Abstract
Electrophysiological responses, accuracy and reaction time were recorded while 7-11-year-olds with typical development (TYP; N=30) and autism spectrum disorder (ASD; N=19) inhibited conflicting information. Relative to the TYP group, children with ASD had larger decrements in accuracy for incongruent trials and were slower. In terms of neural responses, N2 mean amplitude was greater overall for children with ASD relative to TYP children. N2 neural responses related to a behavioral measure of inhibition and cognitive flexibility for TYP children, whereas it related to suppression of interfering information and maintenance of accurate responding for the children with ASD. Results suggest children with ASD recruit more neural resources and perform worse when inhibiting conflicting information relative to TYP peers.
Keywords: autism, N2, event related potential, executive function, executive control, inhibition
Executive function (EF) – the ability to manage complex or conflicting information in the service of a goal – is particularly important to development because EF is related to better academic performance, social skills, lower rates of aggression and disruptive behavior, and better outcomes in adulthood (see Diamond, 2013 for review). By mid-childhood, EF is comprised of inhibition, set-shifting and working memory sub domains (Lehto et al., 2003). Though EF impairment is not a core symptom of the disorder, EF deficits are frequently observed in children with autism spectrum disorder (ASD; Hill, 2003; Kenworthy et al, 2008; Pennington & Ozonoff, 1996) – a common neurodevelopmental disorder that is characterized by reduced social communication skills and the presence of restricted and repetitive behaviors. In particular, set shifting is extremely disrupted in ASD and inhibitory impairments are also present relative to comparison groups (Willcutt, 2008). Inhibition may be further divided into interference control (i.e., suppression of distracting stimuli) and response inhibition (i.e., suppression of dominant responses) (Nigg, 2000). Children with ASD have more difficulty with interference control and less pronounced difficulties with response inhibition relative to comparison groups (Geurts, Verté, Oosterlaan, Roeyers, & Sergeant, 2004; Willcutt et al., 2008).
In the present study, we employed a common measure of interference control, the flanker task (Eriksen & Eriksen, 1974), that involves a central target stimulus flanked by stimuli of the same (congruent) or opposite (incongruent) orientation to examine the neural and behavioral responses of children with ASD and typical development. We used event related potentials (ERP) to examine neural responses that precede behavioral responses on the task. ERP offers a unique perspective because it measures aspects of EF that are difficult to capture behaviorally such as the neural activity underlying correct response inhibition and self-monitoring. We examined three ERP components – the P100, N2, and P3 – during the flanker portion of the developmentally appropriate Child Attention Network (ANT) task (Rueda, Posner, Rothbart, & Davis-Stober, 2004). Each component represents a different aspect of attention and EF skills.
The P100 is an exogenous sensory component that is evoked through passive viewing of sensory information and is known to be modulated by spatial visual attention (Hillyard et al., 1998; Luck et al., 2011 for review). Directed attention to the location of the target generates greater P100 amplitudes. Enhanced P100 amplitudes correlate with better reaction times and target detection across tasks (Hillyard et al., 1998; Eimer et al., 2003). We examined the P100 to determine whether low level attention and sensory processing were similar between the groups.
The N2 component in the Child ANT task is thought to reflect executive attention and monitoring conflicting information between the target and flankers. N2 amplitudes decrease with development and maturation of the network of neural structures (e.g., the anterior cingulate) underlying it (Buss et al., 2011; Espinet et al., 2012; Giedd et al., 1999; Henderson, 2010, Johnstone et al., 2005; Jonkman, 2006; Lamm et al., 2006; Lewis et al., 2006, Lewis & Stieben, 2004). Incongruent flankers represent greater conflict, and relatively larger N2 amplitudes are observed in the incongruent condition (Van Veen & Carter, 2002). Developmentally, the effects of incongruent flankers on the N2 appear to emerge later than the effects of other EF tasks (e.g., the go-nogo), with differences between flanker conditions being more apparent in the N2s of older adolescents and adults but not young children (Checa & Rueda, 2011; Ladouceur et al., 2007; Rueda, Posner, Rothbart, & Davis-Stober, 2004). However, the N2 effect has been detected as early as age 6 (Buss et al., 2011). Controlling for age, children with relatively larger differences in amplitude for incongruent relative to congruent flanker conditions have greater behavioral difficulty with incongruent trials and less effortful control by parent report (Buss et al., 2011). Furthermore, a generalized increase in absolute N2 amplitude (across conditions) is interpreted as “less efficient” processing (Dennis & Chen, 2009).
The P3, which is represented over central parietal leads, is comprised of two subcomponents, the P3a and P3b. The P3a is generated in the frontal lobe and is functionally associated with focal attention. Two main attention networks are activated during the P3a component, the ventral frontoparietal network (VFP), which has been postulated to be engaged in the detection of rare events and thus is modulated by stimulus frequency (Corbetta & Shulman, 2002), and the dorsolateral frontoparietal network (DLFP), which is involved in voluntary shifts of spatial visual attention and top down processing of stimuli (Bledowski et al., 2004; Donner et al., 2002; Goebel et al., 1998; Wojciulik & Kanwisher, 1999). The P3b subcomponent is thought to be associated with memory and event categorization and is generated in the temporal lobe via “target detection” (Baudena et al., 1995; Bledowski et al., 2004; Halgren et al., 1995; Linden et al., 2005; Puce et al., 1989) and “retrieval” or “mental tracking” of the target stimulus compared to memory templates necessary for target detection and memory updating (Jiang et al., 2000; Lepage et al., 2000, see Friedman et al 2000 for review). Together, these components are hypothesized to represent inhibition of irrelevant information that is not directly involved in stimulus evaluation, in order to “amplify” the target signal for enhanced stimulus classification and memory consolidation. The P3 is altered by the frequency of a stimulus, habituation to a stimulus, and the familiarity or novelty of a stimulus (Luck et al., 2011) – all of which were held constant across the flanker conditions in this study; thus any amplitude differences should be a result of the variation between congruent and incongruent flanker conditions. Developmentally, P3 amplitude increases with age in the context of non-flanker tasks (see Luck et al., 2011 for review) and is viewed as a marker of increased inhibitory responding (Hämmerer, Li, Müller, & Lindenberger, 2010). Although the P3 is present in this age range over parietal leads during inhibitory tasks requiring response inhibition, a protracted developmental course involving increased amplitude and scalp distribution is observed starting between age 7-10 and continuing to adulthood (Brydges, Fox, Reid, & Anderson, 2014; Jonkman, 2006). In the flanker task, the P3 is present in 7-14 year olds with larger parietal amplitudes for incongruent trials relative to congruent trials, and it is thought to reflect allocation of effort (Johnstone & Galletta, 2013).
1.1 The Flanker Task in ASD
Behavioral performance on flanker tasks is impaired in adults, adolescents and children with ASD relative to comparison groups without ASD (Adams & Jarrold, 2012; Christ et al., 2011; Dichter & Belger, 2008; Geurts, Luman, van Meel, 2008; but see Sanderson & Allen, 2013). Individuals with ASD have slower reaction times and worse accuracy for the incongruent condition relative to comparison groups, and these effects were apparent even when the task was manipulated in ways that make it easier for youth without autism (Adams & Jarrold, 2012). Although EF is a frequent focus of research, there is little information about the neural profile of children with ASD during EF tasks. ERP provides a window into potential differences in neural processes that underlie behavioral responses on the flanker EF task.
