Abstract
Acute liver failure is an atypical manifestation of dengue with a high mortality. We performed a retrospective cohort study at the Hospital for Tropical Diseases, Bangkok, Thailand. In total, 1,926 patients with serologically confirmed dengue were enrolled in the study from 2011 to 2015. Of these, six patients presented with acute liver failure, four died, and two survived. The incidence of dengue-associated acute liver failure was 0.31%. Dengue-associated acute liver failure was most common among young adults (median age, 29 years). The median duration from onset of fever to development of acute liver failure was 7.5 days. Patients with the severe stage of dengue had a higher risk of developing acute liver failure (P < 0.001). The baseline risk factors associated with the development of acute liver failure were an age of ≤ 40 years (odds ratio [OR] = 1.5, 95% confidence interval [CI] = 1.1–2.0, P < 0.05), a > 10% ratio of atypical lymphocytes (OR = 2.3, 95% CI = 1.8–3.0, P < 0.001), and a platelet count of < 50,000 mm3 (OR = 2.8, 95% CI = 2.2–3.6, P < 0.001). The incidence of acute liver failure in patients with dengue was quite low, but its impact on morbidity, mortality, and poor clinical outcomes was significant. In summary, this study indicates that various baseline risk factors are associated with acute liver failure in patients with dengue.
Introduction
Dengue is an emerging disease and increasing health threat in tropical countries.1 The World Health Organization (WHO) estimates that more than 50 million cases of dengue and 20,000 dengue-related deaths occur annually worldwide. In 2010, approximately 96 million of the 390 million people infected with dengue virus were symptomatic.2 Dengue is classified into three groups according to the WHO 2009 classification: dengue without warning signs, dengue with warning signs, and severe dengue.3 Patients with dengue may develop dysfunction of multiple organs including the heart, muscle, kidney, liver, and brain. Acute liver failure secondary to dengue is rare but has a high mortality rate.4
Acute liver failure is defined as the rapid onset of acute encephalopathy and coagulopathy in a patient without preexisting cirrhosis and with < 26-week duration of illness.5,6 Severe hepatic involvement (acute liver failure) is a rare condition in patients with dengue, but commonly occurs during the second week of illness.3,7 Clinical and laboratory findings include a persistent fever, the appearance of jaundice, hepatomegaly, markedly elevated serum transaminase levels, persistent thrombocytopenia, an increased serum ammonia level, an increased prothrombin time (PT) of > 15 seconds, and an international normalized ratio (INR) of ≥ 1.5.8,9
The pathogenesis of acute liver failure in patients with dengue is controversial, but is thought to be caused by a direct cytopathic effect on liver cells or a dysregulated immune response to the virus.10 Hepatocytes and Kupffer cells are the main targets of the dengue virus. Cellular apoptosis of infected hepatocytes occurs both in vitro and in vivo via apoptosis-inducing ligand pathways. The histological changes associated with dengue occur in the liver parenchyma and are classically characterized by midzonal (zone 2) necrosis of the liver. Councilman bodies, which represent necrosis around viral particles, are also frequently observed in liver biopsy specimens.9
Severe hepatitis is considered to indicate a poor prognosis in patients with dengue, although most patients' illness resolves without treatment. Nonetheless, patients with severe hepatitis can be considered at risk of developing acute liver failure.7,11 In patients with acute liver failure, the PT and activated partial thromboplastin time are prognostic parameters used to assess the severity of liver injury and serve as markers of bleeding.12 Complications of acute liver failure include severe coagulopathy-like disseminated intravascular coagulation (DIC), infection (severe sepsis), renal impairment, increased intracranial pressure leading to cerebral edema, and cardiopulmonary collapse leading to multiple organ failure.13,14
Acute liver failure associated with dengue was first reported during epidemics of dengue in Indonesia in the 1970s; thereafter, it was reported during epidemics in Thailand in 1987 and in Malaysia in 1990.15 Few studies have reported the development of acute liver failure in adult patients with dengue. In addition, few studies have determined the incidence of acute liver failure in adults with dengue or risk factors associated with acute liver failure secondary to dengue.
In a large prospective study in Vietnam, the incidence of acute liver failure in 644 patients with dengue was 0.77% during the 2-year study period.7 Moreover, the study revealed that underlying chronic hepatitis B or C infection can lead to more severe liver dysfunction in patients with dengue,7 although the results of some other studies are contradictory to this finding. Two small studies reported that concomitant hepatitis B or C infection had no effect on the severity of dengue.16,17
The present study was conducted to assess the incidence and clinical outcome of acute liver failure in patients with dengue and to identify parameters associated with acute liver failure secondary to dengue in the Hospital for Tropical Diseases in Bangkok, Thailand.
