvenile allograft chondrocytes transplanted at the lesion site to reproduce the native cartilaginous articular surface.5 The only current graft material available for this procedure, the DeNovo NT Natural Tissue Graft (Zimmer, Warsaw, IN, USA) is a prepackaged allograft substance consisting of immature live chondrocyte cells within their native extracellular matrix from donors typically younger than 13 years. A single package can be used to cover a defect that is 2.0 cm2 as a single-stage procedure for focal defects.7,8 Fibrin adhesive is used to secure these cartilaginous pieces within the subject’s lesion. Its efficacy has been demonstrated for pathology about the knee—such as high-grade cartilage lesions of the patella,9,10 the femoral condyles or trochlear groove11—in quickly and safely relieving pain and improving outcomes at short-term follow-up, but the data have been limited to level IV evidence with only small cohorts of data. In addition to magnetic resonance imaging (MRI) showing good defect fill,12 some of these studies of the knee11 have demonstrated histologically favorable repair tissue of a mixture of hyaline and fibrocartilage with excellent integration of the allograft tissue with the native articular cartilage.
Efficacy of the particulated juvenile articular cartilage allograft technology about the ankle is less well studied. Ideal patients are those with symptomatic, isolated talar osteochondral lesions which have failed prior microfracture; younger (<50 years old) patients with larger lesions (>1.0 cm2) but less than 5 cm2 may benefit more from the procedure, but conclusions are still premature in the published literature.13,14 The purpose of this study was to evaluate the use of juvenile particulate allograft cartilage transplantation for lesions of the talus. Our hypothesis was that its efficacy would be apparent by as early as 6 months of follow-up postoperatively. Because of the relative rarity of the juvenile particulate cartilage allograft transplantation procedure at the talus, it was decided to provide a comprehensive understanding of the currently available evidence via a 2-part study with (1) a systematic review of the literature and (2) a retrospective single-center cohort study of the authors’ patients and their early outcomes.
Methods
Systematic Review of the Literature
A thorough review of the literature was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with a PRISMA checklist. All clinical outcome studies published between the inception of the MEDLINE database and May 15, 2016 were identified. Any clinical study assessing the outcome of juvenile particulate allograft cartilage transplant to the talus was included. Publications that not only described a novel surgical technique for the transplantation of the juvenile particulate cartilage but also reported on the clinical outcome of one or more patients with said technique were included. We excluded those analyses that evaluated other cartilage allograft, autograft or marrow stimulation processes, or nonclinical (review or technique) studies.
The search methodology involved a search of the MEDLINE database through the PubMed interface via a search algorithm with the following search terms: (“talus” OR “talar” OR “ankle”) AND (“juvenile” OR “particulate” OR “DeNovo”) AND (“allograft” OR “cartilage” OR “transplantation”). The search identified 39 studies. Based on the aforementioned criteria, studies were included or excluded after review of the titles and abstracts. Studies with questionable inclusionary status had full-text retrieved for further review. After completion of review of these 39 studies, a total of 4 were identified as appropriate for inclusion in this component of our study ( Fig. 1 ). Manual search of the reference lists of these included studies was performed, with no further inclusion of studies. The primary author reviewed the full text of the 4 articles in order to extract all data into a standardized form created by the authorship team at the onset of this review.
Figure 1.

Study PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Because of the relatively small number of total patients from the literature with the surgical procedure at interest, and the heterogeneity between the patient cohorts in terms of patient demographics, osteochondral lesion characteristics, and surgical approach, a predominantly qualitative, descriptive approach was utilized to describe the collective results from the clinical studies. Highlighted patient and surgical data included the following: mean age, patient gender, mean body mass index (BMI), laterality, etiology (i.e., traumatic or atraumatic), mean duration of preoperative symptoms, frequency and number of previous ankle surgeries, lesion size and location, surgical technique, concomitant surgical procedures, postoperative rehabilitation protocols, reoperation and complication rates, clinical outcome scores, satisfaction, and subjective level of improvement and function.
Cohort Analysis of Authors’ Patients
After institutional review board approval and patient consent, the medical records of 6 ankles in as many total subjects (4 male and 2 female) who were treated with particulated juvenile cartilage (DeNovo NT Natural Tissue Graft, Zimmer, Warsaw, IN) allograft transplantation for osteochondral lesions of the talus between October 16, 2013 and March 24, 2015 were retrospectively reviewed. The patients were treated by 2 senior surgeons at a single academic institution. The preoperative, operative, perioperative, and postoperative records for each of these patients were reviewed. Demographic and preoperative data collected for this study included the patient gender, age, etiology of ankle symptoms, laterality of pathology, location of talar osteochondral lesion, surgical treatment on the ankle prior to the operation of interest, worker’s compensation status, medical comorbidities, MRI findings, utilization of injections, tobacco smoking history, BMI, current work status, physical examination findings, subjective complaints, and various clinical survey scores. Operative data included postoperative diagnosis, surgical approach and technique, and intraoperative findings of lesion size and quality. Postoperative data included subjective patient complaints, objective examination findings, and plain radiographic or MRI findings as available at visits of 6 weeks, 3 months, 6 months, and longest follow-up visit postoperatively.
Authors’ Preferred Surgical Technique
Preoperative regional anesthesia is utilized for the operative leg. In a supine position on the operating table, a bump is applied under the operative side buttocks. A thigh tourniquet is applied. The operative lower extremity is placed on a thigh holder, and the extremity prepped and draped in standard sterile fashion. Preoperative antibiotics are administered. A noninvasive ankle distractor is utilized, and standard anteromedial and anterolateral portal sites are created. Arthroscopy was performed prior to the open portion of the procedure in order to visualize and treat any concurrent pathology; it was also utilized to visualize and document the lesions with images as well as in some cases to confirm that there was a lesion that required the index procedure. Arthroscopy additionally allowed the authors to visualize the exact location of the lesion to determine if maximal plantarflexion or dorsiflexion would allow a simple arthrotomy for access or whether an osteotomy was required to gain access to the lesion with the proper angles and ability to introduce the surgical equipment for intervention.
