Abstract
The 2014 Ebola epidemic has shown the importance of accurate and rapid triage tools for patients with suspected Ebola virus disease (EVD). Our objective was to create a predictive score for EVD. We retrospectively reviewed all suspected cases admitted to the Ebola treatment center (ETC) in Nzérékoré, Guinea, between December 2, 2014, and February 23, 2015. We used a multivariate logistic regression model to identify clinical and epidemiological factors associated with EVD, which were used to create a predictive score. A bootstrap sampling method was applied to our sample to determine characteristics of the score to discriminate EVD. Among the 145 patients included in the study (48% male, median age 29 years), EVD was confirmed in 76 (52%) patients. One hundred and eleven (77%) patients had at least one epidemiological risk factor. Optimal cutoff value of fever to discriminate EVD was 38.5°C. After adjustment on presence of a risk factor, temperature higher than 38.5°C (odds ratio [OR] = 18.1, 95% confidence interval [CI] = 7.6–42.9), and anorexia (OR = 2.5, 95% CI = 1.1–6.1) were independently associated with EVD. The score had an area under curve of 0.85 (95% CI = 0.78–0.91) for the prediction of laboratory-confirmed EVD. Classification of patients in a high-risk group according to the score had a lower sensitivity (71% versus 86%) but higher specificity (85% versus 41%) than the existing World Health Organization algorithm. This score, which requires external validation, may be used in high-prevalence settings to identify different levels of risk in EVD suspected patients and thus allow a better orientation in different wards of ETC.
Introduction
The 2013–2016 Ebola epidemic in west Africa started in December 2013 in rural southern Guinea, and was declared a public health emergency of international concern by the World Health Organization (WHO) in August 2014. As of March 2016, the total number of suspected cases was 28,639 resulting in 11,316 deaths (mortality 40%).1 Early in the course of this epidemic, the increasing number of patients vastly outstripped the available isolation and treatment capacity in the three most affected countries, Guinea, Liberia, and Sierra Leone. One of the main issues that health authorities faced was to rapidly identify and isolate cases to break the chain of Ebola virus disease (EVD) transmission. In the absence of a reliable EVD rapid diagnostic test, accurate screening algorithms, based on epidemiological and clinical characteristics of patient are needed. However, several studies have underlined the difficulty in distinguishing EVD from other causes of febrile illness such as malaria or typhoid.2,3 Of note, clinical symptoms at admission have been also found to be associated with mortality making the assumption that care and more specifically setting in which the patient is hospitalized could be tailored to the clinical presentation.4,5 Clinical case definition has evolved throughout the epidemic and was adapted locally as the outbreak developed.6
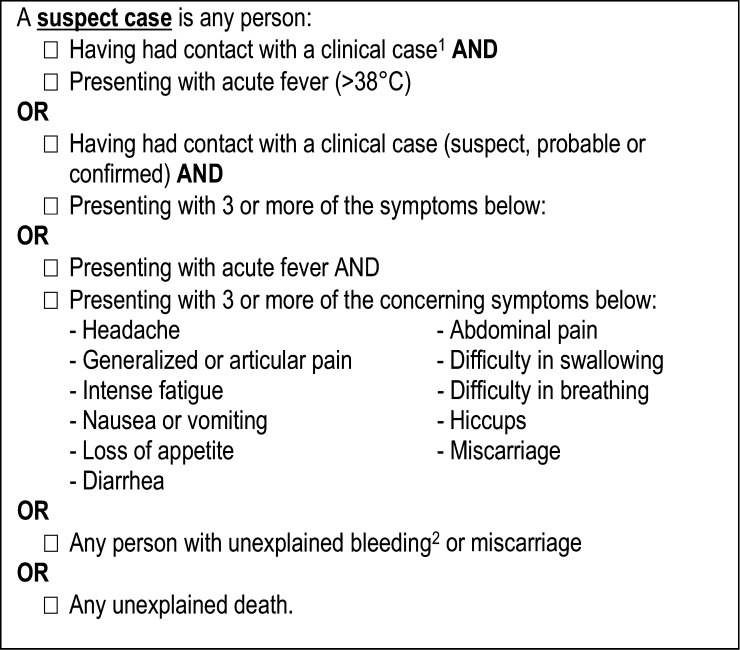
Alliance for International Medical Action (ALIMA) Ebola Treatment Center (ETC) was opened on December 2, 2014, in Nzérékoré. Suspected EVD patients coming from Nzérékoré region were referred to this ETC. At that time, the screening tool used was the WHO definition described as follows: “Any person, suffering or having suffered from a sudden onset of high fever and having had contact with a suspected, probable, or confirmed Ebola case or any person with sudden onset of high fever and at least three of the following symptoms: headaches, vomiting, anorexia/loss of appetite, diarrhea, lethargy, stomach pain, aching muscles or joints, difficulty swallowing, breathing difficulties, hiccup, or any person with inexplicable bleeding.”6
The aim of this study was first to identify epidemiological, sociodemographic, and clinical variables associated with EVD diagnosis and to create, based on these variables, a predictive score for identification of confirmed EVD, using data from confirmed and excluded EVD cases admitted to Nzérékoré ETC during the study period.
Methods
Population of the study.