Materials and Methods
We performed a retrospective cohort study using the medical records of patients with serologically confirmed dengue who were admitted to the Hospital for Tropical Diseases in Bangkok, Thailand, from 2011 to 2015. An immunochromatographic rapid diagnostic test for dengue NS1 antigen and anti-dengue IgM (SD BIOLINE Dengue Duo; Standard Diagnostics, Kyonggi-do, Korea) was used to confirm the diagnosis of dengue. Patient information was deidentified and kept anonymous before data analysis.
We included all male and female patients ≥ 15 years of age who were admitted to the hospital inpatient unit with a diagnosis of dengue. We excluded patients who were concomitantly diagnosed with other causes of liver injury, such as chronic hepatitis B and C, acute hepatitis A and E, other tropical diseases such as malaria and leptospirosis, mushroom poisoning, drug-induced liver failure, pregnancy-induced liver failure, autoimmune hepatitis, or malignant infiltration of the liver.
Evaluation of elevated liver enzymes and severe liver involvement.
Hepatic involvement was classified as follows: Grade A—normal transaminase levels; Grade B—elevation of at least one transaminase level (mild hepatitis); Grade C—elevation of at least one transaminase level to > 3 times the reference value (moderate hepatitis); and Grade D—acute hepatitis with elevation of at least one transaminase level to ≥ 10 times the normal value (severe hepatitis).18
Acute liver failure was defined as evidence of a coagulation abnormality, an INR of ≥ 1.5 in most cases, any degree of mental alteration (encephalopathy) in a patient without preexisting cirrhosis, and an illness duration of < 26 weeks.6,19
Impending liver failure was defined as liver dysfunction that did not meet the criteria for acute liver failure including nausea, vomiting, and laboratory investigations that showed increased serum bilirubin and/or liver enzymes, an increased PT and INR, and thrombocytopenia.18,20
Data collection.
The records of all patients were reviewed from the date of admission to the date of discharge. Each patient record was identified by the patient's hospital registration number. All diagnoses were recorded according to the International Classification of Diseases. After screening the patients against the inclusion and exclusion criteria, we further excluded all patients without evidence of serologically confirmed dengue (i.e., those without positive dengue NS1 antigen or positive dengue IgM antibody). We classified patients with dengue according to the severity of liver involvement.
Statistical analysis.
Raw data were obtained from the case record forms. Data entry was then carried out using Microsoft Excel (Microsoft Corporation, Redmond, WA) and subsequently imported into and analyzed by SPSS version 18 (SPSS Inc., Chicago, IL). All data entry steps and analyses were checked for errors. Descriptive statistics are presented as incidence, mean with standard deviation, median with range, and 95% confidence interval (CI) with 25th–75th percentile or percentage. Categorical variables were tested for associations using either the χ2 test or Fisher's exact test. Student's t test, analysis of variance, or the Mann–Whitney test was used to compare differences between groups. Statistical testing was performed using two-tailed tests. A P value of < 0.05 was considered statistically significant.
All independent variables with or without transformed data were tested for a normal distribution. Qualitative variables such as sex and abdominal pain were recoded before performing the correlation analysis and multiple regression analysis. Multivariate analysis was performed among all variables in patients with dengue with moderate to severe liver involvement and analyzed by multiple regression.
Ethics approval.
This study was conducted with approval from the Hospital for Tropical Diseases, Bangkok, Thailand. The protocol was approved by the International Review Board of the Faculty of Tropical Medicine, Mahidol University, Thailand (MUTM 2015-061-01).
Results
Patient characteristics.
