Abstract
Household members of cholera patients are at a 100 times higher risk of cholera infections than the general population because of shared contaminated drinking water sources and secondary transmission through poor household hygiene practices. In this study, we investigated the bactericidal concentration of free chlorine required to inactivate Vibrio cholerae in household drinking water in Dhaka, Bangladesh. In laboratory experiments, we found that the concentrations of free chlorine required to inactivate 105 colony-forming units (CFU)/mL of V. cholerae serogroups O1 and O139 were 0.1 mg/L and 0.2 mg/L, respectively. The concentration of free chlorine generated by a single chlorine tablet (sodium dichloroisocyanurate [33 mg]) after a 30-minute reaction time in a 10-L sealed vessel containing Dhaka city municipal supply water was 1.8 mg/L; and the concentration declined to 0.26 mg/L after 24 hours. In field measurements, water collected from 165 households enrolled in a randomized controlled trial (RCT) of a chlorine and handwashing with soap intervention (Cholera-Hospital-Based-Intervention-for-7-Days[CHoBI7]), we observed significantly higher free chlorine concentrations in the 82 intervention arm households (mean = 1.12 mg/L, standard deviation [SD] = 0.52, range = 0.07–2.6 mg/L) compared with the 83 control households (0.017 mg/L, SD = 0.01, range = 0–0.06 mg/L) (P < 0.001) during spot check visits. These findings suggest that point-of-use chlorine tablets present an effective approach to inactivate V. cholerae from drinking water in households of cholera patients in Dhaka city. This result is consistent with the findings from the RCT of CHoBI7 which found that this intervention led to a significant reduction in symptomatic cholera infections among household members of cholera patients and no stored drinking water samples with detectable V. cholerae.
Background
Cholera is an acute dehydrating diarrhea, transmitted through contaminated drinking water, with an annual estimate of 3–5 million cases and 120,000 deaths worldwide1 In Bangladesh, cholera is endemic causing an estimated 250,000 cases annually.2 Vibrio cholerae, the bacteria that causes cholera, is frequently isolated from the stool of diarrhea patients presenting in hospitals in Bangladesh.3–5 Furthermore in Dhaka city, millions are estimated to reside in slum areas that lack access to improved water supplies.6 These individuals instead rely on a communal stand pipe which is often an illegal connection to the municipal water supply and highly susceptible to fecal contamination.7,8 Unsafe drinking water and poor sanitation and hygiene is estimated to contribute to over 840,000 deaths every year,9 with 90,000 of these deaths occurring in Bangladesh.10 Therefore, interventions are urgently needed to improve these environmental conditions.
Point-of-use (POU) chlorination of drinking water is a widely used low-cost method for disinfecting bacteriological contamination, and has been shown to reduce disease morbidity from waterborne diseases in low-income countries.11–14 In addition, chlorine has the advantage of reducing the risk of recontamination of drinking water during storage in the home, through the chlorine residual which remains over time.15 There is an extensive literature demonstrating the efficacy of POU chlorination of household drinking water in reducing the incidence of pediatric diarrheal diseases.14,16 However, previous studies have also found that the effectiveness of chlorine in reducing microbial contamination can vary based on source-water turbidity, source-water baseline contamination levels, and in-home contamination.16,17 In rural Ecuador, only 17% of households with high source-water turbidity (> 10 NTU) were able to achieve the World Health Organization guideline for safe drinking water after chlorine treatment with a sodium hypochlorite (NaOCl) solution of 1.875 mg/L.17 In alignment with this finding, a study which analyzed water samples collected from 13 African countries recommended a dosage of 1.875 mg/L NaOCl for water sources with an NTU less than 10, and 3.75 mg/L for water sources between 10 and 100 NTU to meet a free available chlorine concentration greater than 0.2 mg/L after 24 hours of storage.16 Therefore, the chlorine concentration needed to deactivate pathogens in drinking water varies by setting and is based on source-water quality parameters and household drinking water storage and use practices.
