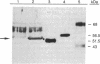
Abstract
We introduced into the CHO cell line the cDNA of the mouse cytochrome P3450 (P450IA2) gene, which oxidizes aromatic amines. A cDNA clone of P3450 was transfected into mutant UV5 cells, which is defective in nucleotide excision repair. Expression of the P3450 cDNA was measured using 9000 x g supernatant (S9) fractions from CHO cells to evaluate Salmonella TA1538 mutagenicity with the mutagen 2-amino-3-methylimidazo[4,5-f]quinoline (IQ). The P3450-expressing clone UV5P3 was reverted to repair proficiency using ethyl methanesulfonate to obtain the UV-resistant clone 5P3R2, which maintained the same level of P3450 protein activity as UV5P3. These genetically similar cell lines were compared for toxicity and mutation induction at the aprt locus. With 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (the most prevalent mutagen found in fried beef) the differential sensitivity due to repair deficiency/proficiency was approximately 40-fold, and with IQ there were smaller, but significant, differences in sensitivity. These genotoxic effects occurred at doses that were approximately 10 times lower than those that previously gave similar effects in S9-mediated assays. Thus, these cell lines should be valuable for genotoxicity analysis as well as important for assessing DNA repair when evaluating compounds that undergo metabolic activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Gelboin H. V., Gonzalez F. J. Mutagenic activation of 2-amino-3-methylimidazo[4,5-f]quinoline by complementary DNA-expressed human liver P-450. Cancer Res. 1990 Apr 1;50(7):2060–2063. [PubMed] [Google Scholar]
- Aoyama T., Gonzalez F. J., Gelboin H. V. Human cDNA-expressed cytochrome P450 IA2: mutagen activation and substrate specificity. Mol Carcinog. 1989;2(4):192–198. doi: 10.1002/mc.2940020405. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Gonzalez F. J., Gelboin H. V. Mutagen activation by cDNA-expressed P(1)450, P(3)450, and P450a. Mol Carcinog. 1989;1(4):253–259. doi: 10.1002/mc.2940010408. [DOI] [PubMed] [Google Scholar]
- Battula N., Sagara J., Gelboin H. V. Expression of P1-450 and P3-450 DNA coding sequences as enzymatically active cytochromes P-450 in mammalian cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4073–4077. doi: 10.1073/pnas.84.12.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookman K. W., Salazar E. P., Thompson L. H. Comparative mutagenic efficiencies of the DNA adducts from the cooked-food-related mutagens Trp-P-2 and IQ in CHO cells. Mutat Res. 1985 Apr;149(2):249–255. doi: 10.1016/0027-5107(85)90031-4. [DOI] [PubMed] [Google Scholar]
- Butler M. A., Iwasaki M., Guengerich F. P., Kadlubar F. F. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Crespi C. L., Langenbach R., Rudo K., Chen Y. T., Davies R. L. Transfection of a human cytochrome P-450 gene into the human lymphoblastoid cell line, AHH-1, and use of the recombinant cell line in gene mutation assays. Carcinogenesis. 1989 Feb;10(2):295–301. doi: 10.1093/carcin/10.2.295. [DOI] [PubMed] [Google Scholar]
- Crespi C. L., Steimel D. T., Aoyama T., Gelboin H. V., Gonzalez F. J. Stable expression of human cytochrome P450IA2 cDNA in a human lymphoblastoid cell line: role of the enzyme in the metabolic activation of aflatoxin B1. Mol Carcinog. 1990;3(1):5–8. doi: 10.1002/mc.2940030104. [DOI] [PubMed] [Google Scholar]
- Davies R. L., Crespi C. L., Rudo K., Turner T. R., Langenbach R. Development of a human cell line by selection and drug-metabolizing gene transfection with increased capacity to activate promutagens. Carcinogenesis. 1989 May;10(5):885–891. doi: 10.1093/carcin/10.5.885. [DOI] [PubMed] [Google Scholar]
- Doehmer J., Dogra S., Friedberg T., Monier S., Adesnik M., Glatt H., Oesch F. Stable expression of rat cytochrome P-450IIB1 cDNA in Chinese hamster cells (V79) and metabolic activation of aflatoxin B1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5769–5773. doi: 10.1073/pnas.85.16.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S., Doehmer J., Glatt H., Mölders H., Siegert P., Friedberg T., Seidel A., Oesch F. Stable expression of rat cytochrome P-450IA1 cDNA in V79 Chinese hamster cells and their use in mutagenicity testing. Mol Pharmacol. 1990 May;37(5):608–613. [PubMed] [Google Scholar]