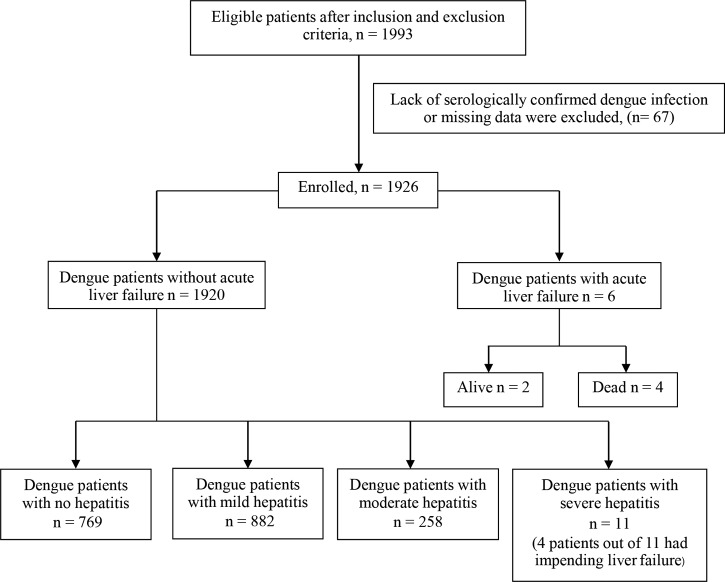
All dengue-positive patients who were admitted to the inpatient unit from 2011 to 2015 were screened. Of these, 1,993 patients were eligible for the study. Sixty-seven patients were excluded due to lack of serologically confirmed dengue or missing data. Thus, 1,926 patients were enrolled in the study. Among these, 1,920 patients (99.69%) had dengue without acute liver failure and six (0.31%) had dengue with acute liver failure. The incidence of dengue-associated acute liver failure was 0.31% in this study. A flowchart of the patients in this study is shown in Figure 1 .
Figure 1.
Study flowchart.
The mean age of the patients was 29.06 ± 12.48 years, and 49.8% were male. The demographic data and baseline clinical characteristics of the patients are shown in Table 1. Most of the patients with dengue-associated acute liver failure were < 40 years of age (66.66%) and male (83.33%). Moreover, among these patients, all cases of liver disease developed secondary to severe dengue hepatitis (100%). There were significant differences between the two groups in the presence of the severe stage of dengue, jaundice, abdominal pain, hepatomegaly, severe dengue hepatitis, days of fever at admission, and laboratory parameters (e.g., hemoglobin, hematocrit, alanine transaminase, and aspartate aminotransferase levels).
Table 1.
Demographic and baseline clinical characteristics of the study cohort
| Baseline characteristics and investigations | All patients (N = 1,926) | Dengue patients without acute liver failure (N = 1,920) | Dengue patients with acute liver failure (N = 6) | P value | |
|---|---|---|---|---|---|
| Age, n (%) | ≤ 40 years | 1,349 (70.04%) | 1,344 (70.00%) | 5 (83.33%) | |
| 41–60 years | 282 (14.64%) | 281 (14.63%) | 1 (16.67%) | 0.580 | |
| > 60 years | 295 (15.32%) | 295 (15.37%) | 0 (0.00%) | ||
| Sex, n (%) | Male | 959 (49.79%) | 954 (49.68%) | 5 (83.33%) | 0.107 |
| Female | 967 (50.21%) | 966 (50.31%) | 1 (16.66%) | ||
| Thai ethnicity, n (%) | 1,687 (87.59%) | 1,682 (87.60%) | 5 (83.33%) | 0.716 | |
| Severe dengue stage,‡ n (%) | 218 (11.32%) | 212 (11.04%) | 6 (100%) | < 0.001* | |
| Jaundice, n (%) | 7 (0.36%) | 4 (0.21%) | 3 (50%) | < 0.001* | |
| Abdominal pain, n (%) | 238 (12.35%) | 233 (12.13%) | 5 (83.33%) | < 0.001* | |
| Hepatomegaly, n (%) | 239 (12.41%) | 233 (12.13%) | 6 (100%) | < 0.001* | |
| Severe dengue hepatitis, n (%) | 17 (0.88%) | 11 (0.57%) | 6 (100%) | < 0.001* | |
| Day of fever at admission (days)† | 4.58 ± 1.29 | 4.57 ± 1.27 | 7.17 ± 3.76 | < 0.001* | |
| Hemoglobin (g/dL)† | 13.89 ± 1.75 | 13.90 ± 1.75 | 12.20 ± 2.93 | 0.018* | |
| Hematocrit† | 41.33 ± 4.82 | 41.35 ± 4.80 | 35.86 ± 8.75 | 0.005* | |
| Total WBC (per mm3) | 3,100.27 (2,187.76–4,265.79) | 3,100.27 (2,187.76–4,265.79) | 2,051.16 (1,174.89–6,165.95) | 0.407 | |
| Atypical lymphocytes (%) | 8.99 (4.89–17.78) | 8.99 (3.98–17.78) | 11.95 (9.77–13.18) | 0.419 | |
| Platelet count (per mm3) | 67,998.60 (40,738.02–102,329.29) | 67,998.60 (40,738.02–102,329.29) | 43,651.58 (21,877.61–75,857.75) | 0.189 | |
| ALT (IU/L) | 59.01 (31.62–125.89) | 59.01 (31.62–125.89) | 2,848.39 (389.04–3,890.45) | < 0.001* | |
| AST (IU/L) | 93.00 (54.95–190.54) | 93.00 (53.70–190.54) | 7,721.47 (2,137.96–16,595.86) | < 0.001* | |
ALT = alanine transaminase; AST = aspartate aminotransferase; n = number; WBC = white blood cells.
