Abstract
The widespread implementation of long-lasting insecticidal nets (LLINs) is a major intervention method for malaria control. Although the LLINs coverage increases, information available on the physical integrity (PI) of implemented LLINs is incomplete. This study aimed to validate human IgG antibody (Ab) response to Anopheles gSG6-P1 salivary peptide antigen, previously demonstrated as a pertinent biomarker of human exposure to Anopheles bites, for evaluating the PI of LLINs in field conditions. We analyzed data from 262 randomly selected children (< 5 years of age) in health districts of Benin. Anti-gSG6-P1 IgG responses were assessed and compared with the PI of LLINs that these same children slept under, and evaluated by the hole index (HI). Specific IgG levels were positively correlated to LLINs HI (r = 0.342; P < 0.0001). According to antipeptide IgG level (i.e., intensity of vector exposure), two categories of LLINs PI were defined: 1) group “HI: [0, 100]” corresponding to LLINs with “good” PI and 2) “HI > 100” corresponding to LLINs with “bad” PI. These results suggest that human Ab response to salivary peptide could be a complementary tool to help defining a standardized threshold of efficacy for LLINs under field use.
Introduction
The absence of an effective malaria vaccine1–3 and the spread of parasite resistance against malaria treatment4 require the strengthening of vector control strategies that reduce vector populations or prevent human–vector contact for the efficient control of malaria. Insecticide-treated nets (ITNs) have proven to be effective against Anopheles vector biting and can considerably prevent malaria transmission.5 To avoid the poor retreatment practices of classical-treated ITNs,6 long-lasting insecticidal nets (LLINs) have been developed since the late 1990s. Since their first evaluation in 1999,7 LLINs have become one of two primary World Health Organization (WHO)–recommended approaches to prevent malaria transmission targeting the mosquito vector.8
The National Malaria Control Program (NMCP) of Benin started distributing LLINs in 2002 with social marketing that promoted subsidized nets within the existing retail sector.9 This was expanded to heavily subsidized nets provided to children and pregnant women through the maternal and child health clinics in 2004. In October 2007, the NMCP, with the support of the World Bank, conducted a nationwide distribution of LLINs to all Beninese children under 5 years of age. More recently, following support from Global Fund, another campaign of mass distribution of free LLINs within households has been organized in 2011.9
To maintain the effectiveness of LLINs to disrupt human–vector contact, regular national distribution of LLINs and their occasional replacement must be programmed in a timely way. Premature replacement is to be avoided for cost-effectiveness reasons and operational risk of failures. Currently, LLIN distributions and replacement needs are based on an assumption that LLINs have an average efficacy of 3–5 years. In Benin, for example, regular mass implementation of LLINs is performed at least every 3 years by the NMCP. However, LLINs efficacy could be overestimated because several factors are involved in net durability such as washing frequency, LLINs upkeep.10,11 Indeed, several studies conducted in Africa on the physical integrity (PI; number and size of holes) of mosquito nets showed different percentages of damaged nets (32–78%) after 12–25 months of use in the field.12–14 Using different cutoffs of the proportionate hole index (HI), other studies conducted in Kenya classified the torn LLINs as either “effective nets” or “ineffective nets.”15,16 These observations raise questions about the exposure of children to Anopheles bites sleeping under damaged LLINs and the need for standardized thresholds at which a given LLIN becomes ineffective (offers little or no protection against mosquito bites) in protecting the sleeper.
Recently, IgG responses to the Anopheles gambiae salivary peptide (gSG6-P1) has been validated as a complementary tool to entomological methods for assessing human–vector contact. A first study in young Senegalese children showed a positive association between anti-gSG6 P1 IgG response and the level of exposure of these children to An. gambiae bites.17 In the same area, another study indicated the potential use of this specific IgG response as a biomarker for low-level exposure to Anopheles bites, settings where conventional entomological methods present limits of sensitivity.18 A second study in northern Senegal demonstrated that the use of this biomarker could discriminate microgeographical heterogeneity of exposure to malaria vectors bites despite the general context of low exposure/transmission.19 In addition, the biomarker seemed to be a useful tool for monitoring the risk of malaria transmission in the dry season (similar context than a pre-elimination area presenting very low exposure to Anopheles vector) and discriminating uninfected children to infected ones.20 Another study evaluated the risk of malaria transmission in children and adults living in Dakar (Senegal) using the salivary biomarker of exposure to Anopheles bites. Results showed considerable variations in anti-gSG6-P1 IgG response levels within and between districts, despite the general context of low exposure to Anopheles.21 Moreover, this biomarker has been used in various areas as a criterion for assessing vector control effectiveness to reduce human–vector contact.22–24 In summary, anti-gSG6-P1 IgG response has been successful, at the population and individual levels, in assessing 1) efficacy of tested LLINs in phase three studies24 and 2) effectiveness of LLINs after mass distribution campaign.23 In addition, the IgG antibody (Ab) response to the gSG6-P1 salivary peptide was not cumulative over time and has a transient life (no detection of IgG after 1 month without, or with very few, Anopheles bites).23,24
The first step of this study was to evaluate the PI, measured by the new WHO HI method, of LLINs used by children, 4 years after LLIN mass distribution in Benin. The second step was then to evaluate the association between human IgG responses to the gSG6-P1 salivary peptide and LLINs PI. The final objective was to assess the potential use of the gSG6-P1 salivary peptide as a pertinent complementary tool that could help define a standardized threshold above which LLINs become ineffective to prevent human–vector contact, subsequently informing the need for replacement.
Materials and Methods
Site description.