Between December 2, 2014, and February 23, 2015, all patients referred to the ALIMA Nzérékoré ETC were included in the study. Patients were either referred by the Ministry of Health Ebola alert system, by health-care settings or came on their own.
Data collection.
At admission, patients or their family members were first asked questions concerning personal information, symptoms, duration of illness, and epidemiological risk factors for possible EVD. Particular importance was accorded to health-care workers, those who had attended a funeral or those having contact with an ill relative, describing symptoms of the WHO definition. Trained Guinean and international nurses or physicians recorded data on standardized paper forms and made the decision to admit to the ETC based on the structured interview and any visual observations that could be made at 3–4 m distance through a Plexiglas wall that prevented direct contact between health workers and those presenting to the ETC. All patients were first admitted to one of two suspect wards. Those who gave a history of vomiting and diarrhea or bleeding were admitted to the “highly suspect” ward, whereas those without these features were admitted to a “suspect” ward. Clinical staff wearing personal protective equipment then entered the isolation zone and directly examined patients for EVD features. Fever was considered as a documented axillary temperature of 37.5°C or higher (axillary temperature plus 0.5°C).
A blood sample was collected from all patients for EVD diagnostic testing by semiquantitative Ebola virus reverse transcription polymerase chain reaction (RT-PCR) in the Pasteur mobile laboratory, in Macenta, for all patients admitted between December 2 and December 25, 2014, and thereafter in the B-Life laboratory located in the Nzérékoré ETC. Patients with a positive test result were moved to the confirmed ward of the ETC and treated according to standardized protocol guidelines.
Patients with initial negative test result were held until a second test done 72 hours after the onset of symptoms was negative. Patients' vital signs and clinical status were transmitted orally from the isolation zone to a nurse or physician outside the zone. Each patient had a written medical record of care, laboratory results, and outcomes maintained in the medical office of the ETC. This paper record was then entered into an individual electronic medical record.
Statistical analysis.
Patients admitted to ETC were categorized as confirmed cases or excluded cases depending on one or more Ebola virus RT-PCR results. Patients with no RT-PCR result were excluded from the analysis.
The relationship between EVD and the 18 sociodemographic, clinical, and epidemiologic variables were assessed using Fisher's exact test or Wilcoxon rank-sum test as appropriate. Epidemiologic variables were defined as being a health worker or having had contact with a person with suspected EVD or having attended funerals. Differences in median time from onset of symptoms to admission were assessed by Wilcoxon rank-sum tests.
The sensitivity and specificity of every clinical characteristic were calculated. The sensitivity and specificity of the WHO suspect case definition (Figure 1 ) were also assessed. We divided the WHO definition into the following three subdefinitions to allow further comparisons: WHO subdefinition1 (risk factor + clinical symptoms), WHO subdefinition2 (risk factor + T° ≥ 37.5°C) and WHO subdefinition3 (T° ≥ 37.5°C + clinical symptoms). In these assessments, the gold standard was considered to be the laboratory-confirmed EVD.
Figure 1.
World Health Organization (WHO) case definitions for Ebola virus disease during an epidemic: Adapted from WHO. Clinical management of patients with viral hemorrhagic fever: a pocket guide for the front-line health worker. 2014:1–113. Available at: http://apps.who.int/iris/handle/10665/130883.
To quantify the prognostic utility of the variables, a multivariate logistic regression with EVD as the dependent variable was performed using a stepwise backward selection algorithm; all collected variables were tested in univariate analysis, then all variables with a P value < 0.20 in the univariate analysis were introduced into the multivariate model. Only variables with a value < 0.05 were retained in the final model except for the variable “presence of an epidemiological risk factor” that we decided to force into the model because of its known relation with EVD transmission in previous studies,7 and because of the key role that these epidemiological factors have in the WHO definition. Concerning fever, a receiver-operating characteristic (ROC) curve with different cutoffs was plotted. In the final model, we tested different cutoffs for fever (37.5; 38; 38.5°C). These cutoffs have been considered relevant for the following reasons: 37.5°C and 38°C, respectively, are the current and prior WHO EVD definition cutoffs, and 38.5°C was arbitrary chosen to have a third cutoff with the same interval between the first two cutoffs. Appropriate fit of the final model was confirmed using the Hosmer–Lemeshow goodness-of-fit test. Subsequently, this temperature cutoff value was substituted in the model's equation.
The final logistic regression model was considered as the risk prediction model and was used to estimate the odds ratio for each parameter included in the prediction equation for EVD. The logistic regression coefficients of each included parameter were converted into an integer risk score by dividing the regression coefficient by a single constant that was then rounded. A risk score was then calculated for each patient by summing the points attributed to each variable according to its presence or not in each patient. This method is further described in the paper by Sullivan and others.8 The population was divided into three categories according to the score: patients at low risk, patients at medium risk, and patients at high risk for EVD.
The discrimination of this scoring system was assessed against the criterion standard of laboratory-confirmed EVD with ROC curves analysis, presenting area under curves (AUCs), and their 95% confidence intervals (CIs) computed after 1,000 stratified bootstrap replicates (with replacement). EVD score has been considered as a whole for AUC estimation. The nonparametric estimate of AUC was performed using the Wilcoxon rank-sum test, namely the proportion of all possible pairs of nondiseased and diseased test subjects for which the diseased result is higher than the nondiseased one plus half the proportion of ties.9,10 All analyses were performed using Stata (V12; StataCorp, College Station, TX).