P < 0.05.
Data are presented as mean ± standard deviation, geometric mean (interquartile range), or number (proportion, %).
Severe dengue stage—evidence of severe plasma leakage, bleeding, and organ impairment.
The baseline parameters between patients with severe hepatitis and those with acute liver failure are compared in Table 2. Hepatomegaly occurred in 100% of the patients with dengue-associated acute liver failure. Moreover, no mortality occurred among patients with dengue-associated severe hepatitis. The atypical lymphocyte and aspartate aminotransferase levels on admission were significantly higher in patients with acute liver failure than in those with severe hepatitis.
Table 2.
Comparison of baseline parameters between patients with severe hepatitis and acute liver failure
| Baseline parameters (N = 17) | Dengue patients with severe hepatitis (N = 11) | Dengue patients with acute liver failure (N = 6) | |
|---|---|---|---|
| Age, n (%) | ≤ 40 years | 8 (72.73%) | 5 (83.33%) |
| 41–60 years | 3 (27.27%) | 1 (16.67%) | |
| > 60 years | 0 (0%) | 0 (0%) | |
| Sex, n (%) | Male | 7 (63.63%) | 5 (83.33%) |
| Female | 4 (36.36%) | 1 (16.66%) | |
| Hepatomegaly, n (%) | 9 (81.81%) | 6 (100%) | |
| Jaundice, n (%) | 1 (9.09%) | 3 (50%) | |
| Outcome (alive), n (%) | 11 (100%) | 2 (33.33%) | |
| Hemoglobin (g/dL) | 13.98 ± 2.01 | 12.23 ± 2.92 | |
| Hematocrit (%) | 40.61 ± 6.39 | 36.68 ± 8.64 | |
| Atypical lymphocytes (%) | 9.37 (4.00–28.00) | 11.71 (10.00–14.25) | |
| Platelet count (per mm3) | 38,681.20 (17,000.00–80,000.00) | 40,784.95 (22,250.00–66,750.00) | |
| ALT (IU/L) | 1,404.42 (1,167.05–1,920.05) | 2,392.76 (1,168.00–3,964.00) | |
| AST (IU/L) | 2,137.96 (1,499.00–3,482.00) | 6,587.18 (2,303.00–16,907.00) | |
ALT = alanine transaminase; AST = aspartate aminotransferase; n = number. Data are presented as mean ± standard deviation, geometric mean (interquartile range), or number (proportion, %).
Baseline demographic data classified by severity of liver involvement.
The baseline demographic data of the patients with dengue with no to mild liver involvement and those with moderate to severe liver involvement are shown in Table 3. There were significant differences in sex, the presence of the severe stage of dengue, hepatomegaly, jaundice, days of fever at admission, and laboratory parameters between the two groups.
Table 3.
Comparison of baseline demographic data classified by severity of liver involvement
| Baseline characteristics (N = 1,926) | Dengue patients with no-to-mild liver involvement (N = 1,651) | Dengue patients with moderate-to-severe liver involvement (N = 275) | P value | |
|---|---|---|---|---|
| Age (years) | 29.08 ± 12.51 | 28.11 ± 10.87 | 0.395 | |
| Age, n (%) | ≤ 40 years | 1,137 (68.86%) | 201 (73.09%) | |
| 41–60 years | 252 (15.27%) | 53 (19.27%) | < 0.001* | |
| > 60 years | 262 (15.87%) | 21 (7.64%) | ||
| Sex, n (%) | Male | 807 (48.87%) | 123 (44.72%) | 0.037* |
| Female | 783 (47.42%) | 152 (55.27%) | ||
| Severe dengue, n (%) | 1 (0.06%) | 17 (6.18%) | < 0.001* | |
| Hepatomegaly, n (%) | 175 (10.59%) | 56 (20.36%) | < 0.001* | |
| Jaundice, n (%) | 2 (0.12%) | 5 (1.81%) | 0.001* | |
| Days of fever at admission (days) | 4.57 ± 1.27 | 4.89 ± 2.35 | < 0.001* | |
| Hemoglobin (g/dL) | 13.91 ± 1.74 | 13.15 ± 2.53 | < 0.001* | |
| Hematocrit (%) | 41.37 ± 4.77 | 39.04 ± 7.38 | < 0.001* | |
| Atypical lymphocytes (%) | 7.94 (4.00–15.99) | 12.53 (7.00–23.49) | < 0.001* | |
| Platelets (per mm3) | 66,069.34 (44,998.70–107,003.99) | 44,668.35 (10,703.99–70,990.46) | 0.284 | |
| ALT (IU/L) | 48.76 (29.00–88.00) | 351.88 (245.01–419.95) | < 0.001* | |
| AST (IU/L) | 80.48 (49.00–132.25) | 513.68 (336.97–698.07) | 0.005* | |
ALT = alanine transaminase; AST = aspartate aminotransferase; n = number. Data are presented as mean ± standard deviation, geometric mean (interquartile range), or number (proportion, %).
