Abstract
Lymph node stromal cells play a role in self-tolerance by presenting tissue antigens to T cells. Yet, immunomodulatory properties of lymphoid tissue stroma, particularly toward CD4+ T cells, remain insufficiently characterized by lack of tools to target antigens for presentation by stromal cells. A lentiviral vector was therefore designed for antigen delivery to MHC class II+ cells of nonhematopoietic origin. Following intravenous vector delivery, the transgene was detected in lymph node gp38+ stromal cells which were CD45- MHCII+ and partly positive for CD86 and CTLA4 or B7-H4. The transgene was not detected in classical dendritic cells of lymph nodes or spleen. Transgene-specific CD4+ and CD8+ T cell responses were primed and regulatory T cells were also induced but effector T cell response did not develop, even after a peptide boost. Antigen-specific CD8+ T cells were not cytolytic in vivo. Thus, expressing a neo-antigen in MHC-II+ lymph node stroma seems to trigger blunt CD4 T cell responses leading to antigen-specific CD8+ T cell anergy. These results open up new perspectives to further characterize lymph node stromal cell functional properties and to develop gene transfer protocols targeting lymph node stroma to induce peripheral tolerance.
Introduction
Antigen presenting cells (APCs) are a functionally heterogeneous population of cells with immunizing or tolerogenic properties. Gene transfer into specific subsets of APC could therefore lead to different immunological outcomes which is important to understand in the context of gene therapy. Professional APCs such as dendritic cells (DC) are major histocompatibility complex (MHC) class II+ mobile sentinels specialized in acquiring antigens from the outside and of integrating various signals to trigger specialized effector T cell responses.1 Stromal cells in tissues or lymphoid organs also function as APCs by presenting endogenous tissue antigens to T cells, playing a key role in self-tolerance in the steady-state.2,3 While most tissue stromal cells only express MHC class I and can only present to CD8 T cells, some subsets of stromal cells express MHC class II. For instance thymic epithelial cells present self-peptides on both MHC class I and class II molecules to shape T cell precursors T cell receptor repertoire by positive selection or clonal deletion.4 Auto-reactive CD4+ T cells, which escape deletion in the thymus may become anergic through additional peripheral tolerance mechanisms, notably in secondary lymphoid organs (SLO).
SLO like spleen or lymph nodes (LN) are highly-organized structures that bring immune cells in close contact within functionally-defined compartments. It is now clear that stromal cells in SLOs are not only important structural elements that define the traffic of cells and of fluids in the organ, but also control lymphoid homeostasis and T cell responses.2 In mice, several models show that peripheral CD8+ T cell tolerance is established in LN by direct presentation of self-antigens on MHC class I by LN stromal cells.5,6,7,8 Recently, SLO stromal cells were also shown to control peripheral CD4+ T cell development. SLO stromal cells can express low levels of constitutive MHC class II molecules, which can be upregulated during immune responses.9,10 The functional role of SLO stromal cells in CD4+ T cell activation has been assessed experimentally with transplants of MHC class II-deficient LN combined with T cell transfer. These experiments showed that MHC class II self-antigen presentation by LN stromal cells controls antigen-specific response by maintaining antigen-specific regulatory T cells.11 It is not known how MHC class II+ stromal cells function in more physiological conditions. Studying antigenic presentation by LN stroma remains challenging3 and has not yet been explored via gene transfer.
Recombinant gene transfer lentiviral vectors (LV) are noninflammatory tools that can be used for in vivo gene delivery. LV can be engineered to express antigens in specific cell types. LV envelope glycoproteins can be engineered for cell-specific entry.12,13,14 In particular, a lentiviral pseudotype specific for murine MHC class II IAd15,16 was used in vivo in mice and was shown to efficiently transduce MHC class II+ DC in SLO.17 The transduction of rare MHC class II+ stromal cells with this pseudotype was not reported in this previous study. LV can also be engineered to regulate cell-specific transgene expression for instance with the use of mir142.3p target sequences which prevent transgene expression in cells of the hematopoietic lineage.18,19 This strategy precludes direct antigenic presentation of the transgene product by DC20 and prevents the induction of antitransgene T cell responses.21,22,23,24
Therefore, we have combined the use of MHC class II+ cell targeting and inhibition of transgene expression in cells of hematopoietic origin to deliver transgene in MHC class II+ stromal cells. Results show that such cells are present in LN of mice in the steady state and that transgene delivery to these cells appears to prime CD4+T cells but prevents the full development of antitransgene effector cells leading to CD8+ T cells anergy.
Results
Transgene expression can be targeted to LN stromal cells with the LV-MHCII-miR vector
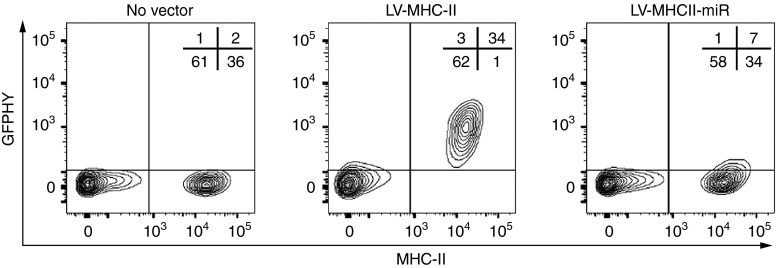
To obtain selective gene expression in MHC class II+ SLO stromal APC in vivo and to measure the induced T cell responses, we engineered a new recombinant LV combining three key features previously tested in other systems. First, the envelope contained MV glycoproteins displaying a scFv specific for murine MHC class II+ cell recognition. Upon systemic administration, this envelope reduces vector biodistribution in the liver and spleen compared with vesicular stomatitis virus G glycoprotein (VSVg), but enables gene transfer into spleen DC.17 Second, the cassette expressed the GFP-HY transgene, a neo-antigenic protein enabling the identification of transduced potential APCs by flow cytometry (FACS). The GFP-HY transgene fuses the green fluorescent protein (GFP) with an HY male murine antigen sequence containing the Dby and Uty T cell epitopes. This sequence permits the detection of male-specific CD4+ and CD8+ T cell responses in female mice.17 Third, GFP-HY transgene expression was regulated by mir142.3p target sequences to prevent antigenic expression in cells of hematopoietic origin and to prevent direct antigenic presentation of the transgene by classical DC.20 In vitro, the mir142.3p target sequences did not alter the expression of GFP-HY in the HEK293T cell line (data not shown) but prevented it in cultured MHC class II+ spleen cells which contain almost exclusively cells of hematopoietic origin (Figure 1). The stroma-targeting vector, abbreviated as LV-MHCII-miR, and its control LV-MHCII previously-characterized,17 differ only by the presence or absence of mir142.3p target sequences. Both vectors were produced using identical methods and titered at similar levels using reverse transcriptase (RT) concentrations, as described previously.17 Vectors were compared side by side using the same amount of RT.
