Abstract
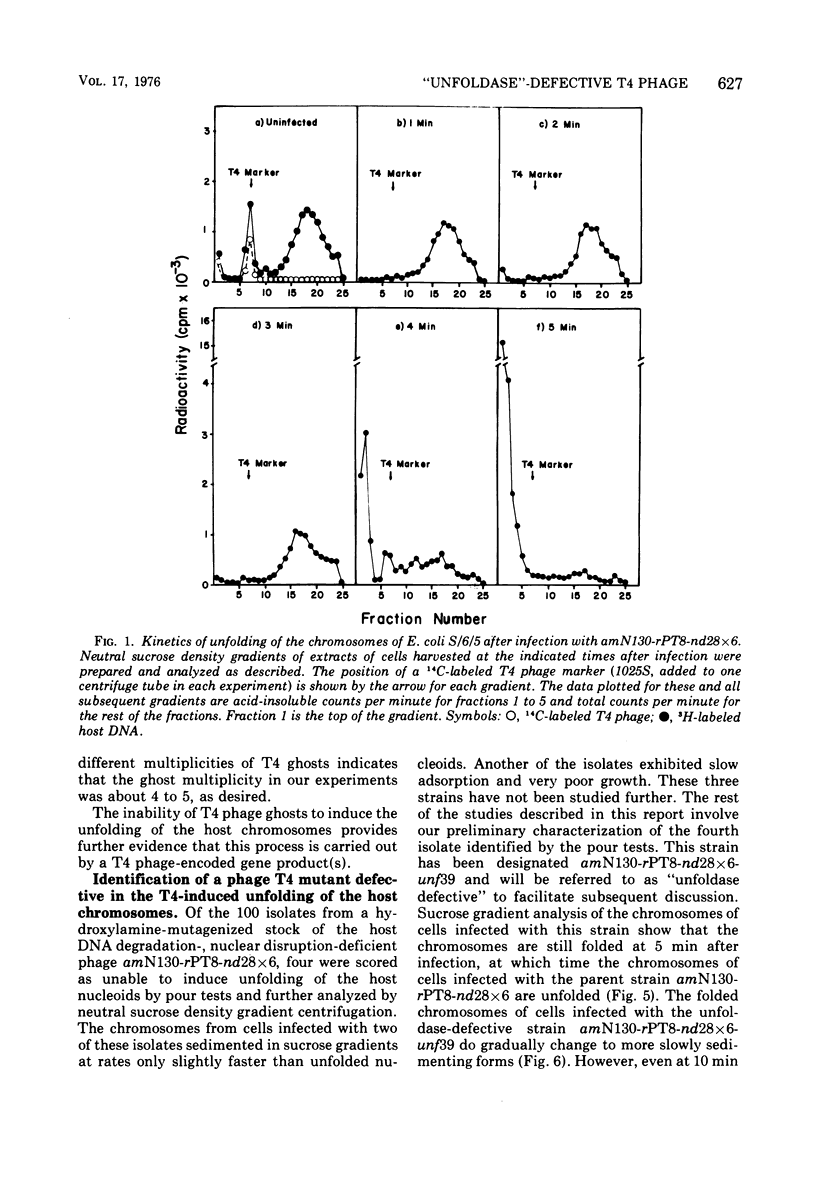
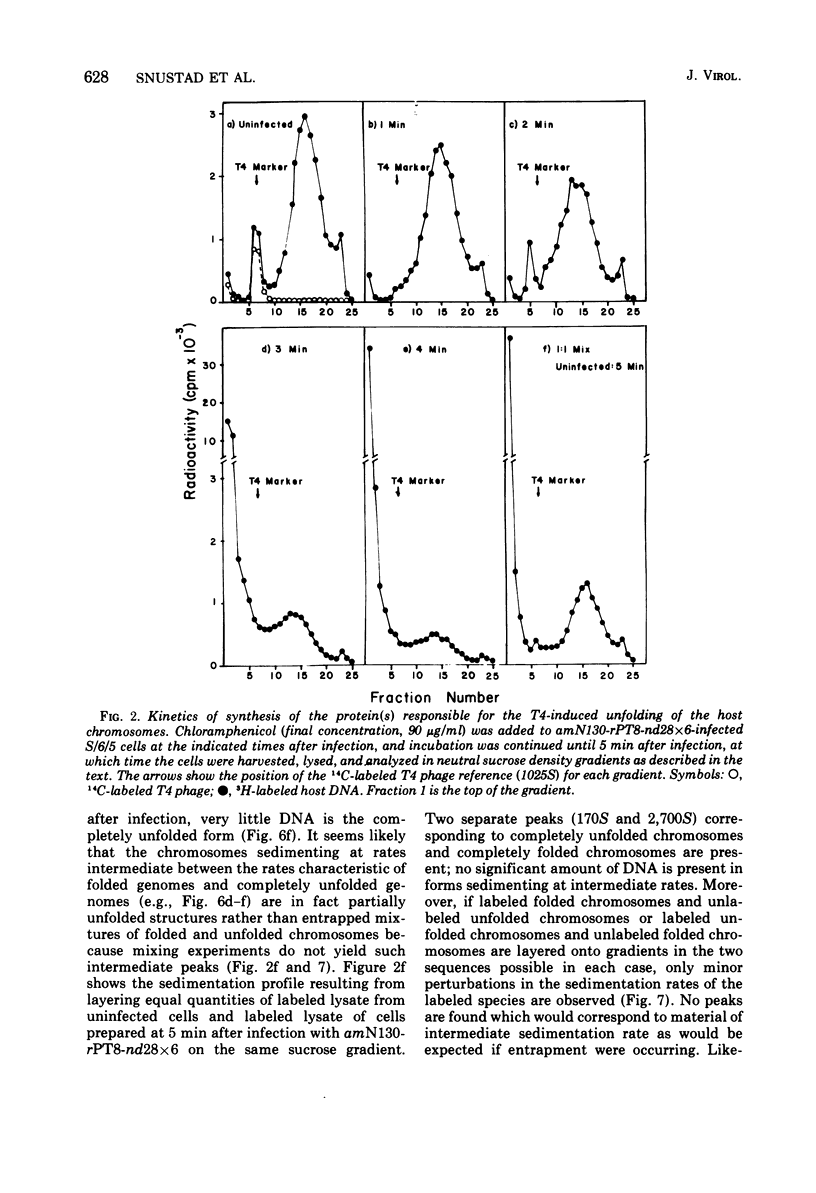
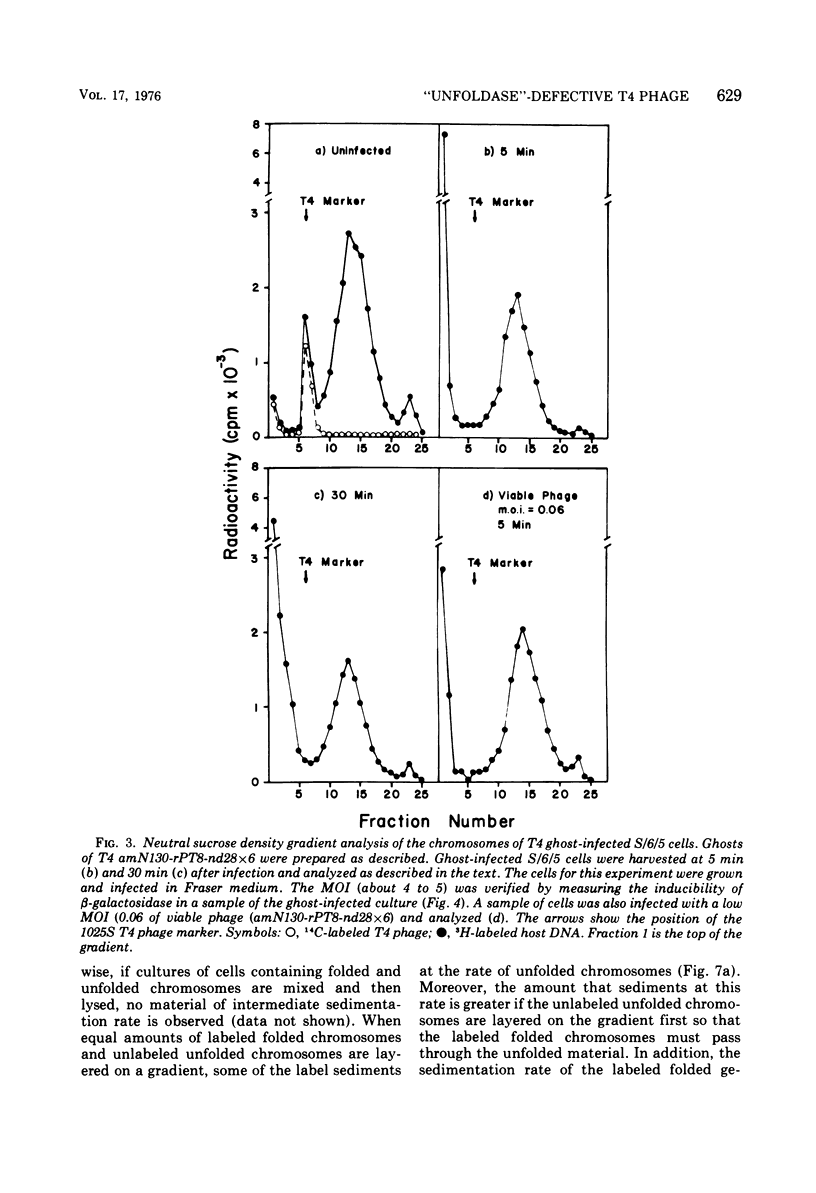
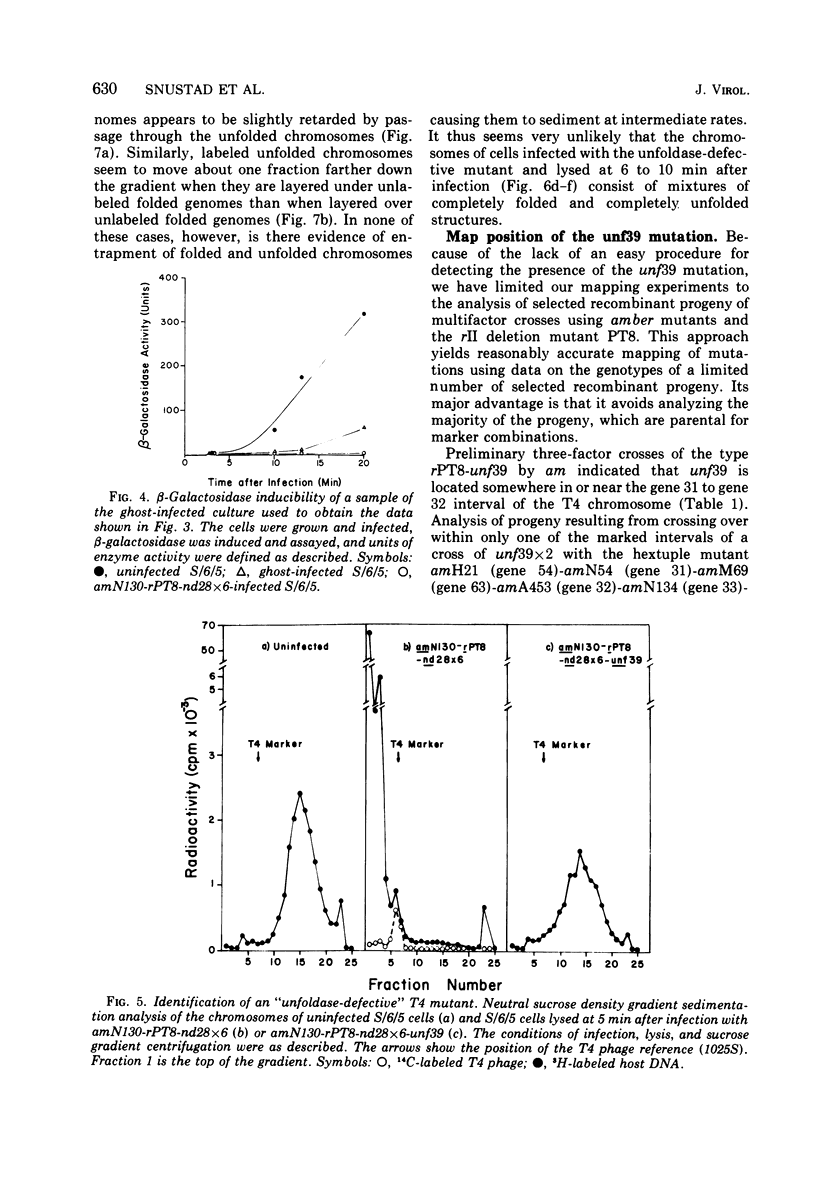
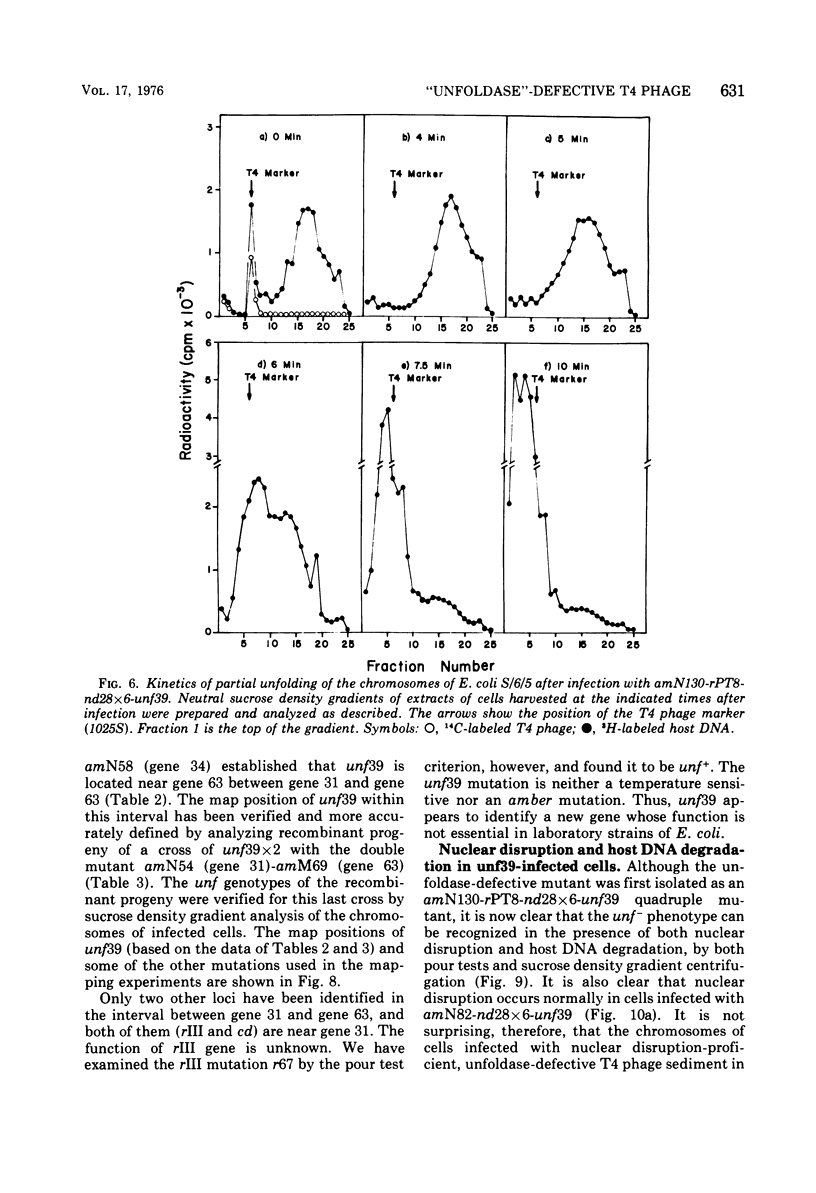
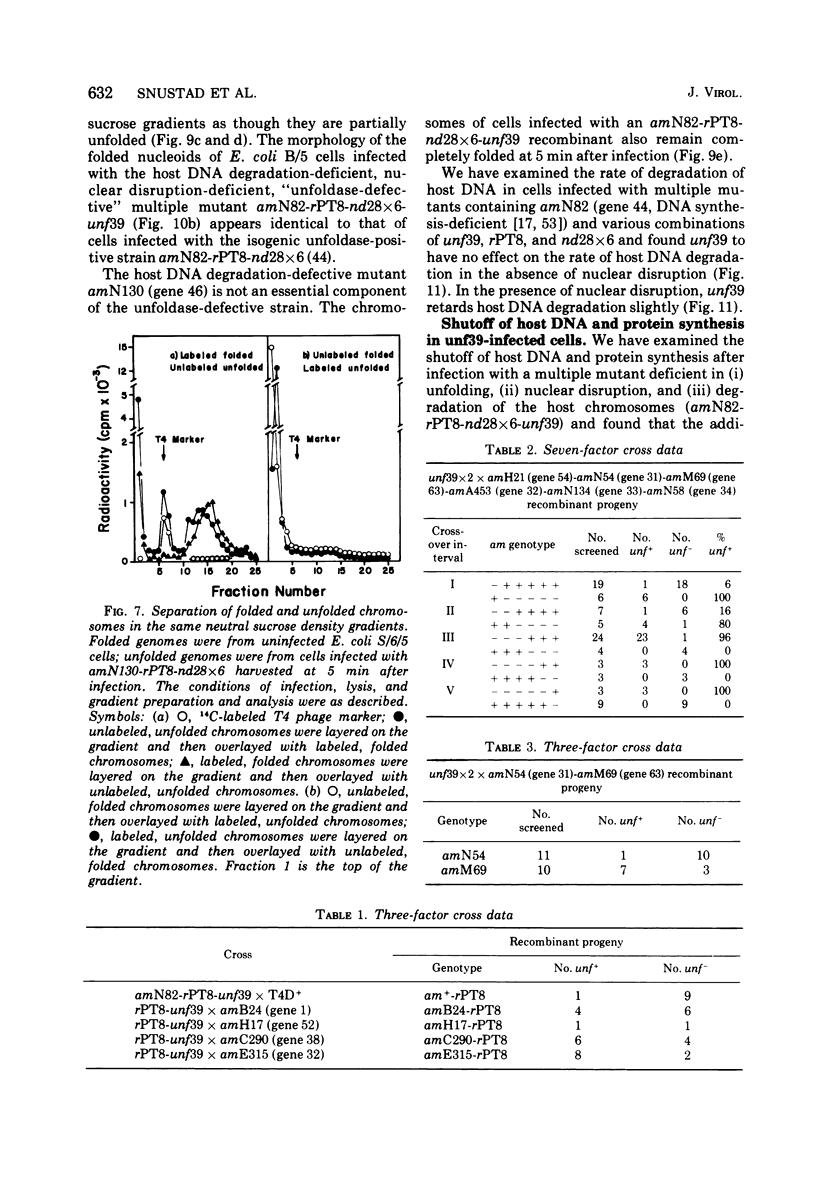
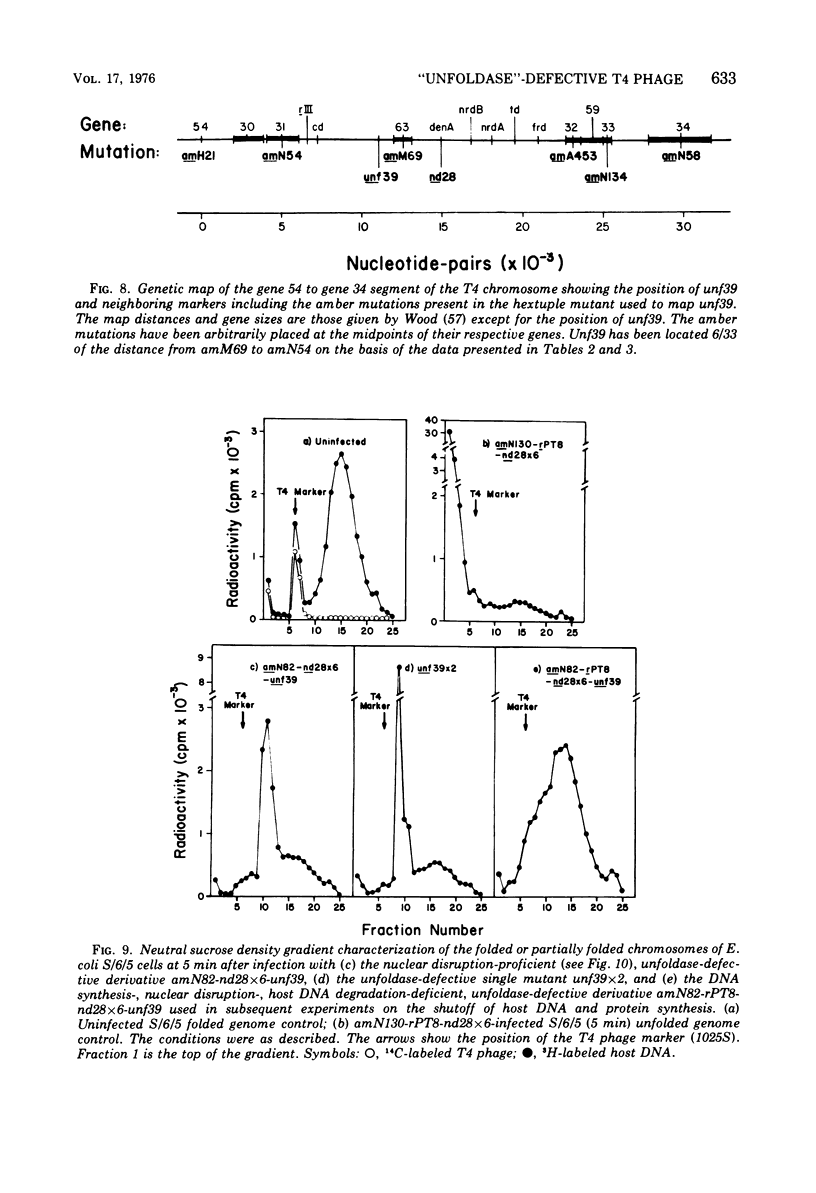
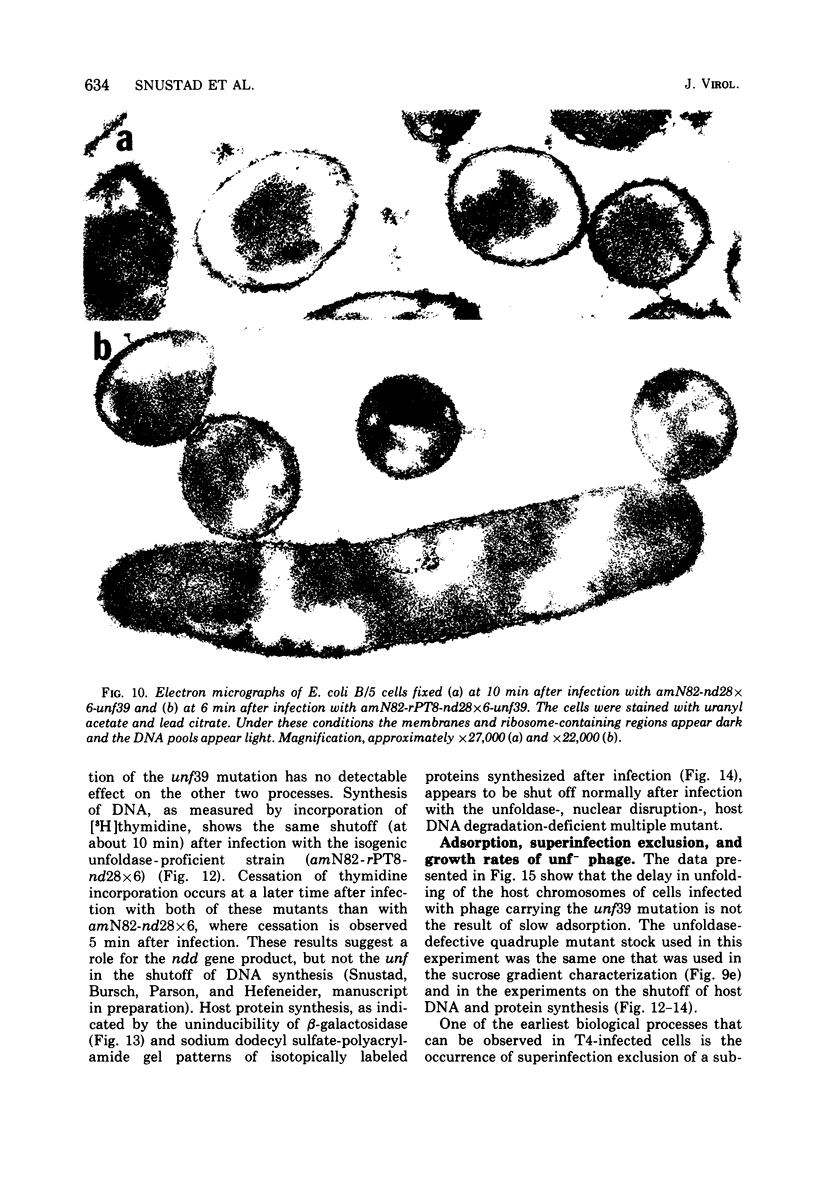
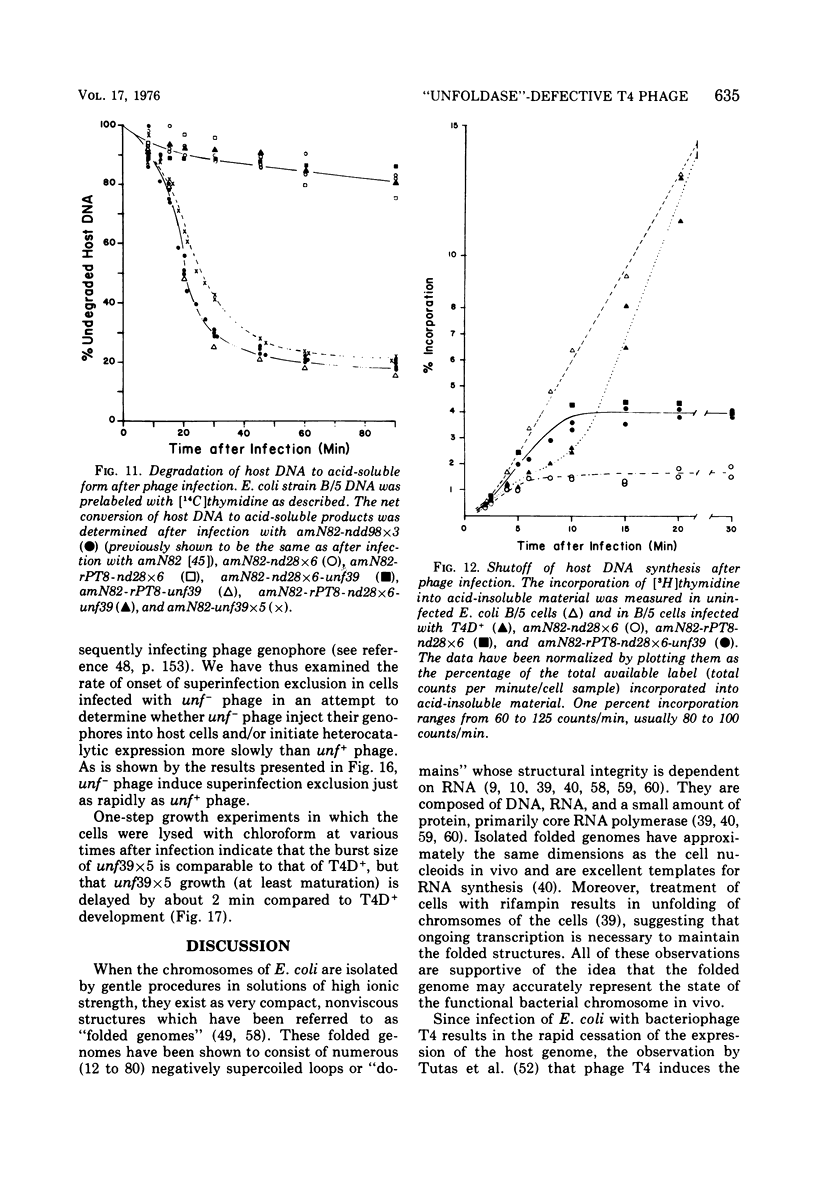
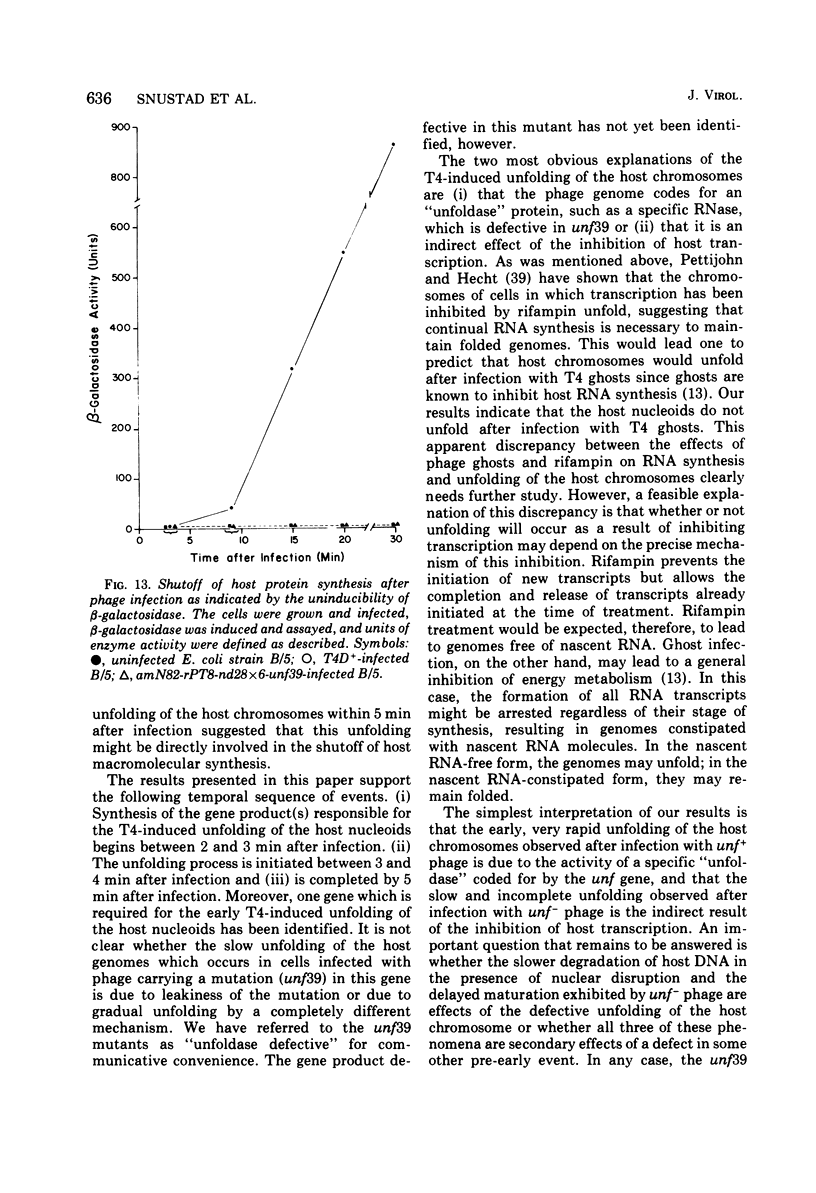
The nucleoids of Escherichia coli S/6/5 cells are rapidly unfolded at about 3 min after infection with wild-type T4 bacteriophage or with nuclear disruption deficient, host DNA degradation-deficient multiple mutants of phage T4. Unfolding does not occur after infection with T4 phage ghosts. Experiments using chloramphenicol to inhibit protein synthesis indicate that the T4-induced unfolding of the E. coli chromosomes is dependent on the presence of one or more protein synthesized between 2 and 3 min after infection. A mutant of phage T4 has been isolated which fails to induce this early unfolding of the host nucleoids. This mutant has been termed "unfoldase deficient" (unf-) despite the fact that the function of