The objective of this study is to determine the free chlorine concentration required to inactivate pandemic serogroups of V. cholerae in municipal water used in Dhaka city and to determine whether this chlorine concentration could be achieved in a field setting to inactivate V. cholerae.
Methods
Laboratory experiments to determine the bactericidal concentrations of free chlorine to inactivate V. cholerae O1 and O139.
Preparation of bacterial stock culture.
Pure colonies of V. cholerae O1 and O139 from gelatin agar plates were separately inoculated in 10 mL Luria-Bertani broth and then incubated at 37°C in a horizontal shaker at 120 rpm for 3 hours. After centrifuging 1 mL culture broth at 5,000 rpm for 5 minutes, pelleted bacterial cells were resuspended in 1 mL phosphate-buffered saline to remove the media. Viable bacterial load in the stock was then cultured on agar plate and using the following formula: stock bacterial load colony-forming units (CFU/mL) = (no. of colonies × dilution factor)/volume of water plated. The stock broth of both V. cholerae O1 and O139 had 107 CFU/mL bacterial cells.
Preparation of different concentrations of chlorine solution.
Previous studies have shown that high turbidity can affect free available chlorine in drinking.17 Therefore, to ensure that our experiments were representative of the drinking water source used by the majority of households in Dhaka city, we prepared chlorine stock solutions using Dhaka Water Supply and Sewerage Authority (DWASA) water. The DWASA water used for laboratory experiments had a turbidity of < 1 NTU, a pH of 7.2, and free available chlorine concentration below the detection limit (< 0.01 mg/L). To prepare the chlorine solutions, 5 L of autoclaved DWASA water were collected in a 10-L sealed water vessel and one chlorine tablet (Aquatabs sodium dichloroisocyanurate [NaDCC, 33 mg]; Medentech, Wexford, Ireland] was added to the water. After dissolving the tablet for 10 minutes, 500 mL chlorinated water was transferred to a conical flask containing 500 mL autoclaved DWASA water. Then, 500 mL water from this conical flask was mixed with next dilution flask containing 500 mL autoclaved DWASA water. Following this method, 2-fold dilutions were performed to produce the following free chlorine concentrations for the V. cholerae O1 inactivation experiments: 2.0, 0.95, 0.47, 0.24, 0.1, 0.05, 0.025, 0.12, 0.006, and 0.003 mg/L. The same procedure was also followed to produce the following free chlorine concentrations for the V. cholerae O139 serogroup inactivation experiments: 1.7, 0.8, 0.4, 0.2, 0.09, 0.045, 0.022, 0.11, 0.005, and 0.002 mg/L. A final flask containing only autoclaved tap water was used as a control. Water in containers 11 and 22 were free of chlorine and used as control (Table 1) and all 22 containers were holding 500 mL water. The free chlorine concentrations of stock solutions were measured using a HACH Pocket Colorimeter™ II (cat. no. 59530-00, CO) which has a detectable range of 0.02 to 5.0 mg/L. The instructions provided in the manufacturer's manual were followed and concentrations below the detection limit were calculated as half of the previous dilution.
Table 1.
