Abstract
Given their high potential to evoke cytolytic T cell responses, tumor antigen-encoding messenger RNA (mRNA) vaccines are now being intensively explored as therapeutic cancer vaccines. mRNA vaccines clearly benefit from wrapping the mRNA into nano-sized carriers such as lipoplexes that protect the mRNA from degradation and increase its uptake by dendritic cells in vivo. Nevertheless, the early innate host factors that regulate the induction of cytolytic T cells to mRNA lipoplex vaccines have remained unresolved. Here, we demonstrate that mRNA lipoplexes induce a potent type I interferon (IFN) response upon subcutaneous, intradermal and intranodal injection. Regardless of the route of immunization applied, these type I IFNs interfered with the generation of potent cytolytic T cell responses. Most importantly, blocking type I IFN signaling at the site of immunization through the use of an IFNAR blocking antibody greatly enhanced the prophylactic and therapeutic antitumor efficacy of mRNA lipoplexes in the highly aggressive B16 melanoma model. As type I IFN induction appears to be inherent to the mRNA itself rather than to unique properties of the mRNA lipoplex formulation, preventing type I IFN induction and/or IFNAR signaling at the site of immunization might constitute a widely applicable strategy to improve the potency of mRNA vaccination.
Introduction
The induction of strong cytolytic CD8+ T cell responses capable of killing transformed cells is considered vital for the success of therapeutic cancer vaccines.1 As CD8+ T cells guard the intracellular proteome, their efficient induction typically requires the presence of antigens in the cellular cytosol, where they can enter the classical route of proteasome degradation and major histocomaptibility complex (MHC-I) mediated antigen presentation. In contrast to protein based vaccines, vaccines based on messenger RNA (mRNA) enable protein expression inside the cytosol of transfected cells and thus show great potential to evoke cytotolytic T cell responses.2 Due to the limited stability of early in vitro transcribed (IVT) mRNAs, mRNA vaccines have been predominantly delivered in the format of ex vivo electroporated dendritic cells (DCs) for most of the time.3 Over the past years, technical improvements in the way IVT mRNAs are prepared (5′ Cap modifications, optimized GC content, improved polyA tails, stabilizing UTRs) have increased the stability of IVT mRNAs to such extent protein expression can now be achieved for days after direct in vivo administration of the mRNA.4,5,6 These breakthroughs have revolutionized the mRNA vaccination allowing direct injection of antigen encoding mRNA to be explored for the treatment of patients with prostate cancer, nonsmall lung cell carcinoma and melanoma.7,8,9,10,11,12,13
When applied directly in vivo, mRNA vaccines strongly benefit from wrapping the mRNA into nano-sized carriers. Within this context, our group previously demonstrated that condensing mRNA into cationic lipoplexes increases the potency of the mRNA vaccine evoked T cell response by several orders of magnitude.14 One of the typical hallmarks of IVT mRNAs condensed into nano-formulations is their capacity to elicit intense secretion of Type I interferons (IFNs) in murine and human DCs.14,15 Indeed, IVT mRNA appears to mimic viral RNA in its capacity to trigger a variety of cellular endosomal and cytosolic RNA sensors that all induce a signaling cascade culminating in the release of type I IFNs.14,15,16,17 Type I IFNs are highly pleiotropic cytokines that can either promote or inhibit T cell responses dependent on the context. Type I IFNs can augment T cell immunity by activating DCs and increasing antigen presentation.
Conversely, the antiviral actions of type I IFN, production of RNAses and instigation of translation arrest might interfere with the expression of the mRNA encoded antigen and therefore negatively impact T cell immunity. Type I IFN signaling on antigen experienced T cells can promote T cell proliferation, survival, and differentiation into effector cells.18 Nevertheless, type I IFN exposure prior to T cell receptor activation can induce antiproliferative and apoptotic programmes in T cells.18,19,20,21 How type I IFNs impact the characteristics of the T cell responses to mRNA lipoplex vaccines and their efficacy to control tumor growth is therefore far from forgone conclusion and constitutes the main goal of this study.
Using an IFN-β reporter mouse strain, we were able to demonstrate that mRNA lipoplexes instigate profound type I IFN responses upon subcutaneous, intradermal, and intranodal injection. In sharp contrast to the beneficial role of type I IFNs in protein and peptide based vaccines,22,23,24,25 type I IFNs severely hampered priming of vaccine specific T cell responses and the generation of antitumor immunity to lipoplex based mRNA vaccination. Preventing type I IFN induced signaling through coadministration of an IFNAR blocking antibody at the site of mRNA based vaccination amplified the cytotolytic T cell response and significantly strengthened vaccine elicited tumor control in prophylactic and therapeutic settings.
Results
mRNA lipoplexes induce a potent type I IFN response in vivo
Cationic liposomes have been reported to increase T cell responses to mRNA encoded antigens.26 In this study, liposomes composed of the cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and the helper lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were used to condense mRNA into lipoplexes. Preliminary research was done to determine the nitrogen/phosphate ratio most suited for in vivo application and is shown as additional data (see Supplementary Figure S1). We evaluated two N/P ratios that give yield to mRNA lipoplexes of similar size (± 300–400 nm) but opposite charge, namely lipoplexes at N/P1 had a negative zeta-potential of −18 mV and N/P10 lipoplexes displayed a positive charge of +32 mV (see Supplementary Figure S1a,b). Further, we addressed mRNA lipoplexes of ratio N/P1 as most suited to yield high expression levels of the delivered mRNA (see Supplementary Figure S1c) and to induce proper induction of IFN-ɣ producing CD8+ and CD4+ T cells upon subcutaneous injection (see Supplementary Figure S1d). As a consequence, N/P1 was selected in all further experiments aimed at addressing the impact of type I IFNs on the efficacy of mRNA lipoplexes to yield T cell immunity.