Figure 1.
LV-MHCII-miR transduces MHC class II+ cells and prevents transgene expression in CD45+ cells in vitro. Expression of the GFP transgene was measured by FACS following in vitro transduction of freshly isolated erythrocyte-lyzed spleen cells with the LV-MHCII and LV-MHCII-miR vectors and results are representative of three separate experiments (in each experiment, splenocytes from one mouse were tested at least in duplicate for each condition). GFP, green fluorescent protein.
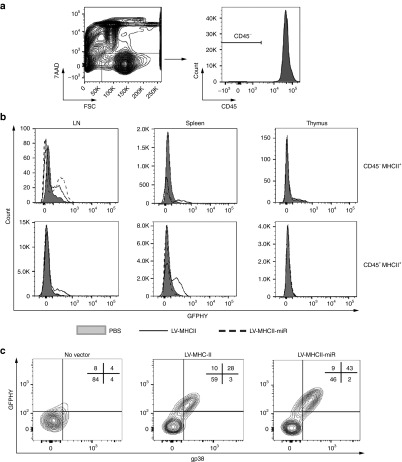
The in vivo biodistribution of MHC class II-targeted vectors was determined 3 days after intravenous (IV) infusion of 50 ng RT of LV into female C57Bl/6 mice. To focus the analysis on MHC class II+ nonhematopoietic (CD45−) stromal cells, which are very rare, peripheral lymphoid tissues (spleen, LN, and thymus) were enzyme-digested and the presence of transgene was examined by FACS gating on CD45- cells (Figure 2a). Marking was compared with that of CD45+ MHCII+ cells of hematopoietic origin. In the conditions used, the GFP-HY signal was undetectable in the thymus but was observed in LN and spleen depending on the cell subsets and presence of miR regulation (Figure 2b). With the LV-MHCII-miR, GFP-HY was found in LN CD45-MHCII+ cells, which were the only detectable transgene-positive cells with this vector in tissues examined. With the LV-MHCII control vector, both CD45- cells and a more abundant population of CD45+ cells were positive for the transgene in LN and spleen (Figure 2b). Such CD45+ MHC class II+ cells included CD11c+ CD11b− DC but not B cells as previously reported.17 Various levels of MHC II were observed on the different cell types analyzed and did not correlate with transduction, as shown in Supplementary Figure S1.
Figure 2.
LV-MHCII-miR transduces gp38+ CD45− stromal cells in vivo. C57Bl/6 female mice were injected intravenously (IV) with 50 ng reverse transcriptase (RT) of LV-MHCII or of LV-MHCII-miR, or with equivalent volume of PBS and 3 days later, spleen, thymus, and LN were digested and analyzed by FACS to measure GFP-HY transgene expression (FL1 channel) and other markers (pool of three mice per group per experiment). (a) Gating strategy to focus on live CD45− cells or live CD45+ cells for the analysis presented in b. (b) Differential expression of the transgene in cellular subpopulations of lymphoid organs following transduction with LV-MHCII and LV-MHCII-miR vectors. Results representative of six independent experiments. (c) Transgene is expressed by CD45− gp38+ LN stromal cells following in vivo transduction with LV-MHCII or with LV-MHCII-miR. Results representative of four independent experiments. GFP, green fluorescent protein; LN, lymph node; LV, lentiviral vectors; PBS, phosphate buffered saline.