Determination of the bactericidal concentration of free chlorine for 105 CFU/mL Vibrio cholerae in drinking water
| Container number | Serogroup | Water volume (mL) | Cl con (mg/L) | Bacterial count, log value (CFU/mL) | Revival after APW enrichment | ||
|---|---|---|---|---|---|---|---|
| Free | Total | Before treatment | After treatment | ||||
| 1 | O1 | 500 | 2 | 2.2 | 105 | 0 | − |
| 2 | O1 | 500 | 0.95 | 1.02 | 105 | 0 | − |
| 3 | O1 | 500 | 0.47 | 0.49 | 105 | 0 | − |
| 4 | O1 | 500 | 0.24 | 0.25 | 105 | 0 | − |
| 5 | O1 | 500 | 0.1 | 0.11 | 105 | 0 | − |
| 6 | O1 | 500 | 0.05 | 0.06 | 105 | 0 | + |
| 7 | O1 | 500 | 0.025 | 0.026 | 105 | 104 | + |
| 8 | O1 | 500 | 0.012 | 0.013 | 105 | 105 | + |
| 9 | O1 | 500 | 0.006 | 0.007 | 105 | 105 | + |
| 10 | O1 | 500 | 0.003 | 0.004 | 105 | 105 | + |
| 11 (control) | O1 | 500 | 0 | 0 | 105 | 105 | + |
| 12 | O139 | 500 | 1.7 | 1.9 | 105 | 0 | − |
| 13 | O139 | 500 | 0.8 | 0.9 | 105 | 0 | − |
| 14 | O139 | 500 | 0.4 | 0.41 | 105 | 0 | − |
| 15 | O139 | 500 | 0.2 | 0.27 | 105 | 0 | − |
| 16 | O139 | 500 | 0.09 | 0.11 | 105 | 0 | + |
| 17 | O139 | 500 | 0.045 | 0.055 | 105 | 104 | + |
| 18 | O139 | 500 | 0.022 | 0.027 | 105 | 105 | + |
| 19 | O139 | 500 | 0.011 | 0.013 | 105 | 105 | + |
| 20 | O139 | 500 | 0.005 | 0.006 | 105 | 105 | + |
| 21 | O139 | 500 | 0.002 | 0.003 | 105 | 105 | + |
| 22 (control) | O139 | 500 | 0 | 0 | 105 | 105 | + |
APW = alkaline peptone water; Cl = chlorine; con = concentration; CFU = colony-forming units. All experiments were repeated thrice; culture positive (+), culture negative (−).
Experiments to inactivate 105 CFU/mL of V. cholerae O1 and O139 cells using 10 chlorine stock solutions.
Previous studies have found environmental water that contains 101–104 CFU/mL V. cholerae cells.18 Therefore, we decided to evaluate the efficacy of chlorine in deactivating V. cholerae one log higher at 105 CFU/mL. Each flask containing 500 mL water with different chlorine concentration was inoculated with 1 mL bacterial stock (107 CFU), which generated a 105 CFU/mL concentration of V. cholerae O1 (containers 1–11) or O139 (containers 12–22) in each flask. After the 30-minutes reaction time recommended by the chlorine tablet manufacturer, 1 mL water from each flask was serially diluted, and 100 μL water from each dilution was spread on Luria agar (LA) plate. The LA plate was incubated at 37°C overnight. The next day, available bacterial load after 30 minutes of treatment was calculated using the formula: stock bacterial load (CFU/mL) = (no. of colonies × dilution factor)/volume of water plated. In addition to directly plating 100 mL water from each flask, water from each flask was also filtered through a 0.22-μm filter paper and the filtrates were enriched in alkaline peptone water (APW, 20 mL) overnight at 37°C. The next day, enriched broths were cultured on LA agar plates for detection of viable bacteria. All experiments were repeated thrice.
Laboratory experiments to determine the free chlorine concentrations over a 24-hour period after the use of a chlorine tablet in DWASA water.
We selected three drinking water vessels: a 16-L locally made plastic bucket with lid, a 10-L aluminum kalshi with no lid (both commonly used in Bangladesh), and a 10-L Topaz™ (Lion Star Plastic, Jakarta, Indonesia) drinking vessel, used in our Cholera-Hospital-Based Intervention for 7 days (CHoBI7) intervention study.19 Topaz is a container made of high-quality plastic that can be sealed by a lid that screws on, and has a tap at the bottom to dispense water. This type of container reduces the chances of secondary contamination by the user and prevents evaporation of chlorine. In contrast, the plastic bucket with a loosely attached lid and the kalshi with no lid allow evaporation of chlorine and recontamination from hands and objects going inside the water vessel during water collection. The manufacturer of the chlorine tablet we used for our experiments recommends the use of one tablet for 3 L water. However, recent studies found that one of these chlorine tablets (NaDCC [33 mg]) can produce a 2 mg/L dose of free chlorine residual in 10 L water.20 Therefore, we decided to use this chlorination ratio in our experiment; each container was filled with 10 L DWASA water and treated with one chlorine tablet. Then, free and total chlorine concentrations were measured before treatment and at seven time points (30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, and 24 hours) after chlorine treatment using the HACH Pocket Colorimeter™ II. The experiment was repeated three times for each container type.