To date, there have been three investigations of electrophysiological responses of children and adolescents with ASD during a flanker task with inconsistent findings. Tye and colleagues (2014) utilized a continuous performance cued-nogo task in which the central target (a letter) was flanked by letters. In this task, the N2, P3 and Contingent Negative Variation (CNV) components were analyzed, and, relative to children aged 8-13 years with ADHD and typical development, children with ASD had reduced absolute N2 amplitudes and increased CNV components, but, in contrast to previous reports (Adams & Jarrold, 2012; Christ et al., 2011; Dichter & Belger, 2008; Geurts, Luman, van Meel, 2008), did not differ in behavioral performance during the task. ERP data suggest that children with ASD may have more efficient conflict monitoring during this task as seen by the reduction in N2 amplitudes (Dennis & Chen, 2009; Buss et al 2011), which might explain the lack of performance deficits. However, analyses focused on the continuous performance aspect of the task and behavioral inhibition during Nogo trials rather than the flanker manipulation making it a less direct measure of interference suppression. Furthermore, the N2 amplitude was measured over a large window, 170-400ms, using peak amplitude rather than mean amplitude, making the N2 susceptible to differences in the number of usable trials per group and condition, which were not controlled in this study. Using a more traditional flanker task, Samyn et al. (2014) found no differences in ERP response at the N2 or P3 component or behavioral performance (i.e., reaction time or number of errors) by 10 to 15-year-olds with ASD relative to an age-matched group without ASD, suggesting either no differences in interference control at the behavioral or neural level for 10-15-year-olds with ASD or poor sensitivity to EF differences. Finally, Larson and colleagues specifically examined the effects of congruent and incongruent flankers in the context of the previous trial condition and conflict adaptation. They found 9 to 17-year-olds with ASD made more errors and the effect of incongruent trials on accuracy was larger for children with ASD than the age-matched comparison group. In terms of N2, the group with ASD had less evidence of conflict adaptation from trial to trial, whereas the comparison group exhibited the expected pattern of conflict adaptation. Larson and colleagues included a relatively large sample size but a wide age range, which may have obscured differences between groups as the comparison group began to produce more consistent and predictable neural responses to the flanker task. As well, experimental design and analyses focused on conflict adaptation rather than interference control.
The current study addresses these inconsistent findings and the limited research focused specifically on the neural underpinnings of interference control in children with ASD by including a narrower age range and focusing exclusively on the flanker condition of a developmentally appropriate task designed to elicit ERP responses in young school-aged children. We selected this age range because there is evidence that neural markers related to processing conflict during the flanker task (i.e., N2, P3) are present for typically developing children (e.g., Buss et al., 2011; Jonkman, 2006), it is a rapid period of behavioral and neural development for the structures underlying the flanker task (Fjell et al., 2012), and evaluating whether responses of children with ASD diverge early in this period of development provides a an opportunity for earlier intervention related to EF for children with ASD. In addition, we sought to build on previous work by examining the relations between the neural responses during this interference control task and behavioral performance across a battery of direct EF assessments rather than parent report measures. This provides the opportunity to examine whether the processes measured by ERPs reflect the same aspects of EF in ASD.
We examined neural responses underlying performance on an interference control task. Although our flanker task was relatively easy in order to facilitate a high number of correct trials for inclusion in ERP analyses, we examined behavioral performance and predicted children with ASD would have reduced behavioral accuracy and slower reaction times, particularly for incongruent trials. In terms of ERP responses, we predicted children with ASD would exhibit immature N2 responses with increased overall amplitude and less differentiation between congruent and incongruent trials, consistent with less efficient monitoring and inhibition of flanker information. For the P3, we expected the typically developing group to have overall larger P3 amplitudes, particularly for incongruent trials, reflecting more mature processing. We also explored the possibility of differences in early visual processing and sensory attention to stimuli at the P100 component, and predicted that groups would not differ at this early stage of processing. Where groups differed in neural response, we examined the pattern of brain-behavior relations using a battery of tasks that measured EF more broadly in order to understand which aspects of EF were most closely related to the neural response of each group. To achieve this goal, we employed a battery of three independent, age-appropriate behavioral measures of EF selected because of their association with the lateral prefrontal cortex and N2 (Lamm, Zelazo, & Lewis, 2006; Zelazo & Muller, 2002): (1) the Color-Word Stroop Task, which measures interference control; (2) the Change Task, which measures response execution, inhibition, and shifting; and (3) the Backward Digit Span, which measures verbal working memory. Finally, we explored the possibility that neural response and behavioral performance related to severity of autism symptoms and individual differences in the degree of co-occurring ADHD symptoms.
Method
2.1 Participants
The overall sample included 33 children with typical development and 28 children with ASD between the ages of 7-11 years who provided behavioral data for the Child ANT task. Of these, 30 children with typical development and 19 with ASD provided adequate ERP data. Groups were matched on age, sex ratio and handedness (see Table 1 for subject characteristics). Recruitment sources included existing registries of families who expressed interest in research and service providers, parent organizations and community events. For both groups, exclusionary criteria included medical disorders or injuries that affect the central nervous system, major physical abnormalities, significant sensory or motor impairments, and seizures. Children were excluded if they currently or had ever taken any anticonvulsant medications, which are known to impact the EEG. Other medications were not exclusionary in accordance with the guidelines for EEG/ERP studies in ASD (Webb et al., 2015). Typically developing children were also excluded if they had a family history of ASD, learning or language impairments, birth or developmental abnormalities, current or past history of psychiatric or neurological disorders, or if they took psychoactive medications. The university Human Subjects Division approved all study procedures and all parents consented for their children to participate.
Table 1.
Participant descriptive characteristics by diagnostic group
| Typically Developing M (SD), range | Autism Spectrum M (SD), range | Significance (t or χ2, p) | |
|---|---|---|---|
| Age (in months) | 115.0 (15.2); 89 to 143 | 110.9 (17.6); 84 to 138 | t=0.96, ns |
| Sex ratio | 3 female: 30 male | 3 female: 25 male | χ2=0.05, ns |
| Handedness | 3 left, 25 right | 5 left: 23 right | χ2=0.58, ns |
| WASI-2 IQ | |||
| Full Scale | 114.5 (10.1); 91 to 132 | 108.2 (16.1); 85 to 153 | t=1.81, ns |
| Verbal Comp. | 112.3 (8.8); 97 to 127 | 106.4 (19.3); 69 to 160 | t=1.49, ns |
| Percept. Reason. | 114.7 (15.0); 88 to 152 | 109.4 (15.3); 86 to 145 | t=1.36, ns |
2.2 Child Descriptive Measurements
All children in both groups had full scale and performance IQ in the average or above average range (i.e. ≥ 85) measured with the WASI-2 (Wechsler, 2011) and groups did not differ significantly in verbal, performance or full scale IQ (see Table 1). Children in the group with ASD had a previous diagnosis of an autism spectrum disorder, which was confirmed using the Autism Diagnostic Observation Schedule, Second edition (ADOS-2; Gotham, Risi, Pickles, & Lord, 2007), the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003), and DSM-5 (American Psychiatric Association, 2013) criteria for Autism Spectrum Disorder. The severity of autism symptoms was computed from the ADOS-2 raw scores following (Hus, Gotham, & Lord, 2014) for two domains that broadly correspond to the existing DSM-5 classification system: Social Affect and Restricted/Repetitive Behaviors.