Ethics.
This is a secondary analysis conducted on data collected for clinical management. All health care was provided free of charge, and patients had the option of refusing treatment at any time. No specific authorizations and consents were collected for this retrospective study. All information had been deidentified before analysis.
Results
Patients' sociodemographic and epidemiological characteristics.
Overall, 147 patients were admitted to Nzérékoré ALIMA ETC, among which 145 patients were included in the study; two patients were excluded because of nonreported RT-PCR results. Of 145 patients enrolled, 69 (48%) were male with a median age at 29 years (interquartile range [IQR] = 20–26). EVD was confirmed in 76 (52%) patients. One hundred and eleven (77%) patients had at least one epidemiological risk factor: 14 (10%) were health-care workers, 37 (25%) attended funerals, and 106 (72%) reported contact with a relative suspected of having EVD. No statistically significant differences were observed between confirmed and excluded EVD cases regarding presence of an epidemiological risk factor (confirmed cases: N = 62, 82% versus excluded cases: N = 49, 71% [P = 0.17]) and time from onset of symptoms to admission to the ETC (3 days [IQR = 1–5] for confirmed cases versus 2 days [IQR = 1–5] for excluded cases [P = 0.20]) (Table 1).
Table 1.
Patients' characteristics admitted to Nzérékoré Ebola Treatment Center; November 2014–February 2015
| Total (N = 145) | EVD-confirmed cases (N = 76) | EVD-excluded cases (N = 69) | Crude OR* (95% CI) | P value | |
|---|---|---|---|---|---|
| Female† | 77 (52%) | 47 (62%) | 29 (42%) | 2.4 (1.2–4.3) | 0.02 |
| Median age, years | 29 (20–46) | 28 (18–48) | 30 (2,245) | – | 0.65 |
| Age groups, years† | 0.65 | ||||
| < 15 | 21 (14%) | 12 (16%) | 9 (13%) | Reference | |
| 15–29 | 57 (39%) | 30 (40%) | 27 (39%) | 0.8 (0.3–2.3) | |
| 30–44 | 36 (25%) | 17 (22%) | 19 (28%) | 0.7 (0.2–2.0) | |
| ≥ 45 | 31 (21%) | 17 (22%) | 14 (20%) | 0.9 (0.3–2.8) | |
| Risk factor† | |||||
| Health-care worker | 14 (10%) | 8 (11%) | 6 (9%) | 1.2 (0.4–3.8) | 0.78 |
| Funeral attendance | 37 (25%) | 21 (28%) | 16 (23%) | 1.3 (0.6–2.7) | 0.57 |
| Contact | 106 (72%) | 58 (76%) | 48 (70%) | 1.4 (0.7–2.9) | 0.45 |
| At least one of the above risk factor | 111 (77%) | 62 (82%) | 49 (71%) | 1.8 (0.8–3.9) | 0.17 |
| Median delay from onset of symptoms to admission, days | 3 (1–5) | 3 (1–5) | 2 (1–5) | – | 0.2 |
| Symptoms | |||||
| Fever (≥ 37.5°C)† | 105 (72%) | 71 (93%) | 34 (49%) | 14.6 (5.3–40.6) | < 0.001 |
| Fever (≥ 38°C)† | 86 (59%) | 67 (88%) | 19 (28%) | 19.6 (8.2–47.0) | < 0.001 |
| Fever (≥ 38.5°C)† | 73 (50%) | 61 (80%) | 12 (17%) | 19.3 (8.3–47.8) | < 0.001 |
| Intense fatigue† | 85 (59%) | 52 (68%) | 33 (49%) | 2.3 (1.2–4.5) | 0.02 |
| Diarrhea and/or vomiting† | 76 (52%) | 47 (62%) | 29 (42%) | 2.2 (1.1–4.3) | 0.02 |
| Headache† | 67 (47%) | 34 (45%) | 33 (49%) | 0.9 (0.5–1.7) | 0.74 |
| Joint pain† | 58 (40%) | 34 (45%) | 24 (35%) | 1.5 (0.8–2.9) | 0.31 |
| Anorexia† | 60 (42%) | 41 (54%) | 19 (28%) | 3.0 (1.5–6.1) | 0.002 |
| Muscular pain† | 47 (33%) | 30 (39%) | 17 (25%) | 2.0 (0.9–4.0) | 0.07 |
| Abdominal pain† | 47 (33%) | 27 (36%) | 20 (29%) | 1.3 (0.7–2.7) | 0.48 |
| Cough† | 33 (23%) | 21 (28%) | 12 (18%) | 1.8 (0.8–4.0) | 0.17 |
| Difficulty in swallowing† | 21 (15%) | 12 (16%) | 9 (13%) | 1.2 (0.5–3.1) | 0.81 |
| Bleeding† | 14 (10%) | 10 (13%) | 4 (6%) | 2.5 (0.7–8.2) | 0.165 |
| Difficulty breathing† | 13 (9%) | 5 (7%) | 8 (11%) | 0.5 (0.2–1.7) | 0.39 |
| Conjunctivitis† | 8 (6%) | 7 (9%) | 1 (1%) | 6.9 (0.8–57.6) | 0.07 |
| Epigastralgia† | 4 (3%) | 3 (4%) | 1 (1%) | 2.8 (0.3–27.5) | 0.62 |
| Death | 53 (37%) | 46 (61%) | 7 (10%) | – | < 0.001 |
CI = confidence interval; EVD = Ebola virus disease; OR = odds ratio. Data are median (interquartile range) or n (%).