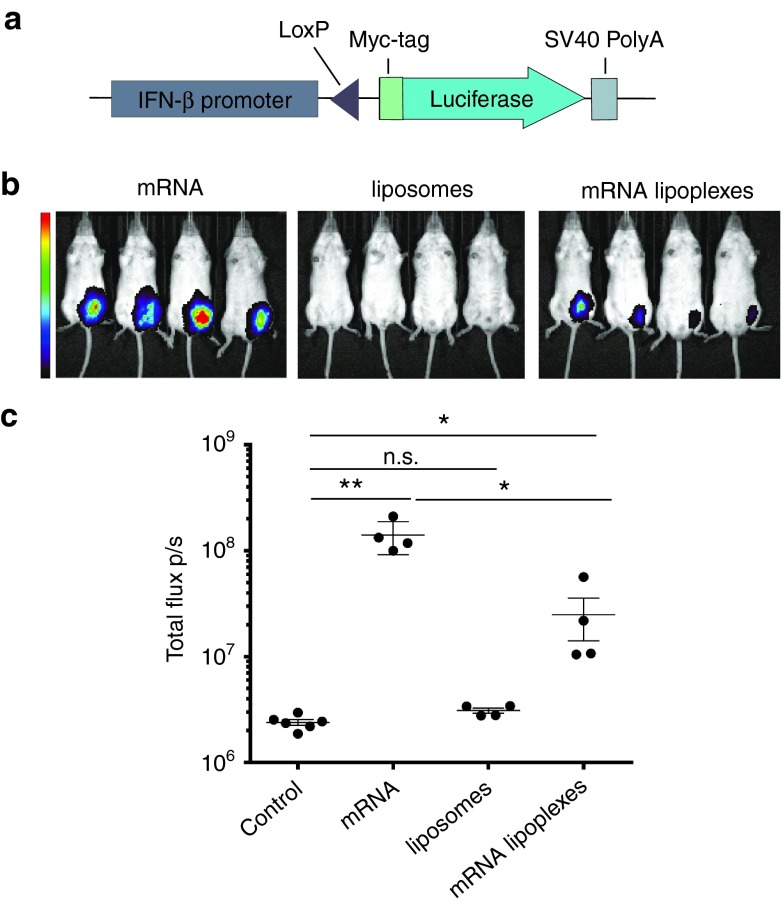
Previously, we have demonstrated that DOTAP-based mRNA lipoplexes elicit strong type I IFN secretion upon incubation with bone marrow derived DCs in vitro.14 To address to which extent mRNA lipoplexes would trigger type I IFNs in vivo upon subcutaneous injection, we used an IFN-β reporter mouse in which a firefly luciferase encoding sequence has been placed under the control of the IFN-β promoter (Figure 1a).27 As type I IFN production is regulated by self-enforcing feedforward loops, heterozygous reporter mice (IFN-β+/Δβ-luc) were used to allow signal amplification by early induced IFN-β. Mice were injected subcutaneously with respectively DOTAP liposomes (no mRNA), unformulated mRNA or mRNA lipoplexes. In vivo bioluminescence imaging revealed a strong induction of the IFN-β promoter to injection of naked mRNA and of mRNA lipoplexes, but not to liposomes without mRNA (Figure 1b,c). Strikingly, naked ovalbumin (OVA) mRNA elicited the most prominent induction of type I IFNs, clearly indicating that type I IFN induction to mRNA is inherent to the mRNA itself rather than to unique features of the mRNA lipoplexes.
Figure 1.
mRNA lipoplexes induce a potent type I IFN response in vivo. (a) Graphical scheme of the IFN-β reporter construct. The myc-tagged luciferase gene is brought under the control of the IFN-β promoter by the Cre-Lox system. (b,c) IFN-β+/Δβ-luc mice were subcutaneous (s.c.) injected with 10 µg of OVA mRNA, mRNA lipoplexes and liposomes. Luminescence was measured 6 hours postinjection. Data are shown as mean ± SD of four mice. **P < 0.001. *P < 0.05 (Mann–Whitney test). Control = 5% glucose water; liposomes = DOTAP/DOPE lipids; mRNA lipoplexes = messenger RNA complexed to liposomes. mRNA, messenger RNA; IFN, interferon, OVA, ovalbumin; SD, standard deviation.
Type I IFNs impact the magnitude and functional characteristics of the vaccine elicited CD8+ T cell response
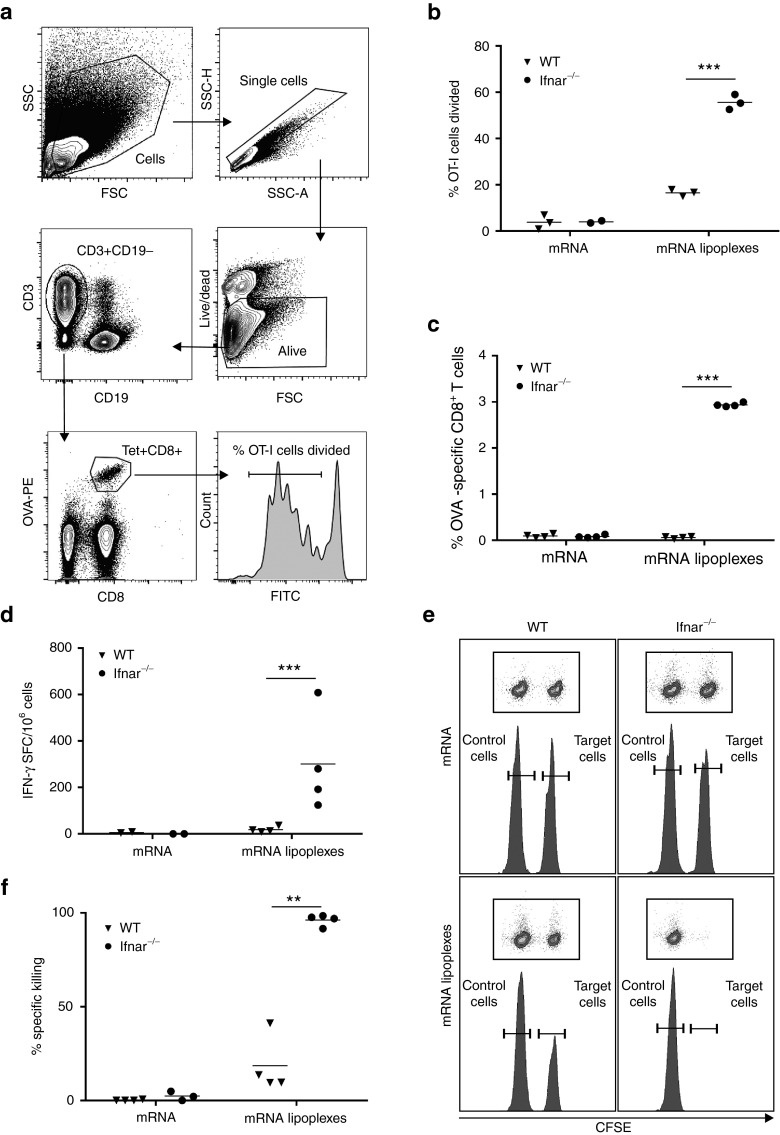
Depending on the context, type I IFNs have been reported to either promote or interfere with the generation of T cell responses. As a consequence, we thoroughly addressed the impact of type I IFN signaling on the magnitude and functionality of the T cell response generated by mRNA lipoplex vaccination through comparative immunization studies in wild type mice and in mice lacking he common IFN-α/β receptor IFNAR1 (Ifnar−/−). First, we addressed the effects of type I IFNs on the initial priming of antigen-specific T cells. To this end, carboxyfluorescein diacetate succinimedyl ester (CFSE) labeled transgenic OVA-specific CD8+ T cells (OT-I T cells) were transferred to respectively wild type and Ifnar−/− mice, which were subsequently immunized with OVA mRNA lipoplexes. Four days postimmunization, the draining popliteal lymph nodes were dissected and OT-I T cell proliferation was analyzed by flow cytometry (Figure 2a). As shown in Figure 2b, Ifnar−/− mice showed strongly elevated OT-I proliferation when compared with wild type mice. This negative impact of type I IFNs on the magnitude of the vaccine evoked CD8+ T cell response was confirmed by quantification of vaccine elicited OVA-specific CD8+ T cells in the blood of wild type versus Ifnar−/− mice (Figure 2c). Five days after immunization, OVA-specific CD8+ T cells were hardly detectable in the blood of wild type mice but reached up to 3% of all CD8+ T cells in the blood of Ifnar−/− mice. No significant numbers of OVA-specific T cells were detected in response to unformulated OVA mRNA. Next, we analyzed the impact of IFNAR deficiency on the functional properties of the vaccine induced CD8+ T cell response. As type I IFNs have been reported to stimulate the differentiation of primed CD8+ T cells into effector cells,20,21,28,29 the increased numbers of vaccine elicited CD8+ T cell response observed in Ifnar−/− mice not necessarily translate into increased effector function in these mice. To address this issue, we compared OVA-specific IFN-γ secretion and target cell specific lysis between immunized wild type and Ifnar−/− mice. Enzyme-linked immunosorbent spot (ELISPOT) assays were performed on splenocytes 2 weeks after the booster immunization with OVA mRNA lipoplexes to quantify the numbers of IFN-γ producing OVA-specific T cells. As depicted in Figure 2d, immunized Ifnar−/− mice showed a strong increase in the numbers of OVA-specific IFN-γ secreting T cells. The cytolytic capacity of the evoked CD8+ T cell response was analyzed through an in vivo killing assay. In brief, 2 weeks after a booster immunization with OVA mRNA lipoplexes, mice were challenged with a 1:1 ratio of OVA peptide-pulsed CFSEhi splenocytes (target cells) and nonpulsed CFSElow splenocytes (nontarget cells). After 2 days, spleens were dissected and the ratio of target cells versus nontarget cells was analyzed by flow cytometry to determine the extent of killing of the target cells. Whereas immunization of wild type mice with OVA mRNA lipoplexes resulted only in a limited killing of the target cells, virtually all target cells were eliminated in immunized Ifnar−/− mice (Figure 2e–f). Taken together, these data clearly demonstrate that IFNAR deficiency increases initial T cell priming to subcutaneously administrated mRNA lipoplex vaccines and that type I IFN are not required for these antigen-experienced T cells to acquire effector function.