Field trial to determine the efficacy of chlorine tablets for the inactivation of V. cholerae.
Field activities.
This study was conducted as a part of a randomized controlled trial (RCT) of a handwashing with soap and water treatment intervention (CHoBI7) given to cholera patients and their household contacts in Dhaka, Bangladesh, from June 2013 to November 2014. The detailed methods for this intervention and trial are published elsewhere.19 The intervention arm received chlorine tablets (NaDCC, 33 mg) for water treatment, a Topaz container (sealed drinking water vessel with tap) for safe water storage, and a handwashing station. The control arm received no intervention hardware. Intervention households were instructed to add one chlorine tablet to 10 L water, and to store this up to 24 hours. Environmental surveillance was conducted in these households at days 1, 3, 5, 7, and 9 after the presentation of the index cholera patient at Dhaka icddr,b Hospital. Household source and stored drinking water was collected from a total of 165 cholera patient households (83 control households and 82 intervention households) at each of these household visits.
All water samples were collected in autoclaved Nalgene bottles (Nalgene Nunc International, St. Louis, MO). The free chlorine concentrations in stored drinking water were measured immediately after collection using a HACH Pocket Colorimeter™ II. Then, the water samples were sent to the laboratory for detection of V. cholerae by bacterial culture. The detailed methods are described elsewhere.21 The findings for V. cholerae in source and stored drinking water for this field trial have been reported previously.19 A two-sample t test was used to compare free chlorine concentrations between the intervention and control arm at baseline and during the intervention period.
Ethics approval and consent.
Informed consent was obtained from all study participants. All study procedures were approved by the research Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) and Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health.
Results
Determination of bactericidal concentration of free chlorine for V. cholerae O1 and O139.
We found that after the 30-minute recommended chlorine tablet reaction time, flasks spiked with free chlorine concentrations ranging from 0.05 mg/L to 2 mg/L and 105 CFU/mL of V. cholerae O1 showed no V. cholerae growth (containers 1–6) (Table 1). For flasks spiked with 105 CFU/mL of V. cholerae O139, those with a free available chlorine concentration ranging from 0.09 mg/L to 1.9 mg/L showed no bacterial growth after the 30-minute reaction time (containers 12–16). However, after overnight enrichment in APW, viable V. cholerae cells were detected in water at chlorine concentrations of 0.05 mg/L for V. cholerae O1 and 0.09 mg/L for V. cholerae O139 (containers 6 and 16). Therefore, the bactericidal concentrations of free chlorine for V. cholerae O1 and O139 were 0.1 mg/L and 0.2 mg/L, respectively.
Free chlorine concentration over time after treatment with chlorine tablets.
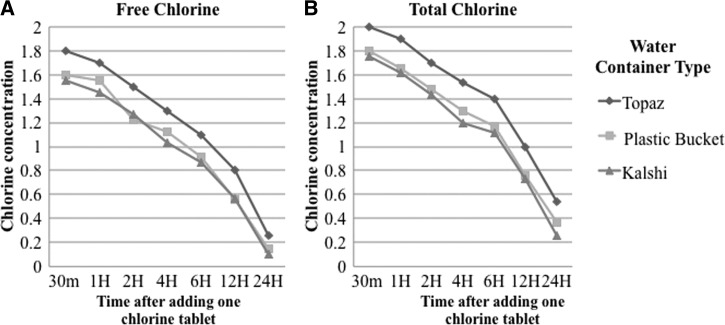
For the experiments to track free chlorine over time, the DWASA water used had an average turbidity of 2.1 NTU, pH.7.7, and a free chlorine concentration of 0.06 mg/L. In the Topaz container, the concentration of free chlorine 30 minutes after adding a single chlorine tablet (NaDCC, 33 mg) was 1.8 mg/L and gradually decreased to 0.26 mg/L after 24 hours (Figure 1 ). The total chlorine was slightly higher than the free chlorine concentration in water, ranging from 2.0 after 30 minutes to 0.54 mg/L after 24 hours. When a single chlorine tablet was added to a 10-L plastic bucket, the free chlorine ranged from 1.6 (after 30 minutes) to 0.145 mg/L (at 24 hours) and total chlorine ranged from 1.8 to 0.37 mg/L over this period. For the aluminum kalshi, the free chlorine and total chlorine concentration was 1.55 mg/L and 1.75 mg/L, respectively, at 30 minutes, and declined to 0.1 mg/L and 0.255 mg/L, respectively, after 24 hours.