Our sample of children with ASD included children with significant symptoms of attention deficit hyperactivity disorder (ADHD), which is currently representative of the population of children with ASD (Leyfer et al., 2006; Simonoff et al., 2008). Among the group with ASD, 8 (29%) scored in the clinically significant range for the Child Behavior Checklist Attention Deficit/Hyperactivity score (Achenbach & Rescorla, 2001). For those with sufficient ERP data for Child ANT, 6 (32%) of the children with ASD and no typically developing children scored in the clinically significant range for ADHD symptoms.
2.3 Electrophysiological Measurement
Electrophysiological responses were continuously recorded during the Child ANT from a 128-channel Net Amps 200 (Electrical Geodesics, Inc.) using the geodesic sensor net 2.0 (GSN) soaked in potassium-chloride electrolyte solution, placed on the participant's head and fitted according to the manufacturer's specifications. Impedances for the electrodes were below 50 kΩ at the start of the session. The signals were recorded online using the vertex reference electrode with hardware filters set at 0.1 Hz high-pass and 200 Hz elliptical low-pass, and a sampling rate of 500 Hz. Data were re-filtered off-line using a low-pass filter to remove electrical noise (Kaiser-type FIR filter, 30 Hz cutoff with 2 Hz rolloff).
2.3.1 Stimuli and experimental procedure
Electrophysiological data were collected at the beginning of a session measuring executive function. The order of tasks was counterbalanced so that half the children in each group completed the Child Attention Network Task (ANT) first and half following another executive function task (not reported). Once EEG was collected during the two tasks, the remainder of the behavioral EF battery was collected in a fixed order.
A simplified version of the Child ANT in which only the flanker portion was presented (Rueda et al., 2004) included 12 practice trials and 108 test trials presented in random order. For each trial, children first heard a beep for 150 ms followed by a fixation cross for 450 ms at the center of the screen. Then, a target and flankers were presented for 2000 ms. Congruent trials (50%) consisted of a central target animal flanked by two flanker animals (on each side) that faced the same direction as the target. Incongruent trials (50%) were identical except that the target and flankers faced opposite directions (see stimuli in Figure 2). All target and flanker stimuli were the same size. Children responded by pressing a button for the direction the target animal faced (50% left, 50% right) and received visual and auditory feedback (i.e., woohoo!) upon responding. In addition to EEG, accuracy and reaction time for correct trials were collected.
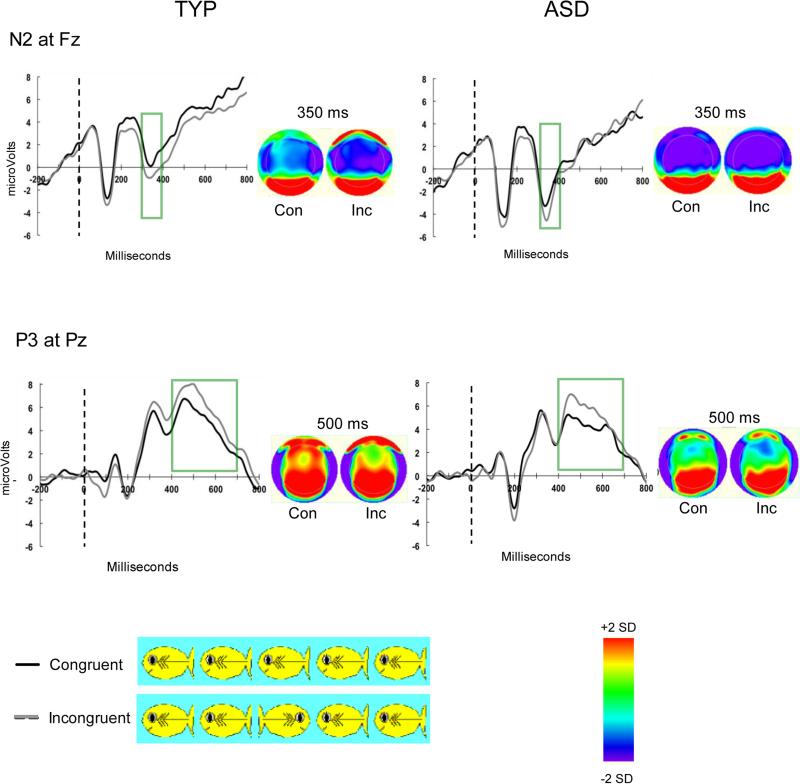
Figure 2.
Graphs for each condition and group representing averaged N2 and P3 ERP responses to the ANT Task as well as topographic maps depicting spatial distribution of responses.
2.3.2 Data Editing and Extraction
EEG data were segmented with a 200 ms baseline period immediately preceding stimulus onset and 800 ms after the onset of the stimulus. Epochs were time-locked to stimulus onset using a photocell. Data were then baseline corrected using the full 200 ms baseline period. Trials with incorrect behavioral responses or artifacts in more than 25 of the channels were excluded from the averages using the following artifact detection criteria: (1) presence of an eye blink using the Netstation Eye Blink algorithm set at 220 μV with an 80 ms moving average and confirmed by visual inspection, (2) having fluctuations exceeding 140 μV with 80 ms moving average, and (3) having no fluctuations of > 1 μV with an 80 ms moving average. Data were visually inspected for additional artifacts and segments were excluded if they contained significant drift, movement artifacts, eye movements, or mechanical artifacts. Any channels marked as having artifact for more than 50% of the trials were replaced using an algorithm that derives values from neighboring electrodes using spherical spline interpolation. Data were then averaged individually for each condition, re-referenced offline to the average of all electrodes minus the four eye channels using the polar average reference effect (PARE) correction to correct for under sampling of the undersurface of the head (Junghöfer, Elbert, Tucker, & Braun, 1999), and baseline corrected again. Given our high-density recording, we created clusters comprised of adjacent electrodes for the N2 (GSN electrodes 20, 11, 4) and the P3 (GSN electrodes 54, 62, 80, 68). Mean amplitude within the time window was examined at the P100 (70-170 ms at Oz), N2 (300-400 ms at the Fz electrode cluster), and P3 (400-700 ms at the Pz electrode cluster). Windows were selected based on previous reports in the literature (P100: Luck et al. 1990 and Rutman et al. 2010; N2: Samyn et al. 2014; P3: Tye et al. 2014) and the appropriateness of these windows for this dataset was confirmed by visual inspection of the grand average waveform and individual averaged data. Electrode sites were chosen based on previous literature (P100; Luck et al. 1990; Rutman et al. 2010; N2: Samyn et al. 2014; Lamm et al., 2006; P3: Tye et al. 2014; Rueda et al., 2004) as well as inspection of the topographical maps given the potential for different activation patterns in ASD (Webb et al. 2015)1.
2.3.3 Included Data
Subjects for whom fewer than 10 trials remained within a condition were excluded from analyses. A 10 trial cut-off was made to include as many participants as possible while maintaining an adequate signal-to-noise ratio, which is consistent with the literature for young children (Lamm et al., 2006; Rueda et al., 2004; Todd, Lewis, Meusel & Zelazo, 2008). Nine children with ASD and 3 children without ASD provided behavioral but not ERP data, χ2 (1, N = 61) = 5.09, p = .024. Nonetheless, diagnostic groups did not differ for age, IQ, sex ratio or handedness when only children with usable ERP were compared. For the subjects with adequate ERP data, 57.1% (SD = 30.7) of trials were included for the group with ASD and 63.8% (SD = 18.7) were included in the comparison group. Groups did not differ in the overall number of trials included in analyses, F(1, 47) = 1.37, p = .25, nor did they differ in the number of good trials per condition, F(1, 47) = .133, p = .72.
