Abstract
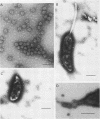
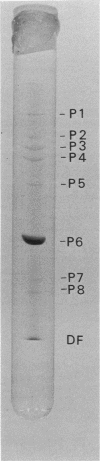
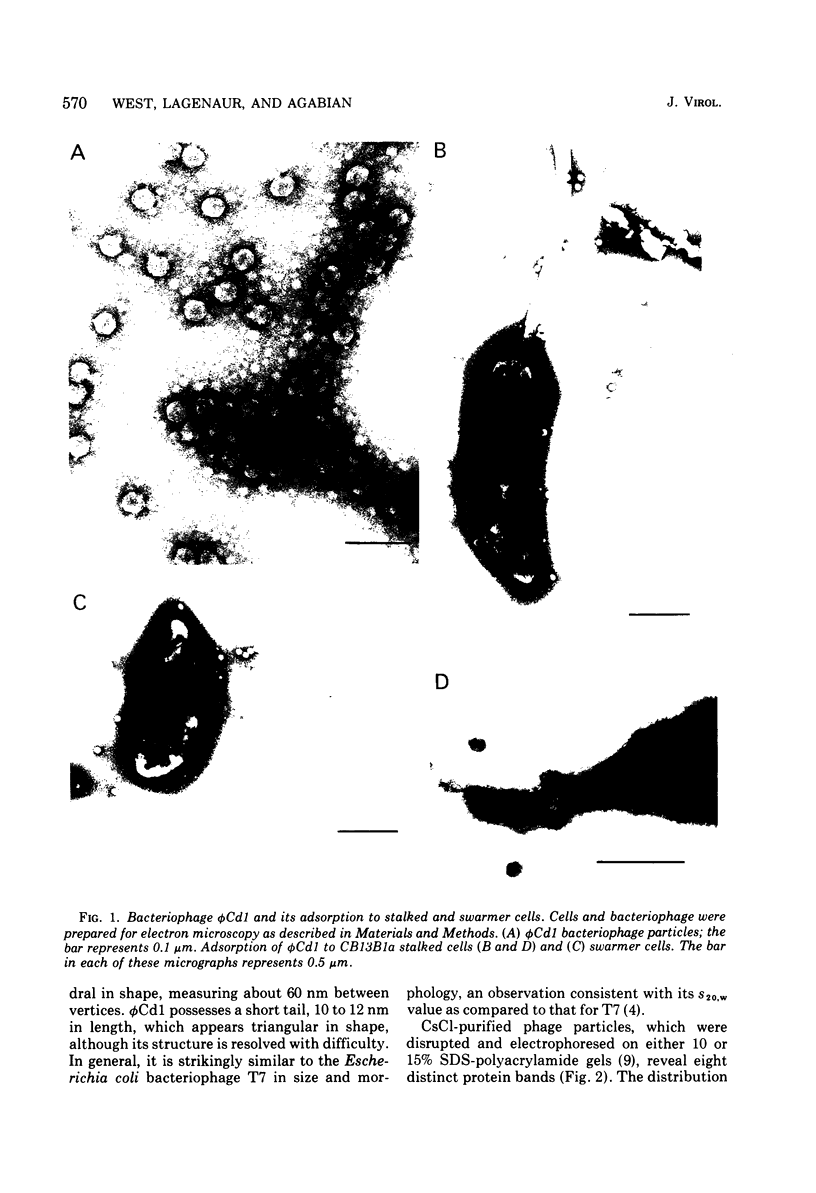
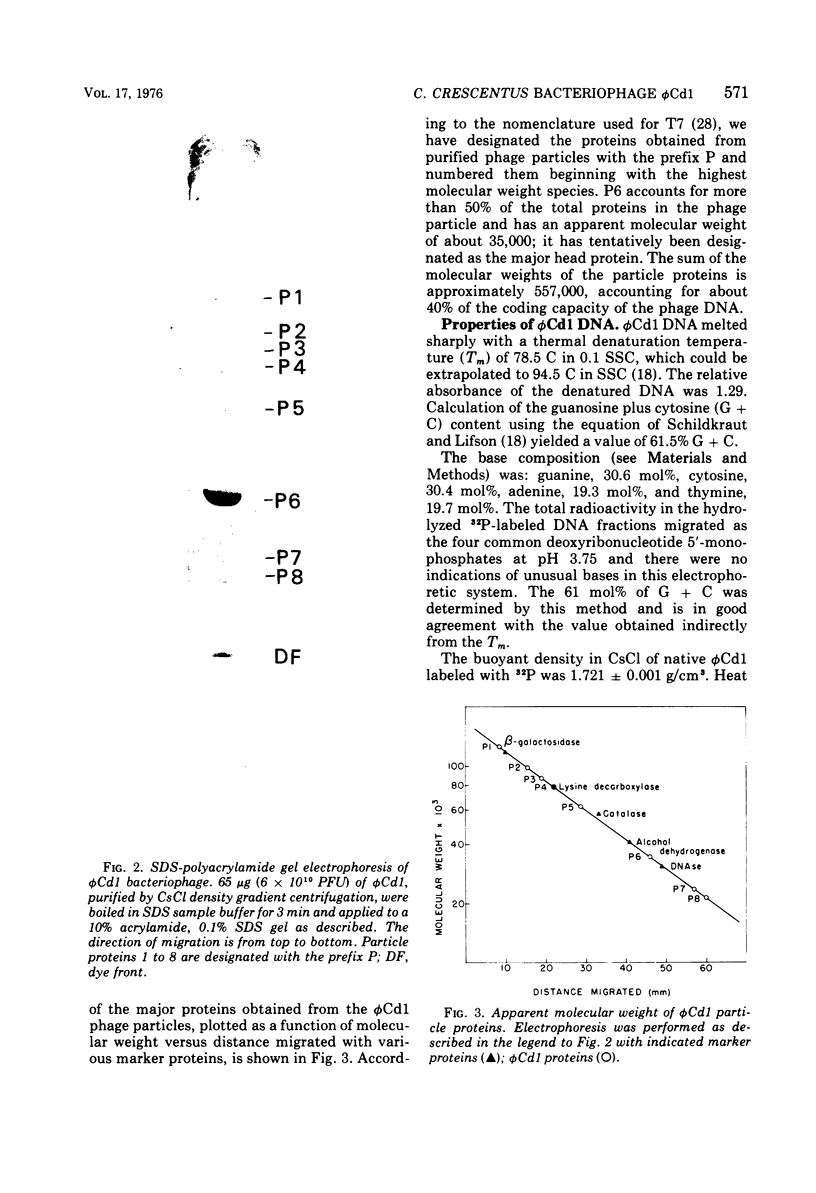
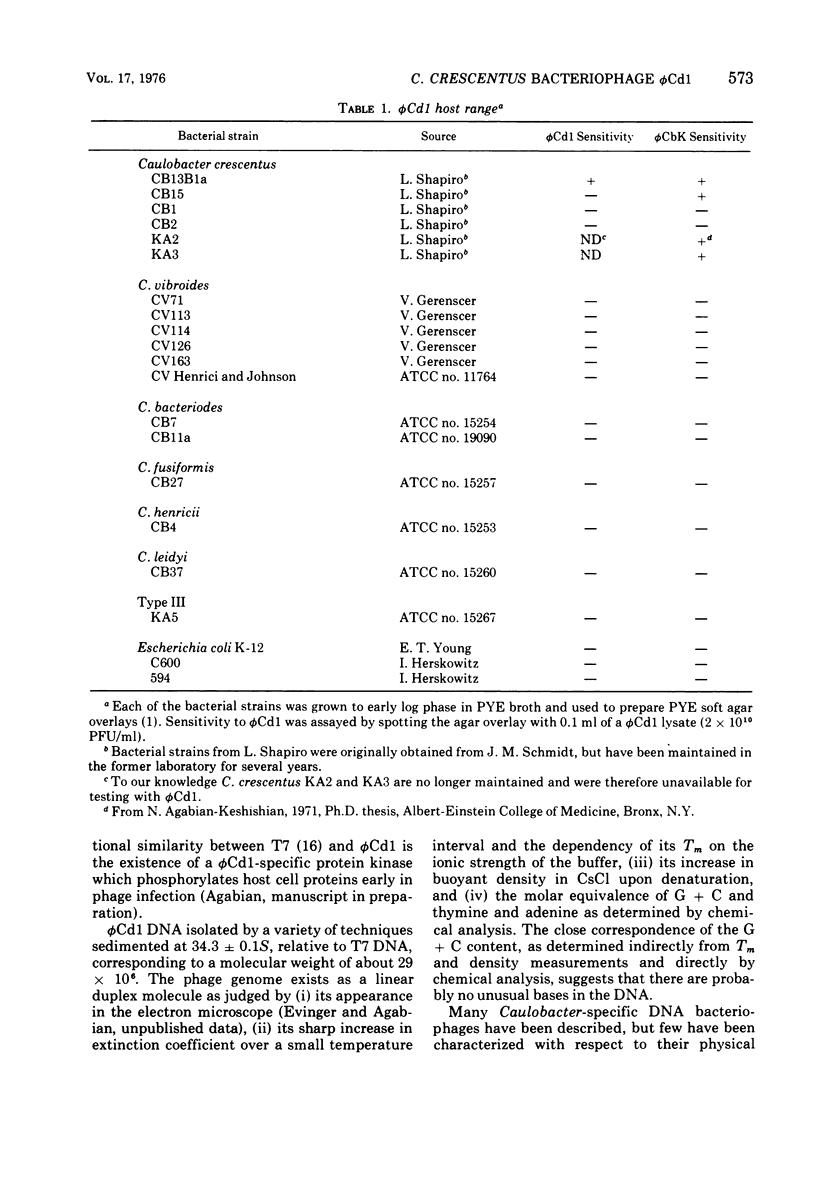
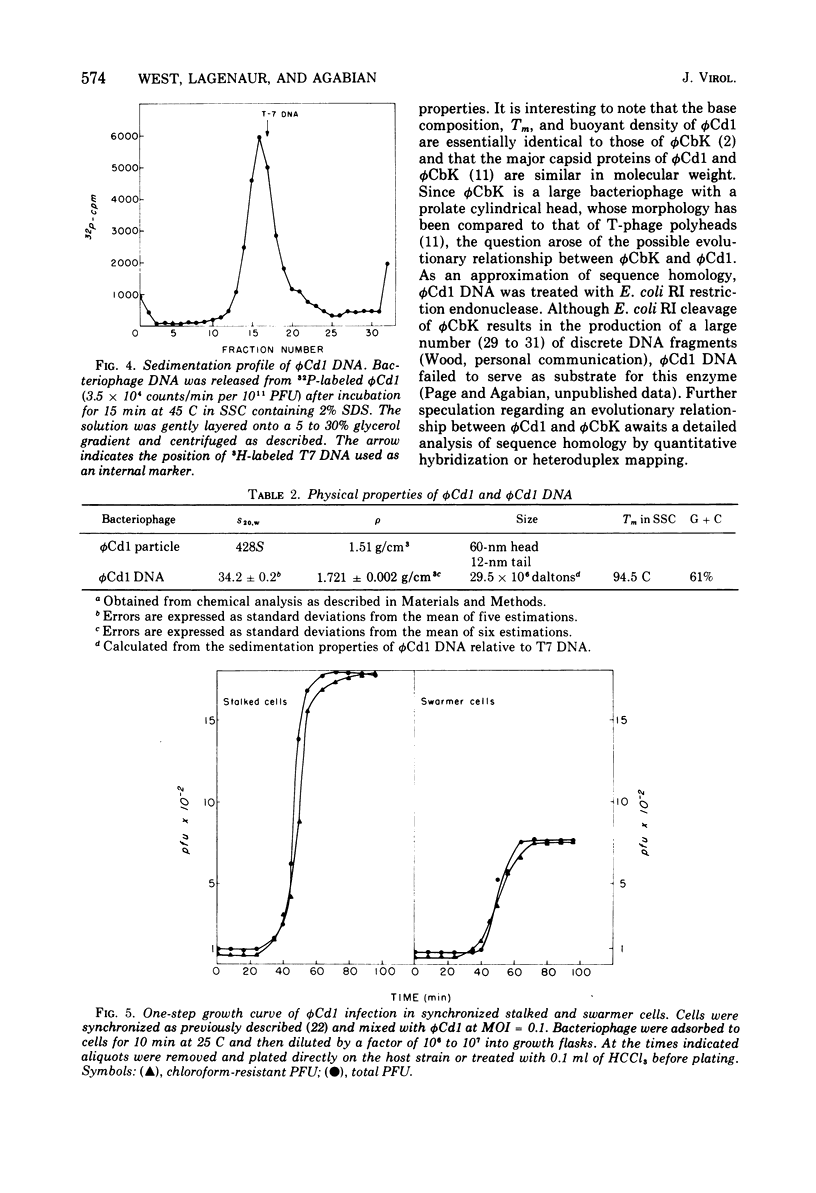
A DNA-containing bacteriophage, phiCd1, was isolated from sewage and shown to infect both stalked and swarmer cells of Caulobacter crescentus strain CB13B1a. phiCd1 is a small, icosohedral bacteriophage, 60 nm in diameter, which possesses a short, noncontractile tail, 10 to 12 nm in length. The bacteriophage particle is composed of at least eight structural proteins. phiCd1 nucleic acid exists as a linear duplex of DNA as judged by: (i) thermal denaturation (Tm), (ii) CsCl density gradient centrifugation, and (iii) chemical analysis of its base composition. The DNA is 61% guanosine plus cytosine, has a buoyant density in CsCl of 1.721 +/- 0.001 g/cm3, and denatures sharply at 78.5 C in 0.1 SSC (standard saline citrate) buffer. The S20, w value for the DNA is 34.3 +/- 0.1S as compared with T7 DNA, indicating a molecular weight of about 29 x 10(6).