Figure 1.
(A) Change of free chlorine concentration and (B) change of total chlorine concentration over time. Three containers, Topaz (10 L), plastic bucket (16 L), and aluminum kalshi (12 L) were filled with 10 L of Dhaka Water Supply and Sewerage Authority water using a measuring cylinder and treated with one chlorine tablet (sodium dichloroisocyanurate [33 mg]). Free and total chlorine concentrations were measured with HACH device at different time points (30 minutes [30m], 1 hour [1H], 2 hours [2H], 4 hours [4H], 6 hours [6H], 12 hours [12H], and 24 hours [24H]).
Field trial to determine efficacy of chlorination treatment.
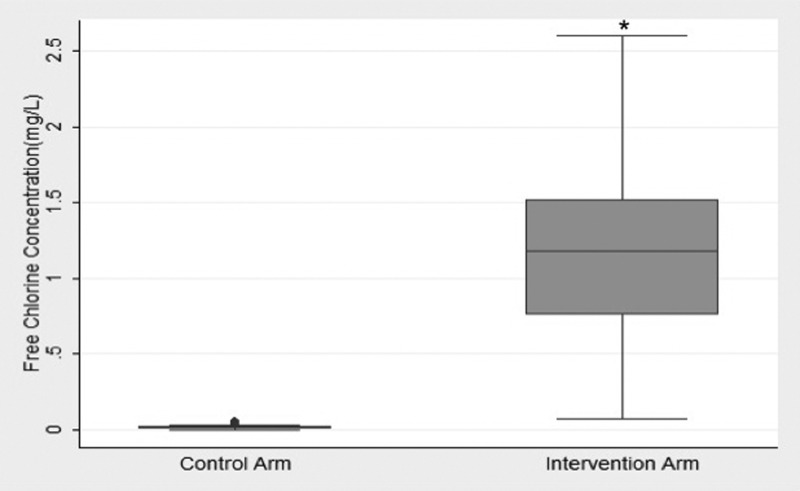
A total of 808 stored water samples (407 control samples and 401 intervention samples) were collected over the study period during the RCT. At the baseline visit before the intervention was delivered, the control and intervention arm had a mean free chlorine concentration for stored drinking water of 0.0175 mg/L and 0.0171 mg/L, respectively (P = 0.81). During the intervention period (surveillance days 3–9), free chlorine in stored drinking water was significantly higher in the intervention arm (1.12 mg/L, standard deviation (SD) = 0.52) compared with the control arm (0.0176 mg/L, SD = 0.01; P < 0.001) (Figure 2 ).
Figure 2.
The baseline (Day 1) mean free chlorine concentration was 0.175 mg/L and 0.171 mg/L in control and intervention household stored water, respectively (P = 0.81). During the intervention period, the free chlorine concentration was significantly higher (P = 0.001) in the intervention arm (1.12 mg/L) compared with the control (0.0176 mg/L). * P = 0.001.