P < 0.05
Risk factors for liver dysfunction.
Univariate and multivariate analyses were performed to determine the association between clinical risk factors and the development of moderate to severe liver involvement. Among patients with moderate to severe liver involvement, the number of patients with acute liver failure, impending liver failure, and moderate and severe dengue hepatitis were summed (N = 275). The univariate analysis revealed the following independent risk factors for the development of moderate to severe liver involvement in patients with dengue: age of ≤ 40 years (odds ratio [OR] = 1.457, CI = 1.045–2.031, P < 0.05), age of 41–60 years (OR = 2.698, CI = 0.929–7.831, P < 0.05), jaundice (OR = 14.704, CI = 2.838–76.172, P < 0.001), ascites (OR = 35.442, CI = 4.250–295.554, P < 0.001), atypical lymphocytes (OR = 2.292, CI = 1.753–2.996, P < 0.001), and platelet count (OR = 2.794, CI = 2.153–3.625, P < 0.001). The use of a univariate P value of < 0.25 had the advantage of allowing inclusion of more variables in the multivariate analysis. The multivariate analysis revealed the following independent risk factors for the development of moderate to severe liver involvement in patients with dengue: age of ≤ 40 years, atypical lymphocytes of > 10%, and a platelet count of < 50,000/mm3 (Table 4).
Table 4.
Predictors for development of moderate to severe liver involvement in patients with dengue
| Variables | Univariate analysis OR (95% CI) | P value | Multivariate analysis AOR (95% CI) | P value | |
|---|---|---|---|---|---|
| Age | ≤ 40 years | 1.457 (1.045–2.031) | 0.018* | 1.457 (1.045–2.031) | 0.027* |
| 41–60 years | 2.698 (0.929–7.831) | 0.039* | 1.303 (0.915–1.856) | 0.143 | |
| > 60 years | 0.548 (0.217–1.384) | 0.132 | – | – | |
| Hepatomegaly | 2.068 (1.482–2.884) | < 0.001* | 1.227 (0.699–2.153) | 0.476 | |
| Jaundice | 4.915 (3.038–7.954) | 0.001* | 3.065 (0.358–26.224) | 0.307 | |
| Atypical lymphocytes (> 10%) | 2.292 (1.753–2.996) | < 0.001* | 1.617 (1.195–2.189) | 0.002* | |
| Platelet count (< 50,000/mm3) | 2.794 (2.153–3.625) | < 0.001* | 2.250 (1.671–3.029) | < 0.001* | |
AOR = adjusted odds ratio; CI = confidence interval; OR = odds ratio.
P < 0.05
Of the six patients with acute liver failure, five were < 40 years of age and one was 51 years of age. None of the six patients had a preexisting chronic liver disease. The maximum duration of illness among the six patients was 41 days. With respect to clinical outcomes, two patients survived and four patients died with complications. The median duration of onset of fever to development of acute liver failure was 7.5 days (range, 5–14 days). The complications of acute liver failure were acute renal failure, sepsis (severe infection), DIC, and multiple organ failure. The mortality rate of acute liver failure in this study was 66.7% (4/6 patients) (Tables 5 and 6).
Table 5.
Detailed characteristics of patients with acute liver failure secondary to dengue
| Patient characteristics (N = 6) | Median | Minimum | Maximum |
|---|---|---|---|
| Duration from onset of fever to development of acute liver failure (days) | 7.5 | 5.0 | 14.0 |
| Peak body temperature (°C) | 38.8 | 37.4 | 39.5 |
| Peak AST (IU/L) | 13,560 | 2,483 | 26,414 |
| Peak ALT (IU/L) | 3,329 | 1,498 | 26,093 |
| Peak direct bilirubin (mg/dL) | 10.72 | 0.70 | 45.28 |
| Peak total bilirubin (mg/dL) | 8.39 | 1.70 | 69.20 |
| Peak INR | 3.14 | 1.79 | 5.40 |
ALT = alanine transaminase; AST = aspartate aminotransferase; INR = international normalized ratio.