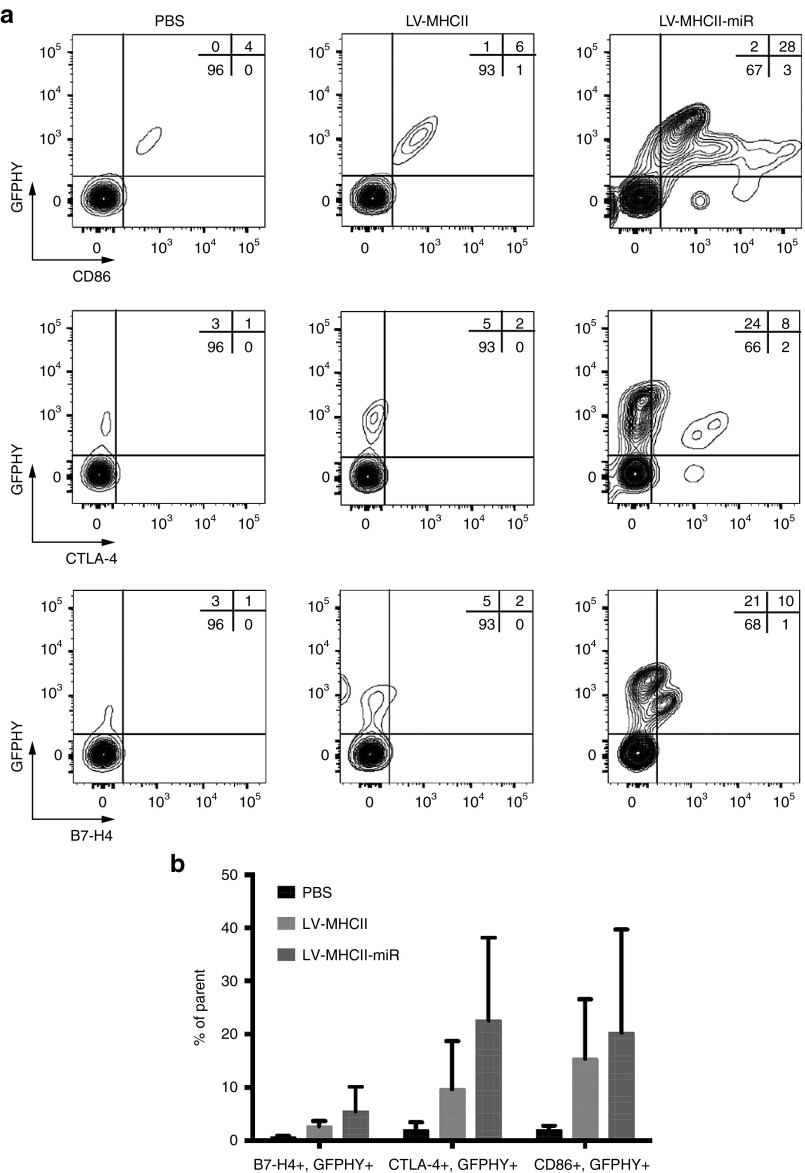
Transgene-positive LN CD45- cells expressed the podoplanin gp38 antigen characteristic of LN stomal cell populations (Figure 2c). They also expressed the costimulatory marker CD86, low levels of CTLA-4 and of B7-H4 (~ 25% of the transduced population) which were seen when the LV-MHCII-miR was used (Figure 3a,b). It appears that if different levels of costimulatory molecules were present on these cells when the control LV-MHCII was injected. Cells appear to express different levels of co-stimulatory molecules depending on the vector used but technical limitations cannot be excluded. Fewer transduced CD45- cells could be analyzed following administration of the LV-MHCII vector which induced an immune response, than with the LV-MHCII-miR vector. However, regardless of vector used, CD45-LN cells expressed similar levels of transgene (Supplementary Figure S2).
Figure 3.
Transduced LN stromal cells are differentially activated following administration of LV-MHCII or LV-MHCII-miR vectors. C57Bl/6 female mice were injected intravenously (IV) with 50 ng reverse transcriptase (RT) of LV-MHCII or LV-MHCII-miR, or PBS as control. After 3 days, LN were harvested and analyzed by FACS (pool of three mice per group per experiment) (a) Representative expression of CD86, CTLA-4, B7-H4 activation markers by transgene expressing cells in LN. Results representative of two (B7-H4) and three (CD86, CTLA-4) independent experiments. (b) Averaged percentage of live CD45− GFP-HY+ cells expressing the indicated markers and SD, from the experiments in a. The parent cell population is CD45− live cells. GFP, green fluorescent protein; LN, lymph node; LV, lentiviral vectors; PBS, phosphate buffered saline; SD, standard deviation.
The LV-MHCII-miR vector leads to T cell hypo-responsiveness
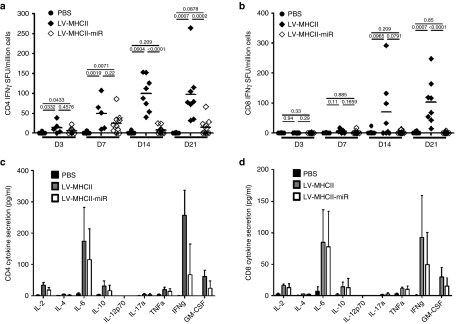
Consistent with its ability to transduce DC, the control LV-MHCII vector induced robust HY antigen-specific T cell responses as previously reported.17 Such T cell responses were higher than those induced by the same amount of LV-MHCII-miR (Figure 4a–d). The LV-MHCII-miR vector induced very low interferon gamma (IFN-γ) CD4+ and CD8+ T cell responses at all time points tested (3-7-14-21 days), with the exception of a small CD4+ T cell response observed at day 7 (Figure 4a,c). The activation of HY-specific T cells at day 7 by the miR vector was confirmed by the detection of cytokine production including interleukin (IL)-2, -10, -6, GM-CSF, and IFN-γ by both CD4+ and CD8+ T cells (Figure 4c,d). Levels of IFN-γ induced by LV-MHCII-miR tended to be lower than those induced by LV-MHCII. However the other cytokines (for instance IL-6) were produced in similar ranges to the control vector indicating that a response had been triggered.
Figure 4.
LV-MHCII-miR vectors induce lower CD4+ and CD8+ T cell responses than LV-MHCII vectors. C57Bl/6 female mice were intravenously (IV) injected in the tail vein with PBS, LV-MHCII or LV-MHCII-miR vectors. Immune responses were measured by interferon-gamma (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) following a 24-hours-Dby or Uty ex vivo stimulation of splenocytes (a,b) or cytometric bead array (c,d). (a,b) Kinetics of response to 50 ng reverse transcriptase (RT) of vector. Immune response to LV-MHCII (a) or LV-MHCII-miR (b) was measured by IFN-γ ELISPOT 3, 7, 14, and 21 days after injection and following Dby or Uty peptide stimulation as indicated (n = 2 separate experiments, 5–10 mice per group). (c,d) Cytokine secreted following immunization. C57Bl/6 female mice were injected IV with PBS, 50 ng RT of LV-VSVg-GFP-HY or LV-MHCII vectors. After 7 days, spleen cells of these mice were harvested and assayed for IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ, TNFα, and GM-CSF secretion after 48 hours stimulation ex vivo with Dby (c) or Uty (d) using the cytometric bead array (n = 5 mice per group). GM-CSF, Granulocyte-macrophage colony-stimulating factor; GFP, green fluorescent protein; IL, interleukein; LN, lymph node; LV, lentiviral vectors; PBS, phosphate buffered saline; TNFα, tumor necrosis factor alpha.