The ankle joint is initially insufflated with approximately 10 mL of normal saline. The skin is then sharply incised, and with a mosquito hemostat the soft tissues are dissected in order to enter the tibiotalar joint. Debridement with a shaver, burr or intra-articular Bovie of arthrofibrosis, osteophytes, and any soft tissue impingement of the anterior tibiotalar joint and medial or lateral gutters ensues. The osteochondral lesion is then directly visualized and probed to distinctly determine if there was an unstable cartilage gap, and removed with a grasper. With use of a curette, the bed of the lesion was fully debrided including the underlying bone to bleeding punctate surfaces in order to complete and establish containment of the osteochondral lesion. Some form of marrow stimulation is necessary; the authors believe it is important to develop vascularity on the talus and propose that curettage accomplishes this well with better punctate bleeding and a more adherent surface.
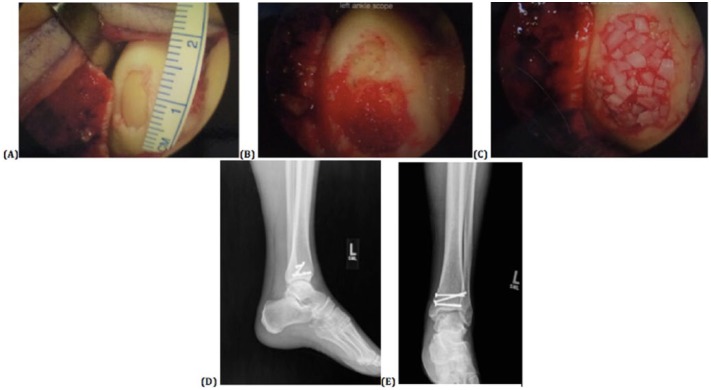
At this point, the arthroscopy equipment is removed, the thigh holder taken down, and depending on the laterality and extent of the lesion, either the lateral or medial portal incision was extended both distally and proximally in order to perform an open arthrotomy, or an osteotomy is performed. The thigh tourniquet is inflated to 275 mm Hg. When an osteotomy is not required, dissection is taken down directly to the level of the joint and the capsule elevated. When a medial malleolus osteotomy is deemed necessary for exposure of the lesion, a long longitudinal medial incision over the distal medial malleolus and metaphyseal region is created. This is taken down sharply through the skin and bluntly through the soft tissue, until the periosteum overlying the ankle joint is identified. Arthrotomies are made in both the anterior and posterior capsule, the posterior approach through the posterior tibial tendon sheath, and Hohmann retractors placed to protect the joint line. A 0.062-inch Kirschner wire is placed in line with the expected osteotomy, and confirmed under multiple views of fluoroscopy. An oscillating saw is used in line with the Kirschner wire to place the distal tibial osteotomy obliquely to the level of the joint with care taken not to violate the articular cartilage. An osteotome is then introduced to complete the remainder of the osteotomy. The osteotomy is booked open in order to gain full visualization of the medial talar osteochondral lesion ( Fig. 2a ).
Figure 2.
Authors’ surgical technique for the placement of the DeNovo NT particulate juvenile cartilage transplantation by open means with medial malleolar osteotomy. (a) The open evaluation is made possible through medial malleolar osteotomy of the medial osteochondral talar lesion after the lesion has arthroscopically been fully debrided down to the underlying bone with established containment of the osteochondral lesion. (b) Further debridement of the lesion ensues to a bleeding subchondral base, and a layer of fibrin glue is placed after the bed is thoroughly dried. (c) The juvenile allograft particulate cartilage is then laid down on top of this bed with full containment and within the confines of the defect, and a second layer of fibrin glue is placed and allowed to dry. (d, e) The osteotomy site is reduced and cannulated screws placed to compress the fracture line with anatomic reduction of the joint.
Additional debridement of the soft tissues is performed as needed. The bed of the lesion is subsequently completely dried, and fibrin glue initially laid on to the surface of the subchondral bone ( Fig. 2b ). The juvenile allograft particulate cartilage is then laid down on top of this bed with full containment and excellent coverage within the confines of the defect. Additional fibrin glue is placed over the top of the particulate cartilage to stabilize the cartilage as well as the graft material ( Fig. 2c ). The arthroscope is used dry to help visualize the particulate allograft cartilage placement as well as to document this portion of the procedure. This is allowed several minutes to set. Concomitant procedures—including lateral ligament reconstruction or os trigonum excision—are then performed. In cases with a medial malleolar osteotomy for access to the joint, the osteotomy site is reduced and 4-0 cannulated guidewires provisionally placed across the osteotomy site, both parallel to the joint line and perpendicular to the osteotomy site. Screws are placed to compress the fracture line with anatomic reduction at the level of the joint and good bony compression and stability ( Fig. 2d and e ). Thereafter the deep capsule and tissue is closed with 3-0 Vicryl suture. The same is used to reapproximate the skin, and finally 3-0 nylon used to close all the skin edges. The wound thereafter was dressed with LiquiBand ointment and dressed in sterile dressing and a hard plaster short leg post mold sugar tong splint in neutral position with nonweightbearing status. Patients received deep venous thrombosis prophylaxis for 3 weeks postoperatively with aspirin 325 mg twice daily.
At 2 weeks postoperative, the patient’s sutures and dressings were removed. A tall CAM boot and compressive stocking are given for the next 6 weeks to allow removal for gentle active range of motion exercises to begin, with continued non-weight bearing. At 6 weeks postoperative, the patient is allowed to bear weight as tolerated in the CAM boot and formal physical therapy is begun to work on stretching, range of motion, and ankle strengthening. At 10 weeks postoperatively, the patient weans from the CAM boot to normal supportive shoe wear. At 16 weeks postoperative, physical therapy is advanced to impact activities and function improvement. At 20 weeks postoperative, a work conditioning program is started as needed based on the patient’s preoperative work status.