Discussion
Despite cholera being endemic in Bangladesh, this is the first study, to our knowledge, to investigate the free chlorine concentrations required to inactivate V. cholerae in DWASA water. We found that 0.1 mg/L of free available chlorine was needed to inactivate V. cholerae O1. The Topaz water storage container was able to achieve a free chlorine concentration of 0.2 mg/L after 24 hours, the Center for Disease Control recommended cutoff, with a single chlorine tablet (33 mg/L). Furthermore, intervention households given chlorine tablets as part of the CHoBI7 intervention had significantly higher free chlorine in the stored drinking water. These findings suggest that POU chlorine tablets present an effective approach to inactivate V. cholerae from Dhaka city municipal drinking water.
Free available chlorine in household stored drinking water in the control arm was very low (mean = 0.0175 mg/L), and was below the threshold found to inactivate V. cholerae. This is alarming given that 30% of cholera patient households had source-water samples with detectable V. cholerae during the surveillance period.19 The majority of study households resided in slum areas of Dhaka city, Bangladesh, and obtained drinking water from illegal connections to the municipal water supply that are susceptible to fecal contamination due to low pressure and breaks in the pipes.22,23 These findings demonstrate the urgent need for POU water treatment methods for these households to prevent transmission of cholera which causes significant morbidity and mortality each year. Furthermore, it is imperative to consider more comprehensive measures to stop illegal water connections, improve the municipal water supply infrastructure, adequately chlorinate the municipal water supply, and to conduct regular water quality monitoring of the distribution network.
In our recent RCT of the CHoBI7 intervention, 94% of intervention households had free chlorine concentrations above the cutoff recommended by the Centers for Disease Control and Prevention of 0.2 mg/L, compared with less than 1% of control households.19 Consistent with this higher free chlorine concentration in intervention households, no stored drinking water samples had detectable V. cholerae in the intervention arm compared with 6% in the control arm during the intervention period. These findings demonstrate the effectiveness of POU chlorine tablets in inactivating V. cholerae in drinking water. Furthermore, we observed a significant reduction in symptomatic cholera infections and a 47% reduction in overall cholera infections.19 Although it is not possible to quantify the health impact of the chlorine tablets alone because this intervention included handwashing with soap, this finding in combination with no V. cholerae being found in stored water in the intervention arms suggests that POU chlorine can provide a promising approach for cholera control for high-risk cholera patient households in Dhaka city.
We determined the number of chlorine tablets to use in our CHoBI7 RCT based the laboratory findings from this study. After 30 minutes of chlorine treatment, water from all three storage containers were found to have free chlorine concentrations between 1.5 and 2 mg/L. Water in the Topaz had slightly higher free chlorine concentration (1.8 mg/L) compared with the plastic bucket (1.6 mg/L) and the aluminum kalshi (1.55 mg/L) at this time point. The lower free chlorine concentration at 24 hours in the plastic bucket and the aluminum kalshi compared with the Topaz was likely attributed to these containers not having a lid with a tight seal leading to evaporation of chlorine. There is also the possibility that the aluminum absorbed some of the chlorine. All three containers retained a free chlorine concentration high enough to deactivate V. cholerae O1 at 24 hours; however, the Topaz performed the best among the three. Previous studies where the kalshi was used for drinking water storage found detectable thermotolerant coliforms by culture even in the presence of higher levels of free chlorine.7 This could be due to high turbidity or contamination level of the source water. The high performance of the Topaz vessel in maintaining a high free chlorine concentration over time suggests it would be a promising water storage vessel to be used for POU chlorination of drinking water in Dhaka city.
The bactericidal concentration of free chlorine was higher for V. cholerae serogroup O139 (0.2 mg/L) compared to O1 (0.1 mg/L). One potential explanation is that the V. cholerae serogroup O139 strains may have gained higher resistance to chlorine because of the presence of extracellular capsular polysaccharide.24 Future studies should investigate chlorine resistance among V. cholerae strains.
This study has limitations. The first is that we did not assess the presence of other enteric pathogens in the drinking water, as the focus of the CHoBI7 RCT was to reduce cholera infection among the household contacts of cholera patients. Second, for bactericidal concentration determination, we only collected DWASA water from one location in Dhaka. Future studies should collect DWASA water from multiple locations in Dhaka city with varying water quality parameters.