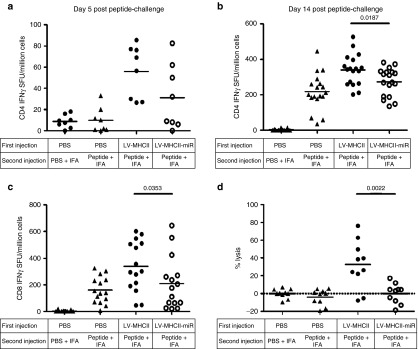
To further characterize the immune reaction induced by the LV-MHCII-miR, we boosted mice by intramuscular (IM) injection of HY peptides complexed with incomplete Freund adjuvant (IFA) and evaluated the kinetics of transgene-specific CD4+ and CD8+ T cell responses using IFN-γ enzyme-linked immunosorbent spot (ELISPOT). In this system, naive mice responded slowly as their CD4+ T cell primary response became detectable only 14 days postchallenge (compare the phosphate-buffered saline (PBS)/peptide+IFA conditions in Figure 5a (5 days postboost) and Figure 5b (2 weeks postboost)). In contrast, nonnaive mice responded more rapidly, mounting CD4+ T cell responses within 5 days of the boost (Figure 5a). This rapid response was observed in mice injected previously with the LV-MHCII-miR even though they remained slightly below that of control vector-treated mice at all time points (Figure 5a,b). These results confirm that the miR-regulated vector had primed a CD4+ T cell immune response as shown in Figure 4a (see day 7 for instance) and Figure 4c. As for antigen-specific IFN-γ CD8+ T cell responses after the boost, they were significantly lower after LV-MHCII-miR administration compared with the control LV-MHC vector (Figure 5c). The boosted response was comparable to that of naive mice injected with peptide and IFA suggesting poor initial CD8+ T cell priming, in coherence with results from Figure 4b. To verify the functionality of the induced CD8+ T cells, we challenged their ability to kill antigen-positive cells by infusing male cells to the treated mice and measuring their specific disappearance (Figure 5d). Female mice that were either naive or primed only by peptide + IFA did not mount effector cytotoxic T cell responses. In contrast, female mice treated with LV-MHCII vector and boosted by peptide + IFA mounted effective cytotoxic responses and a large proportion of the animals were able to kill high levels of injected male cells. When treated with the LV-MHCII-miR vector, in spite of the peptide boost, the mice failed to mount effective cytolytic responses to kill male target cells (Figure 5d).
Figure 5.
Immunization with LV-MHCII-miR does not lead to memory T cell responses. C57Bl/6 female mice were injected IV with PBS, LV-MHCII or LV-MHCII-miR. After 35 days, mice were challenged by an IM injection of a mixture of Dby and Uty peptides complexed with IFA or as control with PBS complexed with IFA. IFN-γ secretion of splenocytes was assayed. (a) Five days after the challenge following a 24 hours Dby restimulation. (b) Fourteen days after the challenge following a 24 hours Dby restimulation or (c) Uty restimulation. Each symbol represents IFN-γ spot-forming unit (duplicate measures) of each mouse from 2 to 3 independent experiments (n = 8– 18 mice per group) (d) C57Bl/6 female mice were injected IV with PBS, LV-MHCII or LV-MHCII-miR. After 35 days, mice were challenged by an IM injection of a mixture of Dby and Uty peptides complexed with IFA or as control with PBS complexed with IFA. After 13 days, a mix of congenic C57Bl/6 CD45.1 male CFSElow labeled cells and female CFSEhigh labeled cells were injected in mice (107 cells per mice). After 42 hours, spleen cells were harvested and the CFSElow C57Bl/6 CD45.1 male cells lysis was assessed by flow cytometry and normalized with the CFSEhigh C57Bl/6 CD45.1 female cells (n = 2, 10 mice per group).CFSE, carboxyfluorescein succinimidyl ester dye; IFA, incomplete Freund adjuvant; IFN, interferon; IV, intravenously; IM, intramuscularly; LV, lentiviral vectors; PBS, phosphate buffered saline.
Overall, our findings show that the LV-MHCII-miR vector which transduced LN stromal cells but not DC, triggered an initial CD4 immune response but little CD8 T cell priming and the responses which failed to develop effectively, led to antigen-specific CD8+ T cell anergy.