Results
Systematic Review of the Literature
Four studies were included: 2 retrospective cohort studies of 23 patients (24 ankles)14 and 7 patients,15 respectively; 1 case report;16 and 1 case report embedded within a novel surgical technique article ( Table 1 ).7 All studies were published in the past 3 years and were level IV or V evidence. In total, there were 32 patients (33 ankles) in 17 males and 15 females. The weighted mean age of the sum of the 32 patients was 35.0 years (range, 17.5-69.0 years) with a weighted mean of 59.4 months (range, 6.4-480 months) of preoperative symptoms in the 26 patients where this information was available. In 20 of 31 (64.5%) ankles with a reported etiology of ankle pain, the etiology was a traumatic inversion sprain or ankle fracture. Eighteen out of 33 (54.5%) ankles had failed a previous bone marrow stimulation procedure. Weighted mean osteochondral lesion area was 117.8 mm2 (range, 15-324 mm2) in the 31 ankles with reported lesion size. Lesion location was lateral in 20 of 32 ankles (62.5%) and medial in 12 of 32 ankles (37.5%) where this information was provided.
Table 1.
Summary of Data from Previously Published Literature Evaluating Particulate Juvenile Articular Cartilage Allograft Transplantation for Osteochondral Lesions of the Talus.
| Primary Author (Year of Publication) | Study Type (Level of Evidence) | No. of Patients (M:F) | Average Patient Age (Years) | Preoperative Demographic Information | Exam/Radiographic Findings before Surgery | Lesion Description | Surgical Technique, Concomitant Procedures and Postoperative Rehabilitation | Results | Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Coetzee (2013)14 | Case Series (Level IV) | 23 (12M:11F) with 24 ankles (1 bilateral female) | 35.0 (range, 17.5-69) | Mean BMI = 28 ± 5.8 kg/m2
14 right, 10 left ankles Traumatic injury in 13/24 ankles, nontraumatic events in 4/24 ankles, and 7/24 unknown etiology Mean 6.9 ± 11 years of preoperative symptoms (range, 0.53-40 years) in 17 patients—71% had symptoms lasting longer than 1 year 14/24 (58%) ankles had failed at least 1 prior bone marrow stimulation procedure 2/24 ankles had failed a prior osteochondral repair for the index lesion 10/24 (42%) ankles had no previous history of operative treatment for the index lesion |
NR | Mean lesion size 125 ± 75 mm2 (range, 50-300 mm2) (n = 22) Mean depth of 7 ± 5 mm (range, 3-20 mm) (n = 19) All lesions had at least 1 dimension ≥10 mm (n = 23) 20/24 ankles were ICRS grade 4 19/24 lesions were medial, and 5/24 lesions were on the lateral talar dome 3/24 lesions located anteriorly, 14/24 centrally and 7/24 posteriorly 6/24 lesions were fully contained (i.e., bordered by a stable rim of articular cartilage), 11/24 were 75%-99% contained, and 5/24 were 50%-74% contained (containment not recorded for 2 lesions) |
12/24 procedures were managed with an open surgery; 3/24 lesions were managed with an arthroscopic procedure; 9/24 lesions were managed with an extended portal procedure 12/24 ankles had osteotomies for lesion access 8/24 ankles underwent a plafondplasty 9/24 ankles (38%) had 1 concomitant procedure; 9/24 ankles (38%) had more than 1 concomitant procedure (included hardware removal, treatment for impingement, synovitis, instability, osteophytes and malalignment) 6/24 ankles (25%) were treated with bone graft prior to placement of the particulate juvenile cartilage Patients immobilized NWB in plaster splint for 2 weeks; at 2 weeks, a removable boot brace was applied and patients remained NWB for 6 weeks total postoperatively; protected activities were continued between 6 and 12 weeks; advancement to running and loading activities was allowed between 24 and 52 weeks |
Mean 16.2 months’ follow-up (range, 10.5-25.6 months) 1 partial graft delamination (~25% of graft) diagnosed at 16 months postoperative, lesion size 180 mm2—converted to revision open osteochondral allograft with a medial malleolus osteotomy 6 additional reoperations at an average of 15 months (range, 2.5-21 months) postsurgery: 5 to remove symptomatic or failed osteotomy hardware, 1 to correct anterior impingement; 1 reoperation in the large lesions group and 5 reoperations in 4 ankles in the moderate lesion group 92% of subjects with moderate-sized lesions demonstrated good to excellent results 56% of subjects with large-sized lesions demonstrated good to excellent results Outcome scores Mean SF12 PCS: 46.4 (95% CI, 42-51; SD 9.96; range, 14.5-60.5) (n = 24) Mean SF12 MCS: 55.1 (95% CI, 52-58; SD 7.12; range, 33.3-65.9) (n = 24) Mean FAAM ADL: 82.4 (95% CI, 76-89; SD 14.4; range, 42.9-100) (n = 24) Mean FAAM Sports: 63.4 (95% CI, 52-75; SD 26.8; range, 5.60-100) (n = 24) Mean VAS Pain: 24 (95% CI, 13-34; SD 25; range, 0-93) (n = 24) Mean AOFAS <80 in 5/23 patients (22%) -Mean AOFAS > 80 in 18/23 patients (78%) Results by lesion size (longest dimension) Moderate lesions between 10 and 15 mm and large lesions greater than 15 mm Mean VAS Pain in moderate lesions: 15 (95% CI, 6-24) (n = 14) Mean VAS Pain in large lesions: 40 (95% CI, 16-64) (n = 9) Mean FAAM ADL in moderate lesions: 87 (95% CI, 81-93) (n = 14) Mean FAAM ADL in large lesions: 76 (95% CI, 62-90) (n = 9) Mean FAAM Sports in moderate lesions: 69 (95% CI, 55-84) (n = 14) Mean FAAM Sports in large lesions: 53 (95% CI, 31-75) (n = 9) Mean AOFAS in moderate lesions: 90 (95% CI, 85-96) (n = 13) Mean AOFAS in large lesions: 76 (95% CI, 58-95) (n = 9) AOFAS <80 in 8% (1/13) of moderate lesions and 44% (4/9) of large lesions AOFAS >80 in 92% (12/13) of moderate lesions and 56% (5/9) of large lesions |
Author relations as designing consultant to Zimmer, Inc. with receipt of financial support for the research Multiple different surgeons and centers (6 surgeons at 5 study centers) Not all data present for all patients Heterogeneity in patient demographics and lesion characteristics Limited subjects Length of follow-up Absence of control group Majority of patients with concomitant procedures Limited information available regarding preoperative outcome scores |
