Abstract
Persistence of human immunodeficiency virus (HIV) in a latent state in long-lived CD4+ T-cells is a major barrier to eradication. Latency-reversing agents that induce direct or immune-mediated cell death upon reactivation of HIV are a possible solution. However, clearance of reactivated cells may require immunotherapeutic agents that are fine-tuned to detect viral antigens when expressed at low levels. We tested the antiviral efficacy of immune-mobilizing monoclonal T-cell receptors against viruses (ImmTAVs), bispecific molecules that redirect CD8+ T-cells to kill HIV-infected CD4+ T-cells. T-cell receptors specific for an immunodominant Gag epitope, SL9, and its escape variants were engineered to achieve supraphysiological affinity and fused to a humanised CD3-specific single chain antibody fragment. Ex vivo polyclonal CD8+ T-cells were efficiently redirected by immune-mobilising monoclonal T-cell receptors against viruses to eliminate CD4+ T-cells from human histocompatibility leukocyte antigen (HLA)-A*0201-positive antiretroviral therapy-treated patients after reactivation of inducible HIV in vitro. The efficiency of infected cell elimination correlated with HIV Gag expression. Immune-mobilising monoclonal T-cell receptors against viruses have potential as a therapy to facilitate clearance of reactivated HIV reservoir cells.
Introduction
Human immunodeficiency virus (HIV) persists in long-lived CD4+ T-cells for the lifetime of the infected individual due to stable integration into host cell chromosomes from the earliest stages of infection. In this form it is inaccessible to immune effector cells and is unperturbed by antiretroviral agents. The apparent cure of a single individual who underwent a CCR5-null hematopoietic stem cell transplantation has provided impetus to develop safer and more scalable therapeutic approaches to HIV cure.1 A two-step approach involving administration of a latency-reversing agent (LRA) together with a therapeutic vaccine or other immunomodulator to engage host immune responses, has gained widespread support; the goal is to reactivate and then eliminate long-lived CD4+ T-cells that harbor latent HIV.2,3 However, LRAs that are both safe and sufficiently potent to induce HIV protein expression without global T-cell activation have yet to be identified.4,5,6 In addition, most therapeutic HIV vaccine candidates tested to date, mobilize only a small fraction of the CD8+ T-cell repertoire, which frequently comprises clones that failed to control the virus in the first place.7,8,9,10,11,12 Ex vivo expanded virus-specific cytotoxic T-cells have been used successfully in the treatment of post-transplant viral infections and were recently shown to eliminate latent HIV-infected CD4+ T-cells after in vitro reactivation13,14,15,16 However, the clinical utility of such an approach for HIV eradication is not yet known.
Engineered T-cell therapy is an alternative immunotherapeutic approach that has been pioneered for cancer treatment. Genetic engineering of T-cells to express specific receptors can overcome the limitations of natural adaptive immune responses to cancer antigens in several ways, including affinity enhancement, redirection of specificity away from self-antigens, proliferation and trafficking (reviewed in ref. 17). As similar considerations apply to genetically unstable viruses such as HIV, redirection of large numbers of effector T-cells toward selected epitopes could provide a critical advantage to the immune system, since ongoing viral escape from CD8+ T-cell responses could be mitigated. In support of this, modification of mature T-cells in the peripheral blood and of hematopoietic stem cells by lentiviral transduction with CD4-ζ or HIV TCRs has been shown to endow them with potent antiviral effector function in vitro and in vivo.18,19,20,21,22 The development of bispecific soluble reagents comprising a TCR fused to a humanized CD3-specific single chain variable fragment (scFv) is a crucial advance on transduced T-cell technology, as ex vivo manipulation of effector cells is not required. These immune-mobilising monoclonal TCRs against cancer (ImmTACs) and viruses (ImmTAVs) have several unique features: targeted modifications of TCR complementarity-determining regions result in extraordinarily high affinity for peptide-MHC class I, in the picomolar range, without loss of specificity; dosing can be tightly controlled and synergized with other therapies; the immune-mobilising moiety enables recruitment of the most potent effector cells. The net result is a rapid and potent polyclonal response that eliminates target cells expressing very low levels of cognate epitope, thus overcoming a major hurdle not only for cancer therapy but also for pathogens such as HIV that downregulate human histocompatibility leukocyte antigen (HLA) class I expression.23,24 Furthermore, selection of TCRs that recognize naturally occurring variants of the cognate epitope potentiates clearance of infected cells harboring virus escape mutants.20,25 ImmTACs have shown promise in vitro and in early clinical trials in melanoma and a similar technology employing antibody-mediated redirection led to development of bispecific T-cell engagers such as blinatumomab, which was recently licensed for of the treatment of certain leukemia.26,27,28,29,30,31
In this study, we investigated the capacity of two ImmTAVs with picomolar affinity for an immunodominant HIV epitope to eliminate HIV gag-expressing CD4+ T-cells, after infection in vitro and after reactivation of latent HIV in CD4+ T-cells from antiretroviral therapy (ART)-treated patients. We show that ImmTAVs are highly effective in mobilising polyclonal CD8+ T-cells to kill infected CD4+ T-cells at low CD8+/CD4+ cell ratios and low epitope densities.
Results
Redirected antigen specificity of human primary CD8+ T-cells by ImmTAVs
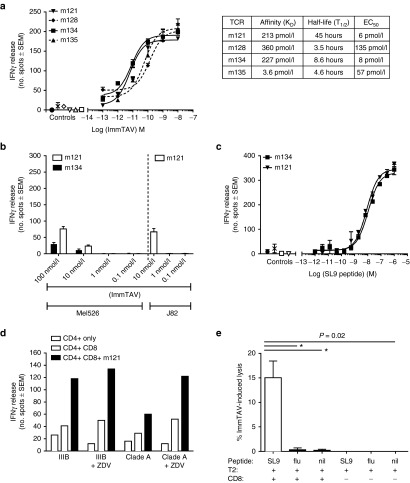
A panel of ImmTAVs with enhanced affinity for the immunodominant HLA-A*02-restricted HIV-1 gag p17 epitope, SLYNTVATL (SL9) was generated by TCR engineering, as described previously.26 Two ImmTAVs, m121 and m134, demonstrated particularly potent biological activity against peptide-pulsed targets, with EC50 values below 10–11 mol/l (Figure 1a), and these were selected for further testing. The target specificity of these ImmTAVs was demonstrated using antigen-negative HLA-A*02-positive cell lines, with which nonspecific T-cell activation was observed only at high ImmTAV concentrations, i.e., at least 1,000-fold >half maximal effective concentration (EC50) values (Figure 1b). A slight loss of specific binding at concentrations >10–9 mol/l has been observed previously with other immune-mobilising TCR reagents and is attributed to HLA-restricted peptide cross-reactivity.26,27 Peptide titration experiments showed that m121 and m134 were sensitive to nanomolar concentrations of exogenously loaded SL9 peptide, indicating recognition of low numbers of epitopes32,33 (Figure 1c). In addition, they could interact with endogenously generated viral peptides presented by HIV-infected primary CD4+ T-cells (Figure 1d), even when the cognate epitope carried a naturally occurring mutation (Y-F at position 3 in the case of the clade A virus) (Figure 1d). We also confirmed that they could induce specific lysis of peptide-loaded targets (Figure 1e).
Figure 1.
Biophysical and functional analysis of affinity-matured immune-mobilising monoclonal T-cell receptors against viruses (ImmTAVs) specific for SL9 epitope. (a) Left: Interferon (IFN)-γ responses of CD8+ T-cells from a healthy donor (8 × 104 cells/well) mediated by titrated concentrations of four SL9-directed ImmTAVs (m121, m128, m134, and m135) when cultured with T2 cells pulsed with 10–9 mol/l SL9 peptide (5 × 104 cells/well), quantified by ELISpot. Negative controls for each ImmTAV at 10–9 mol/l were T2 cells pulsed with an irrelevant peptide (open symbols), CD8+ T-cells in the absence of ImmTAV (cross symbol), and T2 cells only (closed circle). Right: Binding parameters (affinity (KD) and half-life (T1/2)) of each of the four ImmTAVs to SL9 peptide, obtained by surface plasmon resonance analysis. (b) Reactivity of ImmTAVs m121 and m134, against Gag SL9-negative, human histocompatibility leukocyte antigen (HLA) -A*0201-expressing human melanoma cells (Mel526) (5 × 104 cells/well). m121 was also tested against malignant human urothelial cells (J82). ImmTAVs were used at concentrations indicated, with CD8+ T-cells from a healthy donor (8 × 104 cells/well). (c). IFN-γ ELISpot assays showing sensitivity of ImmTAVs, m121, and m134 (10–9 mol/l ImmTAV), to T2 cells (5 × 104/well) pulsed with titrated concentrations of SL9 peptide. Healthy donor CD8+ T-cells and controls were as described in a. (d) Response to human immunodeficiency virus (HIV) -infected (IIIB or A isolates) primary CD4+ T-cells (5 × 104 cells/well) by healthy donor CD8+ T-cells (8 × 104 cells/well) when redirected by m121 ImmTAV (10–8 mol/l). Zidovudine (ZDV) (5 μg/ml) was added as indicated, as we observed that this reduced nonspecific IFN-γ release by CD4+ T-cells when infected with HIV in vitro. (e) CFSE-labelled T2 cells were pulsed with 10 μmol/l SL9 peptide, irrelevant (influenza) peptide or unpulsed, then cultured with healthy donor CD8+ T-cells with or without ImmTAV (1 nmol/l) for 24 hours and analyzed by flow cytometry.