The LV-MHCII-miR vector induces unbalanced effector/regulatory T cells in the periphery
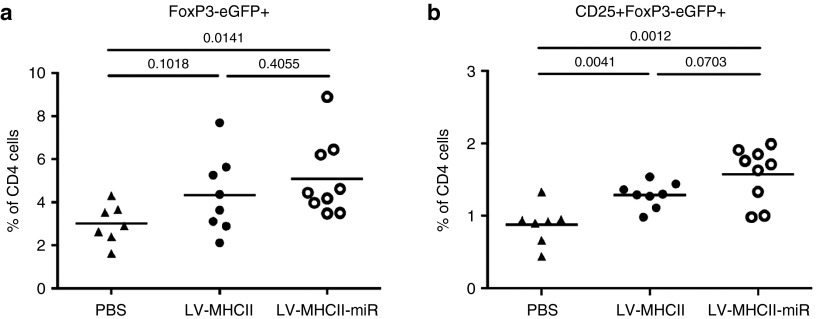
To assess if the T cell hypo-responsiveness induced by LV-MHCII-miR vector was linked to the induction of peripheral regulatory CD4+ T cells (pTregs), we injected vectors into FoxP3-eGFP Marilyn mice. These transgenic mice CD4+ T cells display a T-cell receptor (TCR) specific only for the MHC class II-restricted male epitope DBY and express enhanced GFP (eGFP) under control of the FoxP3 promoter.25 Such mice lack thymic-derived regulatory T cells and have background levels of pTregs. Female FoxP3-eGFP Marilyn mice injected with PBS show very low levels of CD4+ FoxP3-eGFP+ or CD4+ CD25+ FoxP3-eGFP+ pTregs in spleens as seen in Figure 6a,b. However, we detected the induction of CD4+ GFP+ pTregs in their spleen, 28 days after injection of LV-MHCII-miR as well as with LV-MHCII vectors (Figure 6). This conversion was significant when comparing the LV-MHCII-miR and PBS-injected groups but not between the two vector groups. As both effector and regulatory T cells are induced in the course of normal immune responses, the induction of pTregs by the LV-MHCII is not unexpected. However the balance of effector T cells to pTregs is different in the two vector groups. The LV-MHCII-miR vector primes a very low effector T cell response combined with the induction of pTregs therefore, creating a net effect in favor of high antigen-specific regulatory activity.
Figure 6.
Gene transfer vectors induce the conversion of HY-specific CD4+ T cells into regulatory T cells in the periphery. FoxP3-eGFP Marilyn mice were injected IV with PBS, LV-MHCII or LV-MHCII-miR. After 28 days, spleen were harvested and analyzed for presence of (a) converted FoxP3-eGFP+ regulatory T cells and (b) CD25+ FoxP3-eGFP+ cells within CD4+ T cells (n = 8–9 mice per group, three independent experiments). GFP, green fluorescent protein; LV, lentiviral vectors; PBS, phosphate buffered saline.
Discussion
Stromal cell immune regulation of CD4 T cell responses is incompletely understood3 but our results provide a new functional assessment of this question (Supplementary Figure S3). Here, a targeted gene delivery approach which excludes DC has revealed the existence of a very rare gp38+ MHC class II+ LN stromal cell population in steady-state adult mice which is susceptible to express and present the transgene in SLO, contributing to peripheral CD8+ T cells anergy via the induction of CD4+ regulatory T cells and low effector CD4+ T cell responses.
Based on evidence of transgene delivery in MHC class II+ lymph node stromal cells, we postulate that the observed blunted SLO CD4+ T cell responses unable to sustain the full development of a peripheral effector CD8+ T cell response, was the result of direct antigenic presentation by gp38+ stromal cells to CD8 + and CD4+ T cells following transduction. Such primed CD8+ T cells were presumably anergized as a result of insufficient costimulation of CD4+ T cell help combined with high prevalence of induced regulatory T cells. However, we cannot directly prove this point due to technical limitations. While the transgene was clearly detectable in CD45− gp38+ MHC class II+ cells, and low vector copies were found in digested LN stroma (not shown), we were unable to sort sufficient amounts of MHC class II+ LN stromal cells to confirm transduction at the mRNA level or to test direct antigenic presentation by stromal cells to CD4+ T cells (data not shown). We cannot exclude that other cell types in LN or elsewhere, even in limited amounts, contributed to this effect by direct transduction or by passing the antigen to stromal cells. Intense cell–cell interactions occur in LN and for instance stromal follicular DC are known to interact with DC to transfer their antigens for presentation to CD8+ T cells.26 Murine lymphatic endothelial cells have low endogenous basal expression of MHC class II and also acquire peptide-MHC class II complexes from DCs via both cell–cell contact and DC-derived exosomes.10 One advantage of the mir142.3p-target regulation system is that it efficiently excludes transgene from DC (specifically the case in CD45+ MHC class II+ CD11c+ DC (not shown)), thus our system tends to support a direct role of stromal cells for MHC class II presentation. While LN present an ideal environment to exert immune tolerance, this process may also occur elsewhere following systemic vector delivery. For instance, the liver is a tolerogenic organ involving the induction of CD4+ regulatory T cells following gene transfer into hepatocytes.19,21,23,27,28,29,30 As no mechanism has been proposed for the antigen-specific induction of regulatory CD4+ T cells following gene transfer into liver, the possible contribution of transgene presentation by MHCII+ LN stroma following systemic gene delivery could perhaps be considered.
The notion of the existence of MHC class II+ stromal cells in SLO is recent. The role of these cells endogenously expressing, or alternatively acquiring MHC-II molecules from DC10 has been studied in vitro or in vivo through bone marrow transplant chimeras, or through LN or T cell transplant experiments which are not physiological. In these conditions, MHC class II+ LN stromal cells reportedly play a role to maintain FoxP3+ regulatory T cells in LN.11 Regulatory T cells are then able to induce anergy and peripheral deletion of autoreactive cells through cell–cell interaction or by secreting immunosuppressive cytokines. Lymphatic endothelial cells that present MHC class II-complexed peptides acquired from DCs notably following IFNγ activation, also promote T cell apoptosis to induce antigen-specific inhibition of CD4+ T cell survival and proliferation.10 In addition, SLO stromal cells are known to induce tolerance through clonal deletion of antigen-reactive CD8+ T cells.5,7,8 Thus, our data are coherent with these mechanisms and show that blunted effector CD4 T cell responses, induction of antigen-specific regulatory T cells and lack of effector CD8 T cell responses can occur in the steady-state, in the absence of DC-induced immune responses.