| Giza (2013)7 | Case report (Level V) | 1 (1 F) | 36 | 4 years of preoperative symptoms | NR | 18 mm × 18 mm | All-arthroscopic technique Patients kept NWB 6 weeks; at 2 weeks a generic cast book is placed to allow gentle AROM and PROM; strengthening and elastic band resistance exercises with limited plantarflexion (20°). After week 6, advanced to WBAT in a boot with increasing ROM exercises, bike, nonimpact cardio, pool therapy. Weeks 12 to 24, gradual increase in activity. Weeks 24 to 52, return to running and loading activities |
Follow-up of 18 months Foot Function Index: 75 → 25 at 6 months Subjective functional improvement at 6 months Denied pain with stairs or daily activities at 6 months At 18 months, stable function, playing soccer for 90 minutes without pain |
Single patient case with retrospective study Minimal data given for patient, preoperative demographics, surgical or postoperative data Article is predominantly a technique article with single example patient case given |
| Bleazey (2012)15 | Retrospective case series (Level IV) | 7 (5M:2F) | 35.4 | Mean 9.2 months of preoperative symptoms 4/7 (57.1%) had a prior arthroscopic microfracture 7/7 (100%) with history of traumatic inversion sprain or ankle fracture |
NR | Mean, 8.6 mm × 9 mm × 5.9 mm (minimum lesion size, 3 × 5 × 4; maximum lesion size 11 × 13 × 9) Cystic lesion present on medial talus in 6/7 patients and on lateral talus in 1/7 patients All lesions full thickness |
Open procedure 6/7 patients with medial malleolar osteotomy 1/7 patients with fibular osteotomy 7/7 (100%) with subchondral cysts that required decompression with cylindrical sponge cancellous allograft Patient kept NWB in short leg cast for 4 weeks, then transferred into removable boot still NWB until 6 weeks postoperative; patient then transferred to WBAT in boot with initiation of PT for ROM exercises; transition to regular shoe at 8 weeks postoperatively |
Mean 6 months of postoperative follow-up No perioperative complications No inflammatory responses to the cancellous bone sponge or particulate cartilage 1 patient had postoperative MRI performed to evaluate graft incorporation which revealed no subchondral cystic areas and an intact subchondral plate 7/7 patients with clinical improvements in pain scale from preoperative to 6 months postoperative (including parameters of “pain during first step in morning,” “pain during basic walking,” and “pain at end of day”): greatest clinical improvements during first step in the morning and pain at the end of the day 7/7 patients with clinical improvements in activity scale from preoperative to 6 months postoperative (including parameters of “ambulate down stairs,” “ambulate up stairs,” and “ambulate up to 4 city blocks”): greatest clinical improvements walking up to 4 blocks and ambulating down stairs Pain scale improvements from preoperative to 6 months postoperative for first step in a.m. (8.1 → 1.3), during walking (7.7 → 1.9), and end of day (8.3 → 2.4) with 0 being no pain and 10 being pain preventing ambulation 7/7 patients reported that they would do the surgery again at the 6-month postoperative milestone Activity scale improvements from preoperative to 6 months postoperative for down stairs (6.4 → 9.2), up stairs (7.1 → 9.5), and walking up to 4 blocks (4.8 → 9.2) with 0 being unable to perform and 10 being able to perform without pain |
Small sample size Short-term follow-up Concomitant procedure with cancellous cylindrical bone sponge in all patients in addition to the DeNovo NT graft |
| Kruse (2012)16 | Case report (Level V) | 1 (1 F) | 30 | 2 years of preoperative symptoms | Pain on palpation anterolateral ankle Pain on palpation ATFL Increased anterior drawer test and increased talar tilt test Plain films with early arthrosis of tibiofibular articulation and possible loose bodies near medial malleolus tip Stress radiography with increased talar tilt and positive anterior drawer sign MRI with grade 4 OCD of posteromedial shoulder of the talus and complete ATFL rupture and nondisplaced Shepherd’s fracture of talus |
7 mm × 5 mm Lesion located on posteromedial shoulder of talus |
All-arthroscopic technique Patient kept NWB with biweekly cast changes for 4 weeks; then placed in removable cast boot and began ROM activities while still NWB; WBAT in removable cast boot at 6 weeks and transitioned out of boot during the next 2 weeks; at 3 months, returned to light athletic activity (swimming, stationary bike, long walks) |
At 4 months postoperatively, patient began light jogging At 6 months postoperatively, completely pain free with full ROM and full activity; able to walk, jog, and stand for prolonged periods without pain, swelling or stiffness and activity was at preinjury level without any limitations At 2 years postoperatively, reports by phone interview that patient was pain free with no limitation in activity |
Single patient case with retrospective study No standardized pain or functional evaluation Short follow-up period Minimal data given for patient, preoperative demographics, surgical, or postoperative data |
M = male; F = female; NWB = nonweightbearing; AROM = active range of motion; PROM = passive range of motion; ATFL = anterior talofibular ligament; NR = not reported; PT = physical therapy; MRI = magnetic resonance imaging; ICRS = International Cartilage Repair Society; AOFAS = American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scale; SF-12 = Short-Form 12 Health Survey; PCS = physical component score; MCS = mental health composite score; FAAM = Foot and Ankle Ability Measure; ADL = activities of daily living score; VAS = visual analog scale; CI = confidence interval.
