Abstract
Multiple community-based approaches can aid in quantifying mortality in the absence of reliable health facility data. Community-based sentinel site surveillance that was used to document mortality and the systems utility for outbreak detection was evaluated. We retrospectively analyzed data from 46 sentinel sites in three sous-préfectures with a reinforced malaria control program and one sous-préfecture without (Koundou) in Guinea. Deaths were recorded by key informants and classified as due to malaria or another cause. Malaria deaths were those reported as due to malaria or fever in the 3 days before death with no other known cause. Suspect Ebola virus disease (sEVD) deaths were those due to select symptoms in the EVD case definition. Deaths were aggregated by sous-préfecture and analyzed by a 6-month period. A total of 43,000 individuals were monitored by the surveillance system; 1,242 deaths were reported from July 2011–June 2014, of which 55.2% (N = 686) were reported as due to malaria. Malaria-attributable proportional mortality decreased by 26.5% (95% confidence interval [CI] = 13.9–33.1, P < 0.001) in the program area and by 6.6% (95% CI = −17.3–30.5, P = 0.589) in Koundou. Sixty-eight deaths were classified as sEVD and increased by 6.1% (95% CI = 1.3–10.8, P = 0.021). Seventeen sEVD deaths were reported from November 2013 to March 2014 including the first two laboratory-confirmed EVD deaths. Community surveillance can capture information on mortality in areas where data collection is weak, but determining causes of death remains challenging. It can also be useful for outbreak detection if timeliness of data collection and reporting facilitate real-time data analysis.
Background
Malaria is endemic in the Republic of Guinea (Guinea) and was the cause of 34% of all medical consultations in public health facilities in 2012.1 There are, however, important regional differences in malaria endemicity in Guinea; prevalence in children under 5 years of age ranges from 3% in Conakry, the urban capital, to over 55% in the southern, more heavily forested areas of the country.1 Since 2008, every case of uncomplicated malaria should receive oral antimalarial treatment with an artemisinin-based combination therapy (ACT), and in 2013, malaria rapid diagnostic tests (RDTs) were made available free of charge in health facilities.2 However, in the 2012 Demographic and Health Survey, 45% of respondents with a self-reported episode of malaria in the 6 months before the survey neither sought care nor received treatment.1
In areas where civil registration and vital registration systems are inadequate and where health-care seeking at public health facilities is infrequent, surveillance data from health facilities may not accurately reflect community mortality. At present, the majority of reliable data on community mortality come from locations where Health and Demographic Surveillance Systems (HDSS) have been implemented. These systems monitor health and socioeconomic indicators in addition to migration, births, and deaths in a defined population over time.3 Cause of death is attributed according to verbal autopsy (VA), which is the recommended method for determining cause of death in places where vital registration systems are weak. VA uses data from interviews with lay respondents on the signs and symptoms of the decedent before death to attribute cause of death.4 However, the accuracy of the VA result can be hindered by the need for both trained individuals to carry out the interviews and multiple physicians for VA questionnaire review. An additional limitation for malaria-endemic regions in particular is the difficulty of distinguishing a death due to malaria from other common (co-)morbidities.5 With limited resources, VA is limited by the availability of qualified human resources and HDSS site implementation, and follow-up is expensive and difficult to sustain making adoption and implementation of similar systems by governments or local authorities unlikely.
Compounded with weak health facility surveillance in Guéckédou, the reliability of routine health facility–based data was further compromised during the 2013–2016 Ebola virus disease (EVD) epidemic that originated in Guéckédou Préfecture.6,7 Although the majority of health facilities remained open during the epidemic, health-care workers were infected and there was fear of EVD in the community.8 Consequently, patients stayed away from health facilities9 decreasing the reliability of data collected in health facilities during this period. Diagnosis of malaria infections was further complicated during the epidemic due to the overlap of malaria and EVD symptoms10; malaria testing was suspended during the EVD epidemic unless the health-care workers had and used appropriate personal protective equipment.11
Multiple approaches can be used to quantify mortality rates in the absence of reliable data; however, their accuracy in cause of death determination is limited. These approaches include retrospective mortality surveys which are used to monitor mortality in emergencies,12,13 yet they do not provide information on changes over time unless they are repeated. Furthermore, when the recall period is short, large sample sizes are needed to achieve acceptable precision for the estimate.14 Another approach is prospective community-based surveillance which allows for real-time monitoring of trends and can serve as an early warning system for certain diseases depending on the timeliness of data collection, reporting, and evaluation. However, such systems can be labor intensive, expensive, and take time to implement.15 In areas where there is no HDSS and health facility surveillance and vital registration systems are weak or nonexistent, a lack of reliable data hinders the ability to monitor changes in mortality. For organizations working in such areas, alternative means to monitor mortality need to be developed. For the purposes of this project, we aimed to implement a system that was simple and resource light, where data collection occurred in the community by community members and could continue with limited supervision. The primary aim of this project was to monitor community mortality, specifically assessing malaria-attributable mortality in a predominately rural population of the Republic of Guinea using data collected through community-based prospective sentinel site surveillance. Secondarily, we also assessed the ability of community-based sentinel site surveillance to retrospectively detect suspect EVD (sEVD) cases during the 2014–2015 outbreaks.
Methods
Area and study population.
Guéckédou Préfecture is one of 33 préfectures in Guinea and borders Sierra Leone and Liberia. It is subdivided into 10 sous-préfectures, nine rural and one urban, with a total area of 4,400 km2 and a population of 517,572 individuals (2010 estimate). The majority of the inhabitants of this area are subsistence farmers, growing rice and corn, in addition to perennial crops such as coffee and kola nuts. The préfecture has one hospital in its capital, Guéckédou city, 13 health centers (one in each sous-préfecture and four in Guéckédou city) and 46 health posts.