ImmTAV-redirected CD8+ T-cells inhibit exogenous HIV replication in vitro
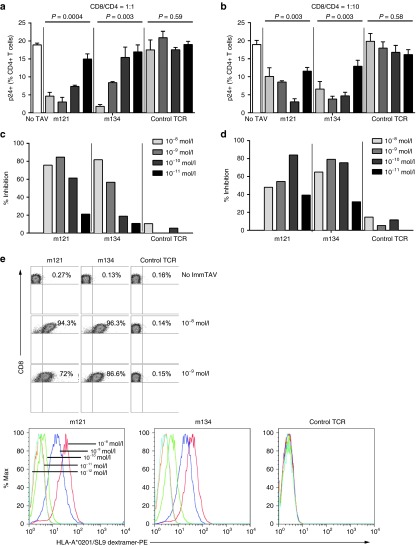
Next, we investigated whether CD8+ T-cells from healthy HLA-A*0201+ donors could inhibit HIV replication in autologous CD4+ T-cells when redirected by ImmTAVs. We have previously established the kinetics of infection with diverse laboratory-adapted and primary HIV isolates in primary CD4+ T-cells: IIIB was selected because infected cell frequencies of ~20% can be sustained at the peak of replication (days 5–7 of culture) without excessive cell death.34 HIV IIIB-infected CD4+ T-cells were cultured alone, with autologous unstimulated CD8+ T-cells or with autologous CD8+ T-cells plus ImmTAVs for 7 days. Consistent with our previous data, the peak Gag p24+ cell frequency was 19% in CD4+ cell-only cultures and as expected, was not reduced by autologous CD8+ T-cells alone.34 However, when redirected by HIV ImmTAVs, CD8+ T-cells reduced infected cell frequencies significantly, by up to 85%, indicating a potent antiviral response. By contrast, an irrelevant TCR-anti-CD3 scFV fusion (control TCR) had negligible effect on HIV replication (Figure 2a–d). The effect of the ImmTAVs was dependent on both their concentration and the CD8+/CD4+ ratio: at a ratio of 1:1, they were most potent at a concentration of 10–8 mol/l, whereas at a CD8+/CD4+ cell ratio of 1:10, potency increased with decreasing ImmTAV concentrations as far as 10−10 mol/l (Figure 2a–d). There were marginally lower percentages of live cells in cultures with HIV ImmTAVs at concentrations of 10 nmol/l compared to 1 nmol/l or no ImmTAV (see Supplementary Figure S1).
Figure 2.
Inhibition of exogenous HIV by ImmTAV-redirected CD8+ T-cells. Primary CD4+ T-cells from healthy HLA-A*0201-positive donors were infected with HIV-IIIB and cultured alone (5 × 104 cells/well), with autologous CD8+ T-cells only or with CD8+ T-cells plus HIV ImmTAVs, m121 and m134, or an irrelevant TCR-anti-CD3 scFV fusion (control TCR). (a, b). Frequencies of HIV-infected (p24 Ag+) CD4+ T-cells and (c, d) percent inhibition after 7 days' culture at CD8+/CD4+ cell ratios of 1:1 a, c and 1:10 b, d; ImmTAVs were tested at concentrations ranging from 10–8 – 10–11 mol/l. Data shown are mean ± SD and are representative of three experiments. Percent inhibition was calculated as described in Materials and Methods. (e) Saturation of CD8+ T-cells with ImmTAVs: CD8+ T-cells were incubated with ImmTAVs at concentrations indicated, before staining with an HLA-A*0201/SL9 dextramer and analysis by flow cytometry. Dextramer binding is shown in dot plots (top) at ImmTAV concentrations of 0, 10–8, and 10–9 mol/l and in histograms (bottom) at ImmTAV concentrations of 10–8 – 10–12 mol/l for each TCR. HLA, human histocompatibility leukocyte antigen; HIV, human immunodeficiency virus; TCR, T-cell receptor.
To assess whether the antiviral efficacy of ImmTAV-redirected CD8+ T-cells was determined by the extent to which available CD8+ T-cells were recruited, purified CD8+ T-cells were loaded with ImmTAVs and then stained with an HLA-A*0201/SL9 dextramer at 37 °C. This showed that the SL9 dextramer bound to ImmTAV-bearing CD8+ T-cells in a dose-dependent manner, with >90% and up to 87% binding the ImmTAV at concentrations of 10–8 mol/l and 10–9 mol/l respectively; no dextramer binding to CD8+ T-cells was observed when loaded with irrelevant TCR (Figure 2e). This indicated that at optimal concentrations for viral inhibition, ImmTAVs engaged the majority of available CD8+ T-cells.
ImmTAVs enhance the capacity of CD8+ T-cells from chronic HIV-infected subjects to reduce autologous HIV spread upon reactivation in vitro
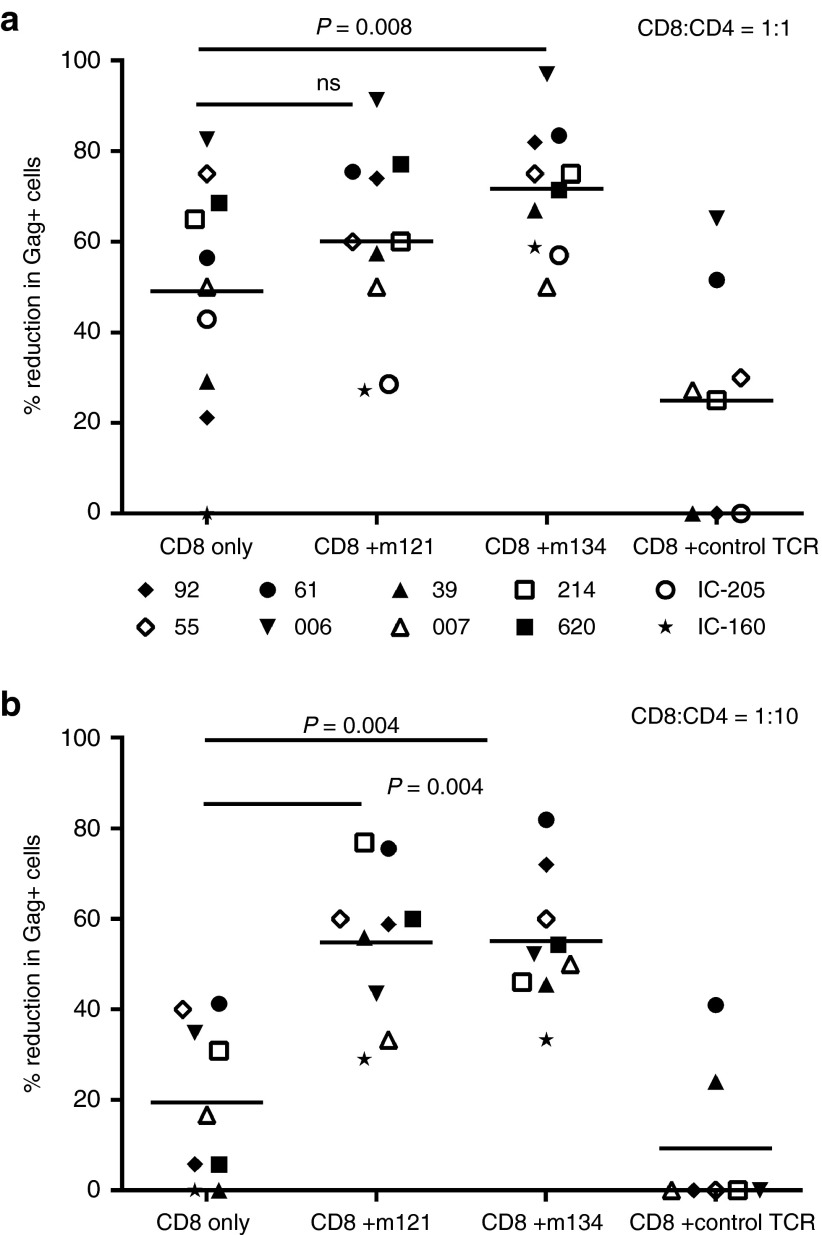
We then assessed the antiviral potency of ImmTAVs against primary HIV isolates in CD4+ T-cells from patients with chronic infection. As phytohemagglutinin (PHA) is reported to induce consistent reactivation of latent HIV in primary cell cultures, we stimulated purified CD4+ T-cells from 10 HLA-A*0201+ chronically infected subjects (8 ART-treated and 2 ART-naive) with PHA for 72 hours and then cultured them with autologous purified CD8+ T-cells, alone or with ImmTAVs for 7 days.35 We have previously observed that PHA stimulation of CD4+ T-cells from 37 ART-treated patients led to detectable HIV reactivation in 46% subjects, defined by Gag+ T-cells acquired in the CD3+CD8− gate exceeding the negative cut-off value of 41, (obtained from the mean +3 SD of Gag+ cells in unstimulated CD4+ T-cell cultures). We have also shown that Gag expression strongly correlated with the level of p24 antigen production measured by enzyme-linked immunosorbent assay.34 To ensure reproducible HIV reactivation prior to ImmTAV exposure, we set a higher threshold requiring at least 100 Gag-positive cells in CD4+/CD8+ cell-only cultures. Both ImmTAVs (used at a concentration of 10–8 mol/l for a CD8+/CD4+ ratio of 1:1 and 10–11 mol/l for a ratio of 1:10) enhanced the capacity of ex vivo CD8+ T-cells to block replication of autologous virus. At a CD8+/CD4+ ratio of 1:1, the mean (SD) reduction in p24 Ag+ cells observed with CD8+ T-cells alone was 49% (26%), which was consistent with data we obtained from 50 chronic ART-treated patients with diverse HLA haplotypes (Yang H. and Dorrell L., unpublished data). In the presence of the ImmTAVs, mean (SD) reduction in p24 Ag+ cells was 60% (20%) for m121 and 72% (14%) for m134 (P = 0.008 for m134) (Figure 3a). At a ratio of 1:10, the ImmTAVs showed similar antiviral effects: 55% (17%) for m121 and 55% (15%) for m134 versus 19% (17%) for CD8+ T-cells alone; P = 0.004 (Figure 3b). The irrelevant TCR-anti-CD3 scFV fusion was tested at 10–9 mol/l because significant nonspecific cell death was observed at 10–8 mol/l in assays with healthy donor T-cells (mean 47% live cells recovered at the end of culture versus 75% for no ImmTAV (P = 0.002, Supplementary Figure S1). At 10–9 mol/l concentration it did not enhance control of viral replication. Similar rates of live cell recovery were observed when comparing cultures with the irrelevant TCR-anti-CD3 scFV fusion at 10–9 mol/l, m121 and m134 at either 10–9 or 10–8 mol/l and CD8+ T-cells alone (Supplementary Figure S1). The irrelevant TCR was therefore used at concentrations of 10–9 mol/l or lower in all subsequent experiments.