*
Crude ORs were obtained through univariate logistic regression.
Variables tested in score-building model.
Clinical symptoms.
At admission to ETC, the most frequent symptoms observed were fever (N = 106, 72%), intense fatigue (85, 59%), headache (67, 47%), anorexia (60, 42%), and joint pain (58, 40%). In the univariate analysis, presence of fever (odds ratio [OR] = 14.6, 95% CI = 5.3–40.6; P < 0.001), anorexia (OR = 3.0, 95% CI = 1.5–6.1; P = 0.002), diarrhea (OR = 2.9, 95% CI = 1.4–6.0; P = 0.003), intense fatigue (OR = 2.3, 95% CI = 1.2–4.5; P = 0.02), and vomiting (OR = 2.1, 95% CI = 1.1–4.2, P = 0.04) were significantly associated with EVD. Conjunctivitis (OR = 6.9, 95% CI = 0.8–57.6; P = 0.07) and muscular pain (OR = 2.0, 95% CI = 0.9–4.0); P = 0.07) were not significantly associated with EVD.
Bleeding was reported in only 14 (10%) EVD cases. Five (7%) EVD patients presented no fever at admission. All EVD cases had either fever or a risk factor for EVD exposure.
Fever appeared in two of them after 4 and 6 days of stay corresponding to 9 and 6 days after onset of symptoms, respectively. In the remaining three, no fever was observed throughout the ETC stay. Of them, two died 3 days after admission and only one 14-year-old patient survived throughout his 15-day stay without fever.
Sensitivity and specificity.
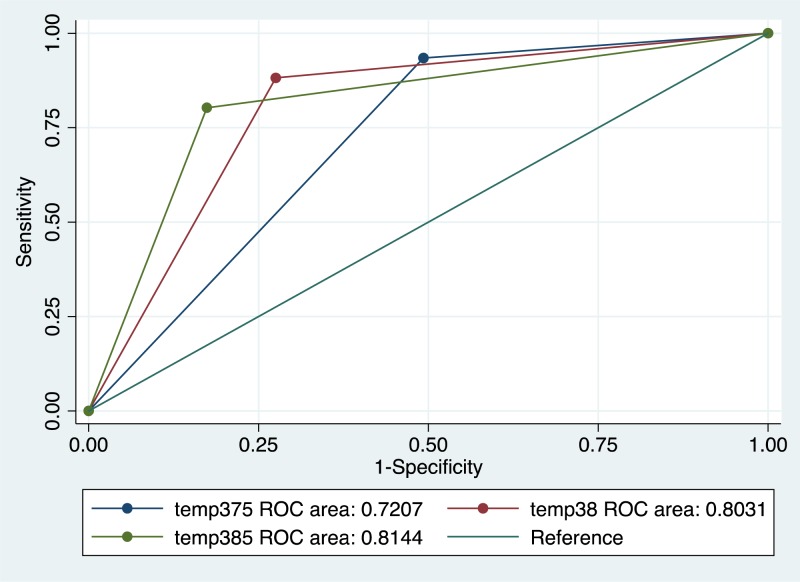
Fever as a unique symptom had a predictive ability to discriminate EVD (Area Under Curve 0.85 (95% CI 0.78–0.91). After multivariate analysis, 38.5°C cut-off was considered the optimal cutoff and used in the prediction model (Figure 2 ).
Figure 2.
Receiver-operating characteristic (ROC) curves of different thresholds of fever predicting Ebola virus disease; Nzérékoré Ebola Treatment Center; November 2014–February 2015. Temperature 37.5°C; 95% confidence interval (CI) = area under curve (AUC) [0.66–0.79]. Temperature 38°C; 95% CI = AUC [0.74–0.87]. Temperature 38.5°C; 95% CI = AUC [0.75–0.88].
Sensitivity and specificity of the 18 variables tested are displayed in Table 2. WHO definition had a sensitivity of 76.8% (95% CI = 66.0–85.1) and specificity of 56.5 (95% CI = 44.0–68.2).
Table 2.