Study setting.
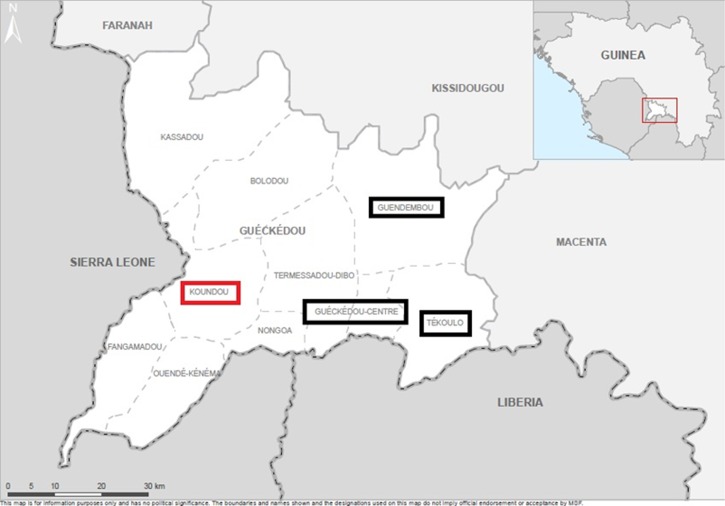
This study was carried out in the Préfecture of Guéckédou in the sous-préfectures of Guéckédou city (Center), Tékoulo, Guendembou, and Koundou as indicated in Figure 1 . These sous-préfectures have a combined area of 1,779 km2 and a population of approximately 297,919 individuals (2010 estimate). Aside from Guéckédou city, Tékoulo, Guendembou, and Koundou are predominately rural, forested sous-préfectures with small villages connected by dirt roads or paths.
Figure 1.
Map of Guéckédou Prefecture. Boxes represent sous-préfectures where community-based sentinel site surveillance was implemented; black boxes are those sous-préfectures with the reinforced malaria control program, the red box is the sous-préfecture that did not receive the reinforced malaria control program.
Reinforced malaria control program.
The reinforced malaria control program began in 2010 as a collaboration between Médecins Sans Frontières (MSF) and the Ministry of Health of Guinea. Planning and recruitment of staff began in 2010; implementation of activities began in 2011 and continued until April 2014. Although information on the program and its impact on malaria parasite prevalence have previously been published,16 briefly, the reinforced malaria control program consisted of interventions in both health facilities and in the community. These interventions included improving detection of clinical malaria cases using RDTs and timely treatment with ACT through health facilities and community health workers, referral of severe cases with pretreatment, in addition to a mass distribution of long-lasting insecticide-treated nets.
The implementation of the reinforced malaria control program began in Guéckédou city, Tékoulo, and Guendembou (N = 3) and roll out was to continue for 3 years in a progressive stepwise manner until all sous-préfectures in Guéckédou Préfecture were receiving the control program (N = 10). Koundou was the next sous-préfecture in which implementation was planned after the three previously mentioned; for this reason, data were collected in Koundou to serve as its preimplementation measurement. Unfortunately, implementation of the program did not move beyond the initial three sous-préfectures due to logistical constraints. Nevertheless, data collection continued in Koundou due to the engagement of the local authorities.
Study design.
The present project was part of a larger study that examined the impact of the reinforced malaria control program on malaria-attributable morbidity, transmission, and mortality in the areas of implementation.
Concurrently, prospective community-based sentinel site (villages or neighborhoods) surveillance was implemented in the three sous-préfectures of Guéckédou Préfecture with the reinforced malaria control program (program area) in addition to the sous-préfecture of Koundou, where the program was to be implemented. Data collected in Koundou served as documentation of mortality in an area where the reinforced malaria control program had not been implemented.
Sample size considerations.
Sample size was calculated based on a hypothesized malaria-attributable mortality of 30% in each sous-préfecture before implementation of the reinforced malaria control program. Detecting a decrease of at least 10% in malaria-attributable mortality after 1 year of intervention with alpha of 10% and 80% power required a minimum of 251 deaths reported per sous-préfecture.
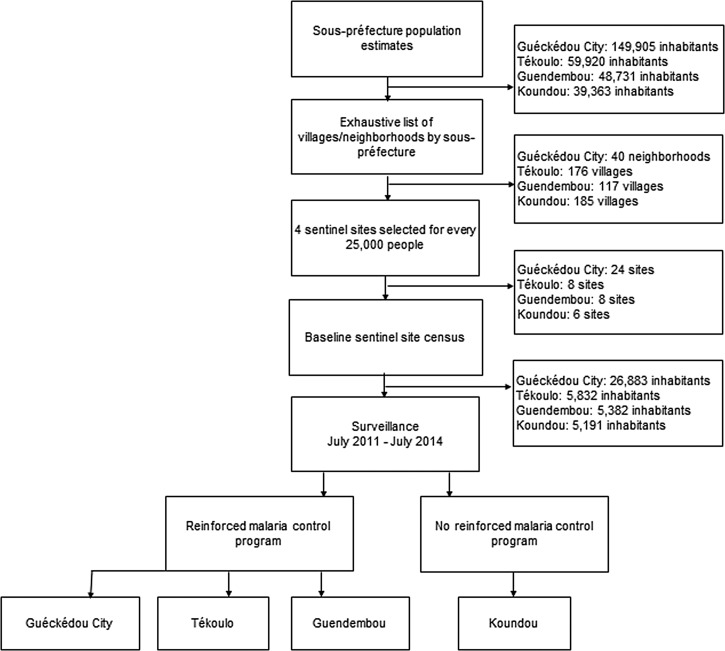
Based on an average village size of 350 people in the rural sous-préfectures, we aimed for each sentinel site to include 400 individuals. With a crude mortality rate of 9.46 deaths/1,000 people/year,17 we anticipated that at least four deaths/month (48/year) would occur in each sentinel site of 400 individuals. To ensure a sufficient number of deaths captured by the surveillance system, at least 251 deaths in 1 year, a minimum of six sentinel sites per sous-préfecture were required.