Nineteen (57.6%) were treated with an open procedure, 5 (15.2%) by arthroscopic means, and 9 of 33 ankles (72.8%) with an extended portal procedure. Nineteen ankles (57.6%) required an osteotomy for lesion access. Sixteen ankles (48.9%) had 1 concomitant procedure, and 9 ankles (72.8%) had 2 or more concomitant procedures; concurrent interventions included things such as decompression with cylindrical or otherwise allograft bone placement, removal of hardware, or treatment means for impingement, synovitis, instability, osteophytes, or malalignment. Rehabilitation protocols are delineated in Table 1 for each of the included studies.
Weighted mean follow-up for the 32 patients was 14.3 months (range, 6.0-25.6 months). There were no reported perioperative complications or inflammatory responses to the implanted materials. Only 1 ankle (3.3%) was converted to a revision open osteochondral allograft with medial malleolar osteotomy at 16 months postoperative; 6 ankles (18.2%) required nonrevision type reoperations at an average of 15 months postoperative with 5 being removal of symptomatic or failed osteotomy hardware, and 1 to correct anterior impingement. Of the 31 ankles with clinical outcome reports, substantial subjective improvements (by reports of “good-to-excellent results”, “improvement in pain scale and activity scale,” and “pain-free ankle without limitations”) were reported in 83.9% of the operative ankles. Seven of 7 ankles (100%) asked if they would do the surgery again in retrospect at the 6-month postoperative milestone said they would.
Analysis of Authors’ Cohort of 6 Patients
A thorough chart review of all 6 patients at our single academic institution who had undergone the surgical procedure of interest was completed ( Table 2 ). The mean (± standard deviation) age of the patients was 35.7 ± 14.4 years (range, 21.2 – 60.5 years). The mean BMI of the patients was 28.82 ± 5.83 kg/m2. There were 2 female (33.3%) patients and 4 male (66.7%) patients, with 4 left (66.7%) ankles and 2 right (33.3%) ankles operated on. Two patient cases (33.3%) were active Worker’s Compensation claims; 1 patient (16.7%) was an active smoker. The etiology of the patients’ ankle pain was traumatic in 5 patients (83.3%), with 3 of those 5 being severe inversion ankle sprains, 1 a nondescript skiing accident, and the final patient a nonoperative distal fibular ankle fracture. Preoperative symptoms were present for a mean 61.2 ± 54.3 months (range, 24.5-158.8 months). Three patients (50.0%) were employed in an occupation considered to be of “heavy manual labor,” of which only 1 was still actively working full duty until the time of surgical intervention. Four (66.7%) of the 6 patients had undergone at least 1 prior operation on the ankle of interest.
Table 2.
Patient Demographic and Preoperative Data.
| Patient No. | Age, Years (Gender)/Side | Duration Preoperative Sx (months) | Lesion Location/Size (mm)a | Worker’s Comp? | Occupation (Heavy Labor?) | Smoker? | Body Mass Index (kg/m2) | Traumatic Etiology? | Prior Ankle Surgery? | Subjective Complaints |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35.1 (M)/left | 29.9 | AL/14 × 15 | Yes | Switchman for railway (yes)—not active | No | 34.04 | Yes—severe inversion ankle sprain | Yes—scope, debridement, loose body excision, microfracture | Pinching ankle pain; difficulty squatting, bending |
| 2 | 21.2 (F)/left | 88.6 | C/12 × 16 | No | Student (no)—still in school | No | 20.07 | Yes—severe inversion ankle sprain | Yes—scope, debridement, microfracture | Pain; mechanical sx (catching, locking) |
| 3 | 22.2 (M)/left | 48.8 | PM/22 × 14 | No | Construction (yes)—active | Yes—½ ppd | 25.80 | Yes—severe inversion ankle sprain | No | Ankle pain, swelling; mechanical sx (clicking, popping); instability |
| 4 | 34.3 (M)/right | 16.5 | AL/11 × 10 | No | Desk job (no)—active | Former—quit 3 years prior to surgery | 28.72 | Yes—skiing accident | Yes—scope, debridement, microfracture ×2 | Mechanical sx (locking, catching); pain with ambulation |
| 5 | 60.5 (M)/left | 158.8 | PM/12 × 8 | No | Periodontist/professor (no)—active | No | 27.98 | No | No | Pain; mechanical sx (catching, locking, popping) |
| 6 | 40.8 (F)/right | 24.5 | PM/10 × 10 | Yes | Unloader (yes) – not active | No | 36.31 | Yes – ankle fracture | Yes – scope, debridement & scope, debridement, drilling | Pain |
| Mean | 35.7 | 61.2 | 13.5 × 12.2 | — | — | — | 28.82 | — | — | — |
| SD | 14.4 | 54.3 | A-P: 4.4 M-L: 3.3 |
— | — | — | 5.83 | — | — | — |
M = male; F = female; ppd = packs per day; SD = standard deviation; AL = anterolateral; C = central; PM = posteromedial; Comp = compensation; “not active” = refers to the patient not currently in active duty work at time of particulated juvenile articular cartilage allograft surgery; “active” = refers to the patient currently in full active duty work at time of particulated juvenile articular cartilage allograft surgery; sx = symptoms; A-P = anteroposterior dimension; M-L = medial-lateral dimension.
Dimensions of the lesion size are given in anteroposterior × medial-lateral directions.
Two of the osteochondral lesions (33.3%) were on the anterolateral talus, 3 (50.0%) were posteromedial, and 1 (16.7%) was central in location. All patients (100%) had preoperative subjective complaints consistent with a cartilaginous pathology—including pain, mechanical symptoms, and swelling—and the pathology was subsequently confirmed with MRI by the presence of a focal osteochondral defect of the talus. The mean dimensions of the patients’ osteochondral lesions were 13.5 ± 4.4 mm in the anteroposterior dimension and 12.2 ± 3.3 mm in the mediolateral dimension. The smallest lesion was measured at 12 × 8 mm, while the largest defect measured 22 × 14 mm.