2.4 Executive Function Behavioral Testing
Three tasks were administered via laptop computer to evaluate different aspects of EF:
2.4.1 Stroop Task
The Stroop Task (Perlstein, Carter, Barch, & Baird, 1999; Stroop, 1935) measures inhibition of interfering information and is has a close theoretical link to flanker tasks. After first screening children for colorblindness, participants (23 with ASD and 24 with typical development) practiced on 20 trials with squares presented one trial at a time in four different colors: red, blue, green and yellow. Then, 16 trials included neutral words (four animal names) equated in length to the four color words in a red, green, blue or yellow font. Finally, in the test block, 96 trials were presented in pseudorandom order with three conditions: (1) congruent trials (25%) presented a color word written in the same color; (2) incongruent trials (25%) presented a color word written in one of the other colors; and (3) neutral trials (50%) presented a non-color word (dog, bear, tiger, monkey) in one of the four colors. Children pressed buttons to indicate the color of the text. The differences between congruent and incongruent conditions for both percent correct and correct reaction time were the dependent variables. Higher scores represented reduced interference control.
2.4.2 Change Task
The Change task (De Jong, Coles & Logan, 1995; Geurts et al., 2004) is adapted from the Stop Task and measures inhibition of dominant responses, response execution (monitoring) and flexibility (shifting). For this lengthy task, 25 children with ASD and 32 with typical development provided adequate data. Three training blocks were initially presented: (1) a reaction time block in which a picture of an airplane was presented on either the right or left side of the screen and children pressed buttons to indicate whether the image appeared on the right or left; (2) a stop block in which a beep preceded a subset of items and children suppressed their responses for trials with beeps; and (3) a change block in which a beep preceded a subset of items and children suppressed the dominant left and right responses and pressed a different button on trials with beeps. In order to adjust for individual differences in reaction time, the mean correct reaction time from the change practice block was used to determine the timing of the first block of test trials and subsequent blocks used the preceding mean correct reaction time from the previous block. Across four test blocks, 25% of trials included beeps and required a change response and 75% were go trials without beeps. An equal number of beeps occurred at 50, 200, 350, and 500 ms before the anticipated response. Three dependent variables were obtained. The mean Stop Signal Reaction Time (SSRT) measured the latency of inhibitory responding (Band, van der Molen, & Logan, 2003). Accuracy (percent incorrect) for trials with no change represented response execution. Accuracy (percent incorrect) collapsed across all trials with changes (regardless of beep duration) represented set shifting. Higher scores indicated more difficulty with inhibition, response execution, and shifting.
2.4.3 Backward Digit Span
Backward Digit Span emphasizes verbal working memory. Participants completed the Numbers subtest of the Children's emory Scale (CMS; Cohen, 1997) administered according to standardized instructions but presented via laptop with pre-recorded audio stimuli. An experimenter recorded the child's verbal responses (for all 28 with ASD and 33 with typical development). For digits forward, children were instructed to repeat strings of digits. Then, for digits backward, children were instructed to say the number strings for each trial opposite the presentation order. For both parts, two trials of the same length were presented before the span increased by one digit. The subtest was discontinued when children made errors on both trials of the same length. To measure working memory, while controlling for baseline verbal memory, a ratio score was calculated from the number of correct trials: [digits forward minus digits backward] divided by digits forward (Lamm et al., 2006). Higher scores indicated worse verbal working memory.
2.5 Analyses
Repeated measures analysis of variance (ANOVA) was used with an alpha level of .05 for all statistical tests, using the Greenhouse-Geisser epsilon correction for nonsphericity (Jennings &Wood, 1976). Analyses for the ANT included a between-subjects factor of group (ASD vs. typical comparison) and a within-subjects factor of flanker type (congruent vs. incongruent). Significant effects were further examined with Bonferroni corrected post hoc tests. In addition, topographical maps were inspected in order to examine response distribution as traditional analyses of these components have not utilized high-density designs. Topographical maps were created by incorporating two standard deviations of the data (p=.05 or 95%) centered around the mean amplitude for the 50 ms at the midpoint of the window of interest for each component and group. Data that fell outside this distribution was not graphically depicted. Mean performance on other EF behavioral tasks was compared between groups using t-tests prior to examining relations with ERPs. Brain-behavior relations were tested by examining bivariate Pearson correlations between the ANT flanker ERP and behavioral measures of EF. Brain-behavior relations were only examined within each group and only for neural components that differed by group. Finally, the impact of comorbid ADHD symptoms on performance was explored dimensionally by examining correlations between parent reported ADHD symptoms, ERP amplitude and behavioral scores. Similarly, we explored the relation between our neural and behavioral measures of EF and observed autism symptoms.
Results
3.1 Behavioral Performance on the Child ANT
Overall, accuracy was higher for congruent than incongruent trials, F(1,59)=16.7, p<.001, ηp2=.22. Post hoc comparisons of each group separately confirmed that both were more accurate for congruent trials (ps<.005). Children with ASD were also less accurate than the typical comparison group, F(1,59)=8.7, p=.005, ηp2=.13. Critically, a significant group by condition interaction was detected, F(1,59)= 4.6, p=.04, ηp2=.07. Post hoc tests indicated this was due to relatively worse accuracy for incongruent relative to congruent trials in the group with ASD. Groups also differed for the incongruent condition, t(59)= −2.7, p=.01, Cohen's d=0.71, but not for the congruent condition, t(59)=−2.0, p=.05, d=0.53. (See Figure 1 and Table 2 for means.)
Figure 1.
Means and Standard Errors for Behavioral Data between Groups on the ANT
Table 2.
Behavioral data by diagnostic group, M (SD), range
| Typically Developing | Autism Spectrum | |
|---|---|---|
| Child ANT Flanker | N=33 | N=28 |
| Overall % Accuracy** | 97.4 (2.9), 85 to 100 | 92.3 (9.4), 65 to 100 |
| Congruent %* | 98.5 (1.9), 93 to 100 | 95.7 (7.0), 74 to 100 |
| Incongruent %** | 96.4 (4.5), 78 to 100 | 88.9 (14.1), 44 to 100 |
| Overall Correct RT* | 689.2 (163.2), 481 to 1120 | 797.1 (230.3), 447 to 1462 |
| Congruent** | 661.2 (158.7), 460 to 1089 | 789.5 (234.2), 430 to 1392 |
| Incongruent | 717.8 (170.4), 502 to 1151 | 807.0 (237.6), 464 to 1573 |
| Stroop | N=24 | N=23 |
| Con-Inc Acc | 4.2 (9.5), −17 to 25 | 5.6 (11.1), −13 to 33 |
| Inc-Con cRT | 97.7 (132.1), −202 to 334 | 116.4 (159.3), −149 to 390 |
| Change | N=32 | N=25 |
| SSRT | 348.5 (210.4), 27 to 1004 | 308.2 (340.2), 5 to 1289 |
| No Change Err* | 8.6 (10.2), 1 to 58 | 15.0 (14.4), 1 to 49 |
| Change Err | 56.6 (14.4), 19 to 83 | 64.3 (16.4), 34 to 92 |
| Backward Digits** | N=33 | N=28 |
| .37 (.20), 0 to .75 | .54 (.25), 0 to 1 |
Note: Significance for t-tests comparing groups is indicated with
p < .05
p < .01
Response times for correct trials were generally faster for congruent than incongruent trials, F(1,59)=21.4, p<.001, ηp2=.27, and the group with ASD was slower overall, F(1,59)=4.6, p=.04, ηp2=.07. A group by condition interaction was detected, F(1,59)=5.9, p=.02, ηp2=.09. Post hoc analyses indicated typical comparison children were faster in the congruent than incongruent condition, t(32)=−7.8, p<.001, d=−0.34, whereas children with ASD were not, t(27)=−1.2, p=.26, d=−0.07 (See Figure 1 and Table 2.) While typical comparison children were slowed only on incongruent trial, children with ASD were less accurate (particularly on incongruent trials) and slower overall.