Figure 3.
ImmTAV-mediated inhibition of autologous HIV spread in CD4+ T-cells from HIV-infected subjects. Purified CD4+ T-cells (1 × 105) from 10 HIV-positive HLA-A*0201+ subjects (8 ART-treated and 2 ART-naive, represented by symbols shown above) were stimulated with PHA for 72 hours to promote replication of endogenous HIV, then cultured with autologous CD8+ T-cells at CD8+/CD4+ cell ratios of 1:1 (a) and 1:10 (b), either alone or with ImmTAVs as indicated (ImmTAVs at 10–8 mol/l for the 1:1 ratio, 10–11 mol/l for the 1:10 ratio). The irrelevant TCR-anti-CD3 scFV fusion (control TCR) was tested at 10–9 mol/l and 10–12 mol/l for the 1:1 and 1:10 ratios respectively because of nonspecific cell death at higher concentrations. Reduction in Gag+ cells was determined on day 7 of coculture. Horizontal lines indicate mean values. HLA, human histocompatibility leukocyte antigen; HIV, human immunodeficiency virus; ImmTAV, immune-mobilising monoclonal T-cell receptors against virus; TCR, T-cell receptor.
ImmTAV-redirected CD8+ T-cells from healthy donors have superior capacity to reduce spread of reactivated HIV in CD4+ T-cells from HIV-positive ART-treated subjects
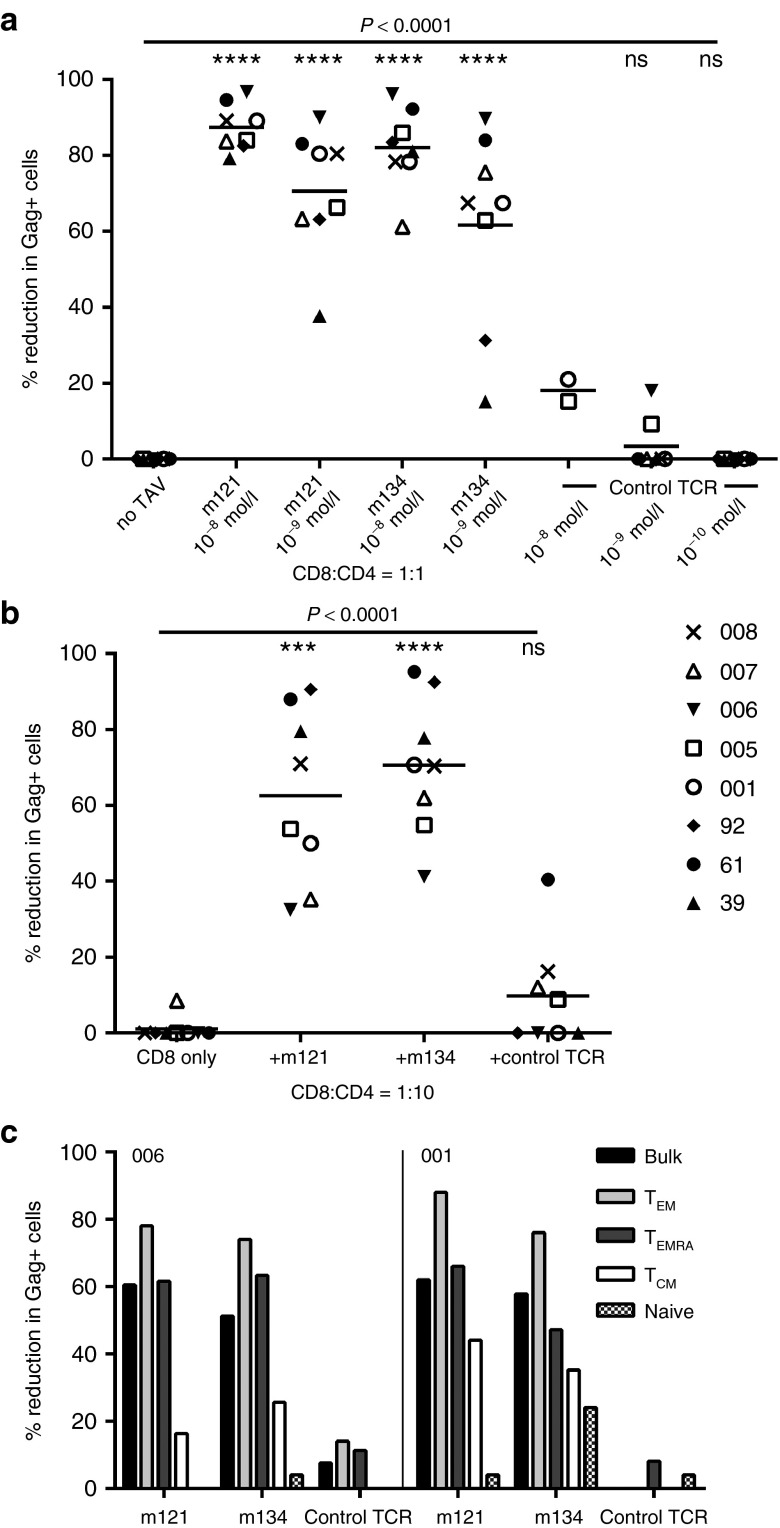
Persistent defects in the effector function of CD8+ T-cells specific for HIV and other antigens are well documented in patients on long-term ART.36,37,38 To investigate whether this could explain the modest effects of the ImmTAVs when used to redirect patient-derived CD8+ T-cells, particularly at the 1:1 CD8+/CD4+ ratio, we cocultured CD8+ T-cells from healthy HIV-uninfected donors with reactivated CD4+ T-cells from the same ART-treated HIV-positive subjects, with or without ImmTAVs. Healthy donor CD8+ T-cells were able to reduce p24 Ag+ cells by >80% when redirected by ImmTAVs at a concentration of 10–8 mol/l and CD8+/CD4+ cell ratio of 1:1 (mean (SD) for m121 – 87% (6%); m134 – 82% (11%); P < 0.0001) (Figure 4a). The ImmTAVs were also tested at 10–9 mol/l to rule out the possibility of any nonspecific binding to HLA-A2-self peptide complexes (which had been observed at 10–8 mol/l with HLA-A*0201-positive human cancer cells, Figure 1b). Spread of infection was reduced by >60% at this concentration (mean (SD) for m121 – 70.5% (17.8%); m134 – 62% (26%), P < 0.0001) (Figure 4a). A similar magnitude of effect was seen with a CD8+/CD4+ cell ratio of 1:10 and ImmTAV concentration of 10–11 mol/l (mean (SD) for m121 − 63% (23%); m134 – 71% (18%); P < 0.0001) (Figure 4b). In all experiments, the irrelevant TCR-anti-CD3 scFV fusion had minimal or no impact on HIV replication, nor did the CD8+ T-cells from healthy donors have any effect in the absence of ImmTAVs, ruling out any CD8+ T-cell killing by an allospecific mechanism.
Figure 4.
Antiviral potency of ImmTAV-redirected healthy donor CD8+ T-cells against HIV-infected CD4+ T-cells from patients. Purified primary CD4+ T-cells (1 × 105) from 8 HIV-positive HLA-A*0201-positive ART-treated subjects were stimulated with PHA to reactivate latent HIV, then cultured with CD8+ T-cells from an HIV-negative donor at (a) a CD8+/CD4+ cell ratio of 1:1, alone or with ImmTAVs at the concentrations indicated; (b) a CD8+/CD4+ ratio of 1:10, alone or with m121 and m134 − 10–11 mol/l, irrelevant TCR-anti-CD3 scFV fusion (control TCR) − 10–12 mol/l. The same subjects are represented in a and b by the symbols in the legend. Reduction in Gag+ cells was determined on day 7 of coculture. Horizontal lines indicate mean values. Note that inhibition in the presence of the control TCR was negligible even when used at the same concentration as the HIV ImmTAVs. (c) Healthy donor CD8+ T-cells were sorted into subsets according to expression of CCR7 and CD45RA and cultured with purified PHA-activated CD4+ T-cells from two ART-treated patients at a ratio of 1:2, in the presence or absence of ImmTAVs (all at 10–9 mol/l) for 7 days. TEM – effector memory, CCR7−/CD45RA−; TEMRA – terminally differentiated effector, CCR7-/CD45RA+; TCM – central memory, CCR7+/CD45RA-; naive, CCR7+/CD45RA+. Reduction in Gag+ cells was determined on day 7 of co-culture. ART, antiretroviral therapy; PHA, phytohemagglutinin; HLA, human histocompatibility leukocyte antigen; HIV, human immunodeficiency virus; ImmTAV, immune-mobilising monoclonal T-cell receptors against virus; TCR, T-cell receptor.
Consequently, we interrogated the flow cytometric data to determine the number of conjugates forming between CD8+ T-cells and HIV-infected CD4+ T-cells (gating strategy is shown in Supplementary Figure S2). In most cases, we observed more frequent conjugates forming in the presence of healthy donor CD8+ T-cells than autologous CD8+ T-cells under all conditions tested; this could have contributed to the greater antiviral effects of the ImmTAVs when redirecting healthy donor CD8+ T-cells (see Supplementary Figure S3).