Performance of clinical and epidemiological characteristics at admission in distinguishing Ebola virus disease confirmed cases vs. excluded cases; Nzérékoré Ebola Treatment Center; November 2014–February 2015
| Se (95% CI) | Sp (95% CI) | |
|---|---|---|
| Presence of a risk factor | 81.5 (44.0–60.7) | 29.0 (19.0–41.3) |
| Fever (≥ 37.5°C) | 93.4 (84.7–97.5) | 50.7 (38.5–62.9) |
| Fever (≥ 38°C) | 88.2 (78.2–94.1) | 72.5 (60.2–82.2) |
| Fever (≥ 38.5°C) | 80.2 (69.2–88.2) | 82.6 (71.2–90.3) |
| Intense fatigue | 68.4 (56.6–78.3) | 51.5 (39.1–63.6) |
| Headache | 45.3 (33.9–57.2) | 51.5 (39.1–63.6) |
| Joint pain | 44.7 (33.4–56.5) | 64.7 (52.1–75.6) |
| Muscular pain | 39.5 (28.7–51.4) | 75.0 (62.8–84.3) |
| Diarrhea | 47.4 (35.9–59.1) | 76.4 (64.3–85.6) |
| Vomiting | 46.0 (34.7–57.8) | 71.0 (58.7–81.0) |
| Abdominal pain | 36.0 (25.5–41.3) | 70.6 (58.1–80.7) |
| Difficulty in swallowing | 15.8 (8.8–26.4) | 86.7 (75.9–93.4) |
| Anorexia | 54.0 (42.2–65.3) | 72.4 (60.2–82.2) |
| Epigastralgia | 4.0 (1.0–12.0) | 98.5 (91.0–99.9) |
| Cough | 27.6 (18.3–39.3) | 82.4 (70.8–90.2) |
| Difficulty breathing | 6.6 (2.4–15.3) | 88.2 (77.6–94.4) |
| Conjunctivitis | 9.2 (4.1–18.6) | 98.5 (91.1–99.9) |
| Bleeding | 13.2 (6.8–23.3) | 94.2 (85.1–98.1) |
| Fever (≥ 38.5°C)+ risk factor | 68.4 (56.6–78.3) | 82.6 (71.2–90.3) |
| WHO definition (Global) | 85.8 (76.7–93.2) | 40.6 (29.1–53.1) |
| WHO subdefinition1 (risk factor + clinical symptoms) | 63.2 (51.3–73.7) | 66.7 (54.2–77.3) |
| WHO subdefinition2 (T° ≥ 37.5°C + risk factor) | 75.0 (63.5–83.9) | 62.3 (49.8–73.5) |
| WHO subdefinition3 (T° ≥ 37.5°C + clinical symptoms) | 67.1 (55.2–77.2) | 76.8 (64.8.0–85.8) |
CI = confidence interval; Se = sensitivity; Sp = specificity; WHO = World Health Organization.
Prediction model.
All sociodemographical variables significantly associated with EVD in univariate analysis with P < 0.2 were included in the multivariate model. Then, a backward stepwise selection procedure was performed to obtain a final model into which “epidemiological risk factor” was forced. At the end of the selection procedure, three variables were retained in the final model: temperature higher than 38.5°C (adjusted OR [aOR] = 18.1, 95% CI = 7.6–42.9), anorexia (aOR = 2.5, 95% CI = 1.1–6.1), and presence of a risk factor (aOR = 1.9, 95% CI = 0.7–5.2). The goodness of fit of the model was 0.69 (Hosmer–Lemeshow test).
The log odds ratios of the three variables selected in the final model were converted into an integer score as described in the methods. Points assigned were 3, 2, and 1 for temperature ≥ 38.5°C, anorexia, and risk factor, respectively. We constructed an individual EVD score, which was calculated by summing the individual points for each of these three variables. This score ranged from 0 to 6.
When applied to the full dataset, the score had an AUC of 0.85 (95% CI = 0.78–0.91) for the prediction of laboratory-confirmed EVD. After performing 1,000 bootstrap samples, the average AUC was 0.82 (95% CI = 0.77–0.87).
We classified patients enrolled in our study in three groups according to their EVD score: Low risk (0–2); medium risk (2–4); high-risk ≥ 4.
Table 3 demonstrates EVD score performance and the proportion of patients with laboratory-confirmed and excluded EVD into each group. Sensibility and specificity of the score for each group were calculated versus all other groups combined. The classification of patients as high-risk level of EVD score demonstrated a lower sensitivity (71% versus 87%) but a higher specificity (85% versus 41%) than the WHO algorithm.
Table 3.
Performance of Ebola score according to a risk level determined by the prediction model; Nzérékoré Ebola Treatment Center; November 2014–February 2015
| EVD-confirmed cases | EVD-excluded cases | Total number of patients | Se 95% CI | Sp 95% CI | |
|---|---|---|---|---|---|
| Low risk | 7 (14%) | 43 (86%) | 50 | 91 (81–96) | 62 (50–73) |
| Medium risk | 15 (48%) | 16 (52%) | 31 | 80 (69–88) | 23 (14–35) |
| High risk | 54 (84%) | 10 (16%) | 64 | 71 (59–81) | 85 (74–92) |
CI = confidence interval; EVD = Ebola virus disease; Se = sensitivity; Sp = specificity. Low risk: patients with Ebola prediction score = 0–2; medium risk = 2–4; high-risk ≥ 4.
Mortality.
Mortality was significantly higher in patients with confirmed EVD cases than in excluded cases (61% versus 10%, P < 0.001). Of the 76 patients with confirmed EVD, 46 died including six children under 5 years of age, leading to a case fatality rate of 61%. Median time from onset of symptoms to death was 4 days (IQR = 2–5). No clinical symptoms were statistically associated with mortality. The following factors were not significantly associated with mortality: bleeding (OR = 2.9, 95% CI = 0.6–15.0, P = 0.18), difficulty breathing (OR = 2.8, 95% CI = 0.3–26.0, P = 0.18), and temperature higher than 38.2°C at admission (OR = 2.5, 95% CI = 0.7–8.8, P = 0.15).