Figure 2.
Type I IFNs impact the magnitude and functional characteristics of the vaccine elicited CD8+ T cell response. (a) Gating strategy used for OVA- specific CD8+ T cell counting and proliferation. Cells are gated based on FSC and SCC, before single cells are gated based on SSC-area and height. Living cells are selected and gated for CD3+CD19- T cells. Within CD8+ T cells, OVA-specificity is gated by labeling with MHC-I SIINFEKL–PE dextramer. Proliferation of CFSE positive OVA-specific CD8+ T cells is shown. (b) Two days prior to immunization CFSE-labeled OT-I cells were adoptively transferred to wild type (WT) and Ifnar−/− mice. Subcutaneous (s.c.) immunization was performed at tail base with 10 µg OVA mRNA lipoplexes, naked mRNA or liposomes alone. Four days after immunization inguinal lymph nodes were isolated and CD8+ T cell proliferation was analyzed by flow cytometry. Data are shown as mean of 2–3 mice. ***P < 0.001 (Chi-square test). (c) Wild type (WT) and Ifnar−/− mice were s.c. injected with 20 µg OVA mRNA lipoplexes or naked OVA mRNA as a control. Blood was isolated 5 days later and the percentage OVA-specific CD8+ T cells was determined by dextramer staining followed by flow cytometry. Data are shown as mean of four mice per group. ***P < 0.001 (Chi-square test). (d) Wild type (WT) and Ifnar−/− mice were immunized s.c. with 20 µg OVA mRNA lipoplexes or naked mRNA as a control. After 2 weeks, mice were boosted with the same formulation. Spleens were isolated 2 weeks after the boost immunization, and the number of OVA-specific interferon-γ spot-forming CD8+ and CD4+ T cells (SFC) was determined by enzyme-linked immunosorbent spot (ELISPOT). Data are shown as mean of 2–4 mice per group. ***P < 0.001 (Chi-square test). (e,f) Wild type (WT) and Ifnar−/− mice were immunized with a two-week interval with naked OVA mRNA or OVA mRNA lipolexes. Two weeks after the boost immunization, a mixture of CFSE-labeled cells pulsed with control (CFSElow) or OVA peptide (CFSEhigh) were adoptively transferred. Specific killing was measured 2 days later by flow cytometry. Data are presented as means of 100 −100x ((CFSEhigh / CFSElow)immunized mice / (CFSEhigh / CFSElow)mock-mice) of 3–4 mice per group. **P < 0.01 (Chi-square test). mRNA = OVA-coding messenger RNA; mRNA lipoplexes = messenger RNA complexed to DOTAP/DOPE liposomes. CFSE, carboxyfluorescein diacetate succinimedyl ester; mRNA, messenger RNA; IFN, interferon, OVA, ovalbumin; SD, standard deviation.
As we observed previously, an increase in the expression of lipoplex delivered mRNA in bone marrow derived DCs lacking IFNAR, we decided to quantify mRNA expression after subcutaneous injection of mRNA lipoplexes in wild type and Ifnar−/− mice. If Ifnar−/− mice would show strongly increased mRNA expression levels, increased antigen expression might well underlie the raise in initial T-cell proliferation we observed in Ifnar−/− mice. To address this issue, luciferase encoding mRNA was condensed into lipoplexes at N/P1 and luciferase expression was assessed through in vivo bioluminescence measurement. Although luciferase expression was slightly elevated in the IFNAR deficient setting, this increase was very subtle and did not reached significance (see Supplementary Figure S2). As a consequence, events downstream of antigen expression must be at the origin of the dramatically raised T cell responses in Ifnar−/− mice.