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian-Keshishian N., Shapiro L. Bacterial differentiation and phage infection. Virology. 1971 Apr;44(1):46–53. doi: 10.1016/0042-6822(71)90151-6. [DOI] [PubMed] [Google Scholar]
- Agabian-Keshishian N., Shapiro L. Stalked bacteria: properties of deoxriybonucleic acid bacteriophage phiCbK. J Virol. 1970 Jun;5(6):795–800. doi: 10.1128/jvi.5.6.795-800.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of T7 bacteriophage. J Mol Biol. 1962 Dec;5:635–642. doi: 10.1016/s0022-2836(62)80091-6. [DOI] [PubMed] [Google Scholar]
- HOUWINK A. L. Caulobacter; its morphogenesis, taxonomy and parasitism. Antonie Van Leeuwenhoek. 1955;21(1):49–64. doi: 10.1007/BF02543799. [DOI] [PubMed] [Google Scholar]
- Jollick J. D., Wright B. L. A flagella specific bacteriophage for caulobacter. J Gen Virol. 1974 Feb;22(2):197–205. doi: 10.1099/0022-1317-22-2-197. [DOI] [PubMed] [Google Scholar]
- Kurn N., Shapiro L. Regulation of the Caulobacter cell cycle. Curr Top Cell Regul. 1975;9:41–64. doi: 10.1016/b978-0-12-152809-6.50009-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton S. B., Rubenstein I. Calibration of molecular weight scales for DNA. J Mol Biol. 1969 Dec 14;46(2):313–328. doi: 10.1016/0022-2836(69)90424-0. [DOI] [PubMed] [Google Scholar]
- Leonard K. R., Kleinschmidt A. K., Agabian-Keshishian N., Shapiro L., Maizel J. V., Jr Structural studies on the capsid of Caulobacter crescentus bacteriophage phiCbK. J Mol Biol. 1972 Nov 14;71(2):201–216. doi: 10.1016/0022-2836(72)90346-4. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S., Hornack P. R., Armstrong P. A. Intracellular development of a large DNA bacteriophage lytic for Caulobacter crescentus. Arch Mikrobiol. 1967;59(1):237–246. doi: 10.1007/BF00406337. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Pai S. H., Ponta H., Herrlich P., Roskoski R., Jr, Schweiger M., Studier F. W. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Feb;71(2):586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Schmidt J. M. Observations on the adsorption of Caulobacter bacteriophages containing ribonucleic acid. J Gen Microbiol. 1966 Nov;45(2):347–353. doi: 10.1099/00221287-45-2-347. [DOI] [PubMed] [Google Scholar]
- Schmidt J. M., Stanier R. Y. The development of cellular stalks in bacteria. J Cell Biol. 1966 Mar;28(3):423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N., Bendis I. Bacterial differentiation. Science. 1971 Sep 3;173(4000):884–892. doi: 10.1126/science.173.4000.884. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N., Hirsch A., Rosen O. M. Effect of dibutyryladenosine 3':5'-cyclic monophosphate on growth and differentiation in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1972 May;69(5):1225–1229. doi: 10.1073/pnas.69.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N. Specific Assay for Differentiation in the Stalked Bacterium Caulobacter crescentus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):200–203. doi: 10.1073/pnas.67.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Wu R., Kaiser A. D. Mapping the 5'-terminal nucleotides of the DNA of bacteriophage lambda and related phages. Proc Natl Acad Sci U S A. 1967 Jan;57(1):170–177. doi: 10.1073/pnas.57.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]