Conclusions
Recurrent cholera causes significant morbidity and mortality in Dhaka city, which is a rapidly growing megacity estimated to have a population of over 20 million. Due to paucity of water, poor infrastructure, and illegal connection, ensuring safe drinking water for such a large population is very challenging. In the present study, we provide data showing evidence on the bactericidal concentration of free chlorine needed to inactivate toxigenic V. cholerae in drinking water in Dhaka city. Our study findings can serve as a guideline for future studies to standardize experiments to assess the efficacy of POU water treatment options using chlorine in other urban settings in Bangladesh or other cities globally.
ACKNOWLEDGMENTS
We thank the study participants and the following research assistants who conducted the fieldwork for this study: Ismat Minhaz Uddin, Rafiqul Islam, Al-Mamun, Maynul Hasan, Kalpona Akhter, Khandokar Fazilatunnessa, Sadia Afrin Ananya, Akhi Sultana, Sohag Sarker, Jahed Masud, Abul Sikder, Shirin Akter, and Laki Das. We also thank Abdus Sadique and Md. Golam Mostafa for their continuous support in the laboratory.
Footnotes
Financial support: This research was supported by the Center for Global Health at Johns Hopkins University and the National Institute of Allergy and Infectious Diseases, National Institutes of Health. icddr,b thanks the governments of Australia, Bangladesh, Canada, Sweden, and United Kingdom for providing core/unrestricted support.
Authors' addresses: Mahamud-ur Rashid, Shirajum Monira, Md. Toslim Mahmud, Zillur Rahman, Munshi Mustafiz, K. M. Saif-Ur-Rahman, Tahmina Parvin, Sazzadul Islam Bhuyian, Fatema Zohura, Farzana Begum, Shwapon Kumar Biswas, Shamima Akhter, and Munirul Alam, International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh, E-mails: mahamudur@icddrb.org, smonira@icddrb.org, palashmahmud@ymail.com, zillur.rahman@icddrb.org, rimonstate@gmail.com, su.rahman@icddrb.org, tparvin@icddrb.org, sazzadul.islam@icddrb.org, fzohura@icddrb.org, farzanab@icddrb.org, drskbiswas2004@yahoo.com, shamima.akhter@icddrb.org, and munirul@icddrb.org. Christine Marie George, Xiaotong Zhang, David Sack, and R. Bradley Sack, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mails: cmgeorge@jhsph.edu, xzhang75@jhmi.edu, dsack1@jhu.edu, and rsack1@jhu.edu.
References
- 1.World Health Organization Diarrhoeal Disease. 2013. http://www.who.int/mediacentre/factsheets/fs330/en/ Available at. Accessed May 4, 2016.
- 2.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, Harris JB, Ryan ET, Cravioto A, Calderwood SB, Qadri F. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg. 2008;79:708–714. [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack DA, Malek MA, Faruque AS, Qadri F, Calderwood SB, Luby SP, Ryan ET. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg. 2006;74:1067–1073. [PMC free article] [PubMed] [Google Scholar]
- 5.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United Nations International Children's Emergency Fund Urban Water Challenges in Bangladesh. 2011. http://www.unicef.org/bangladesh/Urban_water_challenges_in_Bangladesh.pdf Available at. Accessed May 4, 2014.
- 7.Clasen T, Saeed TF, Boisson S, Edmondson P, Shipin O. Household water treatment using sodium dichloroisocyanurate (NaDCC) tablets: a randomized, controlled trial to assess microbiological effectiveness in Bangladesh. Am J Trop Med Hyg. 2007;76:187–192. [PubMed] [Google Scholar]
- 8.Sirajul Islam M, Brooks A, Kabir MS, Jahid IK, Shafiqul Islam M, Goswami D, Nair GB, Larson C, Yukiko W, Luby S. Faecal contamination of drinking water sources of Dhaka city during the 2004 flood in Bangladesh and use of disinfectants for water treatment. J Appl Microbiol. 2007;103:80–87. doi: 10.1111/j.1365-2672.2006.03234.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Mortality and Burden of Disease from Water and Sanitation. 2012. http://www.who.int/gho/phe/water_sanitation/burden/en/ Available at. Accessed May 4, 2014.