At the practical level, our vector has proved useful to detect stromal cell populations in vivo in steady-state adult mice and conditions can be certainly optimized. Other models to explore SLO stroma such as transgenic Cre-podoplaninxR26-YFP mice31 cannot be used in the same functional manner. Several SLO stromal cell populations capable of presenting peripheral tissue restricted antigen have been described, such as fibroblastic reticular cells, lymphatic endothelial cells, blood endothelial cells and double-negative stromal cells among which are extra-thymic Aire-expressing cells.2 The current experimental conditions lead to gene transfer in LN CD45− MHCII+ gp38+ stromal cells, phenotype of both lymphatic endothelial cells and fibroblastic reticular cells, but the exact nature of such cells was not determined. We failed to transduce thymic stromal cells and speculate that this could be due to limited vector amounts. Improvement in vector dose or route of delivery will be needed to explore the range of MHC classII+ stromal cells that can be targeted in various organs (spleen, thymus or organs like liver).
Possibly, clinically-relevant applications in transplantation or auto-immunity could be conceived by targeting a specific antigen to tolerogenic stromal cells to induce peripheral immune tolerance. Further studies are needed to optimize gene delivery in these protocols. In the context of gene therapy and immunotherapy, our results illustrate the complexity of immune responses capable of being induced by various types of APCs expressing a neoantigen, if these were transduced by the vector. In systemic gene transfer protocols, particularly those seeking tolerance, a specific attention should be paid to the possible contribution of MHC classII+LN stromal cells in the observed immune outcome.
Materials and Methods
Construction and plasmids. The GFP-HY gene cassette containing the coding sequences of the Dby peptide (NAGFNSNRANSSRSS) presented by I-Ab and Uty peptide (WMHHNMDLI) presented by H2-Db, has been described in17 . miR142.3p target sequences were obtained from pAAV CMV haSarco_HYmiR142.3pT plasmid24 and inserted in the transgene cassette after WPRE in XbaI restriction site. The targeting measles glycoproteins envelope plasmids pFΔ30 and pHmutΔ18-MHC-II were already described.15,16,32 All plasmids were produced and purified using the Nucleobond PC2000EF kit from Macherey-Nagel (Düren, Germany).
Lentiviral vector production and titration. Batches of LV-MHCII and LV-MHCII-miR were produced following transfection of 293 T cells with five plasmids using the two envelope plasmids already described for the measles pseudotype16: pCG-FΔ30 and pCG-HmutΔ18-ScFV-MHCII (16 µg of each plasmid per 15 cm dish); two plasmids for the accessory functions: pKrev (5.6 µg per 15 cm dish); pKLgagpol (14.6 µg per 15 cm dish); and the transfer plasmid (16 µg per 15 cm dish) which was pRRLsincPPT-PGK-GFP-HY-WPRE (for LV-MHCII) or pRRLsincPPT-PGK-GFP-HY-WPRE-miR142.3p (for LV-MHCII-miR). The next day medium was changed and after 24 hours, the supernatant fluid containing viral particles is collected, filtered and purified by centrifugation, as indicated in figures, and stored frozen at −80°C. Vector batches were titered for P24 and RT content by enzyme-linked immunosorbent assay (ELISA).
Mice. C57Bl/6 (CD45.2) and congenic CD45.1 (PtprcaPep3b/BoyJ (CD45.1)) mice were purchased from Charles River Laboratories (L'Arbresle, France). FoxP3-eGFP Marilyn mice were bred in our facilities, are Rag2−/−, transgenic for TCRValpha 1.1/Vbeta6 receptor specific of the H-Y male antigene presented by I-a b and an ires-EGFP cassette is inserted in the 3′UT region of FoxP3 gene, exon11.25 Mice were housed in our facilities under specific pathogen-free conditions and handled in accordance with French and European directives. Protocols were conducted according to national and international ethical standards and were approved by the local ethics committee (committee C2EA-64). Genetically-modified organisms were handled under appropriate biological containment as per agreement 5244-CAI from the French Ministery of Research and Higher Education.
Cells. Spleen cells for ELISPOT analysis were obtained by mechanic disruption of the organ followed by removal of red blood cells with ammonium chloride/potassium (ACK) lysing buffer.
Cells from the thymus, spleen, and lymph node were obtained following enzymatic digestion for flow cytometry analysis and cell sorting. Diced organs were first agitated in RPMI medium for 10 minutes at 4°C, then medium was replaced with a digesting solution containing Collagenase IV (1mg/ml, Invitrogen, Waltham, MA) and Dnase I (50 µg/ml, Roche, Life Sciences, Meylan, France) and incubated for 15 minutes at 37°C, 900×g. Cells in suspension were collected and the enzymatic reaction was stopped by adding 100 mmol/l Ethylenediaminetetraacetate (EDTA) on this fraction of cells. On the remaining tissue sample, fresh enzymes were added and the digestion procedure was repeated three times. Cell suspensions obtained from each digestion step were washed in PBS. Residual red blood cells present in spleen or thymus were removed with ACK lysing buffer when needed before analysis. Each collected fraction (digested or just agitated) was analyzed individually for GFP expression analysis by flow cytometry.
In vitro splenocyte transduction. Freshly isolated C57Bl/6 mice splenocytes (5 × 105 cells per well) prestimulated with IL-2, IL-4, IL-7 and with 50 ng RT of LV-MHCII or LV-MHCII-miR vectors. After 2 days of transduction, GFP-HY and MHC class II expression are assessed by multicolor flow cytometry.
Immunization with LV. To measure the immunization induced by LV, 6-week-old mice received 50 ng RT of LV or an equivalent volume of PBS IV and the mice were either killed at the indicated time points to measure cytokine responses using ELISPOT or cytokine bead arrays, or they were challenged in vivo by an intramuscular (IM) injection of a mixture of Dby-Uty peptides (100 µmol/l each) (synthesized by Genepep, Montpellier, France) (or PBS in control mice) with IFA (Sigma Aldrich, Saint-Louis, MO) followed or not by in vivo cytotoxic assay (see below) to measure the effect of LV immunization.
IFN-γ ELISPOT. IFN-γ responses were measured by ELISPOT as previously described.33 Results of spot forming units for each mouse were calculated as the average value of duplicate measures after subtraction of background values obtained with in vitro unstimulated splenocytes.