Impact of type I IFNs on the efficacy of antitumor immunity elicited by mRNA lipoplex vaccination
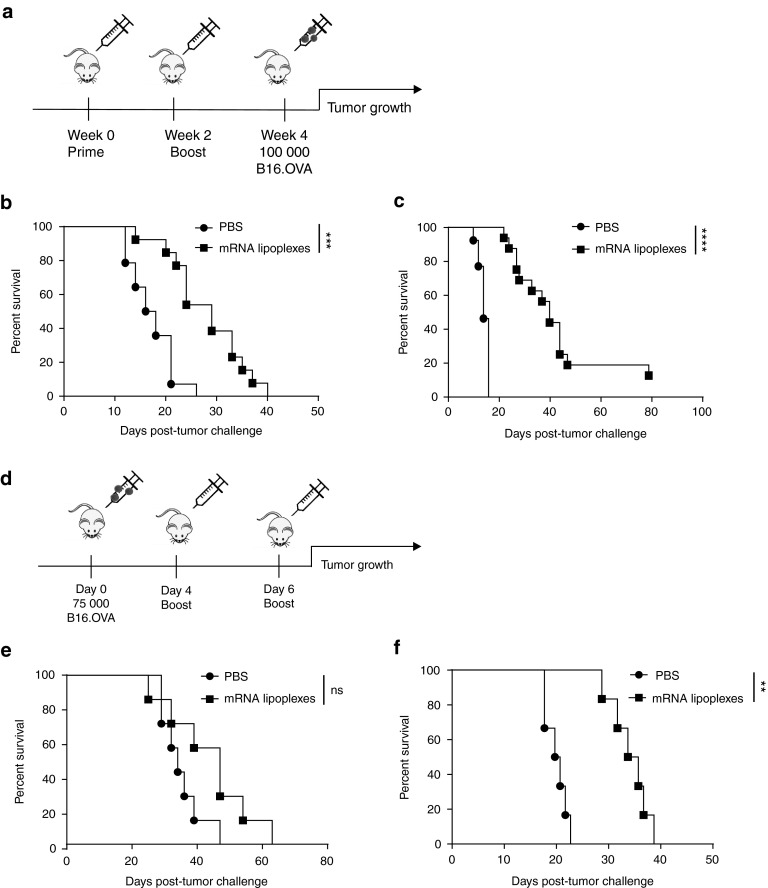
The functional impact of type I IFNs on antitumor immunity mediated by mRNA lipoplex vaccination was addressed in the highly aggressive B16.OVA melanoma model. Mice were either vaccinated prophylactically or therapeutically according to the schedule shown in Figure 3a,d. In wild type mice, prophylactic vaccination significantly increased the median survival time from 17 to 29 days (Figure 3b). In line with their elevated vaccine elicited T cell responses, Ifnar−/− mice benefited even more from vaccination than wild type mice, as the median survival time increased from 14 to 40 days (Figure 3c). This observation is highly striking as Ifnar−/− mice notoriously lack spontaneous antitumor immune responses and succumb much faster to tumors when left untreated.28,29,30,31 Therapeutic vaccination caused a small though nonsignificant improvement in median survival time from 34 to 47 days in wild type mice (Figure 3e). Conversely, therapeutic vaccination yielded a significant survival benefit in Ifnar−/− mice with an increase in median survival time from 20 to 35 days (Figure 3f). Nevertheless, in the therapeutic vaccination setting, vaccinated wild type mice still controlled tumors better than vaccinated Ifnar−/− mice, a feature that can be most likely ascribed to the lack of spontaneous antitumor responses in the IFNAR deficient setting.
Figure 3.
Impact of type I IFNs on the efficacy of antitumor immunity elicited by mRNA lipoplex vaccination. (a) Prophylactic vaccination scheme. Wild type (WT) mice (b) and Ifnar−/− mice (c) were either mock s.c. immunized (i.e., injected with PBS only) or immunized with 20 μg of mRNA lipoplexes. After 2 weeks, mice were boosted with the same formulation. At week 4, mice were inoculated with 100,000 OVA-expressing B16 melanoma cells. (n = 12–16 mice/group). (d) Therapeutic vaccination scheme. Wild type (WT) mice (e) and Ifnar−/− mice (f) were inoculated with 75,000 B16.OVA melanoma cells. After 4 and 6 days, immunization was performed with similar preparations as in the prophylactic setting. (n = 5–6 mice/group). mRNA lipoplexes = OVA-coding messenger mRNA complexed to DOTAP/DOPE liposomes. **P < 0.01; ***P < 0.001; ****P < 0.0001 (Mantel-Cox log-rank test). PBS, phosphate-buffered saline; mRNA, messenger RNA; IFN, interferon, OVA, ovalbumin; SD, standard deviation.
Antibody mediated IFNAR blockade improves the efficacy of the mRNA vaccine evoked antitumor immune response
Results of the experiments in the previous paragraph illustrate that in immunized wild type mice tumor growth control is determined by the combined strength of the spontaneous and vaccine elicited immune responses, whereas in Ifnar−/− mice tumor control will entirely depend on the vaccine elicited immune response. As a consequence, direct comparisons of tumor growth rates between immunized wild type and Ifnar−/− mice do not allow a reliable assessment of the impact of type I IFNs on vaccine mediated tumor control. To circumvent the detrimental effect of genetic IFNAR deficiency on spontaneous antitumor immunity, we therefore, decided to switch to antibody mediated inhibition of IFNAR signaling at the spot of vaccination in wild type mice. Local interference with IFNAR signaling should leave the spontaneous antitumor response intact, and thereby allow us to specifically address the impact of type I IFN signaling on vaccine mediated tumor control.
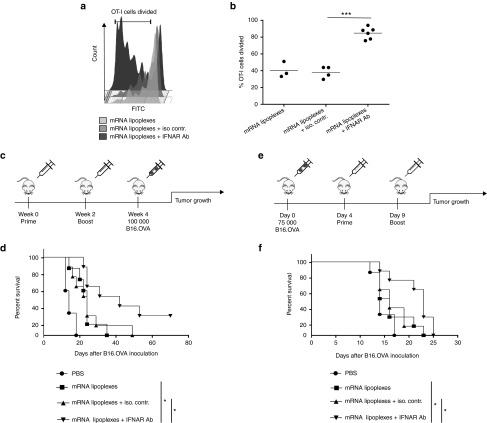
First, we validated whether antibody-mediated IFNAR blockade would indeed amplify the CD8+ T cell response elicited by the mRNA lipoplex vaccine in wild type mice. As can be appreciated from Figure 4a,b coinjection of the IFNAR blocking antibody increased the proliferation of OVA-specific OT-I cells in response to mRNA lipoplexes, while the isotype matched antibody had no impact on OT-I proliferation. Next, we determined if blocking IFNAR at the site of immunization would improve the antitumor efficacy of the lipoplex mRNA vaccines in case of prophylactic (Figure 4c,d) and therapeutic vaccination (Figure 4e,f). In the prophylactic vaccination setting, coinjection of the IFNAR-blocking antibody MAR1-5A3 with the OVA mRNA lipoplexes significantly improved the survival rate of immunized mice (Figure 4d). Importantly, the benefit of blocking IFNAR was preserved in the therapeutic vaccination setting, as mice immunized with mRNA lipoplexes in the presence of MAR1-5A3 displaying an improved outcome compared with mice receiving the same mRNA vaccine alone or combined with an isotype control antibody (Figure 4f). Taken together, these findings demonstrate that type IFNs induced by mRNA lipoplex vaccines negatively impact the vaccine elicit T cell response and its efficacy to control tumor growth upon subcutaneous vaccination.
Figure 4.
Antibody-mediated blocking of IFNAR improves the efficacy of the mRNA vaccine evoked antitumor immune response. (a,b) Two days prior to immunization CFSE-labeled OT-I cells were adoptively transferred to wild type (WT) mice. Immunization was performed in the footpad with 10 µg mRNA lipoplexes in the absence or presence of 20 µg IFNAR blocking antibody or isotype control. Four days after immunization inguinal lymph nodes were isolated and CD8+ T cell proliferation was analyzed by flow cytometry. Data are shown as mean of 3–6 mice per group. ***P < 0.001 (Chi-square test). (a) A representative sample out of 3–6 mice each group is presented. (c) Prophylactic vaccination scheme. (d) Wild type (WT) mice were immunized s.c with 20 μg of mRNA lipoplexes in absence or presence of 20 µg of the IFNAR blocking antibody or isotype control. After 2 weeks, mice were boosted with the same formulation. At week 4, mice were inoculated with 100,000 B16.OVA melanoma cells (n = 6–8 mice/group). *P < 0.05 (Mantel-Cox log-rank test). (e) Therapeutic vaccination scheme. (f) Wild type (WT) mice were inoculated with 75,000 B16.OVA melanoma cells. After 4 and 9 days, immunization was performed using 20 μg of mRNA lipoplexes in absence or presence of the IFNAR blocking antibody or isotype control (20 µg) (n = 6–8 mice/group). *P < 0.05 (Mantel-Cox log-rank test). mRNA lipoplexes = OVA- coding messenger mRNA complexed to DOTAP/DOPE liposomes. CFSE, carboxyfluorescein diacetate succinimedyl ester; mRNA, messenger RNA; IFN, interferon, OVA, ovalbumin; SD, standard deviation.