- Esumi H., Ohgaki H., Kohzen E., Takayama S., Sugimura T. Induction of lymphoma in CDF1 mice by the food mutagen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn J Cancer Res. 1989 Dec;80(12):1176–1178. doi: 10.1111/j.1349-7006.1989.tb01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton J. S., Knize M. G., Shen N. H., Andresen B. D., Bjeldanes L. F., Hatch F. T. Identification of the mutagens in cooked beef. Environ Health Perspect. 1986 Aug;67:17–24. doi: 10.1289/ehp.866717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton J. S., Knize M. G., Shen N. H., Lewis P. R., Andresen B. D., Happe J., Hatch F. T. The isolation and identification of a new mutagen from fried ground beef: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis. 1986 Jul;7(7):1081–1086. doi: 10.1093/carcin/7.7.1081. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Mackenzie P. I., Kimura S., Nebert D. W. Isolation and characterization of full-length mouse cDNA and genomic clones of 3-methylcholanthrene-inducible cytochrome P1-450 and P3-450. Gene. 1984 Sep;29(3):281–292. doi: 10.1016/0378-1119(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Hoy C. A., Salazar E. P., Thompson L. H. Rapid detection of DNA-damaging agents using repair-deficient CHO cells. Mutat Res. 1984 Oct;130(5):321–332. doi: 10.1016/0165-1161(84)90018-9. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Casciano D. A., Couch D. B., Krahn D. F., O'Neill J. P., Whitfield B. L. The use of Chinese hamster ovary cells to quantify specific locus mutation and to determine mutagenicity of chemicals. A report of the gene-tox program. Mutat Res. 1981 Mar;86(2):193–214. doi: 10.1016/0165-1110(81)90024-5. [DOI] [PubMed] [Google Scholar]
- Kato T., Migita H., Ohgaki H., Sato S., Takayama S., Sugimura T. Induction of tumors in the Zymbal gland, oral cavity, colon, skin and mammary gland of F344 rats by a mutagenic compound, 2-amino-3,4-dimethylimidazo[4,5-f]quinoline. Carcinogenesis. 1989 Mar;10(3):601–603. doi: 10.1093/carcin/10.3.601. [DOI] [PubMed] [Google Scholar]
- Kato T., Ohgaki H., Hasegawa H., Sato S., Takayama S., Sugimura T. Carcinogenicity in rats of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. Carcinogenesis. 1988 Jan;9(1):71–73. doi: 10.1093/carcin/9.1.71. [DOI] [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. Mouse cytochrome P3-450: complete cDNA and amino acid sequence. Nucleic Acids Res. 1984 Mar 26;12(6):2917–2928. doi: 10.1093/nar/12.6.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn D. F., Heidelberger C. Liver homogenate-mediated mutagenesis in chinese hamster V79 cells by polycyclic aromatic hydrocarbons and aflatoxins. Mutat Res. 1977 Feb 1;46(1):27–44. doi: 10.1016/0165-1161(77)90108-x. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- McManus M. E., Burgess W., Snyderwine E., Stupans I. Specificity of rabbit cytochrome P-450 isozymes involved in the metabolic activation of the food derived mutagen 2-amino-3-methylimidazo[4,5-f] quinoline. Cancer Res. 1988 Aug 15;48(16):4513–4519. [PubMed] [Google Scholar]
- McManus M. E., Felton J. S., Knize M. G., Burgess W. M., Roberts-Thomson S., Pond S. M., Stupans I., Veronese M. E. Activation of the food-derived mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by rabbit and human liver microsomes and purified forms of cytochrome P-450. Carcinogenesis. 1989 Feb;10(2):357–363. doi: 10.1093/carcin/10.2.357. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B., Levin W. The P450 gene superfamily: recommended nomenclature. DNA. 1987 Feb;6(1):1–11. doi: 10.1089/dna.1987.6.1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Ohgaki H., Kusama K., Matsukura N., Morino K., Hasegawa H., Sato S., Takayama S., Sugimura T. Carcinogenicity in mice of a mutagenic compound, 2-amino-3-methylimidazo[4,5-f]quinoline, from broiled sardine, cooked beef and beef extract. Carcinogenesis. 1984 Jul;5(7):921–924. doi: 10.1093/carcin/5.7.921. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderwine E. G., Battula N. Selective mutagenic activation by cytochrome P3-450 of carcinogenic arylamines found in foods. J Natl Cancer Inst. 1989 Feb 1;81(3):223–227. doi: 10.1093/jnci/81.3.223. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Mooney C. L., Carrano A. V. Hypersensitivity to mutation and sister-chromatid-exchange induction in CHO cell mutants defective in incising DNA containing UV lesions. Somatic Cell Genet. 1982 Nov;8(6):759–773. doi: 10.1007/BF01543017. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Mooney C. L. Repair of DNA adducts in asynchronous CHO cells and the role of repair in cell killing and mutation induction in synchronous cells treated with 7-bromomethylbenz[a]anthracene. Somat Cell Mol Genet. 1984 Mar;10(2):183–194. doi: 10.1007/BF01534907. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Carrano A. V., Salazar E., Felton J. S., Hatch F. T. Comparative genotoxic effects of the cooked-food-related mutagens Trp-P-2 and IQ in bacteria and cultured mammalian cells. Mutat Res. 1983 May-Jun;117(3-4):243–257. doi: 10.1016/0165-1218(83)90125-8. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Rubin J. S., Cleaver J. E., Whitmore G. F., Brookman K. A screening method for isolating DNA repair-deficient mutants of CHO cells. Somatic Cell Genet. 1980 May;6(3):391–405. doi: 10.1007/BF01542791. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Tucker J. D., Stewart S. A., Christensen M. L., Salazar E. P., Carrano A. V., Felton J. S. Genotoxicity of compounds from cooked beef in repair-deficient CHO cells versus Salmonella mutagenicity. Mutagenesis. 1987 Nov;2(6):483–487. doi: 10.1093/mutage/2.6.483. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]