We also examined the effector capability of different CD8+ T-cell memory populations when redirected by an ImmTAV. CD8+ T-cells from one of the healthy donors were sorted into naive, central memory, effector memory and terminally differentiated effector subsets and then added to PHA-activated CD4+ T-cells from two ART-treated patients. Because of the low frequency of central memory cells in the peripheral blood, a CD8+/CD4+ cell ratio of 1:2 was used for all subsets. Effector or effector memory cells showed the strongest capacity to reduce HIV infection when redirected by an ImmTAV, similar to or exceeding the effect observed with bulk CD8+ T-cells, whereas central memory and naive cell subsets were less effective (Figure 4c). These results were consistent with previous observations on ImmTAC killing of cancer cells.26
Antiviral efficacy of ImmTAV-redirected CD8+ T-cells is dependent on rapid killing of infected cells
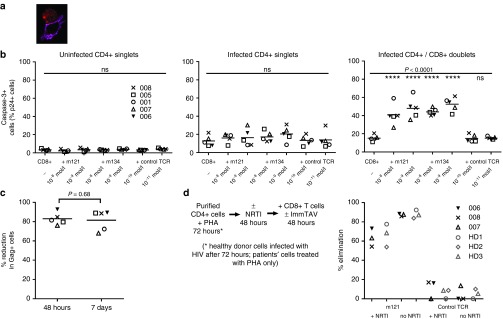
ImmTACs specific for a melanoma antigen have been shown to induce rapid killing of target cells, therefore, we wished to establish whether the antiviral effects of the ImmTAVs involved a lytic mechanism.26,27 We used three approaches to address this. First, we used flow cytometric analysis to identify CD8+/CD4+ T-cell conjugates as before, this time to quantify HIV-infected CD4+ T-cells that expressed caspase-3, which is activated in response to extrinsic apoptotic signals.39,40 We also demonstrated these conjugates by confocal microscopy (Figure 5a). We compared caspase-3 expression in uninfected CD4+ T-cells, infected singlet CD4+ T-cells and infected CD4+/CD8+ T-cell conjugates in the presence or absence of ImmTAVs. HIV-infected singlet CD4+ T-cells showed higher caspase-3 levels than uninfected CD4+ T-cells, suggestive of a recent ImmTAV-mediated death signal or possibly a direct effect of HIV. Caspase-3 expression was significantly increased in infected CD4+ T-cells forming conjugates with CD8+ T-cells exposed to ImmTAVs (mean (SD) for 10–8 mol/l − m121 − 41% (11.5%), m134 – 44% (4%); 10–9 mol/l − m121 − 48% (12%), m134 – 53% (8%); CD4+/CD8+ T-cell conjugates without ImmTAVs – 15% (3%); P < 0.0001) (Figure 5b). Caspase-3 expression was not upregulated above background levels in the presence of the irrelevant TCR-anti-CD3 scFV fusion.
Figure 5.
ImmTAVs mediate killing of infected CD4+ T-cells. (a) Confocal image of a conjugate between an HIV-infected CD4+ T-cell and healthy donor CD8+ T-cells in the presence of an ImmTAV (m121, 10–9 mol/l). Red – p24 Ag; magenta – CD8. (b) Purified activated CD4+ T-cells from 5 HLA-A*0201-positive ART-treated patients were cultured with healthy donor CD8+ T-cells alone or with ImmTAVs at the concentrations indicated. Caspase-3 expression in CD4+ T-cells that were uninfected (left), HIV-infected singlets (middle) or HIV-infected and forming conjugates with CD8+ T-cells (right) was determined by flow cytometric analysis (gating strategy in Supplementary Figure S2). (c) Purified activated CD4+ T-cells from the same five ART-treated patients were cultured with healthy donor CD8+ T-cells (CD8+/CD4+ cell ratio = 1:1) alone or with an ImmTAV (m134, 10–8 mol/l), which was either present throughout the culture period or washed out by replacing the culture medium after 48 hours. Reduction in Gag+ cells, normalized to CD8-only values, on day 7 of coculture is shown. In b and c, horizontal lines indicate mean values. (d) Schema (left) to illustrate the same coculture assay with NRTI-treated CD4+ T-cells. Tenofovir (10 µmol/l) was added to PHA-activated CD4+ T-cells from three ART-treated patients (006, 007, 008, black symbols, immediately after harvesting) and three healthy donors (HD1, HD2, HD3, gray symbols, immediately after spinoculation with HIV IIIB). After 48 hours of drug / mock drug exposure, healthy donor CD8+ T-cells and ImmTAVs were added and cultures were maintained for a further 48 hours. Percent elimination of HIV-infected cells normalized to no TCRs is shown (right). ART, ; PHA, ; HD, ; HIV, human immunodeficiency virus; ImmTAV, immune-mobilising monoclonal T-cell receptors against virus; TCR, T-cell receptor.
Next, we performed cell cultures in which the medium was replaced after 48 hours in order to wash out any unbound TCR; the m134 TCR was used for these experiments because of its shorter binding half-life (8.6 hours) than m121 (Figure 1a). Cultures were maintained for a further 5 days, after which the proportion of infected cells remaining was compared with cultures in which the medium was not changed. Infected cell frequencies at day 7 did not differ significantly between cultures in which the ImmTAVs were present for the duration or for the first 48 hours only (P = 0.68), indicating that the effect of the ImmTAV was irreversible and had reached a maximum by 48 hours (Figure 5c).
Finally, to confirm that Gag-expressing CD4+ T-cells were eliminated upon ImmTAV exposure, we treated HIV-infected cells with a reverse transcriptase inhibitor to stop HIV spread via production of new virions during culture (see Supplementary Figure S4). CD4+ T-cells from 3 ART-treated patients and 3 healthy donors were first stimulated with PHA, to reactivate latent virus or permit in vitro infection, respectively. They were then treated with tenofovir or left untreated for 48 hours, after which they were cocultured with CD8+ T-cells +/− ImmTAV for a further 48 hours (see schema in Figure 5d). The ImmTAV was observed to eliminate 50–78% of HIV-infected cells within 48 hours in the presence of tenofovir, whether they were infected in vitro or in vivo (Figure 5d). A higher proportion of infected cells was eliminated by the ImmTAV when tenofovir was absent (mean 88% versus 66%, P = 0.0006). A likely explanation for this is a reduction in Gag epitope generation following tenofovir treatment, which is an expected downstream effect of inhibition of reverse transcription. Consistent with this, tenofovir had no effect on infected cell elimination by the TCR-anti-CD3 scFV fusion (mean 8% versus 5%, P = 0.48), Figure 5d) and Gag mean fluorescence intensity was slightly lower in tenofovir-treated than untreated cells (see Supplementary Figure S5). Overall, these data confirmed that the HIV ImmTAVs were able to kill in vitro- and in vivo-infected CD4+ T-cells.
The antiviral effect of ImmTAVs is determined by the level of target cell epitope expression
Given that latently infected CD4+ T-cells in the peripheral blood may vary in sensitivity to viral reactivation with a polyclonal stimulus and that some expression of Gag proteins has been demonstrated in the absence of productive infection, ex vivo CD4+ T-cells from ART-treated patients are likely to be heterogeneous with respect to the expression of HIV epitopes.41,42,43 In view of this, we hypothesized that elimination of these cells by the ImmTAVs would depend on both the activation state of the infected cell and the density of cognate epitope on the cell surface. We investigated this in three ways.
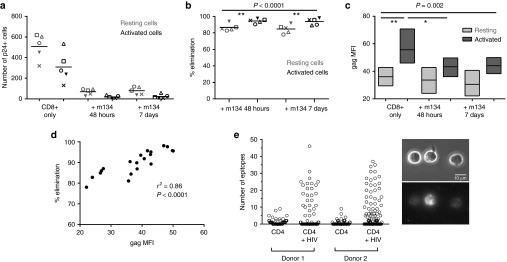
First, we repeated the coculture experiments using PHA-stimulated CD4+ T-cells from 5 HIV-positive ART-treated subjects and CD8+ T-cells from a single healthy donor, with or without ImmTAV. After 7 days, we quantified activated (CD25+/CD69+/HLA-DR+) and resting (CD25−/CD69−/HLA-DR−) CD4+ T-cells in cultures with CD8+ T-cells alone and CD8+ T-cells with ImmTAV. Under all experimental conditions, the proportion of p24 Ag+ cells was higher in activated than resting CD4+ T-cells, yet the absolute number of p24 Ag+ cells among resting cells exceeded that in activated CD4+ T-cell populations because there were many more resting than activated cells overall (Figure 6a and Supplementary Figure S6). Unexpectedly, the ImmTAV eliminated not only the vast majority of activated CD4+ T-cells in cultures exposed to the ImmTAV for only 48 hours (mean (SD) − 95% (3%)) but also most of the resting cells, albeit to a lesser extent (mean (SD) – 87% (4%), P <0.0001). Furthermore, the fraction of infected cells that was eliminated was similar between cultures with ImmTAV for 48 hours and 7 days (mean (SD) for both activated and resting cells at 7 days – 94% (4%) and 85% (5%) respectively, not significant), indicating that the ImmTAV exerted maximal effect in the first 48 hours, regardless of the activation state of the target cell (Figure 6a,b). We analyzed the level of HIV Gag expression in the CD4+ T-cells remaining at the end of 7 days' co-culture with CD8+ T-cells +/− ImmTAV. As expected, Gag expression was significantly lower in resting cells than activated cells overall (P = 0.002), particularly when comparing cultures without ImmTAVs (Figure 6c, ‘CD8+ only'). Of note, the few residual activated cells in the ImmTAV-treated cultures showed significantly lower Gag expression than the remaining activated cells in the CD8+ cell-only cultures, consistent with rapid ImmTAV-mediated elimination of Gaghi cells. By contrast, Gag expression in resting cells was similar among populations cultured with or without ImmTAV and was consistently lower than in activated cells (Figure 6c). Overall, these data suggested that the level of Gag expression in both activated and resting cells was sufficient to render them susceptible to killing but that Gaghi cells were preferentially killed. In support of this, we observed a strong correlation between Gag expression, mean fluorescence intensity and % reduction in infected cells (r2 = 0.86, P < 0.0001) (Figure 6d).