Table 6.
Detailed clinical features of patients with acute liver failure secondary to dengue
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age (years) | 19 | 40 | 51 | 31 | 28 | 22 |
| Sex | Male | Male | Male | Male | Male | Female |
| PELD | No | No | No | No | No | No |
| Days of fever | 7 | 8 | 13 | 6 | 5 | 14 |
| Jaundice | Yes | No | Yes | Yes | Yes | Yes |
| HE during hospitalization | Grade 4 | Grade 3 | Grade 3 | Grade 4 | Grade 2 | Grade 4 |
| Duration of fever (days) | 41 | 11 | 16 | 9 | 18 | 17 |
| Recovery | Yes | No | No | No | Yes | No |
| Outcome | Alive | Dead | Dead | Dead | Alive | Dead |
| Complications | No | Acute renal failure | Acute renal failure | Sepsis | Acute renal failure | DIC, multiple organ failure |
DIC = disseminated intravascular coagulation; HE = hepatic encephalopathy; PELD = preexisting chronic liver diseases.
Discussion
This study revealed a 0.31% incidence of acute liver failure secondary to dengue during a 5-year study period. In previous studies, the incidence of dengue-associated acute liver failure ranged from 0.66% to 0.77%.4,7 Therefore, our study revealed a lower incidence than found in previous studies. In the present study, the incidence of acute liver failure among patients with dengue was 3.1/1,000 patients, which is higher than that found in a previous study in Vietnam (1.5/1,000 patients with dengue).7 The incidence of acute liver failure among dengue-positive patients might vary between studies because of differences in the study design, population, and time period. Differences between studies may also reflect differences in dengue virus serotypes or genotypes circulating in the study areas.21 Previous reports of the incidence and clinical outcomes of acute liver failure resulting from dengue are summarized in Table 7.
Table 7.
Incidence and clinical outcome of acute liver failure secondary to dengue infection
| Study, author, year | Country | Study period | Study population | Age of population | Incidence rate (%) | Mortality rate (%) |
|---|---|---|---|---|---|---|
| Prospective observational study, Itha and others, 2005 | India (dengue outbreak at that time) | September 2003–December 2003 | N = 45 serologically confirmed dengue patients | > 18 years male and female | 4.44 | 100 |
| Prospective observational study, Trung and others, 2010 | Vietnam | 2006–2008 | N = 644 serologically confirmed dengue patients | > 14 years male and female | 0.77 | 20 |
| Prospective observational study, Satva and others, 2014 | India | 2010–2012 | N = 150 serologically confirmed dengue patients | > 18 years male and female | 0.66 | 100 |
| Retrospective cohort study, The present study, 2016 | Thailand | 2011–2015 | N = 1,926 serologically confirmed dengue patients | ≥ 15 years male and female | 0.31 | 66.7 |
n = number.
Previous studies have reported that dengue-induced acute liver failure mostly affects young adults.5 Our results confirm these previous findings; most of our patients with acute liver failure (four of six) were in the adult age group (18–40 years) rather than the older age group (> 40 years).10 A previous study reported that the median duration from onset of fever to development of acute liver failure was 7.5 (5–13) days, which is consistent with our results (7.5 [5–14] days).9 Moreover, we observed the severe stage of the disease in 11.3% of all patients with dengue, and all cases of acute liver failure developed in patients with the severe stage of the disease.
To the best of our knowledge, no study has reported the duration of hospitalization of patients with dengue with liver involvement. In the present study, prolonged hospitalization (> 7 days) was associated with a high mortality rate (66.7%), even for patients under intensive care. Sedhain and others22 reported that a patient who developed acute liver failure (500-fold increase in liver enzyme levels) after dengue infection completely recovered despite the clinical presentation of the disease. Similarly, a case report from India documented complete recovery after acute liver failure following dengue infection.15 In contrast, four of our six patients died and only one patient slowly recovered after 1 month of hospitalization. Although one patient showed normal liver function after 18 days, the fever did not subside. This case was complicated by renal failure and the need for renal dialysis.
Parkash and others11 reported that severe hepatitis was considered a poor prognostic indicator for the development of acute liver failure in patients with dengue. In our study, however, severe hepatitis in patients with dengue was found to be a potential risk factor for the development of acute liver failure. Moreover, our patients developed elevated liver enzymes (60.3%), which supports previous data showing that abnormally high elevations in liver enzyme levels occur more often in young adults than in children with dengue and in at least two-thirds of adult patients with dengue.23 A study by Senaratne and others24 also demonstrated that elevated liver enzymes are associated with increased interleukin-2 levels and predict severe clinical outcomes of dengue disease.