3.2 Electrophysiological Data
There were no significant group, condition, or group by condition interactions for P100 mean amplitude, Fs<0.35, ps >.56, ηp2s<.007, suggesting both groups were experiencing the stimuli in the same way and allocated comparable resources to early sensory and attention processes. The P100 was not included in further analyses.
Overall, N2 mean amplitude was larger for incongruent compared to congruent trials, F(1,47)=5.91, p=.02, ηp2=.11. The group with ASD also had larger absolute amplitude collapsed across conditions, F(1,47)=7.21, p=.01, ηp2=.13 (See Figure 2). However, the interaction between group by condition was not significant, F(1,47)=0.27, p=.61, ηp2=.006. Post hoc analyses revealed this was due to larger N2 amplitudes for children with ASD in both the incongruent condition, t(47)=−2.35, p=.02, d=−0.68, and the congruent condition, t(47)=−2.49, p=.02, d=−0.23. While confirming electrode selection for the N2 component, the typically developing group demonstrated a spatially concentrated negative deflection in the frontal-central region, particularly for the incongruent condition (Figure 2). The group with ASD appeared to have a broader distribution, which encompassed the entire frontal and parietal scalp region, but this effect did not differ statistically between groups when lateral (GSN electrodes 25 (F3), 124 (F4)) or posterior electrodes (GSN electrode 6 (Fcz)) were compared.
For the P3, mean amplitude was larger for incongruent compared to congruent trials, F(1,47)= 5.13, p=.03, ηp2=.10 (See Figure 2). However, there was not a main effect of group, nor a group by condition interaction, Fs<0.37, ps>.55, ηp2s<.008. The spatial distribution of the P3 component appeared more broad and encompassed more anterior electrodes for the typically developing group than the group with ASD (Figure 2). In contrast, the group with ASD had an apparent positive deflection that was more concentrated over the posterior scalp region. Explorations of these regions (GSN electrodes 53 (P3), 87 (P4), 80 (Cpz)) were non-significant for group effects and both groups had maximal activation over the Pz electrode.
3.3 Relations between brain and behavior
General performance on the behavioral battery is summarized in Table 2. In addition to group differences for behavior during the Child ANT, children with ASD were significantly less accurate in response execution (i.e., monitoring) on non-change trials of the Change Task, and had decreased working memory on the Backward Digit Span. Consistent with previous reports, performance on the Stroop was comparable between groups.
The pattern of behavioral correlations was also examined in order to examine whether the N2 reflects the same aspects of EF in ASD as children in the typically developing group given group differences for this component. As shown in Table 3, simple bivariate correlations between behavioral tasks were conducted to first confirm that the battery measured related but independent aspects of EF for both groups. Overall, worse ANT accuracy for incongruent trials related to worse monitoring and response execution (No Change trials of the Change task) for typically developing children and related to reduced working memory for children with ASD. Typically developing children who were slowest on the incongruent ANT trials were less accurate on incongruent trials of another interference suppression task (Stroop) and had worse monitoring and response execution, whereas children with ASD who were slowest on the ANT incongruent trials made fewer errors related to monitoring and response execution. As with the ANT, better interference suppression on the Stroop also related to better monitoring and response execution for typically developing children. Additionally interference suppression directly related to shifting (Change trials of the Change task) and working memory. Among children with ASD, better Stroop interference suppression related to monitoring and response preparation as well as shifting. For typically developing children slower incongruent responses on the Stroop corresponded with fewer shifting errors on the Change task. Finally, for both groups, children who made fewer errors on the Change trials also made fewer errors on the No Change trials. Together, this pattern confirmed that behavioral performance was linked for both groups across interference suppression, response inhibition, and shifting tasks, with working memory being less related to performance as well as some tradeoffs between inhibition and other aspects of EF. The group with ASD had slightly fewer relations between tasks, which may indicate less consistent across the battery.
Table 3.
Behavioral correlations for typically developing children (lower left) and children with autism spectrum disorder (upper right)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. ANT C-I Acc | – | −.15 | −.07 | −.11 | −.32 | .34 | .29 | .59*** |
| 2. ANT I-C cRT | −.12 | – | −.17 | .28 | .11 | −.45* | −.35 | .07 |
| 3. Stroop C-I Acc | .02 | .44* | – | .14 | .20 | .58** | .48* | −.03 |
| 4. Stroop I-C cRT | .10 | −.29 | −.23 | – | −.17 | −.27 | .14 | −.19 |
| 5. Change SSRT | .14 | .24 | .35 | .15 | – | −.14 | −.34 | .15 |
| 6. No Change Err | .38* | .42* | .49* | −.32 | .19 | – | .53** | −.15 |
| 7. Change Err | −.06 | .22 | .52** | −.51** | −.27 | .49** | – | .09 |
| 8. Digit Span BW | .05 | .14 | .42* | −.21 | −.10 | .29 | .22 | – |
Note: Significance for Pearson correlations is indicated with
p < .05
p < .01
p < .001
Then, in order to examine brain and behavior relations at the N2, which differed between groups, a difference score was calculated (incongruent minus congruent) in order to control absolute amplitude differences between individuals and isolate the magnitude of processing conflicting information. Thus, more negative values for the N2 reflected larger negative fluctuations for incongruent relative to congruent trials. Next, bivariate Pearson correlations were computed between behavioral scores and the ERP amplitude difference score. Among typically developing children, larger negative N2 amplitude difference scores related to more shifting errors during the Change Task, r(29)=.43, p=.02, such that a greater negativity for incongruent relative to congruent trials corresponded with more difficulty correctly inhibiting a dominant response and shifting to a different response. No other tasks related to the N2.
For the children with ASD, a larger negative N2 amplitude difference score related to larger differences in accuracy between the congruent and incongruent conditions of the Stroop task, r(17)=.50, p=.04, and more errors during the No Change condition of the Change Task, r(17)=.63, p=.01. That is, children with ASD who had larger differences in amplitude at the N2 between ANT conditions had more difficulty with Stroop interference suppression as well as monitoring performance and sustaining accurate response execution during the Change Task.
3.4 Relations between brain, behavior and clinical symptoms in the group with ASD
The impact of comorbid ADHD symptoms on performance was explored dimensionally by examining correlations between parent-reported ADHD symptoms on the CBCL parent checklist (ADHD scale), the N2, and behavioral performance. ADHD symptoms did not relate to N2 amplitude difference scores. However, among children with ASD, higher levels of ADHD symptoms related to worse inhibitory control as measured by the Stop Signal Reaction Time, r(24)=.54, p=.01, but better shifting as measured by Change accuracy, r(25)=−.50, p=.01, on the Change Task. Conversely, ADHD symptoms were unrelated to performance on the Digit Span, accuracy on the No Change condition, Stroop, or ANT task, rs<0.38, ps>.07.