The study was carried out in rural areas of Ouidah-Kpomassè-Tori Bossito (OKT) and Djougou-Ouaké-Copargo (DOC) health districts located respectively in south and north Benin, western Africa. In the OKT health district, the climate is essentially subequatorial with two rainy seasons (April–July and October–November) and two dry seasons (August–September and December–March). The average annual rainfall is around 1,200 mm, of which 700–800 mm in the first rainy season and 400–500 mm in the second rainy season. February–April are the hottest months (31°C), and July–September are the coldest months (27°C). The OKT health district is a mesoendemic malarious area with a mean annual entomological inoculation rate of 5.3.25,26 The main malaria vectors are An. gambiae s.l. and Anopheles funestus. Their densities vary according to village and with higher densities in villages close to an arm of the Toho Lake than in other villages. Resistance to pyrethroids has shown to be moderate.25,26
The DOC health district has essentially a South Sudanian climate with typically one rainy season (June–October) and one dry season (November–May). The mean monthly temperature ranges between 22°C and 33°C, with an annual mean rainfall of 1.300 mm. In the DOC, malaria transmission is typically seasonal. Anopheles gambiae s.l. is the primary vector species, and resistance to pyrethroids is high.27
Both districts have benefited from mass LLINs distribution by the NMCP in 2007 targeting pregnant women and children under 5 years of age. The study areas do not use indoor residual spraying.
Sampling of LLINs.
This study is a part of the evaluation de la lutte intégrée contre le paludisme au Bénin (EVA-LUT) project, which aimed to evaluate the effectiveness of LLINs in children under 5 years of age.28 Epidemiological data were collected from April to May 2011 in the OKT district and from July to August 2011 in the DOC district, corresponding to the peak of malaria transmission in both districts. Seventy-three villages were randomly selected in these two districts (31 in the OKT and 42 in the DOC). A total of 2,038 children in the OKT district and 1,632 children in the DOC district were enrolled in the investigation. A standardized questionnaire following the Whole Health Organization pesticide evaluation scheme guidelines was used by investigators to collect information about the use of LLINs in both districts. Dried blood spots (DBS; on Whatman 3 MM filter paper, GE Healthcare, Buckinglomshire, United Kingdom) were also collected from all children participating in the EVA-LUT project. DBS were kept at +4°C until use for immunological analysis, as previously described.24
For this study, 3–5 LLINs per village were randomly selected and collected by the medical staff in the households in both health districts. Of the 295 LLINs collected, only 262 were used in the study based on inclusion criteria of discerning net brand. Information about the age of LLINs and the child who slept under the net (whether the child slept under the net the night before, the frequency of net use, etc.) was also collected, and DBS were then available for all children for which LLINs were collected. Each collected bed net was replaced by a new LLIN by the medical staff of the study. The collected “old” LLINs were brought to the laboratory for examination.
LLIN evaluation procedures.
The PI of the collected LLINs was assessed by counting the number of holes (including tears and split seams), their location on the bed net, and their size (by measuring diameter in cm). Holes were classified into four categories according to the WHO procedures29 as follows:
Size 1 or S1 (finger size): smaller than a thumb (0.5–2 cm)
Size 2 or S2 (hand size): larger than a thumb but smaller than a fist (2–10 cm)
Size 3 or S3 (fist size): larger than a fist but smaller than a head (10–25 cm)
Size 4 or S4 (head size): larger than a head (> 25 cm).
Evidence of repairs and the type of repairs were also recorded for collected nets. Using information collected from each LLIN (number and size of holes), two indicators were calculated to analyze the PI of LLINs according the WHOPES procedure: the proportion of nets with holes and the HI.
Proportion of LLINs with any holes (with 95% confidence interval [CI]):
Numerator: Total number of LLINs with at least one hole of size 1–4
Denominator: Total number of LLINs collected in households
The proportionate HI of each net was estimated using the formula29:
HI = (A × no. of Size 1 holes) + (B × no. of Size 2 holes) + (C × no. of Size 3 holes) + (D × no. of Size 4 holes), where A = 1, B = 23, C = 196, and D = 578. A, B, C, and D are weighting factors. B, C, and D factors were estimated from the average diameter of the thumb, fist, and head, respectively, following to the WHO recommendations.29 S1 holes were considered as reference.
For each group of LLINs (dependent on the HI), the mean (and standard deviation) as well as the median (and interquartile range) of HI were determined.
Evaluation of human IgG Ab levels to gSG6-P1 peptide by enzyme-linked immunosorbent assay.
For each selected child (N = 262 sleeping under the selected bed nets), a standardized DBS (1 cm diameter) was eluted by incubation in 350 μL of phosphate buffered saline (PBS–Tween 0.1%) at +4°C for 48 hours. Enzyme-linked immunosorbent assay (ELISA) was carried out on eluates to evaluate the level of IgG responses to the gSG6-P1 peptide antigen, as previously described.24 Briefly, Maxisorp plates (Nunc, Roskilde, Denmark) were coated with gSG6-P1 (20 μg/mL) in PBS. After washing (distilled water + Tween 0.1%), each child's DBS eluate was then incubated (in duplicate) at +4°C overnight at a 1/40 dilution (PBS–Tween 1%). This optimal dilution had been determined by preliminary experiments. Mouse biotinylated Ab against human IgG (BD Pharmingen, San Diego, CA) was incubated at a 1/2,000 dilution in PBS with 1% Tween (1.5 hour at 37°C) and peroxidase-conjugated streptavidin (Amersham, Les Ulis, France) was then added (1/2,000; 1 hour at 37°C). Colorimetric development was carried out using ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium; Sigma, St Louis, MO) in 50 mM citrate buffer (pH = 4) containing 0.003% H2O2. Optical density (OD) was measured at 405 nm, after 2 hours at room temperature in the dark. In parallel, each test sample was assessed in a blank well containing no gSG6-P1 antigen (ODn) to measure nonspecific reactions. Known positive controls were included on each ELISA plate to control for plate-to-plate variation as well as reproducibility of the test. Specific anti-gSG6-P1 IgG levels were also assessed in unexposed individuals to Anopheles (N = 12; negative control from France) to quantify the nonspecific background of the ELISA test. Individual results were expressed as the ΔOD value: ΔOD = ODx − ODn, where ODx represents the mean of individual OD in both antigen wells.
Statistical analysis.