In all, 6 patients (100%), the surgical approach was as described in the previous section of this article—an arthroscopic approach to the ankle for debridement and evaluation of the osteochondral defect was followed with an open procedure to perform the particulate juvenile cartilage allograft transplantation. Two patients (33.3%) required a medial malleolar osteotomy to appropriately access the osteochondral lesion site. Four (66.7%) of the 6 patients underwent concurrent procedures at the time of surgery to address concomitant pathology, including tibiotalar exostectomy, lateral ligament reconstruction, bone grafting of underlying cystic defects, and os trigonum excision. The mean number of concomitant procedures was 1.0 ± 0.89 procedures ( Table 3 ). There were no perioperative complications.
Table 3.
Patient Operative Data.a
| Patient No. | Postoperative Diagnosis | Concomitant Procedures | Surgical Approach to Particulated Juvenile Articular Cartilage Allograft Procedure |
|---|---|---|---|
| 1 | 1. Left ankle synovitis 2. Recurrent anterolateral osteochondral lesion of the talus 3. Impingement |
1. Tibiotalar exostectomy | Open (after initial arthroscopic evaluation and debridement) |
| 2 | 1. Left ankle arthrofibrosis 2. Central osteochondral lesion of the talus 3. Lateral ligament instability |
1. Microfracture of osteochondral lesion 2. Lateral ligament reconstruction |
Open (after initial arthroscopic evaluation and debridement) |
| 3 | 1. Left posteromedial osteochondral defect of the talus 2. Cystic degeneration 3. Posttraumatic arthrosis 4. Symptomatic os trigonum |
1. Bone grafting into underlying cystic defect 2. Os trigonum excision |
Open with medial malleolar osteotomy (after initial arthroscopic evaluation and debridement) |
| 4 | 1. Right recurrent anterolateral talar osteochondral defect | — | Open (after initial arthroscopic evaluation and debridement) |
| 5 | 1. Left posteromedial talar osteochondral lesion 2. Synovitis |
1. Bone grafting into underlying defect | Open with medial malleolar osteotomy (after initial arthroscopic evaluation and debridement) |
| 6 | 1. Right ankle recurrent posteromedial osteochondral lesion of the talus 2. Posttraumatic arthropathy |
— | Open (after initial arthroscopic evaluation and debridement) |
All procedures involved an extensive irrigation and debridement of tissues in addition to the particulate juvenile cartilage allograft transplantation procedure.
Final follow-up for the 6 patients was at a mean 13.04 ± 8.35 months (range, 5.06-25.41 months) postoperative ( Table 4 ). At the time of each patient’s final follow-up, all 6 patients (100%) had reported subjective improvements in pain and range of motion, as well as functional improvement. There have been no reoperations in these patients, and only 1 patient has required any type of repeat intervention to date, namely a trigger point injection into the lateral hindfoot region for new pain symptoms, which was qualitatively different than the patient’s preoperative symptoms and not apparently related. One patient began work at 12 months postoperative in construction without difficulty. Both Worker’s Compensation patients were cleared for full duty return to work in their manual labor occupations by 6 months postoperative.
Table 4.
Patient Postoperative Data.
| Patient No. | Final Follow-up (Months) | Subjective and Objective Improvement from Preoperative Status? | Requirement for Reoperation? | Requirement for Further Intervention? |
|---|---|---|---|---|
| 1 | 21.57 | Yes | No | Yes – trigger point injection into lateral hindfoot region for new pain symptoms |
| 2 | 8.61 | Yes | No | No |
| 3 | 25.41 | Yes | No | No |
| 4 | 5.06 | Yes | No | No |
| 5 | 10.16 | Yes | No | No |
| 6 | 7.43 | Yes | No | No |
| Mean | 13.04 | |||
| SD | 8.35 |
SD = standard deviation.
Postoperative MRI was obtained in three of the 6 patients (at 3 months postoperative in 1 patient, 6 months postoperative in the second patient, and 24 months postoperative in the third). Interestingly, despite the clinical improvements for these 3 patients, the images in each patient demonstrated persistence of a nonuniform chondral surface and subchondral edema at the location of the particulate cartilage placement ( Figs. 3 and 4 ).
Figure 3.
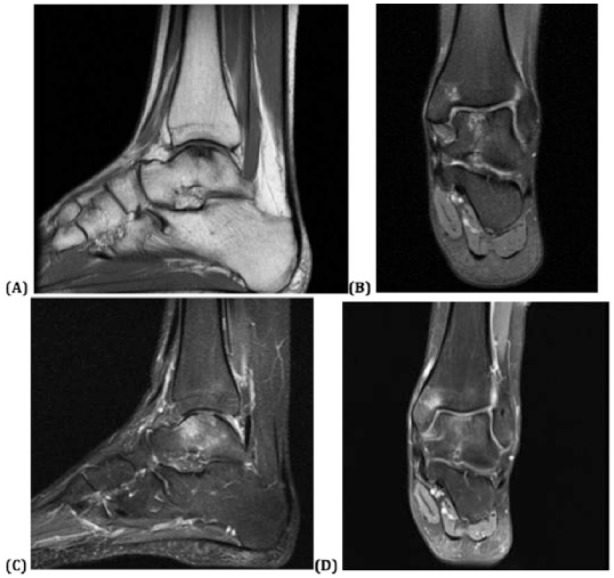
Preoperative magnetic resonance imaging (MRI): (a) T1-weighted sagittal and (b) T2-weighted coronal images demonstrating an initially large osteochondral defect within the central talar dome with subchondral cystic degenerative changes and edema, and 6-month postoperative T2-weighted MRI (c) sagittal and (d) coronal images demonstrating diffuse subchondral bone marrow edema and progressive appearance of chondral loss with early cyst formation.
Figure 4.