Figure 6.
ImmTAV-mediated elimination of activated and resting HIV-infected CD4+ T-cells. Purified PHA-stimulated CD4+ T-cells from the same five HLA-A*0201-positive ART-treated patients as in Figure 5 were cultured with healthy donor CD8+ T-cells alone or with ImmTAV m134 (10–8 mol/l). The ImmTAV was either present for the duration of coculture or washed out after 48 hours. (a) The number of activated (CD25+/CD69+/HLA-DR+) and resting (CD25-/CD69-/HLA-DR-) Gag+ CD4+ T-cells remaining on day 7 of coculture and (b) percent elimination of infected cells, normalized to CD8-only values, within each subset is shown. (c) Mean fluorescence intensity (MFI, arbitrary units) of Gag expression in residual infected activated and resting CD4+ T-cells after culture under the conditions indicated (as for a and b; bars and horizontal lines represent range and mean respectively). Consistency in MFI values was ensured by acquiring all samples in a single run. (d). Correlation between Gag MFI (arbitrary units) and normalized percent elimination of infected cells. (e) Biotinylated m134 ImmTAV was detected by microscopy on the surface of individual HIV-1 IIIB-infected CD4+ T-cells from two healthy donors after staining with streptavidin-PE. Data shown in left panel represent counts of fluorescent spots; the total for each cell was obtained using Z-stack images. Uninfected cells were used to determine background staining. Right panel: representative phase-contrast (top) and corresponding fluorescence images (bottom) of infected CD4+ T-cells from donor 1 stained with m134 ImmTAV. Fluorescence images are 3D reconstructions of individual planes. The brightness/contrast of images was adjusted to optimize epitope visualization. The scale bar represents 10 µm. HLA, human histocompatibility leukocyte antigen; PHA, phytohemagglutinin; HIV, human immunodeficiency virus; ImmTAV, immune-mobilising monoclonal T-cell receptors against virus.
In parallel, we performed a direct quantification of epitope presentation on the surface of infected CD4+ T-cells, comprising activated and resting cells, using a biotinylated version of the m134 ImmTAV to visualize peptide-HLA complexes at the single cell level. This method has been shown previously to be suitable for the detection of low numbers of epitopes on cancer cells (5–150 per cell).32,33 The data shown in Figure 6e were obtained following infection of CD4+ T-cells from two donors; in both cases ~20% of HIV-infected CD4+ T-cells were positive for ImmTAV staining. This was in agreement with the frequency of p24 Ag+ cells detected concurrently by flow cytometry (27.6% p24+ cells for donor 1 and 21.7% p24+ cells for donor 2) and contrasted with the low level of staining observed with uninfected cells. In addition, staining observed with an irrelevant biotinylated TCR-anti-CD3 scFV fusion was similar to that of uninfected cells (data not shown). Of those cells that stained positive with ImmTAV, between 8 and 46 epitopes were observed on individual cells. These data indicate that the ImmTAVs are capable of inducing T-cell killing despite a very low level of epitope presentation on infected cells.
ImmTAV-redirected CD8+ T-cells clear autologous inducible HIV from resting CD4+ T-cells
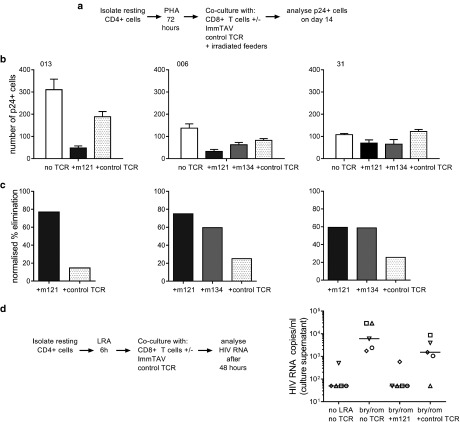
As the previous experiments were performed with unfractionated CD4+ T-cells from ART-treated patients, we modified our coculture assay in order to investigate whether resting HIV-infected cells could be eliminated by the ImmTAVs after reactivation of latent HIV. Resting CD4+ cells were isolated from three patients' peripheral blood mononuclear cells (PBMC) and stimulated with PHA for 72 hours, then cultured with healthy CD8+ T-cells alone, or with ImmTAVs for 14 days. Irradiated feeders were added to support the replication of autologous viruses. Infected cell elimination was again quantified by flow cytometry. In addition, supernatants were tested for p24 Ag release. The supernatants from purified resting CD4+ T-cells cultured alone for 14 days after reactivation were positive for p24 Ag by enzyme-linked immunosorbent assay, confirming that new virions had been produced. ImmTAV-redirected CD8+ T-cells were able to eliminate the majority of reactivated p24 Ag+ CD4+ T-cells in all three subjects (mean 70% for m121 versus 22% for the TCR-anti-CD3 scFV fusion) (Figure 7a–c). Finally, to evaluate ImmTAV-redirected killing after in vitro reactivation of HIV without global T-cell activation, we treated ex vivo purified resting CD4+ T-cells from five ART-treated patients with a combination of LRAs—romidepsin, a histone deacetylase inhibitor and bryostatin, a PKC agonist for 6 hours, followed by culture with healthy donor CD8+ T-cells alone or with an ImmTAV for a further 42 hours. LRA treatment led to induction of HIV replication, indicated by detectable viral RNA in culture supernatants that exceeded >1000 copies/ml in all five patients (Figure 7d). Exposure to ImmTAV after viral reactivation led to a complete abrogation of viral recovery in four out of five patients whereas exposure to the control TCR reduced viral recovery marginally, except in one patient (Figure 7d).
Figure 7.
ImmTAV-mediated clearance of autologous HIV reservoir cells. (a). Schema illustrating assay to quantify ImmTAV-mediated elimination of resting HIV-infected Cd4+ T-cells from ART-treated patients after viral reactivation. CD25-/69-/HLA-DR- CD4+ cells were thoroughly washed after PHA treatment and cultured in duplicate at a density of 3 × 105 cells/well alone, with autologous CD8+ T-cells only or with healthy donor CD8+ T-cells plus ImmTAVs (10–9 mol/l). Irradiated allogeneic PBMC feeders (1.5–3 × 106) were added to all wells. CD8+/CD4+ cell ratios were 1:1 throughout. After 14 days, Gag+ cells were quantified by flow cytometry. (b) Number of Gag+ cells remaining at day 14 of culture (mean of duplicate wells). (c) Percent elimination of Gag+ cells, determined by normalizing to no TCRs. In all subjects, culture supernatants from CD4+ cell-only wells were positive for free p24 Ag and ImmTAV-treated wells were negative (determined by enzyme-linked immunosorbent assay, cut-off 1500 pg/ml). (d) CD25-/69-/HLA-DR- CD4+ cells from five ART-treated HLA-A*0201-positive subjects were thoroughly washed after LRA (bryostatin / romidepsin) treatment and cultured in duplicate with healthy donor CD8+ T-cells (1:1 ratio) ± ImmTAV m121 or control TCR (10–9 mol/l) for a further 42 hours. Supernatants were harvested and viral outgrowth was determined by quantification of HIV RNA, as described in Materials and Methods. LRA, latency-reversing agent; HLA, human histocompatibility leukocyte antigen; ART, antiretroviral therapy; PHA, phytohemagglutinin; HIV, human immunodeficiency virus; ImmTAV, immune-mobilising monoclonal T-cell receptors against virus; PBMC, peripheral blood mononuclear cells; TCR, T-cell receptor.
Discussion
In this study, we have shown that engineered immune-mobilising HIV-specific TCR anti-CD3 fusion proteins mediate killing of CD4+ T-cells from HIV-infected ART-treated individuals upon in vitro reactivation of latent virus. The development of these ImmTAVs was predicated on the generation and selection of TCR candidates with picomolar affinity for pMHC and the capacity to recognize all naturally occurring variants of the cognate epitope. This technology therefore has the potential to address two major challenges that the adaptive immune system faces when responding to antigens derived from reactivated HIV in latently infected cells, namely, the release of viral escape mutants and low levels of antigen expression.20,25 Here, we have shown for the first time that upon specific interaction with cell surface HLA class I-viral peptide complexes, the ImmTAVs were capable of mobilising and activating the majority of effector CD8+ T-cells in the vicinity of the infected cell via CD3 signaling. The capacity to harness a large number of immune effector cells simultaneously is another advantage of ImmTAV technology that may provide superior antiviral efficacy to naturally primed cytotoxic T-cells. In vitro efficacy of a similar technology comprising dual-affinity retargeting proteins, which engage HIV-infected target cells via specific monoclonal antibodies, was demonstrated recently using resting CD4+ T-cells from ART-treated patients.44 However, the affinity of the HIV binding arm of these agents is still considerably lower (50–200 fold) than the TCR component of the ImmTAVs described here. Furthermore, they are targeted to Env epitopes which are expressed at lower densities than Gag on both activated and resting cells and are highly variable in sequence.
We have shown that ImmTAV-redirected polyclonal autologous CD8+ T-cells eliminated HIV-infected CD4+ T-cells more efficiently than the patients' naturally primed CD8+ T-cells specific for the same Gag epitope, SL9, which typically account for ≤ 2% CD8+ T-cells in chronically infected subjects.8,45 These findings were expected in the light of previously reported tumor-lytic effects of melanoma antigen-specific ImmTACs.26 In some individuals, the ImmTAVs conferred on autologous CD8+ T-cells a level of lytic capacity that was similar to naturally primed HIV-specific CD8+ T-cells from elite controllers.46 However, stronger ImmTAV-mediated killing was observed in all subjects when CD8+ T-cells were derived from healthy HIV-uninfected donors, which suggests that persistent functional defects affecting all CD8+ T-cells were present in chronic ART-treated subjects. This is perhaps not surprising, given that ART greatly reduces but does not normalize the expression of immune checkpoint molecules such as PD-1 and TIM-3 in all CD8+ T-cells.47,48 This suggests that synergistic approaches combining ImmTAVs with agents to correct intrinsic CD8+ T-cell dysfunction may be necessary to achieve maximal therapeutic effect.