All data were digitized in an Excel program, and then transferred to GraphPad Prism 5 statistical software (GraphPad, San Diego, CA) for analysis. After confirming that specific IgG response data (expressed in ΔOD) did not fit a Gaussian distribution, the nonparametric Mann–Whitney U test was used for comparison of Ab levels of two independent groups, and the nonparametric Kruskal–Wallis test to compare ΔOD between more than two independent groups. The correlation between LLINs HI and IgG Ab level to the gSG6-P1 peptide was evaluated using the Spearman correlation method. All differences were considered significant at P < 0.05.
Ethics statement.
This study followed ethical principles recommended by the Edinburgh revision of the Helsinki Declaration. The study was approved by the Ethical Committee of Benin Ministry of Health (CNPERS, Institutional Review Board No. 00006860; Number 003 of March 24, 2011) and the “Comité Consultatif de Déontologie et d'Ethique” of Institut de Recherche pour le Développement, of June 20, 2011. The written informed consent of all parents or guardians of children who participated in the study was obtained before inclusion. Final approval was granted by Provisional National Ethics Committee of the Ministry of Health of Benin (no. register: 003-1R800006860).
Results
PI of LLINs.
Among evaluated nets, only 17% (45/262; 95% CI: 12–22%) did not have holes. The great majority of LLINs had holes categorized as S1 (85%; 222/262; 95% CI: 81–89%) or S2 (78%; 205/262; 95% CI: 73–83%) followed by LLINs containing S3 (26%; 69/262; 95% CI: 21–31%) and S4 (5%; 14/262; 95% CI: 2–8%) hole classifications. These results indicate that LLINs collected in the field had a very large number of holes (larger than a thumb) that may allow mosquito entry.
Specific IgG response levels and relationship with HI to determine LLINs PI.
A positive and significant correlation (r = +0.3023, P < 0.0001; Spearman test) between specific IgG level and HI of LLINs was demonstrated (data not shown). These results suggest that, even if correlation value was weak, an increase of the HI values was associated with an increase of specific IgG response levels to the gSG6-P1 peptide.
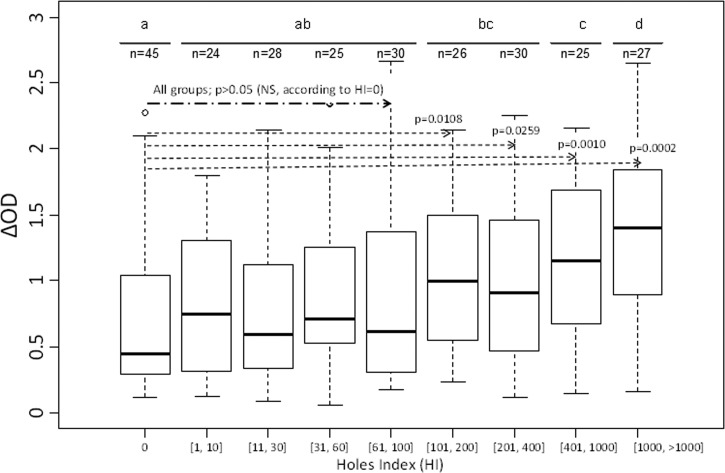
For a step-by-step approach, we then created and clustered amplitude classes to have similar number of individuals between HI classes. Nine classes were obtained (Figure 1 ). Our results showed, overall, an increase of the median level of IgG responses to gSG6-P1 peptide according to the defined HI class. The median value of specific IgG level appeared very low for HI = [0] class and significantly increased for HI = [1–10] (P = 0.011). Between HI = [1–10] and HI = [61–100], the medians of specific IgG levels varied but remained statistically similar (P > 0.05; nonparametric Kruskal–Wallis test). In addition, no significant difference was observed when comparing the first five HI classes (from HI = [0] to HI = [61–100]; P > 0.05). However, a considerable increase in the median of specific IgG level was observed from HI = [101–200] (compared with HI = [61–100]) and progressively increased up to HI = [1,000 to >1,000]. As expected, results validated that significant differences in IgG level were observed between HI = [0] LLINs and each of [101–200], [201, 400], [401, 1,000], and [1,000 > 1,000] classes (P = 0.0108, P = 0.0259, P = 0.0010, and P = 0.0002, respectively; nonparametric Mann–Whitney U test; Figure 1).
Figure 1.
IgG levels to gSG6-P1 salivary peptide according to HI groups. Box plots show anti-gSG6-P1 IgG level (ΔOD) of the different HI groups. The box plots display the median ΔOD value, 25th and 75th percentiles. The dots indicate the outliers, and horizontal bars indicate the median value for each group. Differences between the two groups were tested by using the nonparametric Mann–Whitney U test. HI = hole index; OD = optical density.
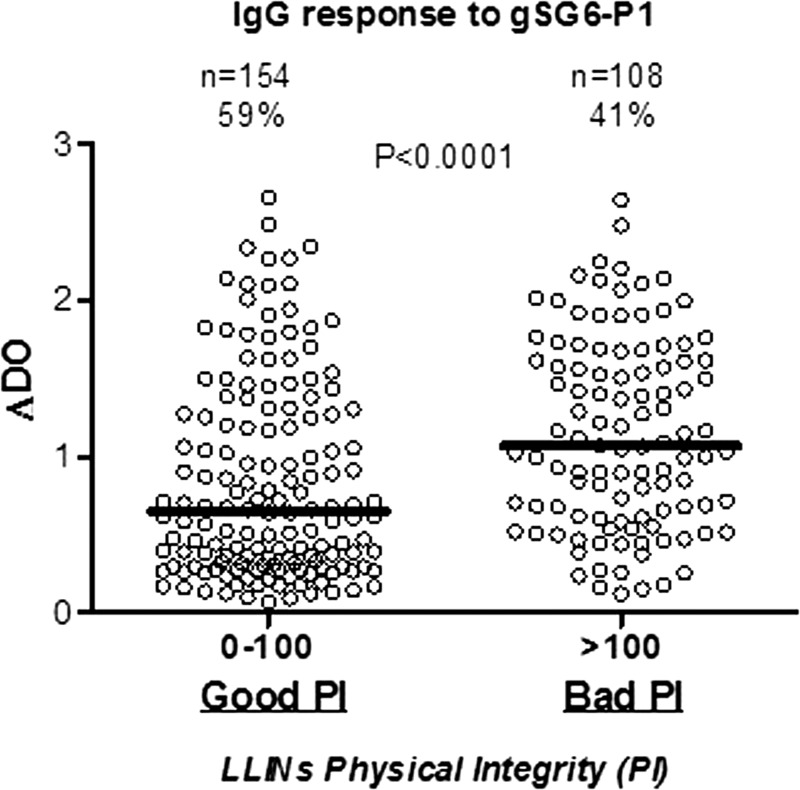
These results seemed to distinguish two groups of LLINs: group 1, corresponding to LLINs with an HI varying between 0 and 100 (good PI or “effective”), and group 2, corresponding to LLINs with an HI > 100 (bad PI or “ineffective”) (Figure 1).