Sentinel site selection.
Population estimates were obtained for each sous-préfecture. As seen in Figure 2 , sentinel site selection by sous-préfecture followed with sites chosen from a sampling frame consisting of an exhaustive list of administrative units (villages or neighborhoods). Implicit stratification was used to improve precision.18 Accordingly, before selection, the administrative units were sorted by sous-préfecture according to urban versus rural and then by driving distance from the nearest health facility. For every 12,500 inhabitants in each sous-préfecture, two sentinel sites were systematically selected from the sampling frame. Forty-six sentinel sites were included in the surveillance system. A baseline census was conducted in each sentinel site to ensure that its population was at least 400 inhabitants. Villages with less than 400 inhabitants were grouped with a neighboring village (or more if necessary) and their populations pooled to form one sentinel site.
Figure 2.
Schematic representation of sentinel site selection.
Implementation.
Each sentinel site was visited before implementation. The implementation team met with village members and leaders and informed them of the project and its objectives. Village members nominated a key informant, a volunteer who was a full-time village resident and could read and write in French, to collect data. The village leader provided consent for their village to participate and a precensus estimate of the population. One supervisor of key informants (supervisor) was hired in each sous-préfecture.
Data collection.
Information on mortality was collected regarding all permanent residents of the sentinel sites. Key informants collected data only when a death occurred. Supervisors visited each sentinel site and collected data from the key informants at least twice per month. Data collection was paper based as many areas of Guéckédou Préfecture were without mobile phone coverage at the time of implementation.
Name, age (< 5, 5–14, 15–44, and ≥ 45 years), sex, cause of death (see below), place of death, and health-seeking behavior before death were collected from the family of the deceased. Data were recorded as reported by the family of the deceased. Data on births and in- and out-migration were not collected as the population was understood to be quite stable. Population estimates were updated every year during an exhaustive census of each sentinel site carried out by the community leader(s), key informant, and their supervisor. VAs were not used because this method was too resource demanding (human and financial) in the context of this program.
Data management.
Supervisors transported the data collection forms to the MSF office in Guéckédou on a monthly basis. The data manager verified and entered the data in a Microsoft Excel spreadsheet and referred any questions regarding inconsistencies to the supervisor and key informants for correction or further precision. Monthly reports were produced and shared within MSF and with the local Ministry of Health officials. Once verified, the data were anonymized and entered in to a project specific Epi Info (version 3.5.4; Centers for Disease Control and Prevention, Atlanta, GA) data mask.
Population and outcomes.
A permanent resident of a sentinel site was considered to be any person who would be counted during the annual sentinel site census. Causes of death were classified as due to 1) “corps chaud”/malaria/fever (considered as a death due to malaria), 2) fever and another specified cause, and 3) another specified cause. A death due to “malaria” was defined as: 1) death due to malaria or naa dialuntouvo/naa hoiyo/yo wo tchouanduni as reported by the family of the deceased, or 2) an individual with a history of fever in the 3 days before death without another known (specified) cause. A death due to “another cause” was one that was either reported as: 1) due to another cause, specified by the family member, or 2) due to another cause with fever before death, or 3) a death with no known cause or fever before death. “EVD suspect deaths” were those reported as not due to malaria and having been due to symptoms listed in the EVD case definition: diarrhea, vomiting, vomiting blood, vomiting, and diarrhea and/or hiccups.6,19
Analysis.
The database was cleaned and statistical analysis was performed using Stata (version 12.1; StataCorp, College Station, TX). All data were analyzed retrospectively after being aggregated into periods corresponding with the rainy and dry seasons: period 1: July–December 2011 (rainy), period 2: January–June 2012 (dry), period 3: July–December 2012 (rainy), period 4: January–June 2013 (dry), period 5: July–December 2013 (rainy), and period 6: January–June 2014 (dry).
All estimates are reported by sous-préfecture and period. Proportional mortality attributable to malaria was calculated as the number of deaths reported as due to malaria over all the deaths reported in the sous-préfecture. The proportion of sEVD deaths were calculated as the number of deaths classified as sEVD deaths over all deaths reported. Descriptive analysis was performed and the results were expressed as frequencies and percentages. Differences in reported place of death, proportional mortality, and the proportion of sEVD deaths, from period 1 to period 6, were compared using a z-test for a difference in proportions. Differences between health-seeking behavior and sEVD deaths by period and sous-préfecture were compared using χ2 or Fisher's exact test, as appropriate. All results are presented with their 95% confidence intervals (CIs) and statistical tests were considered significant at P ≤ 0.05.
Ethical considerations.
The project was carried out with the support of the National Malaria Control Program in Guinea; the surveillance protocol was presented to and approved by both the prefectoral and sous-préfectoral health authorities. Results presented here consist of retrospective analysis of routinely collected program data; as such, they represent a standard component of program monitoring and are considered to be exempt from the MSF Ethical Review Board. Nevertheless, these data were collected in accordance with standards presented in the Declaration of Helsinki and after receipt of verbal informed consent from village leaders. No identifying information was recorded in the database and all individuals were free to refuse contributing information to the surveillance system. During the EVD outbreak, supervisors were included as part of the epidemiological investigation teams and key informants were trained to provide health promotion messages regarding EVD and the prevention of transmission to their and surrounding communities. During the EVD outbreak, data collection continued using strict infection prevention and control procedures which prohibited entrance into households of the individual being interviewed and also limited direct contact between the key informant and the family of the deceased and the supervisor and the key informant.