Discussion
In this study, we compared sociodemographics, clinical, and epidemiological characteristics of confirmed and excluded EVD cases admitted in an ETC. Fever, intense fatigue, anorexia, and gastrointestinal symptoms were the most common symptoms at admission in patients with EVD. After adjustment on the presence of an epidemiological risk factor, we found that presence of temperature higher than 38.5°C and anorexia were associated with increased likelihood of EVD.
In the absence of a reliable rapid diagnostic test for EVD, algorithms based on epidemiological and clinical criteria offer the only option to determine which patients require isolation pending test results.11 Features of a good clinical algorithm for detecting Ebola cases requires taking several aspects into account especially in contexts where there is no choice but to place suspected Ebola patients in the same ward pending test results. This tool needs to be as sensitive as possible to identify all sick patients with a clinical picture consistent with EVD and thus break transmission chains of this highly lethal disease, while retaining a high degree of specificity to minimize EVD transmission within the ETC.12
Anorexia that we found associated with likelihood of EVD support the findings of previous studies.13,14 Anorexia is a subjective symptom and difficult to assess during the first patient interview at admission. One may consider that this symptom may be related to fever and/or gastrointestinal symptoms. In our multivariate model, we tested whether the introduction of “diarrhea and/or vomiting” had an impact on anorexia and EVD. The strength of the association between anorexia and EVD and more precisely the anorexia OR value did not change with and without “diarrhea and/or vomiting” in the model. Of note, no data were available concerning the delay between the occurrence of anorexia, onset of symptoms, and ETC admission. We have decided to adjust our model on the presence of an epidemiological factor because of its important impact on the transmission of EVD and because of its central role in the multilevel EVD WHO definition (Figure 1). This allowed us to compare our score with WHO definitions.
We also found fever associated to EVD unlike Levine and others and Lado and others.14,15 In line with data from the literature, 7% of our patients had no fever at admission. We should emphasize on the fact that fever measurement may be biased by several factors. First, fever occurrence may be variable overtime and during the day in particular. Second, there is an important variability in the delay between onset of symptoms and ETC admission between patients; as a result, there are differences between patients at ETC admission regarding where they are in their Ebola virus life cycle and disease natural history and this may also impact fever onset. Finally, as discussed below, there are also differences in fever measurement and data collection. These factors may have impacted different results in different series reported.
In adjusted multivariate analysis, it appeared that the highest goodness of fit for the model was with the cutoff of 38.5°C. Our results may suggest that the level of temperature in the WHO definition should be raised to at least 38°C as it was in the beginning of the epidemic.
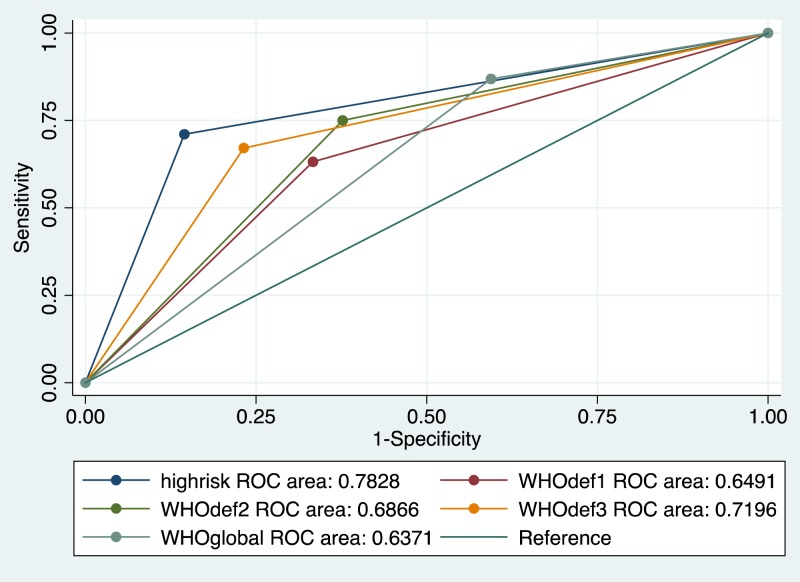
Our estimation of the sensitivity of the WHO definition (86%) is consistent with results of Lado and others (80%) and Levine and others (93%). We found a higher specificity (41%) than both the previous studies (31% and 23%).14,15 Our score has a higher specificity than all the WHO definition and subdefinitions for EVD (Figure 3 ).
Figure 3.
Receiver-operating characteristic (ROC) curves for high-risk group definition of Ebola virus disease (EVD) score compared with different scores based on World Health Organization (WHO) definition for predicting EVD; Nzérékoré Ebola Treatment Center; November 2014–February 2015. High-risk group; 95% confidence interval (CI) = area under curve (AUC) [0.72–0.85]. WHO global definition; 95% CI = AUC [0.57–0.71]. WHO1 (risk factor + clinical symptoms); 95% CI = AUC [0.57–0.73]. WHO2 (T° ≥ 37.5°C + risk factor); 95% CI = AUC [0.65–0.79]. WHO3 (T° ≥ 37.5°C + clinical symptoms); 95% CI = AUC [0.75–0.88].