the gene product defective in this strain is not yet known. Mapping experiments indicate that the unf- mutation is located near gene 63 between genes 31 and 63. The folded genomes of E. coli S/6/5 cells remain essentially intact (2,000-3,000S) at 5 min after infection with unfoldase-, nuclear disruption-, and host DNA degradation-deficient T4 phage. Nuclear disruption occurs normally after infection with unfoldase- and host DNA degradation-deficient but nuclear disruption-proficient (ndd+), T4 phage. The host chromosomes remain partially folded (1,200-1,800S) at 5 min after infection with the unfoldase single mutant unf39 x 5 or an unfoldase- and host DNA degradation-deficient, but nuclear disruption-proficient, T4 strain. The presence of the unfoldase mutation causes a slight delay in host DNA degradation in the presence of nuclear disruption but has no effect on the rate of host DNA degradation in the absence of nuclear disruption. Its presence in nuclear disruption- and host DNA degradation-deficient multiple mutants does not alter the shutoff to host DNA or protein synthesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- BONIFAS V., KELLENBERGER E. Etude de l'action des membranes du bactériophage T2 sur Escherichia coli. Biochim Biophys Acta. 1955 Mar;16(3):330–338. doi: 10.1016/0006-3002(55)90234-1. [DOI] [PubMed] [Google Scholar]
- Bautz F. A., Bautz E. K. Mapping of deletions in a non-essential region of the phage T4 genome. J Mol Biol. 1967 Sep 14;28(2):345–355. doi: 10.1016/s0022-2836(67)80014-7. [DOI] [PubMed] [Google Scholar]
- Berget P. B., Warner H. R. Identification of P48 and P54 as components of bacteriophage T4 baseplates. J Virol. 1975 Dec;16(6):1669–1677. doi: 10.1128/jvi.16.6.1669-1677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruner R., Souther A., Suggs S. Stability of cytosine-containing deoxyribonucleic acid after infection by certain T4 rII-D deletion mutants. J Virol. 1972 Jul;10(1):88–92. doi: 10.1128/jvi.10.1.88-92.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgi A. W., Robinton J., Carlson C. L. Studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:43–51. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- Delius H., Worcel A. Electron microscopic studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:53–58. doi: 10.1101/sqb.1974.038.01.008. [DOI] [PubMed] [Google Scholar]