Finally, to explore the possibility that interference suppression is related to symptoms of ASD, we examined whether severity of autism symptoms related to individual differences in N2 amplitude or performance on the Child Ant and Stroop. The N2 was unrelated to ADOS-2 severity scores for Social Communication or Restricted and Repetitive Behaviors rs<0.17, ps>.50. Behaviorally, children with greater severity for Social Affect symptoms on the ADOS-2 had larger differences in reaction time between congruent and incongruent trials on the Stroop, r(23)=.50, p=.02, and in accuracy for the Child ANT, r(28)=.40, p=.04. No differences were detected for RRB severity scores, rs<0.23, ps>.29.
Discussion
This study investigated neural correlates of EF skills in children with typical development and with ASD using the flanker portion of the Child ANT task, which requires interference control. The results confirm the expected pattern of slower, less accurate responses and more negative N2 amplitude for incongruent trials among typically developing children. For typically developing children, neural responses appear related to a combination of inhibition and shifting.
Children with ASD had reduced accuracy, particularly for incongruent trials, and slower reaction time during the task compared with the typically developing group. Children with ASD also exhibited larger amplitudes at the N2 ERP component, which relates to conflict monitoring. Finally, in the group with ASD, N2 neural responses corresponded to performance on a closely related task that required suppression of interfering information as well as performance on a task that measured the ability to monitor information and sustain accurate responding.
4.1 The Child ANT Behavioral Responses
Overall, for incongruent trials, accuracy was lower and correct responses were slower relative to congruent trials. Typically developing children performed more accurately and efficiently on congruent trials that did not require suppression of incongruent flankers. Although both groups had total accuracy above 90%, children with ASD were generally slower and less accurate. Children with ASD were relatively less accurate on the incongruent condition compared to the congruent condition and had larger decrements in accuracy between conditions. Interestingly, congruent trials did not confer a speed advantage for the group with ASD as they did for the typically developing group. Indeed, the typical comparison group was significantly faster than the group with ASD for congruent trials, while both groups were comparably slow on the incongruent trials. In sum, these data suggest that 7-11 year olds with ASD who had unimpaired general intelligence were able to complete the flanker portion of the Child ANT task, but lacked the same precision and speed as their age and IQ-matched typically developing peers.
4.2 The N2 and Conflict Processing
The N2 is thought to reflect executive attention, and specifically, monitoring the conflict between the target and flankers. For the N2 component, we found larger amplitudes for the incongruent condition relative to the congruent condition indicating that it is sensitive to the degree of conflicting information. Yet, the group with ASD had significantly larger amplitudes across both conditions compared to controls, which suggests more effortful processing of both congruent and incongruent flankers. Although the P3 differed by condition with larger amplitudes for the incongruent flankers, there were no group differences, suggesting both groups generally recruited more neural resources to inhibit incongruent flankers. Thus, increases in amplitude for children with ASD were specific to the N2 component, which suggests neural activity differed specifically at the stage of processing when the conflicting information was first being detected and monitored. Of note, the developmental course of the P3 is more protracted than the N2 (Hämmerer et al., 2010), and individual variability in may have masked effects.
Our data suggest the neural network underlying the N2 component is immature or less efficient for conflict monitoring in ASD. A generalized increase in absolute N2 amplitude (across conditions) is seen as “less efficient” processing (Dennis Chen, 2009). Qualitatively, more diffuse and parietal distributions of the N2, such as those we observed when examining the scalp distribution to confirm electrode selection, are seen in younger children who have immature conflict monitoring (Jonkman, 2006, Lewis et al., 2006, Stieben et al., 2007). Moreover, difficulty with monitoring of conflicting information in ASD builds on previous work that demonstrated atypical error monitoring in individuals with ASD characterized by reduced amplitudes and longer latencies for the Error Related Negativity (ERN) component (Henderson et al., 2006; Sokhadze et al., 2010; Vlamings et al., 2008). Together, these results indicate children with ASD have inefficient neural activation while monitoring both conflicting information as well as their performance.
4.3 Brain-Behavior Relations
Our pattern of correlations among the typically developing group is also consistent with previous reports of the N2 (Buss et al., 2011). More effortful neural monitoring of incongruent trials was specifically linked with greater difficulties with a task that required inhibition of a dominant response and shifting to a different response.
Of note, the pattern of brain-behavior relations differed at the N2 for the two groups, suggesting that children with ASD not only recruit greater magnitude neural responses but also potentially recruit networks that are linked to different aspects of the flanker task. Specifically, individual differences in N2 neural response in the group with ASD related to performance on the Stroop Task. Both the Child ANT and Stroop require suppression of conflicting information. Our findings for children with ASD are consistent with the conclusion that the N2 reflects the efficiency of monitoring conflicting information–to the extent that children with ASD have inefficient N2 responses to incongruent trials, they also struggle to accurately select the relevant stimulus dimension on the Stroop task. Neural responses of the group with ASD to the Child ANT also corresponded to accuracy during the non-change trials of the Change task, which required monitoring the position of a picture on screen and making a corresponding button press. Here again, children with ASD who recruited more neural resources to monitor conflicting information had more difficulty monitoring and sustaining that effort over time. These differences in the pattern of brain-behavior relations suggest that the N2 may be related to flexibility in conflicting conditions in the typically developing children (Espinet et al,, 2012) and monitoring and suppressing interfering information in children with ASD. Following children with ASD over time or providing training may provide an opportunity to explore how these individual differences in processing observed in early childhood develop in the context of ASD.
4.4 Implications for Understanding Executive Function in Autism
Our behavioral findings with the Child ANT replicate previous work (Adams & Jarrold, 2012; Christ et al., 2011; Dichter & Belger, 2008; Geurts et al., 2008). With respect to previous ERP results, our N2 results contrast with those of Tye et al. (2014) who found reduced N2 negativity for children with ASD. Inspection of the means reported indicated this might have been due to reduced differentiation between conditions by children with ASD. Task demands are potentially different for the continuous performance version of the flanker task employed by Tye and her colleagues. Indeed, children with ASD did not differ in their behavioral performance. Additionally, methodological differences in window selection (100 vs. 230 ms) and use of peak relative to mean amplitude may have also contributed to the differences between our findings. Using an older sample than ours (i.e. 10 to 15 year olds), Samyn et al. (2014) found no group differences at the N2 or P3 components, although no differences in accuracy or reaction time were detected either. Thus, it is again possible that task demands may have led to different results. Alternatively, it is possible that children with ASD are initially delayed relative to age-matched peers and develop skills or compensatory processes over time. Finally, Larson and colleagues (2012) specifically examined inter trial adaptation, with typically developing participants exhibiting a relatively more negative N2 for incongruent trials that followed congruent ones–an adaptation not seen in participants with ASD. Unlike other ERP flanker tasks used with ASD, arson's was sensitive to behavioral differences, but they did not compare the overall amplitudes between groups. Nonetheless, they did not find a relatively larger N2 detecting conflicting flankers after congruent trials for the group with ASD. It is possible that these results are consistent with ours in the sense that children with ASD in our younger sample were less efficient overall. As they become more efficient with age, they may fail to exhibit the expected adaptations for conflict reported for 9-17 year olds by Larson et al., (2014). Overall, our study adds information about a slightly younger sample, suggesting that 7-11 year olds with ASD use more neural resources at the N2 regardless of congruency when presented with an interference suppression task that they are less able to perform. Across tasks and ages, differences emerge at the N2 rather than the P3, suggesting that conflict monitoring may be more impaired in individuals with ASD.