It has to be noted that Levine and others used different factors than ours for the creation of an Ebola predictive score based on data from patients of the Bong county treatment center in Liberia. Indeed, they found sick contact, diarrhea, anorexia, and muscle pain associated and abdominal pain as a negative factor. Fever was not used in their score.15 Theses discrepancies, probably due to the differences in patients' characteristics and clinical presentation underline the difficulty in creating a predictive score that can be widely applied.
In the literature, results concerning clinical factors associated with mortality are inconsistent. As in our study, Bah and others16 found no factors significantly associated with mortality. Others studies showed weakness, gastrointestinal symptoms, bleeding, difficulty in breathing, intense fatigue, and myalgia associated with mortality.4,5,17,18 However, unlike the number of patients needed to establish a prediction model, our sample size of confirmed cases and consequently the number of deaths were probably not sufficient to estimate variables associated with mortality.
Limitations should be acknowledged in our study. First, small sample size of our sample may have had an impact on the robustness of the results. Second, it has to be underlined that both factors that we found associated with EVD are uneasy to measure and interpret. Indeed, difficulty in data collection due to the hard circumstances of work in the ETC added to the fact that symptoms that were mostly self-reported may probably have had an impact on the quality of the data. Furthermore, concerning data collection, it has to be stressed that temperature plays an important role in the creation of the score. However, the taking of temperature may have been influenced by several factors including staff training and different ways of taking temperature. These points are important regarding the measuring precision of our cutoff. Third, data were collected from a single treatment center, and may thus not be fully representative of all patients with EVD. Indeed, it seemed that patients recruited in this part of Forrest Guinea may have been reluctant to come to the ETC, and thus were more likely to present severe clinical presentation with late symptoms such as higher temperature and conjunctivitis which appeared to be more specific in our sample. Finally, although showing a good calibration and been internally validated, thanks to bootstrap sampling, our score has not been externally validated and should be ideally tested in a future epidemic in other settings involving new clinicians. It should be emphasized that this score's positive and negative predictive values may be different in other settings than the setting where it was estimated and outside an epidemic context. In addition, it has to be noted that our sample had a high rate of epidemiological risk, mainly contact with an EVD case. Given the sensitivity and specificity of our model, such a score may be only useful in a highly exposed population and if there is no laboratory capacity.
In an outbreak with a high EVD incidence that would require the set-up of community based triage and isolation units, this score could assist health workers in their daily decision to orientate patients in different “suspect wards” according to the level of risk as well as the presence of “wet symptoms (diarrhea and vomiting)” in the clinical presentation. Of note, the pragmatic classification of patients between suspect ward and highly suspect ward in Nzérékoré ETC according to the presence of bleeding or wet symptoms (diarrhea or vomiting) had a sensitivity of 64% and a specificity of 54%. However, this classification was rather used to predict contagiousness than EVD diagnosis.
In conclusion, while rapid diagnostic tests implementation is ongoing, although difficult to design, an optimal clinical algorithm is needed for future EVD epidemics. Rising fever cutoff to 38.5°C should be considered to improve existing algorithms.
ACKNOWLEDGMENTS
We thank the integrality of the Guinean and international staff who worked intensively to treat patients in the ETC. We also thank the Guinean Ministry of Health, the World Food Program for the construction of the ETC, and the NGO Tulipe for its contribution to the project.
Footnotes
Financial support: The Alima ETC project was funded by ECHO (European Commission Humanitarian Office), BMGF (Bill & Melinda Gates Foundation), Avaaz, and OSIWA.
Authors' addresses: Paul Loubet and Yazdan Yazdanpanah, Infectious Diseases Ward, Assistance Publique–Hôpitaux de Paris (AP-HP), Hopital Bichat-Claude Bernard, Paris, France, E-mails: paul.loubet@aphp.fr and yazdan.yazdanpanah@aphp.fr. Romain Palich, Richard Kojan, Olivier Peyrouset, Mamadou Conde, and Augustin Augier, Alliance for International Medical Action (ALIMA), Paris, France, E-mails:romain.palich@gmail.com, richard.kojan@alima-ngo.org, olivier.peyrouset@gmail.com, docteurconde7@gmail.com and aug@alima-ngo.org. Christine Danel, Program PACCI, ANRS, Abidjan, Côte d'Ivoire, E-mail:christinemarie.danel@gmail.com. Sarala Nicholas and Klaudia Porten, Epicentre, Mèdecins Sans Frontières, Paris, France, E-mails:sarala.nicholas@epicentre.msf.org and klaudia.porten@epicentre.msf.org.
References
- 1.World Health Organization Ebola Situation Reports. 2015. http://apps.who.int/ebola/ebola-situation-reports Available at. Accessed September 6, 2015.