Results
Population under surveillance.
For 36 months 43,000 individuals were monitored by the surveillance system and 1,242 deaths were reported. No households refused to take part in the surveillance system and no families refused to have mortality data collected. Deaths of children < 5 years of age and individuals ≥ 45 years of age represented 34.9% (434/1,242) and 36.5% (454/1,242) of all deaths reported through the system, respectively (Table 1). Children < 5 years of age were more frequently reported to have died of malaria (73.5%, 95% CI = 69.3–77.6%) than individuals ≥ 5 years of age (45.4%, 95% CI = 41.9–48.8) (difference 28.1%, 95% CI = 21.0–35.1, P < 0.001).
Table 1.
Administrative division, deaths captured, and location of deaths as reported through sentinel site surveillance by sous-préfecture
| Guéckédou city | Tékoulo | Guendembou | Koundou | Total | |
|---|---|---|---|---|---|
| Population estimate (2010) | 149,905 | 59,920 | 48,731 | 39,363 | 297,919 |
| No. of sentinel sites | 24 | 8 | 8 | 6 | 46 |
| Sentinel site census | |||||
| 2011 | 26,883 | 5,832 | 5,382 | 5,191 | 43,288 |
| 2012 | 26,751 | 6,009 | 5,474 | 5,200 | 43,434 |
| 2013 | 26,524 | 5,943 | 5,405 | 5,474 | 42,999 |
| Deaths captured, N (% malaria) | 594 (56.4) | 204 (50.4) | 208 (51.9) | 236 (59.3) | 1,242 (55.2) |
| < 5, n (% malaria) | 167 (71.3) | 63 (87.3) | 96 (66.7) | 108 (75.0) | 434 (73.5) |
| 5–14, n (% malaria) | 23 (60.8) | 14 (78.5) | 12 (50.0) | 18 (61.1) | 67 (62.7) |
| 15–44, n (% malaria) | 167 (47.3) | 47 (34.0) | 29 (24.1) | 44 (47.7) | 287 (42.9) |
| ≥ 45, n (% malaria) | 237 (51.9) | 80 (26.2) | 71 (43.7) | 66 (40.9) | 454 (44.5) |
| Location of death | |||||
| At home, n (% N) | 406 (68.3) | 169 (82.8) | 178 (85.6) | 181 (76.7) | 934 (75.2) |
| Guéckédou Hospital, n (% N) | 147 (24.7) | 7 (3.4) | 6 (2.9) | 25 (10.6) | 185 (14.9) |
| Public facility, n (% N) | 6 (1.0) | 2 (1.0) | 8 (3.8) | 20 (8.5) | 36 (2.9) |
| Other, n (% N) | 35 (5.9) | 26 (12.7) | 16 (7.7) | 10 (4.2) | 87 (7.0) |
Health-seeking behavior.
Family members frequently reported that the deceased sought care for their illness before their death; 70.1% (871/1,242) of all decedents were reported to have sought care from a public health center or post during the course of the illness that resulted in their death, and 72.1% (495/686) of decedents whose cause of death was reported to be malaria were reported to have sought care at a public health center or post before death. Regardless of age, decedents whose cause of death was classified as malaria frequently sought care for their illness at a public health center or post (< 5 years: 78.6%, 95% CI = 74.1–83.1; 5–14 years: 61.9%, 95% CI = 47.0–76.7; 15–44 years: 74.7%, 95% CI = 67.0–82.5; and ≥ 45 years: 62.3%, 95% CI = 55.6–69.0). There was no significant difference in the number of malaria deaths that sought treatment at a public health center or post by period (P = 0.274); however, individuals in Guendembou sought care less frequently (51.4%) at a public health center or post than the other sous-préfectures (Guéckédou city: 74.5%, Tékoulo: 71.0%, and Koundou: 74.5%).
Reported place of death.
The majority (75.2%) of all deaths reported through the surveillance system occurred at home, whereas 17.8% occurred in a health center, health post, or Guéckédou Hospital (Table 1). From period 1 to period 6, the proportion of deaths occurring at home increased from 68.6% to 79.7% (difference 11.1%, 95% CI = 2.5–19.7, P = 0.011), whereas the proportion of deaths occurring in a health center, health post, or Guéckédou Hospital decreased from 22.0% to 11.7% (difference 10.3%, 95% CI = 3.0–17.6).
Proportional mortality.
Malaria was the most frequently reported cause of death during the surveillance period, 55.2% overall. By sous-préfecture, malaria-attributable mortality accounted for 56.4% of all deaths in Guéckédou city, 50.4% in Tékoulo, 51.9% in Guendembou, and 59.3% in Koundou (Table 1).
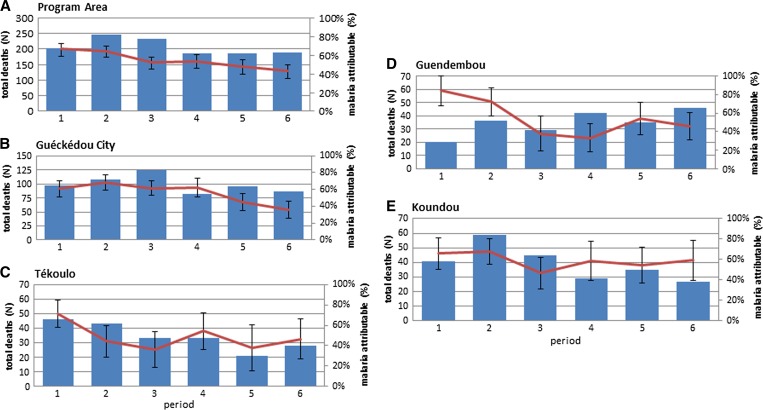
Overall, proportional mortality attributable to malaria decreased steadily from period 1 to period 6 in the program area by 26.4% (95% CI = 15.9–37.0, P < 0.001) (Figure 3 , Panel A). From period 1 to period 6, proportional mortality attributable to malaria decreased by 25.1% (95% CI = 11.1–39.2, P < 0.001) in Guéckédou city, 25.3% (95% CI = 2.3–48.2, P = 0.031) in Tékoulo, 39.3% (95% CI = 17.6–61.0, P < 0.001) in Guendembou, and 6.5% (−17.8% to 30.5%, P = 0.589) in Koundou (Figure 3, Panels B–E).