As expected, the median of specific IgG responses progressively increased according to these two groups (Figure 2 ) and was significantly lower in group 1 compared with group 2 (P < 0.0001; nonparametric Mann–Whitney U test). HI = 100 seemed to be the threshold above which children sleeping under such LLINs became highly exposed to Anopheles vector bites. Then, we suggest the following classification for LLINs according to the immunological results of salivary biomarker and the HI (Figure 2): HI ≤ 100 = “effective” LLINs (offer protection against mosquito bites) and HI > 100 = “ineffective” LLINs (offer no or minimal protection against mosquito bites).
Figure 2.
IgG levels to gSG6-P1 peptide of individuals sleeping under LLINs classified as “Good PI” or “Bad PI.” PI was assessed through HI value associated to 0–100 and > 100 ranges, respectively, for the two status “Good PI” and “Bad PI.” Individual specific IgG level (ΔOD) results are shown for the two groups, and horizontal bars indicate the median value for each PI groups. Statistical significant differences are indicated according to the P value (nonparametric Mann–Whitney U test). HI = hole index; LLINs = long-lasting insecticidal nets; OD = optical density; PI = physical integrity.
PI according to LLINs age and manufacturer type.
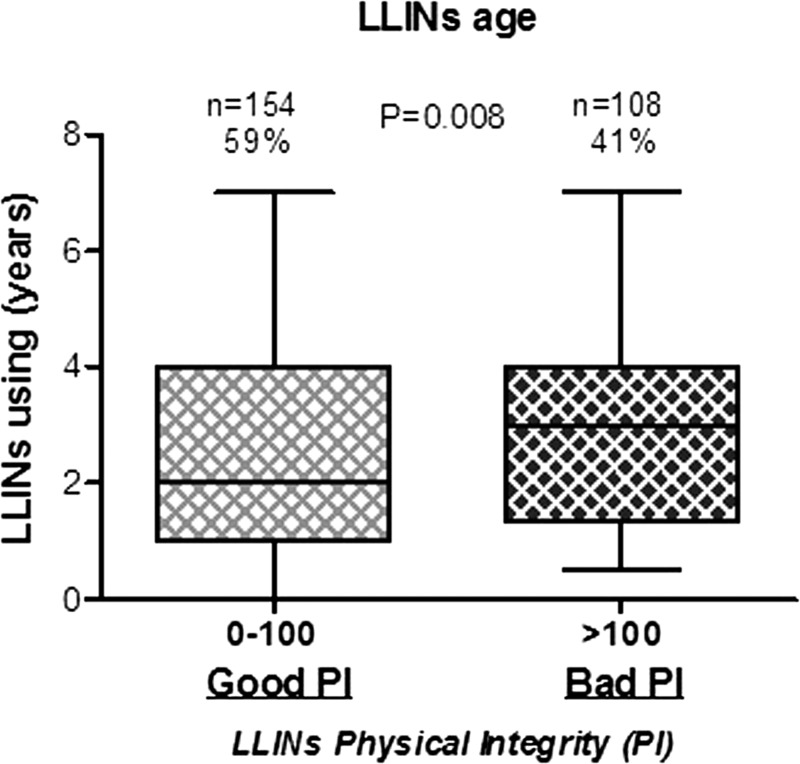
According to PI groups previously defined (effective and ineffective LLINs), the median age (in year) of collected LLINs was first determined (Figure 3 ). The median of LLINs age with “effective” LLINs was low (2.26 years) compared with “ineffective” LLINs (2.75 years; P = 0.0081; nonparametric Mann–Whitney U test). These results suggest that LLINs need replacement after about 2 years of use to keep their protective effect.
Figure 3.
Age of LLINs according to the two defined groups of LLIN PI. Box plots show LLINs age in the two different HI groups associated to 0–100 and > 100 ranges for the two status “Good PI” (light gray) and “Bad PI” (black box), respectively. Horizontal lines in the boxes indicate the median LLIN age value. Boxes display 25th and 75th percentiles. The difference between the two groups was tested by using the nonparametric Mann–Whitney U test. HI = hole index; LLINs = long-lasting insecticidal nets; OD = optical density; PI = physical integrity.
A correlation between specific IgG response to gSG6-P1 peptide and HI was also assessed according to the manufacturer type of LLINs collected in the field (Olyset®, Sumitomo Chemical, Tokyo, Japan, N = 75, PermaNet®, Vestergaard Frandsen, Lausanne, Switzerland, N = 165 and Interceptor®, BASF corporation, Wädenswill, Switzerland, N = 22). Similarly, positive correlations between HI and anti-gSG6-P1 IgG level were observed (Table 1) for the first two LLINs type, with similar coefficients values: Olyset® (r = +0.31, P = 0.0158; Spearman test) and PermaNet (r = +0.35, P = 0.0071). However, no correlation was found between HI and anti-gSG6-P1 IgG level for Interceptor® LLINs. Specific IgG response to the salivary peptide and HI evolution does not seem to depend on LLINs' manufacturer type.
Table 1.