Of all patients with dengue analyzed in the present study, 275 had moderate to severe liver involvement. These patients displayed clinical symptoms associated with a severe stage of the disease, including bleeding manifestations such as epistaxis or gingival bleeding, severe plasma leakage, and organ impairment.
To the best of our knowledge, no previous study has reported the predictors of acute liver failure in patients with dengue. Our findings indicate that a young adult age, > 10% atypical lymphocytes, and a platelet count of < 50,000/mm3 are risk factors for acute liver failure in patients with dengue. However, we identified no risk factors associated with underlying chronic liver disease and acute liver failure. The prognostic factors for acute liver failure depend on many variables including etiology, age, severity of liver dysfunction, nature of the complications, and duration of the illness. Our study findings confirmed these prognostic factors.
A previous study concluded that jaundice and hepatic encephalopathy commonly occur as complications of acute liver failure but that acute renal failure is a less common complication.14 In contrast, we concluded that jaundice, hepatic encephalopathy, and acute renal failure occur as complications of acute liver failure. Moreover, other complications such as acute respiratory failure, DIC, sepsis, and multiple organ failure were also shown to be important in our study.
Many clinical studies have investigated the use of a dengue vaccine to prevent and control dengue. The use of a purified inactivated virus vaccine is currently in the planning stage for clinical testing.25,26 A chimeric dengue vaccine was recently launched following a phase III clinical trial, and a recent study demonstrated the efficacy of that vaccine for symptomatic dengue in up to 65.6% of patients 9–16 years of age.27,28
Clinicians must be aware of the need for early monitoring of patients and measurement of appropriate laboratory parameters to obtain an accurate diagnosis of dengue and avoid misdiagnosis as viral hepatitis in dengue-endemic countries. Early diagnosis and regular monitoring can achieve good clinical outcomes. Therefore, it is important to promptly diagnose dengue and regularly monitor the affected patients' condition, bleeding, and laboratory-determined parameters such as complete blood count, serum transaminase levels, PT, and INR while ensuring good supportive care and management.
In conclusion, although the development of acute liver failure is rare in patients with dengue, it is associated with a high mortality rate. Acute liver failure secondary to dengue occurs more often in young adults than in older patients. Early detection, awareness, and monitoring of laboratory investigations in dengue-confirmed patients are crucial because early detection can prevent deterioration of the severe stage of dengue to acute liver failure. Therefore, well-designed long-term and standardized clinical studies are required to more accurately determine the incidence of acute liver failure in patients with dengue and to establish associated risk factors.
ACKNOWLEDGMENTS
We would like to express our gratitude to the staff of the Hospital for Tropical Diseases, Department of Clinical Tropical Medicine, and the Office of Research Services, Faculty of Tropical Medicine, Mahidol University, Thailand.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by the Faculty of Tropical Medicine, Mahidol University.
Authors' addresses: Khin Kye Mon, Apichart Nontprasert, Chatporn Kittitrakul, Wattana Leowattana, and Kittiyod Poovorawan, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: julymirrormn9@gmail.com, apichart.non@mahidol.ac.th, chatporn.kit@mahidol.ac.th, wattana.leo@mahidol.ac.th, and kittiyod.poo@mahidol.ac.th. Pisit Tangkijvanich, Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, E-mail: pisittkvn@yahoo.com.