Figure 3.
Overall and malaria-attributable mortality by period and sous-préfecture.
Ebola virus disease.
sEVD deaths July 2011–June 2014.
As seen in Table 2, 5.5% (68/1,242) of all deaths reported through the surveillance system were retrospectively classified as sEVD deaths. The number of sEVD deaths increased from period 1 to 6; however, the difference in period-specific estimates was not significant (P = 0.132). EVD suspect proportional mortality increased by 6.1% (95% CI = 1.3–10.8, P = 0.011) from period 1 to period 6, while exhibiting fluctuations probably due to the small number of sEVD deaths per period (range 6–17). The largest number of EVD suspect deaths was reported during period 6 and coincided with the apparition of EVD in Guéckédou.
Table 2.
Deaths retrospectively classified as EVD suspect by reported cause and period, Guéckédou, 2011–2014
| Period 1 | Period 2 | Period 3 | Period 4 | Period 5 | Period 6 | Total | |
|---|---|---|---|---|---|---|---|
| July–December 2011 | January–June 2012 | June–December 2012 | January–June 2013 | July–December 2013 | January–June 2014 | ||
| Diarrhea, n (%) | 4 (66.7) | 8 (57.1) | 6 (66.7) | 8 (66.7) | 2 (20.0) | 8 (47.0) | 36 (52.9) |
| Vomiting, n (%) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Vomiting blood, n (%) | 2 (33.3) | 4 (28.5) | 2 (22.2) | 4 (33.3) | 7 (70.0) | 2 (11.1) | 21 (30.8) |
| Vomiting and diarrhea, n (%) | 0 (0.0) | 1 (7.1) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 4 (23.5) | 6 (8.8) |
| Hiccups, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (5.8) | 2 (2.9) |
| Ebola, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.7) | 2 (2.9) |
| Total | 6 (100) | 14 (100) | 9 (100) | 12 (100) | 10 (100) | 17 (100) | 68 (100) |
| EVD suspect mortality, % (95% CI) | 2.9 (0.6–5.2) | 5.6 (2.7–8.6) | 3.8 (1.3–6.3) | 6.4 (2.8–10.0) | 5.3 (2.1–8.6) | 9.0 (4.9–13.1) | 5.4 (4.2–6.7) |
CI = confidence interval; EVD = Ebola virus disease.
sEVD deaths November 2013–March 2014.
During November 2013–March 2014, the period during which EVD emerged in Guéckédou Préfecture, 131 deaths were reported through the surveillance system. Seventeen (12.9%) were retrospectively classified as sEVD deaths due to the symptoms reported before death for each individual. Fifty-two (39.6%) were reported as due to malaria. The reported causes for deaths classified as sEVD are found in Table 3 and include diarrhea, diarrhea and vomiting, hemorrhagic symptoms, and hiccups. Two of the sEVD deaths captured through this surveillance system were among the first laboratory-confirmed Zaire Ebola cases from the 2013–2016 outbreak.6 Fourteen deaths ccurred in the community, one occurred in Guéckédou Hospital and two occurred in the Guéckédou Ebola Treatment Center and were verified with the EVD patient line listing.
Table 3.
Characterization of EVD suspect deaths occurring between November 2013 and March 2014, Guéckédou, 2011–2014
| Guéckédou city | Tékoulo | Guendembou | Koundou | |
|---|---|---|---|---|
| Vomiting blood | 1 | 0 | 0 | 2 |
| Hiccups | 2 | 0 | 0 | 0 |
| Persistent diarrhea | 0 | 0 | 1 | 1 |
| Diarrhea | 3 | 1 | 1 | 0 |
| Diarrhea and cough | 1 | 0 | 0 | 0 |
| Vomiting and diarrhea | 4 | 0 | 0 | 0 |
| Total | 11 | 1 | 2 | 3 |
EVD = Ebola virus disease.
Discussion
In low-resource settings, the absence of reliable surveillance data and vital registration systems makes it difficult to monitor mortality and determine cause of death, activities that are essential to understanding disease burden,4 and implementing appropriate prevention and control measures. Ascertainment of cause-specific mortality without use of VA poses an additional challenge to surveillance and is further complicated in malaria-endemic areas where malaria is frequently characterized by nonspecific signs including fever.20 In countries without the means to establish an HDSS or maintain health facility–based surveillance, alternative methods of data collection are needed. Herein, we demonstrate that data from prospective community-based mortality surveillance using sentinel sites can provide a means to document mortality and facilitate outbreak detection in low-resource settings, although this remains challenging.