Type I IFNs dampen cytolytic T cell responses to intradermal and intranodal mRNA lipoplex vaccination
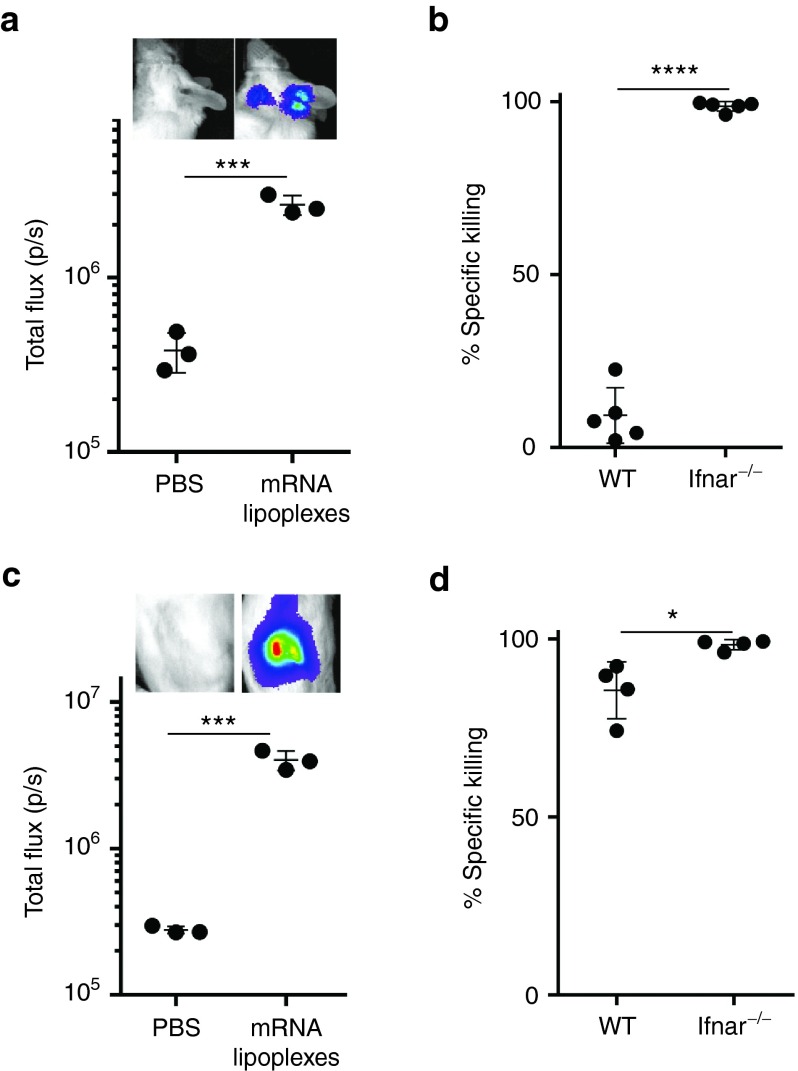
As the route of immunization has a dramatic impact on the type of innate immune cells the mRNA lipoplexes encounter and thereby potentially also on the ensuing T cell response, we decided to evaluate the impact of type I IFNs on the cytolytic T cell response to intradermal and intranodal immunization with mRNA lipoplexes. mRNA lipoplexes also instigated a profound type I IFN response to intradermal (Figure 5a) and intranodal (Figure 5c) injection. In terms of T cell immunity, intradermal immunization with mRNA lipoplexes behaved much alike subcutaneous immunization, with the strength of the cytolytic T cell response shifting from near absent in wild type mice to virtually complete in Ifnar−/− mice (Figure 5b). In line with reports of the Thielemans32 and Sahin33 groups, intranodal immunization turned out to be by far the most potent route of immunization with strong cytolytic T cell responses now being evident in immunized wild type mice (Figure 5d). Nevertheless, even intranodal immunization was aided by IFNAR deficiency, as the cytolytic T cell response was even further enlarged in Ifnar−/− mice. Taken together, these data firmly demonstrate that type I IFNs dampen the strength of the cytolytic T cell response evoked by lipoplex-based mRNA vaccination, regardless of whether the mRNA lipoplexes are delivered subcutaneous, intradermal or intranodal.
Figure 5.
Type I IFNs inhibit the induction of cytolytic T cells regardless of the route of immunization. (a) IFN-β+/Δβ-luc mice were intradermally injected with 10 µg of OVA mRNA lipoplexes complexed or PBS. In vivo bioluminescence was measured 6 hours postinjection. Data are shown as mean ± SD of three mice. ***P < 0,001 (t-test). (b) Wild type (WT) and Ifnar−/− mice were immunized with a two-week interval with 10 µg of mRNA lipoplexes. Two weeks after boost immunization, a mixture of CFSE-labeled cells pulsed with control (CFSElow) or OVA peptide (CFSEhigh) were adoptively transferred. Specific killing was measured after 2 days by flow cytometry. Killing percentages were calculated with the following formula: 100 − 100x ((CFSEhigh/CFSElow)immunized mice/(CFSEhigh/CFSElow)mock-mice) of five mice per group. ****P < 0.0001 (t-test). (c) IFN-β+/Δβ-luc mice were intranodally injected with 10 µg of OVA mRNA lipoplexes or mock treated. In vivo bioluminescence was measured 6 hours postinjection. Data are shown as mean ± SD of three mice. ***P < 0.001 (t-test). (d) Wild type (WT) and IFNAR−/− mice were immunized with a two-week interval with 10 µg of OVA mRNA lipoplexes and killing was performed as previously described. *P < 0.05 (t-test). mRNA lipoplexes = OVA- coding messenger mRNA complexed to DOTAP/DOPE liposomes. CFSE, carboxyfluorescein diacetate succinimedyl ester; mRNA, messenger RNA; IFN, interferon, OVA, ovalbumin; PBS, phosphate-buffered saline; SD, standard deviation.