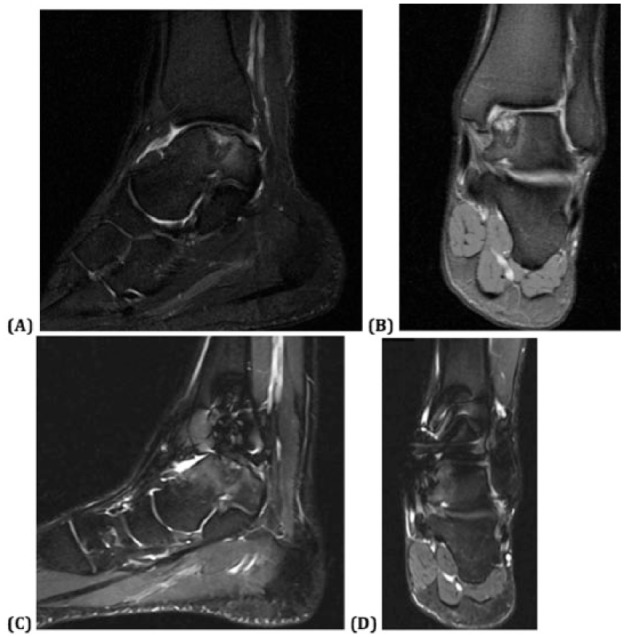
Preoperative T2-weighted magnetic resonance imaging (MRI): (a) sagittal and (b) coronal images demonstrating an initially large osteochondral defect within the posteromedial talar dome with subchondral cystic degenerative changes, and 2-year postoperative T2-weighted MRI (c) sagittal and (d) coronal images demonstrating persistent subchondral bone marrow edema and cystic changes with still loss at the chondral surface.
Discussion
Osteochondral lesions of the talus are a difficult pathology to address for orthopedic foot and ankle surgeons, particularly if the lesions are large and the pathology is recalcitrant to treatment with bone marrow stimulation techniques. As these patients are often young and active—having often sustained the osteochondral defect through a traumatic mechanism—joint preservation techniques continue to evolve, most recently with the use of juvenile cartilage transplantation. The purpose of this study was to attempt to better understand the utility of the fresh particulated juvenile cartilage allograft transplantation for osteochondral lesions of the talus through a systematic review of the literature and a short-term retrospective cohort analysis from patients at a single academic institution.
Within the systematic review, at a weighted mean follow-up of 14.3 months (range, 6.0-25.6 months) for the 32 patients, the revision rate was only 3.3% with a reoperation rate of 18.2%, consisting predominantly of removal of symptomatic or failed hardware used for surgical access (i.e., medial malleolar osteotomy) or concomitant procedure. In total, a high proportion of successful preliminary results were seen in a total of 83.9% of operative ankles. This level IV and V evidence suggests that the particulate juvenile cartilage transplantation for talar osteochondral lesions is an efficacious procedure with good clinical outcomes and low revision and reoperation rates at the short-term follow-up.
Within the retrospective cohort analysis, our preliminary results at the short-term mean 13.04 ± 8.35 months (range, 5.06-25.41 months) postoperative follow-up are promising. This is in light of the relatively large mean osteochondral lesion preoperative size, at 13.5 × 12.2 mm in dimension. There were no perioperative complications or reoperations, although revision procedures may not be likely to require consideration at such an early follow-up. However, patients are subjectively with good clinical outcomes at this time that are superior to their preoperative subjective levels of pain and ankle motion. Predictions on how this procedure will fare in these patients over the long term are suspect at best, but these early results are encouraging.
What is interesting is the postoperative appearance on MRI of the treated talar dome lesions in the three patients with postoperative advance imaging. Along the spectrum from 3 months to 2 years postoperative at the time of MRI being obtained, these patients’ imaging does not demonstrate a substantial improvement in the appearance and contour of the overall cartilage volume at the location of the prior particulated juvenile articular cartilage allograft therapy, with continued subchondral bone marrow edematous changes even as far as 2 years postoperative. This is in spite of the clinical improvements seen in each of these patients, leaving somewhat of a clinical disconnect in these findings, and perhaps providing us with an idea of the natural course on imaging for the particulated juvenile articular cartilage allograft technology which may take years to consolidate despite earlier clinical success.
While we are not able to definitively suggest the reason for these MRI findings, we would propose that it may be related to the performance of marrow stimulation techniques at the time of surgery, the large size of the lesions relative to the articular surface of the talus, or perhaps the fact that many of these patients had undergone prior surgical intervention before the index procedure. The imaging performed at 3 and 6 months postoperative may just be too early, then, to see improvement after allograft juvenile particular cartilage at the talus. Other cartilage restoration means such as autologous chondrocyte implantation of the talus have shown integration of the regenerated tissue similar to native cartilage in the majority of patients, but at longer follow-up times of 5 or 10 years postoperatively17,18 but with persistence of subchondral edema in some patients even 10 years after surgery.17
The imaging findings for the patient who underwent MRI at 2 years postoperative could also be somewhat of an outlier from what would otherwise be found as this patient had a large, cystic quality to his talar lesion prior to intervention; the low sample size makes it difficult, however, to provide firm conclusions. Alternatively, it may just be that the MRI findings should not be expected to show restoration of native anatomy after this procedure. For example, MRI findings of graft instability or failed incorporation after osteochondral allograft of the talus have been reported to not uniformly correlate with procedural failure.19 Similarly, Fraser et al.20 evaluated an athlete patient population who underwent osteochondral transplantation for talar lesions reported the presence of cystic changes in 33% of patients postoperatively, but found no correlation or effect of these findings on patient outcomes.
Our methodology provides insight into the potential of the particulated juvenile articular cartilage allograft technology for use with large, symptomatic osteochondral lesions of the talus, including those patients who have previously failed microfracture. Further data on the topic, including that performed in the setting of a randomized clinical trial, are necessary before any definitive conclusions about its efficacy can be confirmed. In addition, with the high cost of the technology and currently unavailable insurance coverage, particulated juvenile articular cartilage allograft despite its potential efficacy is as of yet still a likely cost-prohibitive surgical intervention for patients unable to pay for the procedure. As was mentioned by Coetzee et al.,6 their data were provided from subjects enrolled in a still ongoing prospective study seeking to enroll approximately 250 subjects with an anticipated 5-year follow-up: The prospectively collected data from this cohort of such a size will be launching point for further discussions of the effectiveness and future utility of the particulated juvenile articular cartilage allograft technology at the talus.