Our brain-behavior relations also add to the existing literature by providing verification that individual differences in the N2 are linked to specific aspects of behavioral performance on EF tasks. Specifically, the N2 related to accuracy of the Stroop task, another similar measure of interference suppression, and the No Change condition of the Change Task, a measure of sustained attention and performance monitoring. A different pattern of correlations was observed for typically developing children, suggesting that the N2 may reflect more general development of executive skills that are captured by inhibiting a dominant response and shifting to a different one on the Change condition of the Change Task. Further exploration within the group with ASD indicated that ADHD symptoms were not related to the N2 or behavioral responses to interference suppression tasks. Instead, ADHD symptoms related to behavioral measures of response inhibition (SSRT latency) and the combination of inhibition and shifting (Change condition of the Change Task). Although ADHD symptoms did not relate to interference suppression tasks, symptoms of ASD did.
Taken together, these findings suggest that there is continuity between various aspects of EF for both groups, but the diversity of EF domains is meaningful in understanding neural and behavioral responses for both groups (Miyake & Friedman, 2012). Examining specific aspects of EF, particularly distinct inhibitory task demands (Nigg, 2000), appears especially important for understanding symptoms and comorbidity in ASD. Our results demonstrate less efficient neural responses during a measure of interference suppression for children with ASD, less accurate and rapid behavioral responses, and a correspondence between larger neural responses to incongruent flankers and reduced performance on a similar behavioral measure of interference suppression. In the context of previous work, these neural differences are detected in a task that is sensitive to behavioral differences between children with ASD and typical development and in a relatively younger sample than previously reported, highlighting the importance of examining developmental changes and task demands in future work.
4.5 Limitations and Future Directions
Future work should also address the limitations of our investigation. First, we selected a relatively narrow age range of children early in the development of the N2 effect. Though our task selection was intended to be inclusive, allowing most children to provide accurate responses for a majority of the trials, our age range precludes us from determining whether our results represent a delay or a deficit. Our functioning inclusion criteria reduce cognitive confounds and allowed for comparisons of neural structures related to EF in the absence of general cognitive delay. Yet, despite overall high performance in our young sample, our behavioral findings suggest the task was sensitive to group and condition differences. Future work including children with ASD who also have general cognitive delays is needed to determine whether these differences exist throughout the autism spectrum.
Our sample size did not permit us to investigate for differences between children with and without comorbid ADHD. Only Tye et al. (2014) has compared a group with ASD + ADHD to a group with ADHD alone using a continuous performance task with flankers. Nonetheless, roughly one third of our sample of children with ASD had clinically significant symptoms of ADHD and examination of the relations between these scores and our battery suggested that inclusion of individuals with ADHD symptoms cannot account for the pattern of findings for the N2 or behavioral performance on the working memory, monitoring/sustained attention, or interference suppression tasks. Interestingly, ADHD symptoms were related to inhibitory latency (SSRT) and shifting (change accuracy) on the Change Task, which measured inhibition of a dominant motor response and the ability to shift to a non-dominant response. Future work will benefit from separation of these groups and the comparison of children with ASD to other clinical conditions. Indeed, this will be extremely beneficial for diagnosing the nature of executive function impairments and providing more targeted treatments.
Finally, our sample size for children who provided adequate ERP data may have limited our findings. For example, we did not find a significant group by condition interaction and replication with a larger sample will allow for stronger conclusions about the relative N2 amplitudes on congruent versus incongruent trials within groups. Likewise, replication with a larger sample will be important for confirming the pattern of relations across our battery.
4.6 Conclusion
Our investigation provides additional information about the brain and behavioral relations for conflict processing in autism and typical development, paralleling a previous investigation linking neural activity during response suppression to specific aspects of EF behavior in typically developing children (Lamm et al., 2006). For the typically developing group, this study revealed that individual differences in the magnitude of neural response to conflicting information during an interference suppression task related to better ability to inhibit one response and shift to a different response. In addition, this study examined processes that underlie impaired behavioral inhibition of interfering information among children with ASD with average intelligence. Our ERP data suggest that differences in neural response arise at a higher level of processing rather than early sensory responses to visual input. N2 ERP responses suggest that a less efficient pattern of responding is present when monitoring conflicting information, consistent with our behavioral findings of delayed reaction times and reduced accuracy.
Clinically, our findings provide important clues for where and when to intervene with children on the autism spectrum. Young, school aged children with ASD may benefit from practice targeting the efficiency of filtering conflicting information and increasing executive inhibition of attention given the role of EF in academic performance, social skills and disruptive behavior. Intervention may contribute to better social functioning as a result of being less bogged down by filtering out irrelevant information and focusing on key aspects of the social interaction. Finally, ERPs may be an important source of information about early neural functioning in the prefrontal cortex and anterior cingulate and useful as a biomarker for assessment of EF skills.
Highlights.
■ Children are faster and more accurate, with less negative N2 for congruent flankers.
■ Children with ASD are less accurate and slower, particularly for incongruent trials.
■ Children with ASD have inefficient N2 neural responses to conflict monitoring.
■ N2 neural responses correspond to inhibition and monitoring behavior.
■ Symptoms of ADHD relate to response inhibition and ASD to interference suppression.
Acknowledgements
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number K99HD071966 (Faja). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the BASIC Study team for research assistance, Wendy Stone, Michael Posner, and M. Rosario Rueda for consultation on study design, and the UW Center on Human Development and Disability (CHDD) for support. We especially thank the participants and their families. These data were presented at the Society for Research in Child Development in Philadelphia, PA in 2015.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Consistent with previous literature, we also examined results from single electrodes N2 (Fz - GSN200 11) and for the P3 (Pz GSN200 62) and the pattern of findings for group x condition comparisons and correlations between brain and behavior were identical.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth & Families; Burlington, VT: 2001. [Google Scholar]
- Adams NC, Jarrold C. Inhibition in autism: Children with autism have difficulty inhibiting irrelevant distractors but not prepotent responses. Journal of Autism and Developmental Disorders. 2012;42:1052–1063. doi: 10.1007/s10803-011-1345-3. doi: 10.1007/s10803-011-1345-3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Author; Washington, D.C.: 2013. [Google Scholar]
- Band GP, Van Der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalography and Clinical Neurophysiology. 1995;94:251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–12. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DE. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Brydges CR, Fox AM, Reid CL, Anderson M. Predictive validity of the N3 and P3 ERP components to executive functioning in children: a latent-variable analysis. Frontiers in Human Neuroscience. 2014;8:1–10. [Google Scholar]
- Buss KA, Dennis TA, Brooker RJ, Sippel LM. An ERP study of conflict monitoring in 4–8-year old children: Associations with temperament. Developmental Cognitive Neuroscience. 2011;1:131–140. doi: 10.1016/j.dcn.2010.12.003. doi: 10.1016/j.dcn.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa P, Rueda MR. Behavioral and Brain Measures of Executive Attention and School Competence in Late Childhood. Developmental Neuropsychology. 2011;36:1018–1032. doi: 10.1080/87565641.2011.591857. doi: 10.1080/87565641.2011.591857. [DOI] [PubMed] [Google Scholar]
- Christ SE, Kester LE, Bodner KE, Miles JH. Evidence for selective inhibitory impairment in individuals with autism spectrum disorder. Neuropsychology. 2011;25:690–701. doi: 10.1037/a0024256. doi: 10.1037/a0024256. [DOI] [PubMed] [Google Scholar]