Correlation between anti-gSG6-P1 IgG levels and HI according to manufacturer type
| N | Correlation HI with IgG | |||
|---|---|---|---|---|
| r | 95% CI | P value | ||
| LLINs (total) | 262 | 0.30 | 0.18–0.41 | < 0.0001 |
| Olyset® | 75 | 0.31 | 0.05–0.52 | 0.0158 |
| PermaNet® | 165 | 0.35 | 0.09–0.57 | 0.0071 |
| Interceptor® | 22 | 0.03 | −0.40–0.45 | 0.8845 |
CI = confidence interval; HI = hole index; LLINs = long-lasting insecticidal nets; r = correlation coefficient obtained by Spearman test, P value.
Discussion
The measure of a child's exposure to Anopheles bites using a salivary biomarker, as presented in this study, could be a pertinent indicator for monitoring the risk of malaria after LLIN mass distribution campaigns. Indeed, anti-gSG6-P1 IgG response levels were positively correlated with the HI, corresponding to the potential deterioration of LLIN efficacy. These data suggest that children sleeping under deteriorated LLINs were more exposed to Anopheles vector bites, thus at risk of malaria. A recent work has shown a higher human–vector contact from 0 to an average of 5 bites/man/night in association with the deterioration of LLINs.30 This demonstrates the importance of monitoring LLINs PI as a measure of malaria prevention. Our results also showed that LLINs deterioration, which induces the loss of the ability to prevent human–vector contact, seemed to occur more rapidly (within 2 years) than the predicted 3–5 years provided by manufacturers to guide NMCPs for their replacement. A recent study conducted in Benin31 has also found a rapid deterioration of LLINs distributed by the NMCP in 2011, due to washing frequency. Elsewhere in Africa, Ritmeijer and others32 reported 44% of fine-mesh LLINs reasonably intact without holes after 2 years of use during a leishmaniasis control program in Sudan. Another study found 78% of polyester nets with holes, 12–15 months after LLINs distribution in a refugee camp in western Uganda.33 They ranged from 45% severely damaged (> 7 holes larger than 2 cm) in the cross-sectional net survey10 to 33.2% with > 5 holes after 2 years in Sudan32 and to 28% with at least one hole of 40 cm2 after 12–15 months in Uganda.33 Taken together, these data show that a 2-year replacement of LLINs would be more relevant than an every 3–4 years to avoid a potential malaria rebound after 2 years of use, due to LLINs that did not offer substantial protection against mosquito bites.
This study has also explored the methodological aspects for determining the PI of a given net. Indeed, a standardized HI29 was developed so that the PI of nets could be categorized, by using a weight factor corresponding to each category of hole (thumb, fist, head, or larger than head). This weight is approximately proportional to the average surface area of each hole category. Then, the HI includes not only the presence or absence of holes but also takes into account their number and size. For this reason, several studies use this HI for monitoring LLINs PI after mass distribution campaigns by NMCPs in several African countries.34–36 However, little data are available to decide on a threshold at which a given LLIN is considered as “good” (effective/no replacement) or “bad” (ineffective/need replacement), because each study used a different HI. The use of biomarker for evaluating the real human–vector contact could be therefore an alternative to define an adequate threshold. Indeed, some authors classify LLINs into four categories according to their HI: good condition (HI < 25), fair condition (25 ≥ HI ≤ 174), mediocre condition (175 ≥ HI ≤ 299), and poor condition (HI > 300).37,38 Other authors propose a classification into three groups: good condition (HI < 64), serviceable (64 ≥ HI ≤ 768), and needs replacement (HI > 768).39 In a previous study in Tanzania, an “intact net” was defined as nets with less than 20 holes, which are smaller than 2 cm in diameter.40 By this definition, 83% of nets in our study were “intact” or in “Good PI,” assuming that finger-sized holes were smaller than 2 cm in diameter. Therefore, additional tools are needed to better evaluate LLINs PI so as to help NMCPs deciding when to replace LLINs in the field.
To provide a more standardized and continuous measure of LLINs PI that takes into account human–vector contact, the HI was combined with the salivary biomarker of exposure to Anopheles vector bites (human anti-gSG6-P1 IgG responses), which was routinely validated as an indicator of the “real” human–vector contact. In addition, assessing number and size of holes represent a considerable work, not possible to perform directly in the bedroom of inhabitants (because of the LLINs have to be removed from the bed), and is time consuming. For these reasons, we estimate that the proposed salivary biomarker could be an interesting and adequate alternative strategy to evaluate, even if indirect, the PI of LLINs. Our results showed that two groups of LLINs could be distinguished according to the level of specific IgG, that is, exposure to Anopheles bites, of children sleeping under these net: 1) nets with a “good” PI/effective (HI ≤ 100; 59%) and 2) nets with a “poor or bad” PI/ineffective (HI > 100; 41%). In addition, HI > 100 seems to be the threshold at which LLINs should be replaced when regarding the high level of specific IgG observed in this group compared with the other. This result appeared to be confirmed by LLINs mean age, which showed that LLINs with a “good/effective” PI were implemented or used only since 2.26 years, whereas higher mean age were observed for LLINs in “bad/ineffective” PI (> 2.6 years). These results reflect similar trends as those obtained elsewhere in Africa. In Benin, a study30 has shown that at an HI of 276, several mosquito species were able to enter the LLINs through the holes to bite. Another study, in Kenya, had shown that the mean number of Anopheles mosquitoes found within nets increased in nets with hole sizes above 50 cm.2,41 In addition, the main malaria vector, An. gambiae s.l., was the most collected mosquito species that entered the torn LLINs to bite, and some were even tested positive for Plasmodium falciparum sporozoites. Our results suggest the relevance of using the salivary biomarker of exposure to Anopheles vector bites as a tool for defining a threshold at which LLINs become ineffective, that is, not protecting against mosquito bites. Indeed, exposure to malaria vectors changes as children sleep under LLINs with a “good” or a “bad” PI. Previous studies on LLINs have shown a rapid drop in anti-gSG6P1 IgG responses after implementation of LLINs in a semiurban area, Angola. This specific IgG response was positively associated with a correct use of LLINs that were in good condition.24 But, an increase in anti-gSG6-P1 IgG responses was observed 8 months after LLINs implementation suggesting that individuals are reexposed to Anopheles bites. This biomarker seems to be a pertinent tool to evaluate the variations in human exposure to Anopheles bites after LLINs mass distribution campaigns.