The surveillance system as it was implemented in Guéckédou was intentionally simple and resource light, a model that would both serve the purposes of the project in Guéckédou while also allowing it to be adapted to different contexts. In some contexts, monitoring all population movements and attributing cause of death by VA may not be feasible due to limited financial resources and/or difficulties finding qualified human resources. Implementation of VA, the gold standard for cause of death attribution, requires review of interviews by two or more trained clinicians, individuals that are rare in rural areas in addition to being difficult to find in countries like Guinea where there are very few.21 An additional limitation to the use of VA is its ability to accurately attribute cause of death, particularly for malaria-related deaths.22 As a result, after investigating the community understanding of malaria and the different terms they use to signify malaria, cause of death was classified as reported by the deceased's next-of-kin. Using this method for cause of death attribution, deaths reported as due to fever or malaria were considered as a malaria-attributable death. If no other cause was specified, deaths resulting from conditions unrelated to malaria that presented with fever (e.g., typhoid) with no other known cause were likely to have been classified as “due to malaria.” Although use of this proxy may have overestimated the number of malaria-attributable deaths, the use of fever as a proxy for malaria is not without precedent,23–25 and has also been proposed by the World Health Organization as the case definition for malaria to be used in community-based surveillance for individuals in malaria-endemic areas.26
The importance of data from community-based surveillance is apparent in areas like Guéckédou Préfecture where data collection in health facilities is weak and many deaths occur outside of health facilities. Indeed the majority of decedents were reported to have died at home and increased significantly from period 1 to period 6, whereas the proportion reported to have died in a public health center or post decreased. The increase may be due to community fear of visiting public health facilities during period 6 when the EVD outbreak was declared in Guéckédou, a consequence of the 2014–2015 EVD epidemic documented in Guinea,9 Sierra Leone,27 and Liberia.28
Notwithstanding, 72.1% of decedents classified as having died of malaria reportedly sought care for their illness at a health center or post before death. As the national malaria control guidelines emphasize systematic testing before treatment,2 an unknown number of these deaths may have been reported as due to malaria after receiving confirmation at the health facility; however, we were unable to verify this data in health facility records. Although the confirmed cases of malaria should be reflected in the national statistics, had the surveillance system not been in place, the deaths captured through the community surveillance system would not have been documented.
The deaths documented through the surveillance system occurred during the implementation of a reinforced malaria control program and was used to provide sous-préfecture-specific estimates of the number of deaths occurring in addition to malaria-attributable mortality. Yet, few deaths occurred each month requiring the data to be aggregated into 6-month periods and not providing enough power with which to make annual comparisons. Consequently, the estimates of malaria-attributable mortality in the rural sous-préfectures fluctuated from period to period. In addition to the small number of deaths reported, the fluctuations may have been the result of factors that are difficult to quantify retrospectively including periodic population movements and variations in completeness of reporting. Seasonality of malaria may also have played a role in the fluctuations with periods 1, 3, and 5 encompassing the rainy season when more cases of malaria tend to occur. Nevertheless, from period 1 to period 6, proportional mortality attributable to malaria decreased in Guéckédou city, Tékoulo, and Guendembou, the three sous-préfectures where the reinforced malaria control program was implemented. Although the decrease in malaria-attributable proportional mortality reported here could also be due to changes in other causes of death that present with fever, we are unaware of any other large health intervention that addressed causes of febrile illness in this area during the surveillance period. Furthermore, this assertion is corroborated by results from a cross-sectional study of malaria prevalence in the same areas, during the same period which documented a significant increase in the coverage of malaria control interventions in addition to a decrease in malaria prevalence linked to the reinforced malaria control interventions.16 Although the system seemed to adequately detect mortality, more work would be needed to ensure improved cause of death attribution.
Cause of death attribution could be improved through use of refined syndrome definitions. Clusters of deaths presenting with similar syndromic presentations could be detected by community surveillance and provide valuable information for outbreak detection.29 Retrospective analysis of the data described above was conducted to ascertain if the EVD outbreak that was laboratory confirmed in Guéckédou in March 20146 could have been detected earlier if the data had been monitored for causes of death other than malaria. There was a statistically significant increase in the number of sEVD deaths, those with at least one of the symptoms found in the WHO EVD case definition,10 from period 1 to period 6, whereas differences in sEVD case numbers between periods other than 1 and 6 were not significant. Similar to malaria, the symptoms of Ebola aside from hiccups and bleeding of unknown origin which generally indicate severe illness are nonspecific. Despite using a sensitive sEVD case definition which captures both specific and nonspecific EVD symptoms and led to the detection of numerous suspect cases before the outbreak, the number of sEVD cases identified per period were probably too small to detect a significant difference between periods. Consequently, to operationalize the use of such definitions, additional work would need to be done to determine the appropriate syndrome definition in addition to defining a threshold which determines when follow-up in the community would be required. Despite the fact that this community-based surveillance was in place, EVD was not considered a possibility until later in time. Furthermore, to improve the utility of community surveillance data, timely reporting and analysis in addition to an improved understanding of the communities and their understanding of illness is essential. Similar surveillance systems, particularly for Ebola, are already in place but use predefined triggers or events that serve as an alert.30 Although event-based surveillance is useful for identifying events during an outbreak, community surveillance using syndrome definitions derived from case definitions is a proactive approach and may result in earlier outbreak detection.
There are several important limitations to this data. Causal inference between changes in mortality and the implementation of the reinforced malaria control program should be made with caution as the program components were implemented in field conditions and not as a controlled trial. Nevertheless, data from sentinel sites that did not receive the malaria control program were included for the purpose of comparison. When evaluating changes in mortality, data on preprogram mortality are not available in the sentinel sites themselves, thus the only comparison that can be made is between period-specific mortality in the individual sous-préfectures themselves, which are further limited by the rare occurrence of death. These analyses also did not take into consideration population movements, births, arrivals, and departures; however, the population in the area is quite stable and the impact of these movements on the overall denominator is probably quite small. The result of the annual census in each site confirms this supposition. We attempted to validate the data from the system using capture recapture31 for the individuals who were reported to have died at health facilities; however, when visiting the named health facility, we were unable to match a sufficient number of individuals, probably due to the fact that deaths are not always recorded in the register. Finally, despite information sessions with local community leaders in each sentinel site, due to the size of some of the urban sentinel sites, it is possible that the key informants were not aware of all deaths occurring in the site and may also have encountered difficulties enumerating the population of their site.