This study has several important limitations. Limitations of the systematic review include a scarcity of clinical studies, which limits the quantity of primary data available for analysis. As with any review, the quality of our conclusions is limited by the quality of the primary data—that is, of the 4 studies included, 2 were single case reports, and the third a case series of only 7 patients. Additionally, all included studies were retrospective in methodology. Heterogeneity in the surgical techniques used by the different authorship groups may additionally limit the summative conclusions of the grouped cohort. Limitations of the retrospective case series, first and foremost, are related to the small cohort size and retrospective methodology. Additionally, the presence of more objective clinical outcome findings reported in the postoperative period would more adequately allow for a more analytical assessment of this cohort’s postoperative outcomes. The fact that multiple patients had concurrent pathology addressed with concomitant procedures may additionally confound the postoperative results of the juvenile particulate cartilage allograft transplantation procedure.
Overall, preliminary data thus suggest that treatment of large, traumatic or atraumatic, symptomatic osteochondral defects of the talus with particulated juvenile cartilage transplantation may improve patient subjective complaints of pain and function. Systematic review of the available literature highlights the need for future prospective, larger cohort studies of particulated juvenile articular cartilage allograft use on the talus but suggests a similar potential for the technology.
Footnotes
Authors’ Note: The work for this report was performed at: Rush University Medical Center in Chicago, Illinois.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Unrelated to the work of this manuscript, B.M.S. received publishing royalties from Nova Science Publishers and Postgraduate Institute for Medicine; J.L. receives research support from Arthrex, Inc. and is on the editorial/governing board for Foot and Ankle International; S.L. receives publishing royalties from SLACK Incorporated, and is on the governing board for American Orthopaedic Foot and Ankle Society and Foot and Ankle International.
Ethical Approval: Ethical approval for this study was obtained from Rush University Medical Center Office of Research Affairs, Institutional Review Board. ORA#: 15040906-IRB01. Exemption from IRB Review.
Informed Consent: Informed consent was not sought for the present study because this exemption was granted in accordance with 45CFR46.101 (b) (4) - This research involves the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are either publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
Trial Registration: Not applicable.
References
- 1. Badekas T, Takvorian M, Souras N. Treatment principles for osteochondral lesions in foot and ankle. Int Orthop. 2013;37:1697-706. doi: 10.1007/s00264-013-2076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36:1750-62. doi: 10.1177/0363546508316773. [DOI] [PubMed] [Google Scholar]
- 3. McGahan PJ, Pinney SJ. Current concepts review: osteochondral lesions of the talus. Foot Ankle Int. 2010;31:90-101. doi: 10.3113/FAI.2010.0090. [DOI] [PubMed] [Google Scholar]
- 4. Adams SB, Jr, Demetracopoulos CA, Parekh SG, Easley ME, Robbins J. Arthroscopic particulated juvenile cartilage allograft transplantation for the treatment of osteochondral lesions of the talus. Arthrosc Tech. 2014;3:e533-7. doi: 10.1016/j.eats.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giza E, Delman C, Coetzee JC, Schon LC. Arthroscopic treatment of talus osteochondral lesions with particulated juvenile allograft cartilage. Foot Ankle Int. 2014;35:1087-94. doi: 10.1177/1071100714548704. [DOI] [PubMed] [Google Scholar]
- 6. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7:414-422. doi: 10.1177/1938640014543362. [DOI] [PubMed] [Google Scholar]
- 7. Giza E, Howell S. Allograft juvenile articular cartilage transplantation for treatment of talus osteochondral defects. Foot Ankle Spec. 2013;6:141-4. doi: 10.1177/1938640013479934. [DOI] [PubMed] [Google Scholar]
- 8. Hatic SO, 2nd, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3:361-4. doi: 10.1177/1938640010388602. [DOI] [PubMed] [Google Scholar]
- 9. Buckwalter JA, Bowman GN, Albright JP, Wolf BR, Bollier M. Clinical outcomes of patellar chondral lesions treated with juvenile particulated cartilage allograft. Iowa Orthop J. 2014;34:44-9. [PMC free article] [PubMed] [Google Scholar]
- 10. Tompkins M, Hamann JC, Diduch DR, Bonner KF, Hart JM, Gwathmey FW, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29:1661-70. doi: 10.1016/j.arthro.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 11. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42:1417-27. [DOI] [PubMed] [Google Scholar]
- 12. Farr J, Cole BJ, Sherman S, Karas V. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg. 2012;25:23-30. [DOI] [PubMed] [Google Scholar]
- 13. Cerrato R. Particulated juvenile articular cartilage allograft transplantation for osteochondral lesions of the talus. Foot Ankle Clin N Am. 2013;18:79-87. doi: 10.1016/j.fcl.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 14. Coetzee JC, Giza E, Schon LC, Berlet GC, Neufeld S, Stone RM, et al. Treatment of osteochondral lesions of the talus with particulated juvenile cartilage. Foot Ankle Int. 2013;34:1205-11. doi: 10.1177/1071100713485739. [DOI] [PubMed] [Google Scholar]
- 15. Bleazey S, Brigido SA. Reconstruction of complex osteochondral lesions of the talus with cylindrical sponge allograft and particulate juvenile cartilage graft: provisional results with a short-term follow-up. Foot Ankle Spec. 2012;5:300-5. [DOI] [PubMed] [Google Scholar]
- 16. Kruse DL, Ng A, Paden M, Stone PA. Arthroscopic De Novo NT® juvenile allograft cartilage implantation in the talus: a case presentation. J Foot Ankle Surg. 2012;51:218-21. doi: 10.1053/j.jfas.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 17. Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513-7. [DOI] [PubMed] [Google Scholar]
- 18. Battaglia M, Vannini F, Buda R, Cavallo M, Ruffilli A, Monti C, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19:1376-84. doi: 10.1007/s00167-011-1509-x. [DOI] [PubMed] [Google Scholar]
- 19. El-Rashidy H, Villas D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93:1634-40. doi: 10.2106/JBJS.J.00900. [DOI] [PubMed] [Google Scholar]
- 20. Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24:1272-9. doi: 10.1007/s00167-015-3606-8. [DOI] [PubMed] [Google Scholar]