However, our results showed that some children sleeping under LLINs with “good/effective” PI presented positive anti-gSG6-P1 IgG response, which was quite high and varied according to the child. This suggests that they were also exposed to Anopheles vector bites when they were out of LLINs before sleeping. A study in Kenya has shown a rise in vector biting activity (An. funestus) between 19:30 and 20:30 outdoors and 18:30 and 19:30 indoors.42 We can assume that a mother who puts her child to bed (under a net) a little later than another mother who puts her child to bed earlier (under a net) may increase the child's probability of exposure to Anopheles vector bites. Other factors could influence anti-gSG6P1 IgG responses. A study in young Beninese children has shown that the anti-gSG6-P1 IgG response levels increased with age, which corresponds to a progressive acquisition of antisaliva immunity.43 In Kenya, a study reported the presence of An. gambiae s.l. within nets without holes.41 So, children sleeping under LLINs with a “good/effective” PI could be also exposed to Anopheles bites if nets were not tucked properly underneath the mattress, leaving openings for mosquitoes to enter. Currently, such biomarker appeared therefore to be adequate at the population level, to define “effective” versus “not effective” LLINs. It appeared then essential to determine and to apply a threshold of HI corresponding to a threshold of salivary biomarker level at which an observed LLIN is not very effective against Anopheles vectors. Other studies in the future must be performed in different settings to determine the individual applicability of the biomarker and threshold suggested here to a broader range of malaria transmission settings. A more focused LLIN distribution/replacement scheme that takes into account standardized measure of “ineffective” PI is required. As the roll back malaria partnership aims to attain LLINs coverage among vulnerable groups, the benefits acquired through scaling up LLINs coverage may be lost if the LLINs are either damaged or not effectively used.
Conclusions
New indicators are urgently needed alone or in combination with existing ones to assess the PI of LLINs for malaria control and prevention. This study points out the association between the HI of LLINs and a salivary biomarker of human exposure to Anopheles bites. Findings reported here highlight the ability of using this new immunoepidemiological indicator to define categories of PI to include “effective” or “ineffective” (LLIN needs replacement). Future studies are needed to refine indicator thresholds and subsequent definitions of LLINs PI. Nevertheless, preliminary results represent an important step toward the establishment of using epidemiological indicators for monitoring the PI of LLINs. The development and use of salivary biomarkers could represent an alternative mechanism for measuring the PI of LLINs distributed by NMCPs. For large scale use, future development of such individual biomarker concerns its application under rapid diagnostic test (i.e., dipstick), which could considerably increase its use directly in the field, as a point-of-care tool.
ACKNOWLEDGMENTS
We thank the collaboration of National Malaria Control Program of Benin. We also thank the populations participating in the EVA-LUT project and authorities of the OKT and DOC health district for their kind support and collaboration. We are grateful to Marie-Claire Henry to have initiated this project and all medical and epidemiological staff of EVA-LUT project.
Footnotes
Financial support: The study was supported by NMCP of Benin (PALP project no. 555 MS/DC/SGM/DNPS/PNLP/PALP/DNMP DU 30/12/2010) and Institut de Recherche pour le Développement (IRD).
Authors' addresses: Mahoutin H. Noukpo, Georgia B. Damien, and Emmanuel Elanga-N'Dille, UMR IRD 224–CNRS 5290–Universités Montpellier Maladies Infectieuses et Vecteurs: Ecologie, Génétique, Evolution et Contrôle (MIVEGEC), Cotonou, Bénin, and Centre de Recherche Entomologique de Cotonou (CREC), Cotonou, Bénin, E-mails: hnoukpo@yahoo.fr, georgia.damien@ird.fr, and emmsdille@yahoo.fr. André B. Sagna, Papa M. Drame, and Franck Remoue, Institut Pierre Richet (IPR), Institut Nationale de la Santé Publique (INSP), Bouaké, Côte d'Ivoire, and UMR IRD 224–CNRS 5290–Universités Montpellier Maladies Infectieuses et Vecteurs: Ecologie, Génétique, Evolution et Contrôle (MIVEGEC), Cotonou, Bénin, E-mails: sagna.ab@gmail.com, papamak2002@yahoo.fr, and franck.remoue@ird.fr. Evelyne Chaffa, Programme National de Lutte Contre le Paludisme (PNLP), Ministère de la Santé, Cotonou, Bénin, E-mail: evechaff2000@yahoo.f. Olayidé Boussari, Centre de Recherche Entomologique de Cotonou (CREC), Cotonou, Bénin, E-mail: olayideb@yahoo.fr. Vincent Corbel, Department of Entomology, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Nakhon Pathom, Thailand, E-mail: vincent.corbel@ird.fr. Martin Akogbéto, Centre de Recherche Entomologique de Cotonou (CREC), Cotonou, Bénin, and Faculté des Sciences et Techniques (FAST), Université d'Abomey Calavi (UAC), Abomey, Bénin, E-mail: akogbetom@yahoo.fr.