- 2.MacNeil A, Farnon EC, Morgan OW, Gould P, Boehmer TK, Blaney DD, Wiersma P, Tappero JW, Nichol ST, Ksiazek TG, Rollin PE. Filovirus outbreak detection and surveillance: lessons from Bundibugyo. J Infect Dis. 2011;204((Suppl 3)):S761–S767. doi: 10.1093/infdis/jir294. [DOI] [PubMed] [Google Scholar]
- 3.Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204((Suppl 3)):S810–S816. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 4.Barry M, Touré A, Traoré FA, Sako F-B, Sylla D, Kpamy DO, Bah EI, Bangoura M, Poncin M, Keita S, Tounkara TM, Cisse M, Vanhems P. Clinical predictors of mortality in patients with Ebola virus disease. Clin Infect Dis. 2015;60:1821–1824. doi: 10.1093/cid/civ202. [DOI] [PubMed] [Google Scholar]
- 5.Qin E, Bi J, Zhao M, Wang Y, Guo T, Yan T, Li Z, Sun J, Zhang J, Chen S, Wu Y, Li J, Zhong Y. Clinical features of patients with Ebola virus disease in Sierra Leone. Clin Infect Dis. 2015;61:491–495. doi: 10.1093/cid/civ319. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Case Definition Recommendations for Ebola or Marburg Virus Diseases. 2015. http://www.who.int/csr/resources/publications/ebola/case-definition/en/ Available at. Accessed September 6, 2015.
- 7.Brainard J, Hooper L, Pond K, Edmunds K, Hunter PR. Risk factors for transmission of Ebola or Marburg virus disease: a systematic review and meta-analysis. Int J Epidemiol. 2015;45:102–116. doi: 10.1093/ije/dyv307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan LM, Massaro JM, D'Agostino RB. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 9.Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh F, Turnbull BW. Nonparametric and semiparametric estimation of the receiver operating characteristic curve. Ann Stat. 1996;24:25–40. [Google Scholar]
- 11.Pittalis S, Fusco FM, Lanini S, Nisii C, Puro V, Lauria FN, Ippolito G. Case definition for Ebola and Marburg haemorrhagic fevers: a complex challenge for epidemiologists and clinicians. New Microbiol. 2009;32:359–367. [PubMed] [Google Scholar]
- 12.Dhillon RS, Srikrishna D, Sachs J. Controlling Ebola: next steps. Lancet. 2014;384:1409–1411. doi: 10.1016/S0140-6736(14)61696-2. [DOI] [PubMed] [Google Scholar]
- 13.Maganga GD, Kapetshi J, Berthet N, Kebela Ilunga B, Kabange F, Mbala Kingebeni P, Mondonge V, Muyembe J-JT, Bertherat E, Briand S, Cabore J, Epelboin A, Formenty P, Kobinger G, González-Angulo L, Labouba I, Manuguerra J-C, Okwo-Bele J-M, Dye C, Leroy EM. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med. 2014;371:2083–2091. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- 14.Lado M, Walker NF, Baker P, Haroon S, Brown CS, Youkee D, Studd N, Kessete Q, Maini R, Boyles T, Hanciles E, Wurie A, Kamara TB, Johnson O, Leather AJM. Clinical features of patients isolated for suspected Ebola virus disease at Connaught Hospital, Freetown, Sierra Leone: a retrospective cohort study. Lancet Infect Dis. 2015;15:1024–1033. doi: 10.1016/S1473-3099(15)00137-1. [DOI] [PubMed] [Google Scholar]
- 15.Levine AC, Shetty PP, Burbach R, Cheemalapati S, Glavis-Bloom J, Wiskel T, Kesselly JKT. Derivation and internal validation of the Ebola prediction score for risk stratification of patients with suspected Ebola virus disease. Ann Emerg Med. 2015;66:285–293. doi: 10.1016/j.annemergmed.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Bah EI, Lamah M-C, Fletcher T, Jacob ST, Brett-Major DM, Sall AA, Shindo N, Fischer WA, Lamontagne F, Saliou SM, Bausch DG, Moumié B, Jagatic T, Sprecher A, Lawler JV, Mayet T, Jacquerioz FA, Méndez Baggi MF, Vallenas C, Clement C, Mardel S, Faye O, Faye O, Soropogui B, Magassouba N, Koivogui L, Pinto R, Fowler RA. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–47. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 17.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, Sealfon RSG, Kanneh L, Moigboi A, Momoh M, Fullah M, Moses LM, Brown BL, Andersen KG, Winnicki S, Schaffner SF, Park DJ, Yozwiak NL, Jiang P-P, Kargbo D, Jalloh S, Fonnie M, Sinnah V, French I, Kovoma A, Kamara FK, Tucker V, Konuwa E, Sellu J, Mustapha I, Foday M, Yillah M, Kanneh F, Saffa S, Massally JLB, Boisen ML, Branco LM, Vandi MA, Grant DS, Happi C, Gevao SM, Fletcher TE, Fowler RA, Bausch DG, Sabeti PC, Khan SH, Garry RF. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan T, Mu J, Qin E, Wang Y, Liu L, Wu D, Jia H, Li Z, Guo T, Wang X, Qin Y, Li Y, Chen S, Zhang Y, Zhang J, Wu Y, Wang S, Li J. Clinical characteristics of 154 patients suspected of having Ebola virus disease in the Ebola holding center of Jui Government Hospital in Sierra Leone during the 2014 Ebola outbreak. Eur J Clin Microbiol Infect Dis. 2015;34:2089–2095. doi: 10.1007/s10096-015-2457-z. [DOI] [PubMed] [Google Scholar]