Mortality data from prospective, community-based sentinel site surveillance can be used to document community mortality. However, improved methods for cause of death attribution are needed to enhance cause-specific mortality measurement in low-resource settings. Herein, we suggest a derivation of prospective community-based sentinel site surveillance that could be useful for outbreak detection if timeliness of data collection and reporting were improved, facilitating real-time data analysis. In such cases, standard predefined profiles for specific causes of death of interest and thresholds of acceptability should be developed. Prospective mortality surveillance using sentinel sites is not only a manner in which to document community mortality, it can also compliment health facility surveillance particularly in areas where data collection is weak.
ACKNOWLEDGMENTS
We thank the supervisors, the key informants, and sentinel site communities for their contributions to this work.
Footnotes
Financial support: Médecins Sans Frontières Operational Center, Geneva, financed this work. Epicentre (Amanda Tiffany and Rebecca F. Grais) received core funding from Médecins Sans Frontières.
Authors' addresses: Amanda Tiffany, Epicentre, Geneva, Switzerland, E-mail: amanda.tiffany@geneva.msf.org. Faya Pascal Moundekeno, World Health Organization, Fria, Guinea, E-mail: moundekenofp84@yahoo.fr. Alexis Traoré, Direction Préfectorale de la Santé, Guéckédou, Guinea, E-mail: alexistraore60@gmail.com. Melat Haile, Médecins sans Frontières, Nairobi, Kenya, E-mail: melatreta5u@yahoo.com. Esther Sterk and Micaela Serafini, Médecins sans Frontières, Geneva, Switzerland, E-mails: esther.sterk@geneva.msf.org and micaela.serafini@geneva.msf.org. Timothé Guilavogui, National Malaria Control Program, Conakry, Guinea, E-mail: gui_timothee@yahoo.fr. Blaise Genton, University Hospital, Lausanne, Switzerland, E-mail: blaise.genton@unibas.ch. Rebecca F. Grais, Epicentre, Paris, France, E-mail: rebecca.grais@epicentre.msf.org.
References
- 1.Measure DHS. Guinée Enquête Démographique de la Santé. 2012. http://dhsprogram.com/pubs/pdf/FR280/FR280.pdf Available at. Accessed January 12, 2016.
- 2.Ministere de la Santé et de l'Hygiene Publique–Republique de la Guinée . Politique Nationale de Lutte Contre Le Paludisme. Conakry, Guinea: National Malaria Control Program; 2014. [Google Scholar]
- 3.Streatfield PK, Khan WA, Bhuiya A, Alam N, Diboulo E, Sié A, Yé M, Compaoré Y, Soura AB, Bonfoh B, Jaeger F, Ngoran EK, Utzinger J, Melaku YA, Mulugeta A, Weldearegawi B, Gomez P, Jasseh M, Hodgson A, Oduro A, Welaga P, Williams J, Awini E, Binka FN, Gyapong M, Kant S, Misra P, Srivastava R, Chaudhary B, Juvekar S, Wahab A, Wilopo S, Bauni E, Mochamah G, Ndila C, Williams TN, Desai M, Hamel MJ, Lindblade KA, Odhiambo FO, Slutsker L, Ezeh A, Kyobutungi C, Wamukoya M, Delaunay V, Diallo A, Douillot L, Sokhna C, Gómez-Olivé FX, Kabudula WC, Mee P, Herbst K, Mossong J, Chuc NTK, Arthur SS, Sankoh OA, Tanner M, Byass P. Malaria mortality in Africa and Asia: evidence from INDEPTH health and demographic surveillance system sites. Glob Health Action. 2014;7:25369. doi: 10.3402/gha.v7.25369. http://www.documentation.ird.fr/hor/fdi:010063236 Available at. Accessed March 22, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Verbal Autopsy Standards : The 2014 WHO Verbal Autopsy Instrument. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 5.Garenne M, Fauveau V. Potential and limits of verbal autopsies. Bull World Health Organ. 2006;84:3–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keïta S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traoré A, Kolié M, Malano ER, Heleze E, Bocquin A, Mély S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Günther S. Emergence of Zaire Ebola virus disease in Guinea: preliminary report. N Engl J Med. 2014;371:1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Ebola Situation Report: 25 November 2015. 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-25-november-2015 Available at. Accessed November 28, 2015.
- 8.Bogus J, Gankpala L, Fischer K, Krentel A, Weil GJ, Fischer PU, Kollie K, Bolay FK. Community attitudes toward mass drug administration for control and elimination of neglected tropical diseases after the 2014 outbreak of Ebola virus disease in Lofa County, Liberia. Am J Trop Med Hyg. 2015;94:497–503. doi: 10.4269/ajtmh.15-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plucinski MM, Guilavogui T, Sidikiba S, Diakité N, Diakité S, Dioubaté M, Bah I, Hennessee I, Butts JK, Halsey ES, McElroy PD, Kachur SP, Aboulhab J, James R, Keita M. Effect of the Ebola-virus-disease epidemic on malaria case management in Guinea, 2014: a cross-sectional survey of health facilities. Lancet Infect Dis. 2015;15:1017–1023. doi: 10.1016/S1473-3099(15)00061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Case Definition Recommendations for Ebola or Marburg Virus Diseases. Interim Guideline. 2014. http://apps.who.int/iris/bitstream/10665/146397/1/WHO_EVD_CaseDef_14.1_eng.pdf?ua=1 Available at. Accessed January 12, 2016.