References
- 1.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood BM, Targett GA. Malaria vaccines and the new malaria agenda. Clin Microbiol Infect. 2011;17:1600–1607. doi: 10.1111/j.1469-0691.2011.03612.x. [DOI] [PubMed] [Google Scholar]
- 3.Rogier C, Orlandi-Pradines E, Fusai T, Pradines B, Briolant S, Almeras L. Malaria vaccines: prospects and reality [in French] Med Mal Infect. 2006;36:414–422. doi: 10.1016/j.medmal.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien C, Henrich PP, Passi N, Fidock DA. Recent clinical and molecular insights into emerging artemisinin resistance in Plasmodium falciparum. Curr Opin Infect Dis. 2011;24:570–577. doi: 10.1097/QCO.0b013e32834cd3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Guillet P, Alnwick D, Cham MK, Neira M, Zaim M, Heymann D, Mukelabai K. Long-lasting treated mosquito nets: a breakthrough in malaria prevention. Bull World Health Organ. 2001;79:998. [PMC free article] [PubMed] [Google Scholar]
- 7.Doannio JM, Dossou-Yovo J, Diarrassouba S, Chauvancy G, Darriet F, Chandre F, Henry MC, Nzeyimana I, Guillet P, Carnevale P. Efficacy of permethrin-impregnated Olyset net mosquito nets in a zone with pyrethroid resistant vectors. I–Entomologic evaluation [in French] Med Trop (Mars) 1999;59:349–354. [PubMed] [Google Scholar]
- 8.World Health Organization . WHO Releases New Guidance on Insecticide-Treated Mosquito Nets. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 9.Ministry of Health of Benin . Annuaire des Statistiques Sanitaires 2011: Direction de la Programmation et de la Prospective. Cotonou City, Benin: Ministry of Health of Benin; 2012. p. 122. [Google Scholar]
- 10.Erlanger TE, Enayati AA, Hemingway J, Mshinda H, Tami A, Lengeler C. Field issues related to effectiveness of insecticide-treated nets in Tanzania. Med Vet Entomol. 2004;18:153–160. doi: 10.1111/j.0269-283X.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- 11.Kilian A. How long does a long-lasting insecticidal net last in the field? Public Health J. 2010;21:43–47. [Google Scholar]
- 12.Kilian A, Byamukama W, Pigeon O, Atieli F, Duchon S, Phan C. Long-term field performance of a polyester-based long-lasting insecticidal mosquito net in rural Uganda. Malar J. 2008;7:49. doi: 10.1186/1475-2875-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilian A, Byamukama W, Pigeon O, Gimnig J, Atieli F, Koekemoer L, Protopopoff N. Evidence for a useful life of more than three years for a polyester-based long-lasting insecticidal mosquito net in western Uganda. Malar J. 2011;10:299. doi: 10.1186/1475-2875-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin A, Azondekon R, Gueye S, Green M, Beach R. Tracking Long-lasting Insecticidal (Mosquito) Nets (LLINs) Distributed via National Campaign: Assessing LLIN Loss, Physical Deterioration, and Insecticidal Decay in Benin: Retrospective Study. Cotonou, Benin: PMI/USAID/CREC; 2011. pp. 10–28. [Google Scholar]
- 15.Mejia P, Teklehaimanot HD, Tesfaye Y, Teklehaimanot A. Physical condition of Olyset® nets after five years of utilization in rural western Kenya. Malar J. 2013;12:158. doi: 10.1186/1475-2875-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutuku FM, Khambira M, Bisanzio D, Mungai P, Mwanzo I, Muchiri EM, King CH, Kitron U. Physical condition and maintenance of mosquito bed nets in Kwale County, coastal Kenya. Malar J. 2013;12:46. doi: 10.1186/1475-2875-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poinsignon A, Cornelie S, Mestres-Simon M, Lanfrancotti A, Rossignol M, Boulanger D, Cisse B, Sokhna C, Arca B, Simondon F, Remoue F. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One. 2008;3:e2472. doi: 10.1371/journal.pone.0002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poinsignon A, Cornelie S, Ba F, Boulanger D, Sow C, Rossignol M, Sokhna C, Cisse B, Simondon F, Remoue F. Human IgG response to a salivary peptide, gSG6-P1, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malar J. 2009;8:198. doi: 10.1186/1475-2875-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagna AB, Sarr JB, Gaayeb L, Drame PM, Ndiath MO, Senghor S, Sow CS, Poinsignon A, Seck M, Hermann E, Schacht AM, Faye N, Sokhna C, Remoue F, Riveau G. gSG6-P1 salivary biomarker discriminates micro-geographical heterogeneity of human exposure to Anopheles bites in low and seasonal malaria areas. Parasit Vectors. 2013;6:68. doi: 10.1186/1756-3305-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagna AB, Gaayeb L, Sarr JB, Senghor S, Poinsignon A, Boutouaba-Combe S, Schacht AM, Hermann E, Faye N, Remoue F, Riveau G. Plasmodium falciparum infection during dry season: IgG responses to Anopheles gambiae salivary gSG6-P1 peptide as sensitive biomarker for malaria risk in northern Senegal. Malar J. 2013;12:301. doi: 10.1186/1475-2875-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drame PM, Machault V, Diallo A, Cornelie S, Poinsignon A, Lalou R, Sembene M, Dos Santos S, Rogier C, Pages F, Le Hesran JY, Remoue F. IgG responses to the gSG6-P1 salivary peptide for evaluating human exposure to Anopheles bites in urban areas of Dakar region, Senegal. Malar J. 2012;11:72. doi: 10.1186/1475-2875-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbel V, Remoue F, Rogier C. Synergies in integrated malaria control—authors' reply. Lancet Infect Dis. 2013;13:112–113. doi: 10.1016/S1473-3099(12)70340-7. [DOI] [PubMed] [Google Scholar]