Cytometric bead array. Splenocytes were cultured in duplicate wells of round bottom plates (1 × 106 cells/well) in presence of Dby peptide (2 µmol/l) or Uty peptide (2 µmol/l) during 48 hours. Splenocyte secretion of IL-2, IL-4, IL-6, IL-10, IL-12p70, IL-17A, TNFα, IFN-γ, and GM-CSF was quantified by flow cytometry (LSRII) using the corresponding flex set of cytometric bead array kit (BD-Biosciences, Le Pont de Claix, France) using the manufacturer protocol and its FCAP software.
In-vivo cytotoxic assay. Splenocytes from congenic C57Bl/6 CD45.1 male and female mice were respectively labeled with 0.2 µmol/l or 2µmol/l of carboxyfluorescein succinimidyl ester dye (CFSE) in RPMI during 8 minutes in obscurity at room temperature followed by a 2-minutes incubation at 37°C. The reaction was stopped by adding cold RPMI 10% fetal bovine serum (FBS) and placing the cells at 4°C during 5 minutes. Cells were then washed in RPMI 10% FBS and incubated 20 minutes at 37°C to stabilize the marking. Before injection into recipient mice, CFSE-labeled splenocytes were washed two times, counted and resuspended at the desired concentration (5 × 106 CFSElow male cells + 5 × 106 CFSEhigh female cells per 100 µl). Cells were injected IV into congenic C57Bl/6 CD45.2 recipient mice and 42 hours following the injection, splenocytes from injected mice were obtained and analyzed by flow cytometry to assess the specific male target cell lysis level based on the comparison of remaining levels of adoptively-transferred CD45.1 CFSElow male cells with CD45.1 CFSEhigh female cells. The percentage (%) of male target cells lysis was calculated according to the following formula:
% of lysis = ((CD45.1 male cells mean % / CD45.1 female cells mean %)PBS CD45.1 male cells % / CD45.1 female cells %))LV) / CD45.1 male cells mean % / CD45.1 female cells mean %))PBS)*100
Flow cytometry. All reagents used for flow cytometry were purchased from BD-Biosciences. Cell suspensions were first incubated with anti-FcγRIII/II (2.4G2) monoclonal antibodies (mAb) for 15 minutes at 4°C and then stained for 30 minutes at 4°C in PBS with 0.1% bovine serum albumin (BSA) using saturating amounts of the following mAbs: biotinylated anti-CD11c, biotinylated anti-CD45.1, phycoerythrin (PE)-conjuguated anti-CD11b, allophycocyanin-conjuguated anti-MHC-II, PE-conjugated anti-gp38 (eBioscience, San Diego, CA) and PE-cyanin 7 conjuguated streptavidin (BD-Biosciences). Cells were analyzed on a LSRII cytometer using the Diva software (BD-Biosciences) or FlowJo software version 6.1.3 (FlowJo, Ashland, OR). The gating strategies are shown in figures and all analyses exclude dead cells based on 7-actinomycin D (Sigma Aldrich) staining.
Statistical analysis. Significant differences between mean values were determined using unpaired two-tailed Student's test or Mann-Whitney depending on normality using GraphPad Prism software version 7.6.5. P-value under 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. C57Bl/6 female mice were injected IV with 50 ng RT of LV-MHCII or of LV-MHCII-miR and 3 days later, LN were digested and analyzed by FACS to measure GFP-HY transgene and MHCII expression level in 3 distinct LN cellular subpopulations following transduction with LV-MHCII and LV-MHCII-miR vectors : B cells (CD3-CD19+), DC (CD11b-CD11c+) and lymph node stromal cells (CD45-) (pool of 3 mice per group per experiment). Results representative of 6 independent experiments. Figure S2. CD45- transduction efficiency avaluated in the LV-MHCII or LV-MHCII-miR- injected C57Bl/6 mice from the experiments shown in figure 3b. A representative gating strategy is shown on the left and the 3 independent experiments transduction mean and SD on the right. Figure S3. C57Bl/6 female mice were injected IV with 50 ng RT of LV-MHCII or of LV-MHCII-miR and 3 days later, LN, spleen and thymus were digested and analyzed by FACS to measure GFP-HY transgene expression in DC (CD11b-CD11c+) (pool of 3 mice per group per experiment). Results are representative of 6 independent experiments.
Acknowledgments
The authors are grateful to their colleagues at Genethon in the groups “Bioexperimentation”, “Animal Facility”, and “Flow cytometry” for help with experiments. The authors are also grateful to CJ Buchholz for criticial review of the manuscript. Financial support for this study was obtained from PERSIST (EC FP7 large-scale integrating project GA number 222878), from AFM/Telethon, from “Fondation pour la Recherche Médicale” and from the French Ministry of Research via Ecole Doctorale B2T, University Paris Diderot.