- Delius H., Worcel A. Letter: Electron microscopic visualization of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):107–109. doi: 10.1016/0022-2836(74)90577-4. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Snopek T. J., Cozzarelli N. R. Characterization of a new class of deletions of the D region of the bacteriophage T4 genome. Virology. 1975 Mar;64(1):144–145. doi: 10.1016/0042-6822(75)90086-0. [DOI] [PubMed] [Google Scholar]
- Dove W. The extent of rII deletions in phage T4. Genet Res. 1968 Apr;11(2):215–219. doi: 10.1017/s001667230001140x. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. Assay for the Killing Properties of T2 Bacteriophage and Their "Ghosts". J Bacteriol. 1965 Sep;90(3):724–728. doi: 10.1128/jb.90.3.724-728.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H. Biological activity of bacteriophage ghosts and "take-over" of host functions by bacteriophage. Bacteriol Rev. 1970 Sep;34(3):344–363. doi: 10.1128/br.34.3.344-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Fabricant R., Kennell D. Inhibition of host protein synthesis during infection of Escherichi coli by bacteriophage T4. 3. Inhibition by ghosts. J Virol. 1970 Dec;6(6):772–781. doi: 10.1128/jvi.6.6.772-781.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coliphage T2. I. Preparation, assay, and some chemical properties. J Gen Physiol. 1957 May 20;40(5):809–825. doi: 10.1085/jgp.40.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., DIXON J., CHASE M. Nucleic acid economy in bacteria infected with bacteriophage T2. I. Purine and pyrimidine composition. J Gen Physiol. 1953 Jul;36(6):777–789. doi: 10.1085/jgp.36.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants in a nonessential gene of bacteriophage T4 which are defective in the degradation of Escherichia coli deoxyribonucleic acid. J Virol. 1971 Jan;7(1):95–105. doi: 10.1128/jvi.7.1.95-105.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- KOCH A. L., PUTNAM F. W., EVANS E. A., Jr Biochemical studies of virus reproduction. VIII. Purine metabolism. J Biol Chem. 1952 May;197(1):113–120. [PubMed] [Google Scholar]