We observed that ImmTAVs eliminated the majority of HIV-infected CD4+ T-cells that expressed markers of recent activation, CD25, CD69 and HLA-DR. This was expected, since HIV replication, and consequently antigen expression, is greatest in activated CD4+ T-cells.49 A surprising observation was that the majority of resting infected cells present in the same cultures were also killed. Consistent with their lack of expression of activation markers, expression of Gag was also lower in these cells compared with activated cells, which could explain their relatively reduced susceptibility to killing. However, we did not observe a linear association between the expression of Gag and of activation markers on endogenously HIV-infected CD4+ T-cells. Expression of Gag in the absence of productive infection has been reported in a superinfection model and in ex vivo CD4+ T-cells from ART-treated patients. These so called “Gag-positive reservoir cells” appear to be rare but were nevertheless susceptible to CD8+ T-cell killing, which suggests a possible explanation for our results.43,50 Alternatively, the Gag-positive resting cells that we identified could represent cells that were infected while in transition to the resting state and the Gag proteins detected were from incoming virions.51
We observed a strong correlation between Gag mean fluorescence intensity and susceptibility of Gag-positive cells to ImmTAV-mediated killing, which suggested that intracellular Gag was a useful proxy for cognate Gag epitope density on the infected cell surface. Direct quantification of HIV SL9 epitopes on the cell surface indicated that <50 were present on primary CD4+ T-cells that were infected in vitro after polyclonal activation. It was not possible to perform this analysis on ex vivo CD4+ T-cells from patients due to the limits of detection with the fluorescence microscopy approach used. However, by purifying resting CD4+ T-cells from ART-treated patients and reactivating latent HIV in vitro, we were able to confirm that ImmTAV-redirected CD8+ T-cells were able to reduce viral recovery from these latently infected cells even though the density of SL9 epitopes was likely to be considerably lower under such conditions than after in vitro infection. Collectively, these observations indicated that ImmTAV-mediated killing was highly efficient, although nevertheless dependent on the density of HLA class I-HIV peptide complexes on the infected cell surface. This has implications for the use of ImmTAVs to kill infected CD4+ T-cells in vivo. Circulating HIV-infected CD4+ T-cells are largely in a resting state and may be heterogeneous in terms of viral protein expression when treated with LRAs. To summarize, ImmTAVs are most effective against activated cells though they also mediate elimination of resting cells that are expected to present very low viral antigen concentrations, which may reflect the level of antigen expression on a subset of HIV reservoir cells in vivo.
Our study has shown the translational potential of ImmTAVs for combating HIV infection; however, for this to be fully realized, several issues need to be resolved. First, we assessed killing of infected cells upon recognition of a single HLA class I-bound HIV epitope. To achieve clinically useful effects on the latent reservoir, multiple TCRs targeting diverse viral epitopes might be required. However, this must be balanced with the risk of off-target effects when using a cocktail of ImmTAVs, which would need careful consideration during development. Second, the potency of the ImmTAVs was lower when they were used at a concentration of 10–9 mol/l, despite similar levels of caspase-3 expression in targeted CD4+ T-cells to those observed at ImmTAV concentrations of 10–8 mol/l. HLA restricted cross-reactivity can be detected with tumor targeting ImmTACs at concentrations >10–9 mol/l. The potential for cross-reactivity is determined in part by the extent to which peptide mimics are expressed in the human genome, which can be assessed using a combination of in silico and in vitro methods.26,27,52 A systematic approach involving a molecular level analysis of TCR binding to peptide variants to define a recognition motif, database searches to identify proteins containing the motif and empirical testing for TCR reactivity using a panel of HLA-matched antigen-negative primary cell lines for TCR reactivity is being used routinely as a predictor of clinical safety, with clinical evidence supporting this strategy.53 Assays to screen for reactivity against HLA-mismatched cell lines, platelets and even whole blood can also minimize the risk of induction of alloreactivity or a cytokine storm. These approaches may be more informative than preclinical testing in animal models for HIV. For example, an ImmTAV specific for a monkey MHC allele would be required for evaluation in the SIV model and would have limited predictive value for human HLA-TCR interactions. HLA-humanized mouse models circumvent this particular issue but cannot provide adequate assurance of safety in humans since variation at a single residue within the target peptide can be sufficient to abrograte TCR binding.53 An important distinction between soluble bispecific TCRs and TCR-transduced T-cells (which led to fatality) is that dosing can be more tightly controlled with the former.52 Safe dosing may involve multiple administrations of the ImmTAV in order to achieve a clinically significant impact on the viral reservoir, in a similar manner to the treatment of melanoma.30 Finally, in vivo studies may need to address whether adequate ImmTAV concentrations can be attained in relevant tissues. Murine and non-human primate models have shown that the outcome of persistent viral infections was highly dependent on the number and timing of CD8+ T-cell effectors that colocalized with virus-infected targets in lymphoid tissues.54 The frequency of circulating HIV-infected cells is very low (from <1/million to ~1/10,00055) but the majority of HIV-infected cells are found in lymphoid tissues in vivo, particularly in germinal centers where they may be inaccessible to CD8+ T-cells, and also in other tissue compartments that are known to be poorly penetrated by antiretroviral drugs.56,57 Reassuringly, ImmTACs against melanoma antigens were able to localize to tumor sites after intravenous (i.v.) administration in murine models and tumor shrinkage with an ImmTAV has been observed in the clinic.27,30
In summary, we have shown in a clinically relevant in vitro system that ImmTAVs specific for an immunodominant HIV Gag epitope have potent antiviral effects against CD4+ T-cells harboring diverse primary HIV isolates. We conclude that ImmTAVs are promising agents that could form a component of HIV eradication strategies.
Materials and Methods
Cell lines and primary cells. Mel526 cells were obtained from Thymed, J82 and T2 cells were obtained from American Type Culture Collection. PBMC were isolated by density gradient separation. CD4+ and CD8+ T-cells were enriched from PBMC by negative selections using magnetic bead immunodepletion, in accordance with the manufacturer's instructions (MACS, Miltenyi Biotech, Surrey, UK). In selected experiments, freshly isolated CD8+ T-cells were sorted into memory subsets on a MoFlo cell sorter (Beckman Coulter, Brea, CA) using CD8-APC, CCR7-PE-Cy7, and CD45RA-PE antibodies (BD Biosciences, Oxford, UK). Resting CD4+ T-cells were isolated from ART-treated HLA-A*0201-positive subjects by depletion of CD8+ T-cells as described above, followed by labelling of cells expressing CD25, CD69, and HLA-DR using PE-conjugated antibodies (BD Biosciences) and depletion using anti-PE beads (Miltenyi Biotec).
Surface plasmon resonance. Purified ImmTAVs were subjected to surface plasmon resonance (SPR) analysis on a BIAcore3,000 as described previously.26 Briefly, biotinylated pMHC monomers were immobilized on streptavidin-coupled CM-5 sensor chips up to 100 RU and biotin-blocked. Equilibrium binding constants and dissociation rate constants were calculated using the BIAevaluation software.
Production of ImmTAVs. TCR isolation and engineering to produce ImmTAV reagents have been described previously.26,58 In brief, phage display affinity maturation was performed using the native SL9 peptide to select alternative affinity enhanced variants of the wild-type TCR described in Varela-Rohena et al. (2008). To produce ImmTAVs, soluble, disulphide linked TCRs fused to anti-CDR3 and incorporating the affinity-enhanced alpha and beta chains were expressed as inclusions bodies in Escherichia coli and subsequently refolded and purified (reviewed in ref. 53). Recognition of the following SL9 escape variants: SLYNTIATL, SLYNTIAVL, SLYNTVAVL, SLFNTIATL, SLFNTIAVL, SLFNTVATL, and SLFNTVAVL by the wild-type TCR has been demonstrated previously.20
IFN-γ ELISpot assays. Interferon (IFN)-γ ELISpot assays were performed as previously described and according to the manufacturer's instructions.26 Briefly, target cells and effector cells were cultured at 5 × 104 cells/well and 8 × 104 cells/well respectively. Effector cells were tested with and without ImmTAVs at various concentrations. Plates were incubated overnight at 37 °C / 5% CO2 and quantified after development using an automated ELISpot reader (Immunospot Series 5 Analyzer, Cellular Technology). EC50 values were determined from nonlinear fitting of data obtained with titrated ImmTAV concentrations using Graphpad Prism version 6 software.
CFSE-based cytolytic T-cell assay. T2 cells were labelled with Carboxyfluorescein succinimidyl ester (CFSE) (1μmol/l) for 5 minutes and 37 °C. Labelling was quenched with warm RPMI supplemented with 10% fetal calf serum (R10) and cells were pulsed with peptide (10 μmol/l) in serum-free medium for 5 hours. After washing off unbound peptide, they were cocultured with healthy donor CD8+ T-cells (1:1 ratio) +/− ImmTAV (1 nmol/l) for 24 hours and then analyzed by flow cytometry. Labelling efficiency was confirmed to be 100%. Specific lysis was determined from the formula: 1 – (% CFSEhi cells in ImmTAV-treated sample) / (% CFSEhi cells in sample without ImmTAV) × 100%.