Supplementary Material
References
- Adiko, AC, Babdor, J, Gutiérrez-Martínez, E, Guermonprez, P and Saveanu, L (2015). Intracellular transport routes for MHC I and their relevance for antigen cross-presentation. Front Immunol 6: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, D, Fletcher, AL and Turley, SJ (2013). Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev 251: 160–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosue, S and Dubrot, J (2015). Modes of antigen presentation by lymph node stromal cells and their immunological implications. Front Immunol 6: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, G and Takahama, Y (2012). Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol 33: 256–263. [DOI] [PubMed] [Google Scholar]
- Cohen, JN, Guidi, CJ, Tewalt, EF, Qiao, H, Rouhani, SJ, Ruddell, A et al. (2010). Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med 207: 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, AL, Lukacs-Kornek, V, Reynoso, ED, Pinner, SE, Bellemare-Pelletier, A, Curry, MS et al. (2010). Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med 207: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, JW, Epardaud, M, Sun, J, Becker, JE, Cheng, AC, Yonekura, AR et al. (2007). Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol 8: 181–190. [DOI] [PubMed] [Google Scholar]
- Magnusson, FC, Liblau, RS, von Boehmer, H, Pittet, MJ, Lee, JW, Turley, SJ et al. (2008). Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterol 134: 1028–1037. [DOI] [PubMed] [Google Scholar]
- Malhotra, D, Fletcher, AL, Astarita, J, Lukacs-Kornek, V, Tayalia, P, Gonzalez, SF et al.; Immunological Genome Project Consortium. (2012). Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol 13: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrot, J, Duraes, FV, Potin, L, Capotosti, F, Brighouse, D, Suter, T et al. (2014). Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J Exp Med 211: 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista, AP, Roozendaal, R, Reijmers, RM, Koning, JJ, Unger, WW, Greuter, M et al. (2014). Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. Elife 3:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz, CJ, Friedel, T and Büning, H (2015). Surface-engineered viral vectors for selective and cell type-specific gene delivery. Trends Biotechnol 33: 777–790. [DOI] [PubMed] [Google Scholar]
- Goyvaerts, C, Kurt, de G, Van Lint, S, Heirman, C, Van Ginderachter, JA, De Baetselier, P et al. (2014). Immunogenicity of targeted lentivectors. Oncotarget 5: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch, RC, Mühlebach, MD, Schaser, T, Kneissl, S, Jost, C, Plückthun, A et al. (2011). DARPins: an efficient targeting domain for lentiviral vectors. Mol Ther 19: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari, F, Lopes, L, Verhoeyen, E, Marasco, W and Collins, MK (2009). Single-chain antibodies that target lentiviral vectors to MHC class II on antigen-presenting cells. Hum Gene Ther 20: 554–562. [DOI] [PubMed] [Google Scholar]
- Ageichik, A, Buchholz, CJ and Collins, MK (2011). Lentiviral vectors targeted to MHC II are effective in immunization. Hum Gene Ther 22: 1249–1254. [DOI] [PubMed] [Google Scholar]
- Ciré, S, Da Rocha, S, Yao, R, Fisson, S, Buchholz, CJ, Collins, MK et al. (2014). Immunization of mice with lentiviral vectors targeted to MHC class II+ cells is due to preferential transduction of dendritic cells in vivo. PLoS One 9: e101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, BD and Naldini, L (2009). Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet 10: 578–585. [DOI] [PubMed] [Google Scholar]
- Brown, BD, Venneri, MA, Zingale, A, Sergi Sergi, L and Naldini, L (2006). Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med 12: 585–591. [DOI] [PubMed] [Google Scholar]
- Ferrand, M, Galy, A and Boisgerault, F (2014). A dystrophic muscle broadens the contribution and activation of immune cells reacting to rAAV gene transfer. Gene Ther 21: 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni, A, Brown, BD, Cantore, A, Sergi, LS, Naldini, L and Roncarolo, MG (2009). In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood 114: 5152–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, BD, Cantore, A, Annoni, A, Sergi, LS, Lombardo, A, Della Valle, P et al. (2007). A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood 110: 4144–4152. [DOI] [PubMed] [Google Scholar]
- Matsui, H, Hegadorn, C, Ozelo, M, Burnett, E, Tuttle, A, Labelle, A et al. (2011). A microRNA-regulated and GP64-pseudotyped lentiviral vector mediates stable expression of FVIII in a murine model of Hemophilia A. Mol Ther 19: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgerault, F, Gross, DA, Ferrand, M, Poupiot, J, Darocha, S, Richard, I et al. (2013). Prolonged gene expression in muscle is achieved without active immune tolerance using microrRNA 142.3p-regulated rAAV gene transfer. Hum Gene Ther 24: 393–405. [DOI] [PubMed] [Google Scholar]
- Carpentier, M, Chappert, P, Kuhn, C, Lalfer, M, Flament, H, Burlen-Defranoux, O et al. (2013). Extrathymic induction of Foxp3+ regulatory T cells declines with age in a T-cell intrinsic manner. Eur J Immunol 43: 2598–2604. [DOI] [PubMed] [Google Scholar]
- McCloskey, ML, Curotto de Lafaille, MA, Carroll, MC and Erlebacher, A (2011). Acquisition and presentation of follicular dendritic cell-bound antigen by lymph node-resident dendritic cells. J Exp Med 208: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata, A, Mimuro, J, Mizukami, H, Kashiwakura, Y, Takano, K, Ohmori, T et al. (2009). Liver-restricted expression of the canine factor VIII gene facilitates prevention of inhibitor formation in factor VIII-deficient mice. J Gene Med 11: 1020–1029. [DOI] [PubMed] [Google Scholar]
- Cooper, M, Nayak, S, Hoffman, BE, Terhorst, C, Cao, O and Herzog, RW (2009). Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther 20: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátrai, J, Cantore, A, Bartholomae, CC, Annoni, A, Wang, W, Acosta-Sanchez, A et al. (2011). Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology 53: 1696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi, A, Battaglia, M, Lombardo, A, Annoni, A, Roncarolo, MG and Naldini, L (2004). Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood 103: 3700–3709. [DOI] [PubMed] [Google Scholar]
- Onder, L, Scandella, E, Chai, Q, Firner, S, Mayer, CT, Sparwasser, T et al. (2011). A novel bacterial artificial chromosome-transgenic podoplanin-cre mouse targets lymphoid organ stromal cells in vivo. Front Immunol 2: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke, S, Maisner, A, Mühlebach, MD, Koehl, U, Grez, M, Cattaneo, R et al. (2008). Targeted cell entry of lentiviral vectors. Mol Ther 16: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudres, M, Ciré, S, Vasseur, V, Brault, L, Da Rocha, S, Boisgérault, F et al. (2012). MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol Ther 20: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.