- 23.Drame PM, Diallo A, Poinsignon A, Boussari O, Dos Santos S, Machault V, Lalou R, Cornelie S, LeHesran JY, Remoue F. Evaluation of the effectiveness of malaria vector control measures in urban settings of Dakar by a specific Anopheles salivary biomarker. PLoS One. 2013;8:e66354. doi: 10.1371/journal.pone.0066354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drame PM, Poinsignon A, Besnard P, Cornelie S, Le Mire J, Toto JC, Foumane V, Dos-Santos MA, Sembene M, Fortes F, Simondon F, Carnevale P, Remoue F. Human antibody responses to the Anopheles salivary gSG6-P1 peptide: a novel tool for evaluating the efficacy of ITNs in malaria vector control. PLoS One. 2010;5:e15596. doi: 10.1371/journal.pone.0015596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djenontin A, Bio-Bangana S, Moiroux N, Henry MC, Bousari O, Chabi J, Osse R, Koudenoukpo S, Corbel V, Akogbeto M, Chandre F. Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vectors in Ouidah-Kpomasse-Tori district from Benin (west Africa): a pre-intervention study. Parasit Vectors. 2010;3:83. doi: 10.1186/1756-3305-3-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djogbenou L, Pasteur N, Akogbeto M, Weill M, Chandre F. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med Vet Entomol. 2011;25:256–267. doi: 10.1111/j.1365-2915.2010.00925.x. [DOI] [PubMed] [Google Scholar]
- 27.Djogbenou L, Pasteur N, Bio-Bangana S, Baldet T, Irish SR, Akogbeto M, Weill M, Chandre F. Malaria vectors in the Republic of Benin: distribution of species and molecular forms of the Anopheles gambiae complex. Acta Trop. 2010;114:116–122. doi: 10.1016/j.actatropica.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Damien GB, Djenontin A, Chaffa E, Yamadjako S, Drame PM, Ndille EE, Henry MC, Corbel V, Remoue F, Rogier C. Effectiveness of insecticidal nets on uncomplicated clinical malaria: a case-control study for operational evaluation. Malar J. 2016;15:102. doi: 10.1186/s12936-016-1156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . Guidelines for Monitoring the Durability of Long-Lasting Insecticidal Mosquito Nets under Operational Conditions. Geneva, Switzerland: World Health Organization; 2011. p. 44. [Google Scholar]
- 30.Gnanguenon V, Azondekon R, Oke-Agbo F, Sovi A, Osse R, Padonou G, Aikpon R, Akogbeto MC. Evidence of man-vector contact in torn long-lasting insecticide-treated nets. BMC Public Health. 2013;13:751. doi: 10.1186/1471-2458-13-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnanguenon V, Azondekon R, Oke-Agbo F, Beach R, Akogbeto M. Durability assessment results suggest a serviceable life of two, rather than three, years for the current long-lasting insecticidal (mosquito) net (LLIN) intervention in Benin. BMC Infect Dis. 2014;14:69. doi: 10.1186/1471-2334-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritmeijer K, Davies C, van Zorge R, Wang SJ, Schorscher J, Dongu'du SI, Davidson RN. Evaluation of a mass distribution programme for fine-mesh impregnated bednets against visceral leishmaniasis in eastern Sudan. Trop Med Int Health. 2007;12:404–414. doi: 10.1111/j.1365-3156.2006.01807.x. [DOI] [PubMed] [Google Scholar]
- 33.Spencer S, Grant AD, Piola P, Tukpo K, Okia M, Garcia M, Salignon P, Genevier C, Kiguli J, Guthmann JP. Malaria in camps for internally-displaced persons in Uganda: evaluation of an insecticide-treated bednet distribution programme. Trans R Soc Trop Med Hyg. 2004;98:719–727. doi: 10.1016/j.trstmh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Craig AS, Muleba M, Smith SC, Katebe-Sakala C, Chongwe G, Hamainza B, Walusiku B, Tremblay M, Oscadal M, Wirtz R, Tan KR. Long-lasting insecticidal nets in Zambia: a cross-sectional analysis of net integrity and insecticide content. Malar J. 2015;14:239. doi: 10.1186/s12936-015-0754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakizimana E, Cyubahiro B, Rukundo A, Kabayiza A, Mutabazi A, Beach R, Patel R, Tongren JE, Karema C. Monitoring long-lasting insecticidal net (LLIN) durability to validate net serviceable life assumptions, in Rwanda. Malar J. 2014;13:344. doi: 10.1186/1475-2875-13-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wills AB, Smith SC, Anshebo GY, Graves PM, Endeshaw T, Shargie EB, Damte M, Gebre T, Mosher AW, Patterson AE, Tesema YB, Richards FO, Jr, Emerson PM. Physical durability of PermaNet 2.0 long-lasting insecticidal nets over three to 32 months of use in Ethiopia. Malar J. 2013;12:242. doi: 10.1186/1475-2875-12-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batisso E, Habte T, Tesfaye G, Getachew D, Tekalegne A, Kilian A, Mpeka B, Lynch C. A stitch in time: a cross-sectional survey looking at long lasting insecticide-treated bed net ownership, utilization and attrition in SNNPR, Ethiopia. Malar J. 2012;11:183. doi: 10.1186/1475-2875-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilian A. LLIN Durability: Where We are and Where We Want to Go?; 6th RBM Vector Control Working Group Meeting; Geneva, Switzerland. February 7–9, 2011.2011. [Google Scholar]
- 39.Allan R, O'Reilly L, Gilbos V, Kilian A. An observational study of material durability of three World Health Organization-recommended long-lasting insecticidal nets in eastern Chad. Am J Trop Med Hyg. 2012;87:407–411. doi: 10.4269/ajtmh.2012.11-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maxwell CA, Msuya E, Sudi M, Njunwa KJ, Carneiro IA, Curtis CF. Effect of community-wide use of insecticide-treated nets for 3–4 years on malarial morbidity in Tanzania. Trop Med Int Health. 2002;7:1003–1008. doi: 10.1046/j.1365-3156.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- 41.Ochomo EO, Bayoh NM, Walker ED, Abongo BO, Ombok MO, Ouma C, Githeko AK, Vulule J, Yan G, Gimnig JE. The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malar J. 2013;12:368. doi: 10.1186/1475-2875-12-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, Nyangau D, Abel L, Atieno E, Awuor S, Drakeley C, Cox J, Stevenson J. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259. doi: 10.1186/s12936-015-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drame PM, Poinsignon A, Dechavanne C, Cottrell G, Farce M, Ladekpo R, Massougbodji A, Cornélie S, Courtin D, Migot-Nabias F, Garcia A, Remoue F. Specific antibodies to Anopheles gSG6-P1 salivary peptide to assess early childhood exposure to malaria vector bites. Malar J. 2015;14:285. doi: 10.1186/s12936-015-0800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]