Dextramer analysis. Purified CD8+ T-cells from a healthy donor were incubated with ImmTAVs at various concentrations, together an HLA-A*0201-SL9 dextramer conjugated to PE (Immudex, Copenhagen, Denmark) for 20 minutes at 37 °C. Cells were then stained with Aqua live/dead (Invitrogen, Carlsbad, CA), CD3-APC-Cy7 (Biolegend, London, UK) and CD8-APC (BD Biosciences) and analyzed by flow cytometry. All cytometric analyses were performed on a CyAn flow cytometer and analyzed using FlowJo (version 9.2).
Exogenous HIV infection and reactivation of endogenous HIV in patient-derived CD4+ T-cells. The laboratory-adapted CXCR4-tropic clade B isolate, IIIB, was obtained from the Programme EVA Centre for AIDS Reagents, National Institute for Biological Standards and Control (NIBSC), a center of the Health Protection Agency, UK. CD8-depleted PBMC (hereafter referred to as CD4+ cells) were stimulated with PHA (5 µg/ml) in RPMI-1640 medium supplemented with 10% FCS (R10) for 3 days, washed and infected with HIV IIIB at a multiplicity of infection of 1 × 10−2 by spinoculation for 2 hours at 25 °C, as described previously.34 Endogenous HIV in PBMC from HIV-positive subjects was reactivated by stimulation of purified CD4+ T-cells with PHA (5 µg/ml) in R10 for 3 days. In selected experiments, reactivation of latent HIV in purified resting CD4+ T-cells was achieved by treatment with bryostatin-1 (10 nmol/l, Sigma-Aldrich, Dorset, UK) and romidepsin (40 nmol/l, Cambridge Bioscience, Cambridge, UK) at 37 °C for 6 hours.
Viral inhibition / infected cell elimination assay. After spinoculation or reactivation, HIV-infected CD4+ T-cells were washed and cultured in duplicate (1 × 105 cells/well) in R10 supplemented with IL-2 (20 IU/ml), either alone or with purified CD8+ T-cells with and without ImmTAVs for 7 days. An irrelevant tumor-specific ImmTAC was used as a control for specificity. The frequency of infected cells was determined by intracellular staining for Gag p24 Ag, optimized for sensitivity and specificity, as described previously.34,59 Viral inhibition / infected cell elimination was calculated by normalizing to data obtained with no ImmTAVs, using the formula: (fraction of Gag+ cells in CD4+ T-cells cultured with CD8+ T-cells alone – fraction of Gag+ in CD4+ T-cells cultured with CD8+ cells plus ImmTAV) / fraction of p24+ cells in CD4+ T-cells cultured with CD8+ T-cells alone × 100%. In selected experiments, CD4+ T-cells were analyzed for expression of caspase-3 or activation markers CD25, CD69, and HLA-DR using PE-conjugated antibodies (BD Biosciences). To confirm productive infection in patients' CD4+ T-cells after in vitro reactivation of latent HIV, culture supernatants were tested for free p24 Ag by enzyme-linked immunosorbent assay, as described previously.34 In experiments with latency reversing agents, viral outgrowth was assessed by amplification of HIV RNA in culture supernatants using QIAamp Viral Mini Kit (QIAGEN, Manchester, UK) and quantification by reverse-transcriptase polymerase chain reaction (RT-PCR) (RealStar HIV RT-PCR Kit 1.0, Altona Diagnostic, Hamburg, Germany).
Confocal imaging. HIV IIIB-infected CD4+ T-cells were cultured for 6 days, subjected to dead cell removal and then cocultured with purified CD8+ T-cells (1:2 ratio) +/− ImmTAVs (10–9 mol/l) on poly-L-lysine coated cover slips for 30 minutes at 37 °C. Cells were permeabilized and fixed with 4% paraformaldehyde followed by 0.2% Triton-X 100, blocked with 2.5% goat serum for 45 minutes, then stained with primary antibodies to p24 Ag (37G12 directly conjugated to Aberior STAR 635P, St Louis, MO) and CD8 (rabbit anti-CD8a, followed by secondary goat anti-rabbit Alexa568, Abcam, Cambridge, UK). Cover slips were mounted on slides using VectaShield mounting buffer and sealed. HIV infected CD4+ / CD8+ T-cell conjugates were visualized using a Zeiss LSM510 confocal microscope with 63× oil objective.
Quantification of epitope presentation by microscopy. CD4+ T-cells obtained from two healthy volunteer donors were infected in vitro with HIV-1 IIIB, as described earlier, and stained with biotinylated m134 ImmTAV (5 µg/ml) or irrelevant TCR in phosphate-buffered saline supplemented with 0.5% bovine serum albumin, 400 nmol/l CaCl2 and 400 nmol/l MgCl2 for 30 minutes at RT. Stained cells were then incubated with PE-conjugated streptavidin (10 µg/ml) at RT for 20 minutes (BD Bioscience), fixed with 2% paraformaldehyde for 30 minutes, resuspended in phosphate-buffered saline and plated on glass coverslip chambers. Phase-contrast and PE-fluorescence images were acquired as previously described using a Zeiss 200 mol/l/Universal Imaging system with a 63× objective.32 Z-stack fluorescent images were taken (27 individual planes, 0.7 µm apart) to cover the entire 3D surface of the cell. The fluorescent spots corresponding to ImmTAV-PE bound to peptide-HLA complexes on each Z-stack were counted and summed to obtain the total count for each cell. In each experiment epitopes were quantified on at least 50 individual cells.
Statistical analysis. Statistical analysis was performed using GraphPad Prism software (version 6.0). ImmTAV effects at different concentrations were analyzed using one-way analysis of variance with Dunnett's post-test correction for multiple comparisons. Paired samples were analyzed using a paired t-test. Correlations were explored by determining the Pearson correlation coefficient. All tests were two-tailed and P values < 0.05 were considered significant.
Ethics statement and study participants. Blood samples from HIV-positive subjects in this study were obtained with approval from UK Research Ethics Committees (Oxfordshire Research Ethics Committee & Gene Therapy Advisory Committee) and written informed consent from all participants, prior to transfer to the Weatherall Institute of Molecular Medicine (WIMM, University of Oxford) Licensed Tissue Bank, in compliance with the Human Tissue Act 2004. All except two subjects had received combination antiretroviral therapy for at least 12 months at the time of sampling, and had maintained undetectable plasma HIV-1 RNA (<50 copies/ml) and CD4+ cell counts > 350 cells/µl. Two subjects were ART-naive with CD4+ cell counts > 350 cells/µl. PBMC were stored in vapour phase liquid nitrogen until use. Healthy HIV-uninfected donor PBMC were obtained from buffy coats supplied by the National Health Service Blood Transfusion Service, Bristol, UK or from the WIMM Licensed Tissue Bank.
SUPPLEMENTARY MATERIAL Figure S1. Percentage of live cells after exposure to titrated concentrations of ImmTAVs. Figure S2. Gating strategy for identification of HIV-infected caspase-3-positive CD4+ T-cells that formed doublets with CD8+ T-cells. Figure S3. Number of conjugates formed between Gag+ CD4+ T-cells and autologous or healthy donor CD8+ T-cells in the presence of ImmTAVs. Figure S4. Dose-dependent Inhibition of HIV spread in CD4+ T-cells by tenofovir. Figure S5. Gag mean fluorescence intensity (MFI) in HIV-infected CD4+ T-cells after culture with tenofovir. Figure S6. Distribution of HIV-uninfected CD4+ T-cells among resting and activated subsets.
Acknowledgments
This work was funded by Oxford NIHR Biomedical Research Centre. We thank the patients who donated blood samples for this study. We are grateful to Craig Waugh, MRC Human Immunology Unit, WIMM, University of Oxford, for assistance with cell sorting. L.D. is a Jenner Investigator. S.B., G.B., R.A., A.V., T.M., P.M., J.O., S.J.P., M.A., N.H., and B.K.J. are employees of Immunocore Ltd. ImmTAVs m121 and m134 used in this study were produced by Immunocore Ltd.