- KOZLOFF L. M. Origin and fate of bacteriophage material. Cold Spring Harb Symp Quant Biol. 1953;18:209–220. doi: 10.1101/sqb.1953.018.01.032. [DOI] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. II. Induction of host messenger ribonucleic acid and its exclusion from polysomes. J Virol. 1970 Aug;6(2):208–217. doi: 10.1128/jvi.6.2.208-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner J. F. Enzymes of nucleic acid metabolism. Annu Rev Biochem. 1970;39:291–322. doi: 10.1146/annurev.bi.39.070170.001451. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. Chromatin staining of bacteria during bacteriophage infection. J Bacteriol. 1950 Apr;59(4):551–560. doi: 10.1128/jb.59.4.551-560.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G. E., GILLEN D. H., HEAGY F. C. Cytological changes in Escherichia coli produced by infection with phage T2. J Bacteriol. 1950 May;59(5):603–615. doi: 10.1128/jb.59.5.603-615.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Parson K. A., Snustad D. P. Host DNA degradation after infection of Escherichia coli with bacteriophage T4: dependence of the alternate pathway of degradation which occurs in the absence of both T4 endonuclease II and nuclear disruption on T4 endonuclease IV. J Virol. 1975 Jan;15(1):221–224. doi: 10.1128/jvi.15.1.221-224.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. D., Vetter D. Control of T4 endonuclease IV by the D2a region of bacteriophage T4. Virology. 1973 Aug;54(2):544–546. doi: 10.1016/0042-6822(73)90165-7. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Warner H. R., Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants of bacteriophage T4 defective in the induction of T4 endonuclease II. J Biol Chem. 1971 May 25;246(10):3431–3433. [PubMed] [Google Scholar]
- Snustad D. P., Conroy L. M. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption. I. Isolation and genetic characterization. J Mol Biol. 1974 Nov 15;89(4):663–673. doi: 10.1016/0022-2836(74)90043-6. [DOI] [PubMed] [Google Scholar]
- Snustad D. P. Differential transmission of amber and wild-type alleles of bacteriophage T4 in mixedly infected cells of Escherichia coli. Virology. 1970 May;41(1):52–65. doi: 10.1016/0042-6822(70)90053-x. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Parson K. A., Warner H. R., Tutas D. J., Wehner J. M., Koerner J. F. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption. II. Physiological state of the host nucleoid in infected cells. J Mol Biol. 1974 Nov 15;89(4):675–687. doi: 10.1016/0022-2836(74)90044-8. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Warner H. R., Parson K. A., Anderson D. L. Nuclear disruption after infection of Escherichia coli with a bacteriophage T4 mutant unable to induce endonuclease II. J Virol. 1972 Jul;10(1):124–133. doi: 10.1128/jvi.10.1.124-133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Tessman I. Mutagenic treatment of double- and single-stranded DNA phages T4 ans S13 with hydroxylamine. Virology. 1968 Jun;35(2):330–333. doi: 10.1016/0042-6822(68)90275-4. [DOI] [PubMed] [Google Scholar]
- Tutas D. J., Wehner J. M., Koerner J. F. Unfolding of the host genome after infection of Escherichia coli with bacteriophage T4. J Virol. 1974 Feb;13(2):548–550. doi: 10.1128/jvi.13.2.548-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEED L. L., COHEN S. S. The utilization of host pyrimidines in the synthesis of bacterial viruses. J Biol Chem. 1951 Oct;192(2):693–700. [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]