Discussion
Condensing mRNA into lipoplexes significantly improves the strength of the T cell response against the mRNA encoded antigen upon in vivo immunization. Nevertheless, the key innate host factors that determine the potency of lipoplex mRNA vaccines and their efficacy to instigate antitumor immunity have remained unresolved. Earlier, we have shown that type I IFNs are the most prominent cytokines secreted by DCs when incubated with mRNA lipoplexes.14,15 As type I IFNs are major regulators of T cell immunity to viruses and to tumors, we decided to address their functional impact on the T cell response to mRNA lipoplex vaccines. Vaccination studies in Ifnar−/− mice revealed a dramatically increased priming of vaccine specific T cells in the absence of IFNAR signaling. These vaccine primed T cells acquired full effector function and efficiently eliminated target cells. When challenged with the highly aggressive B16 melanoma model, vaccinated Ifnar−/− mice benefited more from mRNA lipoplex vaccination compared with wild type mice in terms of increase in survival time to nontreated controls. Nevertheless, therapeutically vaccinated Ifnar−/− mice still succumbed earlier to B16 challenge when compared with vaccinated wild type mice. Ifnar−/− mice however lack spontaneous antitumor immunity, making direct comparisons between Ifnar−/− en wild type mice concerning the effects of vaccination on tumor control difficult to interpret. To avoid any confounding effects of genetic IFNAR deficiency on spontaneous versus vaccine elicited antitumor immunity, we therefore shifted to coadministration of an IFNAR blocking antibody at the time and spot of immunization in wild type mice.
Blocking IFNAR at the vaccination site conferred a substantial survival benefit in response to both prophylactic and therapeutic vaccination, thereby establishing type I IFNs as host factors that severely hamper the efficacy of mRNA lipoplexes as antitumor vaccines.
The exact mechanism by which type I IFNs exert their negative impact remains largely unresolved. Type I IFNs can affect the instigation of effector T cell immunity at multiple levels. First, as type I IFNs are potent antiviral cytokines that typically activate RNAses and block translation to prevent viral replication,34 they might hamper T cell immunity to mRNA vaccines by lowering the amount of antigen expressed, a feature we have reported on using in vitro BM-DCs incubated with mRNA lipoplexes.14 Nevertheless, the impact of IFNAR deficiency on the mRNA expression level in vivo was very limited and thus most likely does not constitute the major factor behind the dramatically improved cytolytic T cell response in Ifnar−/− mice. A potential explanation is that type I IFNs exert their negative impact directly at the level of the T cell. Indeed, whereas type IFNs can clearly act as signal 3 cytokines that promote the differentiation of antigen primed CD8+ T cells into cytolytic effectors,29 they can also block T cell proliferation and even instigate T cell apoptosis.19,20,21 Which of these opposing effects prevails, depends on the kinetics of T cell exposure to type I IFNs.17 If IFNAR triggering precedes T-cell receptor triggering, the T cell inhibitory properties prevail. In case of mRNA lipoplex vaccination, type I IFN release occurs rapidly, TLRs and other RNA sensing receptors can be triggered in the endosomal compartments even before the mRNA leaves the endosomes for translation, and most likely before DCs that have taken up the mRNA lipoplexes have reached the lymph nodes to present the antigen. Nevertheless, studies using mice selectively deficient in IFNAR in DCs or in T cells are required to shed further light at which stage type I IFNs exactly interfere with T cell immunity to mRNA lipoplexes.
In general, our findings regarding the negative impact of type I IFNs on T cell immunity to mRNA lipoplex vaccines are in sheer contrast with two recent reports by Kranz35 and Broos.36 Although speculative, we believe that these discrepancies can be largely attributed to the different route (intravenous) of vaccine delivery applied in these studies. Intravenous injection of mRNA lipoplexes will result in different cell types targeted and in an altered kinetics of antigen expression and type I IFN induction. As explained above, the stimulatory versus inhibitory effects of type I IFNs on T cell immunity largely depend on the timing of T cell exposure to type I IFNs. Intravenous injection of mRNA lipoplexes thereby might result in an improved convergence of antigen expression and type I IFN induction, causing the beneficial effects of type I IFNs to prevail.
In summary, we have firmly established type I IFNs as host factors that negatively regulate the capacity of mRNA lipoplex vaccines to instigate cytolytic T cells upon subcutaneous, intradermal, and intranodal administration. As type I IFN induction is inherent to IVT mRNAs, our findings are of importance to many other nano-formulations explored for mRNA vaccination. If so, strategies to prevent or reduce type I IFNs might be of great value to improve the clinical efficacy of mRNA vaccines.
Additional experimental characterization data are provided in Supplementary Data.
Materials and Methods
Mice. Female wild type C57BL/6 mice were purchased from Janvier (Le Genest Saint Isle, France). OT-I mice carrying a transgenic CD8+ T cell receptor specific for the MHC I-restricted OVA peptide SIINFEKL were donated by Bart Lambrecht from Ghent University (Ghent, Belgium). Ifnar1−/−mice were bred at the breeding facility of the Vlaams Instituut voor Biotechnolgoy (VIB, Ghent, Belgium). C57BL/6 luciferase reporter mice (IFN-β+/Δβ-luc) were bred at the Helmholtz Center for Infection Research (HZI). All mice were 7–12 weeks old at the start of the experiment and maintained under specific pathogen-free conditions. Animals were treated according to the European guidelines for animal experimentation. All experiments were approved by the local ethical committee for animal experiments of Ghent University (Ghent, Belgium) or of the Helmholtz Center for Infection Research (Braunschweig, Germany).
Production of in vitro transcribed mRNA. The pGEM4Z-OVA-A64 and the pGEM4Z-EGFP-A64 plasmids were kindly donated by David Boczkowski from Duke University (Durham, NC). The pBluescript-luc-A64 plasmid was provided by Joanna Rejman from Ghent University (Ghent, Belgium). All plasmids were propagated in E. coli competent cells (Stratagene, La Jolla, CA) and purified using endotoxin-free QIAGEN-tip 500 columns (Qiagen, Chatsworth, CA). The pGEM4-OVA-A64 and pGEM4Z-EGFP-A64 plasmids were linearized with SpeI (MBI Fermentas, St Leon-Rot, Germany), whereas the pBluescirpt-luc-A64 plasmid was linearized with DraI (MBI Fermentas). Linearized plasmids were purified using a PCR purification kit (Qiagen, Venlo, The Netherlands) and RNA was transcribed using the T7 mMessage Machine Kit (Ambion, Austin, TX) according to the manufacturer's instructions. The in vitro transcribed mRNA was purified by lithium chloride precipitation.
Immunizations and injections of mRNA lipoplexes. Subcutaneous immunizations were performed in C57BL/6 mice twice at tail base in a two-week interval. According to the experiment 10 or 20 μg of OVA-encoding mRNA was complexed with DOTAP/DOPE lipids in a N/P ratio of 1 (Avanti Polar Lipids, Alabaster, AL) and injected in a total volume of 40 μl of 5% glucose water (Ambion, Thermo Fisher, Waltham, MA). For intranodal delivery of mRNA, C57BL/6 mice were anesthetized with ketamine (70 mg/kg; Ceva) and xylazine (10 mg/kg; Bayer). The inguinal lymph node was surgically exposed and injected with 10 µg RNA lipoplexes in a total volume of 15 µl. Subsequently, the wound was closed. For intradermal immunization, 10 µg of mRNA lipoplexes was injected into the ear dermis in a total volume of 20 µl. Accordingly to the experiments, the total vaccine volume included 20 μg of MAR1-5A3 (antimouse IFNAR) or mouse IgG1 isotype control (both from Leinco Technologies, St. Louis, MO). For in vivo measuring of mRNA expression levels wild type and Ifnar−/− mice were injected s.c. with 10 µg of luciferase encoded mRNA. mRNA expression levels were measured 8 hours after injection via in vivo biolumenescence.