References
- 1.Malavige GN, Fernando S, Fernando DJ, Seneviratne SL. Dengue viral infections. Postgrad Med J. 2004;80:588–601. doi: 10.1136/pgmj.2004.019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villar LA, Rojas DP, Besada-Lombana S, Sarti E. PLoS Negl Trop Dis. 2015;9:e0003499. doi: 10.1371/journal.pntd.0003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 4.Nimmagadda SS, Mahabala C, Boloor A, Raghuram PM, Nayak U. Atypical manifestations of dengue fever (DF): where do we stand today. J Clin Diagn Res. 2014;8:71–73. doi: 10.7860/JCDR/2014/6885.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polson J, Lee WM. American Association for the Study of Liver Diseases AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 6.Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. Alexandria, VA: American Association for the Study of Liver Diseases; 2011. [DOI] [PubMed] [Google Scholar]
- 7.Trung DT, Thao Le TT, Hien TT, Hung NT, Vinh NN, Hien PT, Chinh NT, Simmons C, Wills B. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774–780. doi: 10.4269/ajtmh.2010.10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itha S, Kashyap R, Krishnani N, Saraswat VA, Choudhuri G, Aggarwal R. Profile of liver involvement in dengue virus infection. Natl Med J India. 2005;18:127. [PubMed] [Google Scholar]
- 9.Gasperino J, Yunen J, Guh A, Tanaka KE, Kvetan V, Doyle H. Fulminant liver failure secondary to haemorrhagic dengue in an international traveller. Liver Int. 2007;27:1148–1151. doi: 10.1111/j.1478-3231.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 10.Tan SS, Bujang MA. The clinical features and outcomes of acute liver failure associated with dengue infection in adults: a case series. Braz J Infect Dis. 2013;17:164–169. doi: 10.1016/j.bjid.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkash O, Almas A, Jafri SM, Hamid S, Akhtar J, Alishah H. Severity of acute hepatitis and its outcome in patients with dengue fever in a tertiary care hospital Karachi, Pakistan (south Asia) BMC Gastroenterol. 2010;10:43. doi: 10.1186/1471-230X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squires RH. Acute liver failure in children. Semin Liver Dis. 2008;28:153–166. doi: 10.1055/s-2008-1073115. [DOI] [PubMed] [Google Scholar]
- 13.Gotthardt D, Riediger C, Weiss KH, Encke J, Schemmer P, Schmidt J, Sauer P. Fulminant hepatic failure: etiology and indications for liver transplantation. Nephrol Dial Transplant. 2007;22((Suppl 8)):viii5, viii8. doi: 10.1093/ndt/gfm650. [DOI] [PubMed] [Google Scholar]
- 14.Gill RQ, Sterling RK. Acute liver failure. J Clin Gastroenterol. 2001;33:191–198. doi: 10.1097/00004836-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Vinodh B, Bammigatti C, Kumar A, Mittal V. Dengue fever with acute liver failure. J Postgrad Med. 2005;51:322. [PubMed] [Google Scholar]
- 16.Kuo CH, Tai DI, Chang-Chien CS, Lan CK, Chiou SS, Liaw YF. Liver biochemical tests and dengue fever. Am J Trop Med Hyg. 1992;47:265–270. doi: 10.4269/ajtmh.1992.47.265. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TL, Nguyen TH, Tieu NT. The impact of dengue haemorrhagic fever on liver function. Res Virol. 1997;148:273–277. doi: 10.1016/s0923-2516(97)88364-1. [DOI] [PubMed] [Google Scholar]
- 18.American Gastroenterological Association American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology. 2002;123:1364–1366. doi: 10.1053/gast.2002.36060. [DOI] [PubMed] [Google Scholar]
- 19.Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol. 2013;19:349–359. doi: 10.3350/cmh.2013.19.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchman AL, Iyer K, Fryer J. Parenteral nutrition–associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology. 2006;43:9–19. doi: 10.1002/hep.20997. [DOI] [PubMed] [Google Scholar]
- 21.Yung CF, Lee KS, Thein TL, Tan LK, Gan VC, Wong JG. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am J Trop Med Hyg. 2015;92:999–1005. doi: 10.4269/ajtmh.14-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedhain A, Adhikari S, Regmi S, Chaudhari SK, Shah M, Shrestha B. Fulminant hepatic failure due to dengue. Kathmandu Univ Med J. 2011;9:73–75. doi: 10.3126/kumj.v9i2.6293. [DOI] [PubMed] [Google Scholar]
- 23.Treeprasertsuk S, Kittitrakul C. Liver complications in adult dengue and current management. Southeast Asian J Trop Med Public Health. 2015;46((Suppl 1)):99–107. [PubMed] [Google Scholar]
- 24.Senaratne T, Carr J, Noordeen F. Elevation in liver enzymes is associated with increased IL-2 and predicts severe outcomes in clinically apparent dengue virus infection. Cytokine. 2016;83:182–188. doi: 10.1016/j.cyto.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Thomas SJ, Endy TP. Vaccines for the prevention of dengue: development update. Hum Vaccin. 2011;7:674–684. doi: 10.4161/hv.7.6.14985. [DOI] [PubMed] [Google Scholar]
- 26.Tang KF, Ooi EE. Diagnosis of dengue: an update. Expert Rev Anti Infect Ther. 2012;10:895–907. doi: 10.1586/eri.12.76. [DOI] [PubMed] [Google Scholar]
- 27.Sinha G. Sanofi's dengue vaccine first to complete phase 3. Nat Biotechnol. 2014;32:605–606. doi: 10.1038/nbt0714-605a. [DOI] [PubMed] [Google Scholar]
- 28.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortes M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M. CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]