- 11.World Health Organization Global Malaria Programme Guidance on Temporary Malaria Control Measures in Ebola-Affected Countries. 2014.
- 12.Valenciano M, Gergonne B, Morgan O, Aramburu C, Cawthorne A, D'Ancona FP, Doyle A, Fotiadis M, Payne L. WHO Report: Retrospective Mortality Survey among the Internally Displaced Population, Greater Darfur, Sudan, August 2004. 2004. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2548 Available at. Accessed March 22, 2016.
- 13.Carrión Martín AI, Bil K, Salumu P, Baabo D, Singh J, Kik C, Lenglet A. Mortality rates above emergency threshold in population affected by conflict in North Kivu, Democratic Republic of Congo, July 2012–April 2013. PLoS Negl Trop Dis. 2014;8:e3181. doi: 10.1371/journal.pntd.0003181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Checchi F, Roberts L. Documenting mortality in crises: what keeps us from doing better? PLoS Med. 2008;5:e146. doi: 10.1371/journal.pmed.0050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Checchi F, Roberts L. Interpreting and Using Mortality Data in Humanitarian Emergencies. A Primer for Non-Epidemiologists. London: Humanitarian Practice Network; 2005. [Google Scholar]
- 16.Tiffany A, Moundekeno FP, Traoré A, Haile M, Sterk E, Guilavogui T, Genton B, Serafini M, Grais RF. Encouraging impact following 2.5 years of reinforced malaria control interventions in a hyperendemic region of the Republic of Guinea. Malar J. 2016;15:298. doi: 10.1186/s12936-016-1353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Central Intelligence Agency The World Factbook, Guinea. 2015. https://www.cia.gov/library/publications/the-world-factbook/geos/gv.html Available at. Accessed November 30, 2015.
- 18.Levy PS, Lemeshow S. Sampling of Populations: Methods and Applications. 4th edition. New York, NY: Wiley; 2008. [Google Scholar]
- 19.Team WER Ebola virus disease in west Africa: the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowe AK, Steketee RW, Arnold F, Wardlaw T, Basu S, Bakyaita N, Lama M, Winston CA, Lynch M, Cibulskis RE, Shibuya K, Ratcliffe AA, Nahlen BL. Roll Back Malaria Monitoring and Evaluation Reference Group Viewpoint: evaluating the impact of malaria control efforts on mortality in sub-Saharan Africa. Trop Med Int Health. 2007;12:1524–1539. doi: 10.1111/j.1365-3156.2007.01961.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization WHO Global Health Workforce Statistics, 2014 Update. 2014. http://www.who.int/hrh/statistics/hwfstats/en/ Available at. Accessed March 15, 2016.
- 22.Anker M, Black RE, Coldham C, Kalter HD, Quigley MA, Ross D, Snow RW. A Standard Verbal Autopsy Method for Investigating Causes of Death in Infants and Children. Geneva: Switzerland; 1999. [Google Scholar]
- 23.Okiro EA, Hay SI, Gikandi PW, Sharif SK, Noor AM, Peshu N, Marsh K, Snow RW. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooth I, Bjorkman A. Fever episodes in a holoendemic malaria area of Tanzania: parasitological and clinical findings and diagnostic aspects related to malaria. Trans R Soc Trop Med Hyg. 1992;86:479–482. doi: 10.1016/0035-9203(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 25.Olaleye BO, Williams LA, D'Alessandro U, Weber MM, Mulholland K, Okorie C, Langerock P, Bennett S, Greenwood BM. Clinical predictors of malaria in Gambian children with fever or a history of fever. Trans R Soc Trop Med Hyg. 1998;92:300–304. doi: 10.1016/s0035-9203(98)91021-5. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization Regional Office for Africa . Integrated Disease Surveillance and Response in the African Region: A Guide for Establishing Community Based Surveillance. Brazzaville: Republic of the Congo; 2014. [Google Scholar]
- 27.Bolkan HA, Bash-Taqi DA, Samai M, Gerdin M, von Schreeb J. Ebola and indirect effects on health service function in Sierra Leone. PLoS Curr Outbreaks. 2014;12:4–9. doi: 10.1371/currents.outbreaks.0307d588df619f9c9447f8ead5b72b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ly J, Sathananthan V, Griffiths T, Kanjee Z, Kenny A, Gordon N, Basu G, Battistoli D, Dorr L, Lorenzen B, Thomson DR, Waters A, Moore UG, Roberts R, Smith WL. Facility-based delivery during the Ebola virus disease epidemic in rural Liberia: analysis from a cross-sectional, population-based household survey. PLoS Med. 2016;13:1–17. doi: 10.1371/journal.pmed.1002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henning KJ. What is syndromic surveillance? Morbidity and Mortality Weekly: 2004. Overview of syndromic surveillance.http://www.cdc.gov/mmwr/preview/mmwrhtml/su5301a3.htm Available at. Accessed March 22, 2016. [Google Scholar]
- 30.Crowe S, Hertz D, Maenner M, Ratnayake R, Baker P, Lash RR, Lee-Kwan SH, Williams C, Jonnie GT, Gorina Y, Anderson A. A plan for community event-based surveillance to reduce Ebola transmission: Sierra Leone, 2014–2015. Morbidity and Mortality Weekly. 2015. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6403a7.htm Available at. Accessed March 22, 2016. [PMC free article] [PubMed]
- 31.Roberts B, Morgan OW, Sultani MG, Nyasulu P, Rwebangila S, Myatt M, Sondorp E, Chandramohan D, Checchi F. A new method to estimate mortality in crisis-affected and resource-poor settings: validation study. Int J Epidemiol. 2010;39:1584–1596. doi: 10.1093/ije/dyq188. [DOI] [PMC free article] [PubMed] [Google Scholar]