Flow cytometry. All flow cytometric experiments were performed on a triple-laser (B-V-R) LSR-II (Becton Dickinson, San Jose, CA) and analyzed with FlowJo (Treestar, Ashland, OR). Cells were stained with α-CD16/CD32 (BD Biosciences, San Diego, CA) to block nonspecific FcR binding, and with Live/Dead Fixable Aqua stain (Invitrogen, Merelbeke, Belgium) to eliminate dead cells from analysis. Antibodies used are α-CD8 PerCP, α- CD3 pacific blue, α-CD19 APC-Cy7, α-CD11c PerCP-Cy5.5, α-F4/80 APC (all BD Biosciences), and MHC dextramer H-2 Kb/SIINFEKL-PE (Immudex, Copenhagen, Denmark).
In vivo imaging of IFNβ induction. Heterozygous luciferase reporter mice (IFN-β+/Δβ-luc) were injected subcutaneously, with phosphate-buffered saline (PBS), 10 µg of OVA-mRNA complexed with DOTAP/DOPE liposomes at an N/P ratio of 1 (Avanti Polar Lipids), DOTAP/DOPE alone or naked OVA-mRNA in a total volume of 20 μl 5% glucose water. Intradermal or intranodal injections were performed with 10 µg of mRNA lipoplexes at an N/P ratio of 1 (Avanti Polar Lipids), DOTAP/DOPE alone or naked OVA-mRNA in a total volume of 10–20 μl 5% glucose water. IFNβ induction was measured at 0, 3, and 6 hours after injection via in vivo biolumenescence.
In vivo bioluminescence imaging. For in vivo imaging, mice were injected intravenously with 150 mg/kg of D-luciferin (PerkinElmer, Waktham, MA) in PBS and monitored using an IVIS lumina II imaging system. Photon flux was quantified using the Living Image 4.4 software (all from Caliper Life Sciences, Hopkinton, MA).
Enzyme-linked immunosorbent spotC57BL/6 mice were immunized twice with 20 µg of DOTAP/DOPE-complexed OVA-encoding mRNA in a two-week interval. Two weeks after the boost immunization, spleens were isolated and passed through 70 μm nylon strainers (BD Biosciences) to obtain single cell suspensions. Red blood cells were lysed using ACK red blood cell lysis buffer (BioWhittaker, Wakersville, MD) and 2.5 × 105 cells were cultured for 24 hours on IFN-γ (Diaclone, Besançon, France) precoated 96-well plates in the presence of 10 μg/ml OVA peptides (Anaspec, Fremont, CA). To quantify the amount of OVA-specific CD8+ and CD4+ T cells we pulsed the splenocytes with respect to 10 µg/ml MHC-I and MHC-II OVA peptides. Spots were analyzed according to the manufacturer's instructions using enzyme-linked immunosorbent spot (ELISPOT) reader.
CD8+ T cell dextramer staining. Mice were immunized twice with 20 µg of DOTAP/DOPE complexed OVA-encoding mRNA as described previously. After 5 days, blood samples were taken and red blood cells were removed using ACK lysis buffer (BioWhittaker). Cells were stained with α-CD16/CD32 (BD Biosciences), Live/Dead Fixable Aqua stain (Invitrogen), α-CD8 PerCP, α- CD3 pacific blue, α-CD19 APC-Cy7 (all BD Biosciences) and MHC dextramer H-2 Kb/SIINFEKL-PE (Immudex).
In vivo T cell proliferation assay. Two days before immunization OT-I cells were labeled with 5 µmol/l CFSE (Invitrogen). Two million CFSE-labeled OT-I cells were i.v. injected into wild type and Ifnar−/− mice 2 days before immunization. Immunization was performed as previously described. Four days after immunization draining lymph nodes were isolated and CD8+ T cell division was analyzed by flow cytometry. Cells were stained with α-CD16/CD32 (BD Biosciences), Live/Dead Fixable Aqua stain (Invitrogen), α-CD8 PerCP, α- CD3 pacific blue, α-CD19 APC-Cy7 (all BD Biosciences), and MHC dextramer H-2 Kb/SIINFEKL-PE (Immudex).
In vivo killing assay. Splenocytes from female wild type mice were pulsed with 1 µg/ml of MHC-I OVA peptide or HIV-1 Gag peptide as a control before labeling with 5 µmol/l or 0.5 µmol/l CFSE (Invitrogen), respectively. Labeled cells were mixed at a 1:1 ratio, and a total of 1.5 × 107 cells mixed cells were adoptively transferred into immunized mice 2 weeks after boost. Splenocytes from host mice were analyzed 2 days later by flow cytometry after staining with α-F4/80 (BD Biosciences) to exclude auto-fluorescent macrophages. Percentage antigen-specific killing was determined using the following formula: 100 – 100* ((% CFSEhi cells / % CFSElowcells)immunized mice /(% CFSEhi cells / % CFSElow cells)nonimmunized mice.
Tumor challenge. For the prophylactic tumor experiments, immunized mice were inoculated s.c. in the flank with 105 B16-OVA melanoma cells (VIB cell bank) in 200 µl PBS 2 weeks after boost immunization. Immunizations were performed as described above. Tumor growth was followed by measuring the tumor size index, i.e., the product of the largest perpendicular diameters, with a caliper. For assessment of therapeutic efficacy, 7.5 × 104 B16-OVA melanoma cells in 200 µl PBS were administered 4 days prior to immunization. Boost immunizations were given 2–5 days after priming.
SUPPLEMENTARY MATERIAL Figure S1. Characterization of mRNA lipoplexes at nitrogen/phosphate (N/P) ratio 1 and 10. Figure S2. Luciferase expression was measured in wild type (WT) en Ifnar−/− mice after s.c injection of 10 µg luciferase encoding mRNA lipoplexes at ratio N/P1. Supplementary Data.
Acknowledgments
This work was supported by grants from the Interuniversitary Attraction Pole consortium IUAPVII/63-120C07812W-B/12917/01IUAP, the UGhent Concerted Research Consortium BOF12/GOA-B/12424/01, the Fund for Scientific Research Flanders project G.0226.10, the Strategic Basic Research Program of the Flanders government project 80016, the Europrise (European vaccines and microbicides Enterprise) project 037611, the UGhent Methusalem project BOF09/01M00709, and the SOFI-B (Secondary Research Funding) at the Institute of Tropical Medicine. De B.A. acknowledges IWT (Agentschap voor innovatie door wetenschap en technologie, Vlaanderen) for a PhD scholarship. Pollard C. was supported by a PhD scholarship from SOFI-B. Roose K. is supported by EC FP7 project FLUNIVAC. De K.S. acknowledge FWO Flanders for funding and Van Lint S. acknowledge BOF-UGhent. The authors declare no conflict of interest.