- 10.World Health Organization Inadequate Water, Sanitation and Hygiene in Low- and Middle-Income Countries. 2012. http://apps.who.int/gho/data/view.main.INADEQUATEWSHv?lang=en Available at. Accessed May 4, 2014.
- 11.World Health Organization Progress on Drinking Water and Sanitation: Special Focus on Sanitation. 2008. http://www.who.int/water_sanitation_health/monitoring/jmp2008/en/ Available at. Accessed May 4, 2014.
- 12.Arnold BF, Colford JM. Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg. 2007;76:354–364. [PubMed] [Google Scholar]
- 13.Clasen T, Edmondson P. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at the household level. Int J Hyg Environ Health. 2006;209:173–181. doi: 10.1016/j.ijheh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ. 2007;334:782. doi: 10.1136/bmj.39118.489931.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright J, Gundry S, Conroy R. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health. 2004;9:106–117. doi: 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 16.Lantagne DS. Sodium hypochlorite dosage for household and emergency water treatment. J Am Water Works Ass. 2008;100:106–119. doi: 10.2166/wh.2017.012. [DOI] [PubMed] [Google Scholar]
- 17.Levy K, Anderson L, Robb KA, Cevallos W, Trueba G, Eisenberg JN. Household effectiveness vs. laboratory efficacy of point-of-use chlorination. Water Res. 2014;54:69–77. doi: 10.1016/j.watres.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer S, Sommer R, Farnleitner AH, Kirschner AK. Rapid and sensitive quantification of Vibrio cholerae and Vibrio mimicus cells in water samples by use of catalyzed reporter deposition fluorescence in situ hybridization combined with solid-phase cytometry. Appl Environ Microbiol. 2012;78:7369–7375. doi: 10.1128/AEM.02190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George CM, Monira S, Sack DA, Rashid MU, Saif-Ur-Rahman KM, Mahmud T, Rahman Z, Mustafiz M, Bhuyian SI, Winch PJ, Leontsini E. Randomized controlled trial of hospital-based hygiene and water treatment intervention (CHoBI7) to reduce cholera. Emerg Infect Dis. 2016;22:233. doi: 10.3201/eid2202.151175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ercumen A, Naser AM, Unicomb L, Arnold BF, Colford JM, Jr, Luby SP. Effects of source-versus household contamination of tubewell water on child diarrhea in rural Bangladesh: a randomized controlled trial. PLoS One. 2015;10:e0121907. doi: 10.1371/journal.pone.0121907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huq A, Haley BJ, Taviani E, Chen A, Hasan NA, Colwell RR. Detection, isolation, and identification of Vibrio cholerae from the environment. Curr Protoc Microbiol. 2012;6:6A.5. doi: 10.1002/9780471729259.mc06a05s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque R, Mondal D, Kirkpatrick BD, Akther S, Farr BM, Sack RB, Petri WA., Jr Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am J Trop Med Hyg. 2003;69:398–405. [PubMed] [Google Scholar]
- 23.Chowdhury F, Rahman MA, Begum YA, Khan AI, Faruque AS, Saha NC, Baby NI, Malek MA, Kumar AR, Svennerholm AM, Pietroni M. Impact of rapid urbanization on the rates of infection by Vibrio cholerae O1 and enterotoxigenic Escherichia coli in Dhaka, Bangladesh. PLoS Negl Trop Dis. 2011;5:e999. doi: 10.1371/journal.pntd.0000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faruque SM, Sack DA, Sack RB, Colwell RR, Takeda Y, Nair GB. Emergence and evolution of Vibrio cholerae O139. Proc Natl Acad Sci USA. 2003;100:1304–1309. doi: 10.1073/pnas.0337468100. [DOI] [PMC free article] [PubMed] [Google Scholar]