Supplementary Material
References
- Allers, K, Hütter, G, Hofmann, J, Loddenkemper, C, Rieger, K, Thiel, E et al. (2011). Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 117: 2791–2799. [DOI] [PubMed] [Google Scholar]
- Shan, L, Deng, K, Shroff, NS, Durand, CM, Rabi, SA, Yang, HC et al. (2012). Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autran, B, Descours, B and Bacchus, C (2013). Immune control of HIV-1 reservoirs. Curr Opin HIV AIDS 8: 204–210. [DOI] [PubMed] [Google Scholar]
- Archin, NM, Liberty, AL, Kashuba, AD, Choudhary, SK, Kuruc, JD, Crooks, AM et al. (2012). Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487: 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanham, G and Van Gulck, E (2012). Can immunotherapy be useful as a “functional cure” for infection with Human Immunodeficiency Virus-1? Retrovirology 9: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen, CK, Laird, GM, Durand, CM, Siliciano, JD and Siliciano, RF (2014). New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 20: 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell, L, Yang, H, Ondondo, B, Dong, T, di Gleria, K, Suttill, A et al. (2006). Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J Virol 80: 4705–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H, Dong, T, Turnbull, E, Ranasinghe, S, Ondondo, B, Goonetilleke, N et al. (2007). Broad TCR usage in functional HIV-1-specific CD8+ T cell expansions driven by vaccination during highly active antiretroviral therapy. J Immunol 179: 597–606. [DOI] [PubMed] [Google Scholar]
- Autran, B, Murphy, RL, Costagliola, D, Tubiana, R, Clotet, B, Gatell, J et al.; ORVACS Study Group. (2008). Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452). AIDS 22: 1313–1322. [DOI] [PubMed] [Google Scholar]
- Schooley, RT, Spritzler, J, Wang, H, Lederman, MM, Havlir, D, Kuritzkes, DR et al.; AIDS Clinical Trials Group 5197 Study Team. (2010). AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis 202: 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza, JP, Bowman, KA, Adzaku, S, Smith, EC, Enama, ME, Bailer, RT et al.; VRC 101 Study Team. (2013). Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J Infect Dis 207: 1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, RB, Rockstroh, JK, Pantaleo, G, Asmuth, DM, Peters, B, Lazzarin, A et al. (2014). Safety and efficacy of the peptide-based therapeutic vaccine for HIV-1, Vacc-4x: a phase 2 randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 14: 291–300. [DOI] [PubMed] [Google Scholar]
- Leen, AM, Myers, GD, Sili, U, Huls, MH, Weiss, H, Leung, KS et al. (2006). Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 12: 1160–1166. [DOI] [PubMed] [Google Scholar]
- Leen, AM, Bollard, CM, Mendizabal, AM, Shpall, EJ, Szabolcs, P, Antin, JH et al. (2013). Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121: 5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, S, Sung, J, Cruz, C, Castillo-Caro, P, Ngo, M, Garrido, C et al. (2015). Broadly-specific cytotoxic T cells targeting multiple HIV antigens are expanded from HIV+ patients: implications for immunotherapy. Mol Ther 23: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, JA, Lam, S, Garrido, C, Archin, N, Rooney, CM, Bollard, CM et al. (2015). Expanded cytotoxic T-cell lymphocytes target the latent HIV reservoir. J Infect Dis 212: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw, MH, Westwood, JA and Darcy, PK (2013). Gene-engineered T cells for cancer therapy. Nat Rev Cancer 13: 525–541. [DOI] [PubMed] [Google Scholar]
- Mitsuyasu, RT, Anton, PA, Deeks, SG, Scadden, DT, Connick, E, Downs, MT et al. (2000). Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood 96: 785–793. [PubMed] [Google Scholar]
- Scholler, J, Brady, TL, Binder-Scholl, G, Hwang, WT, Plesa, G, Hege, KM et al. (2012). Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 4: 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Rohena, A, Molloy, PE, Dunn, SM, Li, Y, Suhoski, MM, Carroll, RG et al. (2008). Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med 14: 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, A, Zheng, JH, Follenzi, A, Dilorenzo, T, Sango, K, Hyman, J et al. (2008). Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol 82: 3078–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen, SG, Levin, BR, Bristol, G, Rezek, V, Kim, S, Aguilera-Sandoval, C et al. (2012). In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS Pathog 8: e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, O, Maréchal, V, Le Gall, S, Lemonnier, F and Heard, JM (1996). Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2: 338–342. [DOI] [PubMed] [Google Scholar]
- Rajapaksa, US, Li, D, Peng, YC, McMichael, AJ, Dong, T and Xu, XN (2012). HLA-B may be more protective against HIV-1 than HLA-A because it resists negative regulatory factor (Nef) mediated down-regulation. Proc Natl Acad Sci USA 109: 13353–13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, K, Pertea, M, Rongvaux, A, Wang, L, Durand, CM, Ghiaur, G et al. (2015). Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddy, N, Bossi, G, Adams, KJ, Lissina, A, Mahon, TM, Hassan, NJ et al. (2012). Monoclonal TCR-redirected tumor cell killing. Nat Med 18: 980–987. [DOI] [PubMed] [Google Scholar]
- McCormack, E, Adams, KJ, Hassan, NJ, Kotian, A, Lissin, NM, Sami, M et al. (2013). Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother 62: 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, M, Evans, J, Steven, N, Corrie, P, Mulatero, C, Sznol, M, et al. (2014). IMCgp100: a novel bispecific biologic for the treatment of malignant melanoma. . 105th Annu. Meet. Am. Assoc. Cancer Res., San Diego, California: p CT329.
- Bossi, G, Buisson, S, Oates, J, Jakobsen, BK and Hassan, NJ (2014). ImmTAC-redirected tumor cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol Immunother 63: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, M, Corrie, P, Sznol, M, Infante, J, Mulatero, C, Evans, J, et al. (2015). A phase I/IIa study of IMCgp100: Partial and complete durable responses with a novel first-in-class immunotherapy for advanced melanoma. Proc. 106th Annu. Meet. Am. Assoc. Cancer Res., Philadelphia: p CT106.
- Przepiorka, D, Ko, CW, Deisseroth, A, Yancey, CL, Candau-Chacon, R, Chiu, HJ et al. (2015). FDA Approval: Blinatumomab. Clin Cancer Res 21: 4035–4039. [DOI] [PubMed] [Google Scholar]
- Purbhoo, MA, Sutton, DH, Brewer, JE, Mullings, RE, Hill, ME, Mahon, TM et al. (2006). Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol 176: 7308–7316. [DOI] [PubMed] [Google Scholar]
- Bossi, G, Gerry, AB, Paston, SJ, Sutton, DH, Hassan, NJ and Jakobsen, BK (2013). Examining the presentation of tumor-associated antigens on peptide-pulsed T2 cells. Oncoimmunology 2: e26840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H, Yorke, E, Hancock, G, Clutton, G, Sande, N, Angus, B et al. (2013). Improved quantification of HIV-1-infected CD4+ T cells using an optimised method of intracellular HIV-1 gag p24 antigen detection. J Immunol Methods 391: 174–178. [DOI] [PubMed] [Google Scholar]
- Spina, CA, Anderson, J, Archin, NM, Bosque, A, Chan, J, Famiglietti, M et al. (2013). An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 9: e1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles, SA, Weeks, KA, Nou, E, Berkley, AM, Rood, JE, Osborne, CM et al. (2009). Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol 83: 11876–11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, EM, Milush, JM, Ho, EL, Batista, MD, Holditch, SJ, Keh, CE et al. (2012). Expansion of CD8+ T cells lacking Sema4D/CD100 during HIV-1 infection identifies a subset of T cells with decreased functional capacity. Blood 119: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor, MJ, Cope, A, Gay, CL, Ferrari, G, McGee, KS, Kuruc, JD et al. (2013). Antiretroviral therapy initiated during acute HIV infection fails to prevent persistent T-cell activation. J Acquir Immune Defic Syndr 62: 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry, NA and Lazebnik, Y (1998). Caspases: enemies within. Sci 281: 1312–1316. [DOI] [PubMed] [Google Scholar]
- de Oliveira Pinto, LM, Lecoeur, H, Ledru, E, Rapp, C, Patey, O and Gougeon, ML (2002). Lack of control of T cell apoptosis under HAART. Influence of therapy regimen in vivo and in vitro. AIDS 16: 329–339. [DOI] [PubMed] [Google Scholar]
- Wang, FX, Xu, Y, Sullivan, J, Souder, E, Argyris, EG, Acheampong, EA et al. (2005). IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest 115: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo, AR, Sobolewski, MD, Bosch, RJ, Fyne, E, Piatak, M Jr, Coffin, JM et al. (2014). Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA 111: 7078–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf, EH, Pace, MJ, Peterson, BA, Lynch, LJ, Chukwulebe, SB, Mexas, AM et al. (2013). Gag-positive reservoir cells are susceptible to HIV-specific cytotoxic T lymphocyte mediated clearance in vitro and can be detected in vivo [corrected]. PLoS One 8: e71879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, JA, Pickeral, J, Liu, L, Stanfield-Oakley, SA, Lam, CY, Garrido, C et al. (2015). Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest 125: 4077–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, CM, Lawrence, J, Schapiro, JM, Altman, JD, Winters, MA, Crompton, M et al. (1999). Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART). J Immunol 162: 1780–1788. [PubMed] [Google Scholar]
- Buckheit, RW 3rd, Salgado, M, Silciano, RF and Blankson, JN (2012). Inhibitory potential of subpopulations of CD8+ T cells in HIV-1-infected elite suppressors. J Virol 86: 13679–13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehr, M, Cahenzli, J, Haas, A, Price, DA, Gostick, E, Huber, M et al. (2008). Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J Virol 82: 3391–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassu, A, Marcus, RA, D'Souza, MB, Kelly-McKnight, EA and Palmer, BE (2011). Suppression of HIV replication by antiretroviral therapy reduces TIM-3 expression on HIV-specific CD8(+) T cells. AIDS Res Hum Retroviruses 27: 1–3. [DOI] [PubMed] [Google Scholar]
- Stevenson, M, Stanwick, TL, Dempsey, MP and Lamonica, CA (1990). HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J 9: 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace, MJ, Graf, EH, Agosto, LM, Mexas, AM, Male, F, Brady, T et al. (2012). Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog 8: e1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckheit, RW 3rd, Siliciano, RF and Blankson, JN (2013). Primary CD8+ T cells from elite suppressors effectively eliminate non-productively HIV-1 infected resting and activated CD4+ T cells. Retrovirol 10: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, BJ, Gerry, AB, Dukes, J, Harper, JV, Kannan, V, Bianchi, FC et al. (2013). Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5: 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates, J, Hassan, NJ and Jakobsen, BK (2015). ImmTACs for targeted cancer therapy: Why, what, how, and which. Mol Immunol 67: 67–74. [DOI] [PubMed] [Google Scholar]
- Li, Q, Skinner, PJ, Ha, SJ, Duan, L, Mattila, TL, Hage, A et al. (2009). Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Sci 323: 1726–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, S, Graf, EH, Dahl, V, Strain, MC, Yukl, SA, Lysenko, ES et al. (2013). Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 9: e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, JJ, Amancha, PK, Rogers, K, Ansari, AA and Villinger, F (2012). Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol 188: 3247–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa, Y, Lum, R, Okoye, AA, Park, H, Matsuda, K, Bae, JY et al. (2015). B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y, Moysey, R, Molloy, PE, Vuidepot, AL, Mahon, T, Baston, E et al. (2005). Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol 23: 349–354. [DOI] [PubMed] [Google Scholar]
- Yang, H, Wu, H, Hancock, G, Clutton, G, Sande, N, Xu, X et al. (2012). Antiviral inhibitory capacity of CD8+ T cells predicts the rate of CD4+ T-cell decline in HIV-1 infection. J Infect Dis 206: 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.