- Cohen MJ. Children's Memory Scale. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Developmental changes in real life decision making: Performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Developmental Neuropsychology. 2004;25:251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Chen CC. Trait anxiety and conflict monitoring following threat: an ERP study. Psychophysiology. 2009;46:122–131. doi: 10.1111/j.1469-8986.2008.00758.x. doi: 10.1111/j.1469-8986.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive Functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Atypical modulation of cognitive control by arousal in autism. Psychiatry Research: Neuroimaging. 2008;164:185–197. doi: 10.1016/j.pscychresns.2007.12.005. doi: 10.1016/j.pscychresns.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage. 2002;15:16–25. doi: 10.1006/nimg.2001.0951. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A, McGlone FP. The role of spatial attention in the processing of facial expression: an ERP study of rapid brain responses to six basic emotions. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Espinet SD, Anderson JE, Zelazo PD. N2 amplitude as a neural marker of executive function in young children: An ERP study of children who switch versus perseverate on the dimensional change card sort. Developmental Cognitive Neuroscience. 2012;2:S49–S58. doi: 10.1016/j.dcn.2011.12.002. doi: 10.1016/j.dcn.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd B, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Jr., et al. Multimodal imaging of the self-regulating developing brain. Proceedings of the National Academy of Sciences. 2012;48:19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microscopy Research and Technique. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Luman M, Van Meel CS. What's in a game: the effect of social motivation on interference control in boys with ADHD and autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2008;49:848–857. doi: 10.1111/j.1469-7610.2008.01916.x. doi: 10.1111/j.1469-7610.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verté S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goebel R, Linden DE, Lanfermann H, Zanella FE, Singer W. Functional imaging of mirror and inverse reading reveals separate coactivated networks for oculomotion and spatial transformations. Neuroreport. 1998;9:713–719. doi: 10.1097/00001756-199803090-00028. [DOI] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, et al. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalography and Clinical Neurophysiology. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Li S-C, Müller V, Lindenberger U. An electrophysiological study of response conflict processing across the lifespan: Assessing the roles of conflict monitoring, cue utilzation, response anticipation and response suppression. Neuropsychologia. 2010;48:3305–16. doi: 10.1016/j.neuropsychologia.2010.07.014. doi: 10.1016/j.neuropsychologia.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Henderson HA. Electrophysiological correlates of cognitive control and the regulation of shyness in children. Developmental Neuropsychology. 2010;35:177–193. doi: 10.1080/87565640903526538. doi: 10.1080/87565640903526538. [DOI] [PubMed] [Google Scholar]
- Henderson H, Schwartz C, Mundy P, Burnette C, Sutton S, Zahka N, Pradella A. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain and Cognition. 2006;61:96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Sciences. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders. 2014;44:2400–12. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Wood CC. The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Galletta D. Event-rate effects in the flanker task: ERPs and task performance in children with and without AD/HD. International Journal of Psychophysiology. 2013;87:340–348. doi: 10.1016/j.ijpsycho.2012.07.170. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL. Development of inhibitory processing during the Go/NoGo Task: A behavioral and event-related potential study of children and adults. Journal of Psychophysiology. 2005;19:11. [Google Scholar]
- Jonkman LM. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood: A Go/Nogo ERP study. Brain Research. 2006;1097:181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 1999;110:1149–55. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review. 2008;18:320–338. doi: 10.1007/s11065-008-9077-7. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Larson MJ, South M, Clayson PE, Clawson A. Cognitive control and conflict adaptation in youth with high-functioning autism. Journal of Child Psychology and Psychiatry. 2012;53:440–448. doi: 10.1111/j.1469-7610.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- Lehto JE, Juujärvi P, Kooistra L, Pulkkinen L. Dimensions of executive functioning: Evidence from children. British Journal of Developmental Psychology. 2003;21:59–80. [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proceedings of the National Academy of Sciences. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, Stieben J. Emotion regulation in the brain: Conceptual issues and directions for developmental research. Child Development. 2004;75:371–376. doi: 10.1111/j.1467-8624.2004.00680.x. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18:430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Leyfer O,T, Folstein SE, Bacalman S, Davis SO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Linden DE. The P300: where in the brain is it produced and what does it tell us? The Neuroscientist. 2005;11:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. Oxford University Press; New York, NY: 2011. [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalography and clinical neurophysiology. 1990;75:528–542. doi: 10.1016/0013-4694(90)90139-b. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive Functions and Developmental Psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Barch DM, Baird JW. The Stroop Task and attention deficits in schizophrenia: A critical evaluation of card and single-trial Stroop methodologies. Neuropsychology. 1999;12:414–425. doi: 10.1037//0894-4105.12.3.414. [DOI] [PubMed] [Google Scholar]
- Puce A, Kalnins RM, Berkovic SF, Bladin PF. Limbic P3 potentials, seizure localization, and surgical pathology in temporal lobe epilepsy. Annals of Neurology. 1989;26:377–385. doi: 10.1002/ana.410260311. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cogndon R. HLM 6 for Windows. Scientific Software International, Inc.; Skokie, IL: 2004. [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discouting in a model of impulsive behavior: Effect of or probability discounting in a model of impulsive behavior: Effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK, Davis-Stober CP. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neuroscience. 2004;5:39. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview–Revised (ADI–R) manual. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top–down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience. 2010;22:1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samyn V, Wiersema JR, Bijttebier P, Roeyers H. Effortful control and executive attention in typical and atypical development: an event-related potential study. Biological Psychology. 2014;99:160–171. doi: 10.1016/j.biopsycho.2014.03.006. doi: 10.1016/j.biopsycho.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Sanderson C, Allen ML. The specificity of inhibitory impairments in autism and their relation to ADHD-type symptoms. Journal of Autism and Developmental Disorders. 2013;43:1065–1079. doi: 10.1007/s10803-012-1650-5. doi: 10.1007/s10803-012-1650-5. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, El-Baz A, Horrell T, Sokhadze G, Carroll T, et al. Impaired Error Monitoring and Correction Function in Autism. Journal of Neurotherapy. 2010;14:79–95. doi: 10.1080/10874201003771561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Todd RM, Lewis MD, Meusel L-A, Zelazo PD. The time course of social-emotional processing in early childhood: ERP responses to facial affect and personal familiarity in a Go-Nogo task. Neuropsychologia. 2008;46:595–613. doi: 10.1016/j.neuropsychologia.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Tye C, Asherson P, Ashwood KL, Azadi B, Bolton P, McLoughlin G. Attention and inhibition in children with ASD, ADHD and co-morbid ASD+ADHD: An event-related potential study. Psychological Medicine. 2014;44:1101–1116. doi: 10.1017/S0033291713001049. doi: 10.1017/S0033291713001049. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vlamings PH, Jonkman LM, Hoeksma MR, Van Engeland H, Kemner C. Reduced error monitoring in children with autism spectrum disorder: An ERP study. European Journal of Neuroscience. 2008;28:399–406. doi: 10.1111/j.1460-9568.2008.06336.x. doi: 10.1111/j.1460-9568.2008.06336.x. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Bernier R, Henderson HA, Johnson MH, Jones EJ, Lerner MD, Westerfield M. Guidelines and best practices for electrophysiological data collection, analysis and reporting in autism. Journal of Autism and Developmental Disorders. 2015;45:425–443. doi: 10.1007/s10803-013-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence-Second edition. Psychological Corporation; San Antonio, TX: 2011. [Google Scholar]
- Willcutt E, Sonuga-Barke E, Nigg J, Sergeant J. Banaschewski T, Rohde LA, editors. Recent developments in neuropsychological models of childhood psychiatric disorders. Biological Child Psychiatry. Recent Trends and Developments. 2008:195–226. [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23:747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of Childhood Cognitive Development. Blackwell; Oxford: 2002. p. 445. doi: 10.1002/9780470996652.ch20. [Google Scholar]