Supplementary Material
References
- Appay, V, Douek, DC and Price, DA (2008). CD8+ T cell efficacy in vaccination and disease. Nat Med 14: 623–628. [DOI] [PubMed] [Google Scholar]
- Sahin, U, Karikó, K and Türeci, Ö (2014). mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov 13: 759–780. [DOI] [PubMed] [Google Scholar]
- Palucka, K and Banchereau, J (2013). Dendritic-cell-based therapeutic cancer vaccines. Immunity 39: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp, S, Kreiter, S, Selmi, A, Simon, P, Koslowski, M, Huber, C et al. (2006). Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108: 4009–4017. [DOI] [PubMed] [Google Scholar]
- Anderson, BR, Muramatsu, H, Jha, BK, Silverman, RH, Weissman, D and Karikó, K (2011). Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res 39: 9329–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, BR, Muramatsu, H, Nallagatla, SR, Bevilacqua, PC, Sansing, LH, Weissman, D et al. (2010). Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res 38: 5884–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carralot, JP, Weide, B, Schoor, O, Probst, J, Scheel, B, Teufel, R et al. (2005). Developments in immunotherapy for gastrointestinal cancer, production and characterization of amplified tumor-derived, cRNA libraries to be used as vaccines against metastatic melanomas. Genet Vaccines Ther 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benteyn, D, Van Nuffel, AM, Wilgenhof, S, Corthals, J, Heirman, C, Neyns, B et al. (2013). Characterization of CD8+ T-cell responses in the peripheral blood and skin injection sites of melanoma patients treated with mRNA electroporated autologous dendritic cells. Biomed Res Int 976383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch, S, Schwentner, C, Stenzl, A and Bedke, J (2014). mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum Vaccin Immunother 10: 3146–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgenhof, S, Corthals, J, Van Nuffel, AM, Benteyn, D, Heirman, C, Bonehill, A et al. (2015). Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunol Immunother 64: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig, SM, Haentschel, M, Weimer, KJ, Heine, A, Muller, MR, Brugger, W et al. (2011). Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther 19: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, Z, Dannull, J, Yang, BK, Dahm, P, Coleman, D, Yancey, D et al. (2005). Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol 174: 3798–3807. [DOI] [PubMed] [Google Scholar]
- Kübler, H, Scheel, B, Gnad-Vogt, U, Miller, K, Schultze-Seemann, W, Vom Dorp, F et al. (2015). Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, C, Rejman, J, De Haes, W, Verrier, B, Van Gulck, E, Naessens, T et al. (2013). Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol Ther 21: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel, B, Teufel, R, Probst, J, Carralot, JP, Geginat, J, Radsak, M et al. (2005). Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur J Immunol 35: 1557–1566. [DOI] [PubMed] [Google Scholar]
- Scheel, B, Aulwurm, S, Probst, J, Stitz, L, Hoerr, I, Rammensee, HG et al. (2006). Therapeutic anti-tumor immunity triggered by injections of immunostimulating single-stranded RNA. Eur J Immunol 36: 2807–2816. [DOI] [PubMed] [Google Scholar]
- Andries, O, De Filette, M, De Smedt, SC, Demeester, J, Van Poucke, M, Peelman, L et al. (2013). Innate immune response and programmed cell death following carrier-mediated delivery of unmodified mRNA to respiratory cells. J Control Release 167: 157–166. [DOI] [PubMed] [Google Scholar]
- Crouse, J, Kalinke, U and Oxenius, A (2015). Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol 15: 231–242. [DOI] [PubMed] [Google Scholar]
- van Boxel-Dezaire, AH, Rani, MR and Stark, GR (2006). Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25: 361–372. [DOI] [PubMed] [Google Scholar]
- Tanabe, Y, Nishibori, T, Su, L, Arduini, RM, Baker, DP and David, M (2005). Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol 174: 609–613. [DOI] [PubMed] [Google Scholar]
- Gimeno, R, Lee, CK, Schindler, C and Levy, DE (2005). Stat1 and Stat2 but not Stat3 arbitrate contradictory growth signals elicited by alpha/beta interferon in T lymphocytes. Mol Cell Biol 25: 5456–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeestraten, EC, Speetjens, FM, Welters, MJ, Saadatmand, S, Stynenbosch, LF, Jongen, R et al. (2013). Addition of interferon-α to the p53-SLP® vaccine results in increased production of interferon-γ in vaccinated colorectal cancer patients: a phase I/II clinical trial. Int J Cancer 132: 1581–1591. [DOI] [PubMed] [Google Scholar]
- Kameshima, H, Tsuruma, T, Kutomi, G, Shima, H, Iwayama, Y, Kimura, Y et al. (2013). Immunotherapeutic benefit of α-interferon (IFNα) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci 104: 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, JE, McNeel, DG and Olson, BM (2012). Vaccination using peptides spanning the SYT-SSX tumor-specific translocation. Expert Rev Vaccines 11: 1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuhn, L, Vanparijs, N, De Beuckelaer, A, Lybaert, L, Verstraete, G, Deswarte, K et al. (2016). pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc Natl Acad Sci USA 113: 8098–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midoux, P and Pichon, C (2015). Lipid-based mRNA vaccine delivery systems. Expert Rev Vaccines 14: 221–234. [DOI] [PubMed] [Google Scholar]
- Lienenklaus, S, Cornitescu, M, Zietara, N, Łyszkiewicz, M, Gekara, N, Jabłónska, J et al. (2009). Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J Immunol 183: 3229–3236. [DOI] [PubMed] [Google Scholar]
- Fuertes, MB, Kacha, AK, Kline, J, Woo, SR, Kranz, DM, Murphy, KM et al. (2011). Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 208: 2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger, JM, Valenzuela, JO, Agarwal, P, Lins, D and Mescher, MF (2005). Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 174: 4465–4469. [DOI] [PubMed] [Google Scholar]
- Fuertes, MB, Woo, SR, Burnett, B, Fu, YX and Gajewski, TF (2013). Type I interferon response and innate immune sensing of cancer. Trends Immunol 34: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, MS, Kinder, M, Matsushita, H, Mashayekhi, M, Dunn, GP, Archambault, JM et al. (2011). Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 208: 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint, S, Goyvaerts, C, Maenhout, S, Goethals, L, Disy, A, Benteyn, D et al. (2012). Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res 72: 1661–1671. [DOI] [PubMed] [Google Scholar]
- Kreiter, S, Selmi, A, Diken, M, Koslowski, M, Britten, CM, Huber, C et al. (2010). Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res 70: 9031–9040. [DOI] [PubMed] [Google Scholar]
- Schoggings, JW, Wilson, SJ, Panis, M, Murphy, MY, Jones, CT, Bieniasz, P et al. (2011). A divers range of gene products are effectors of the type I interferon antiviral response. Nature 472: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz, LM, Diken, M, Haas, H, Kreiter, S, Loquai, C, Reuter, KC et al. (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534: 396–401. [DOI] [PubMed] [Google Scholar]
- Broos, K, Van der Jeught, K, Puttemans, J, Goyvaerts, C, Heirman, C, Dewitte, H et al. (2016). Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of Type I interferon. Mol Ther Nucleic Acids 5: e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.