Abstract
Accurate, reliable and reproducible quantification of nucleic acids (DNA/RNA) is important for many diagnostic applications and in routine laboratory testing, for example, for pathogen detection and detection of genetically modified organisms in food. To ensure reliable nucleic acid measurement, reference materials (RM) that are accurately characterised for quantity of target nucleic acid sequences (in copy number or copy number concentration) with a known measurement uncertainty are needed. Recently developed digital polymerase chain reaction (dPCR) technology allows absolute and accurate quantification of nucleic acid target sequences without need for a reference standard. Due to these properties, this technique has the potential to not only improve routine quantitative nucleic acid analysis, but also to be used as a reference method for certification of nucleic acid RM. The article focuses on the use and application of both dPCR and RMs for accurate quantification.
Keywords: Digital PCR, Reference material
In the past decade molecular biology research has rapidly expanded with new high-throughput technologies becoming routine laboratory methods. Until recently, real-time quantitative polymerase chain reaction (qPCR) has been the gold standard for nucleic acid quantification. However, this form of quantification requires comparison of an unknown to a reference standard [1], [2], which means it is often difficult to compare results from different laboratories because of the use of diverse standards or calibrators. To ensure reliable nucleic acid measurement, RMs or controls that are accurately characterised for quantity of target nucleic acid sequences (in copy number) with a known measurement uncertainty are needed to improve accuracy, comparability and traceability in nucleic acid testing.
First introduced in the 1990s [3], [4], dPCR is increasingly being utilised for quantification of DNA targets and rare events [2], [5], [6], pathogen detection [7], [8], viral load testing [9], [10], detection of genetically modified organisms in food [11] and quantification of massively parallel sequence libraries [12]. More recently, an international comparison study [13] conducted between metrology institutes demonstrated excellent comparability between dPCR and qPCR results, and, in general, dPCR resulted in better precision. Digital PCR is now being used as a reference method by several metrology institutes to value assign copy number concentration to RMs [5], [14], [15] providing traceability of calibrant materials used in routine quantitative molecular assays to the mole in International Systems of Units (SI) [16]. Digital PCR involves random distribution of a reaction mix containing target nucleic acid, primers, probe and mastermix into hundreds to millions of uniformly sized nanoliter or picoliter partitions such that some of the partitions contain no copies of the target molecule. Following PCR amplification in each individual partition, the target nucleic acid copy number concentration is calculated using Eq. (1) [2], [6]. Some of the key properties of dPCR compared to qPCR are listed in Table 1.
Table 1.
Key properties of digital PCR compared to real-time PCR.
| Factor | Potential for |
|---|---|
| Direct counting—no calibrant | Improved reproducibility between labs, across time etc. Traceability to SI as copy number units |
| Poisson model | Better measurement precision Better resolving power |
| Partitioning | Increased sensitivity and less assay competition Improved accuracy since less impacted by inhibitors |
Although dPCR is an absolute measurement method, there are a number of factors that could affect the reliability of dPCR quantification [2], [6]. For use as a high accuracy reference method, each factor which could impact on the accuracy of the measurement or that could create a bias in the measurement result needs to be controlled and uncertainty associated with that factor minimised. The equation that is used to calculate the target DNA concentration has three key components Eq. (1): the Poisson model which is derived from number of positive reactions (P) and total number of reactions (R); the reaction volume (VR) and the dilution factor (D) which includes dilution of sample in preparation of PCR and subsequent dilution into the PCR mastermix. Accuracy of measurement of each factor in Eq. (1) will affect accuracy of concentration estimate. Several studies [2], [6], [17], [18] have shown the impact of reaction number, percentage of positive partitions and accuracy of reaction volume on concentration estimates by dPCR. For most precise dPCR measurements, experiments should be designed so that the percentage of positive partitions falls with the optimum window. To minimise potential bias from volumetric pipetting, dilutions of target DNA solution can be prepared gravimetrically. A recent study by Corbisier et al. [17], demonstrated the effect of assigned droplet volume on copy number data and showed that the copy number concentration varies depending on the assigned droplet volume. The study found that the droplet volume determined by optical microscopy (0.834 nL) was smaller than the droplet volume used (0.91 nL) in Quantasoft version 1.3.2.0 (Bio-Rad). In addition to the components in Eq. (1), external factors such as sample preparation, homogeneity and stability of the stock solution and any intermediate dilution solutions, DNA conformation and thermal temperature variability can also impact on dPCR data. At the National Measurement Institute Australia (NMIA) [2], [6] and other metrology institutes, these factors have been investigated and are controlled carefully in the dPCR measurement process when assigning a reference value to a reference material. When dPCR is used as a routine diagnostic tool, a higher measurement uncertainty would normally be acceptable. Hence, some of the factors listed above may not need to be addressed [19]. The dPCR −specific MIQE checklist provides recommendations on essential and desirable information for reporting dPCR results [20].
| (1) |
What is a reference material? The terms ‘reference material’ (RM) and ‘certified reference material’ (CRM) have specific definitions to outline their different properties. A RM is a “material, sufficiently homogenous and stable with respect to one or more specified properties, which has been established to be fit for its intended use in a measurement process” and a CRM is a “reference material characterised by a metrologically valid procedure for one or more specified properties, accompanied by an RM certificate that provides the value of the specified property, its associated uncertainty, and a statement of metrological traceability” [21]. The ISO Guides 34 and 35 detail the requirements for the management system of RM producers and RM certification, respectively [22], [23]. Producing RMs of a high enough quality to allow comparison of results between laboratories and over time requires a significant amount of work. Production of a CRM includes planning, material processing, storage and handling, assessment of homogeneity and stability. The certification process involves characterisation, assignment of a property value, estimation of uncertainty, and preparation of a certificate or report [23]. At NMIA, we have implemented and validated two dPCR systems, the Fluidigm chamber dPCR system [5], [6] and the Bio-Rad droplet dPCR system [2] and applied this technology to value assign copy number concentration to CRMs produced as per the recommendations in ISO Guides 34 and 35 [22], [23]. Table 2 illustrates the steps involved in the CRM production adopted at NMIA. In order to use this technique as a high accuracy reference method for quantification of nucleic acid RMs, at NMIA, in addition to the steps outlined in Table 2, partition volume is measured by optical microscopy imaging and associated uncertainty is determined; target DNA concentration is adjusted so that the proportion of positive partitions is optimal for best precision; and the number of technical replicates is increased to gain confidence in the data generated.
Table 2.
Steps involved in nucleic acid CRM production at NMIA.
| Step | Process |
|---|---|
| Design and synthesis | In silico design and synthesis of candidate DNA RM |
| Verification of sequence | Sanger sequencing |
| Purity of DNA | Massive parallel sequencing, 16S ribosomal RNA gene (16S) gene dPCR, UV spectrophotometry (A230, A260, A280nm) |
| Quantification of bulk DNA | Digital PCR; Isotope dilution mass spectrometry |
| Dilution | Gravimetric dilution |
| Dispensing of aliquots | Manual pipetting, High accuracy acoustic dispensing technology; Robotics |
| Characterisation (copy numbers, ratios) | Digital PCR |
| Homogeneity testing | Digital PCR |
| Stability monitoring Short-term (transport conditions) Long term (storage conditions) |
Digital PCR |
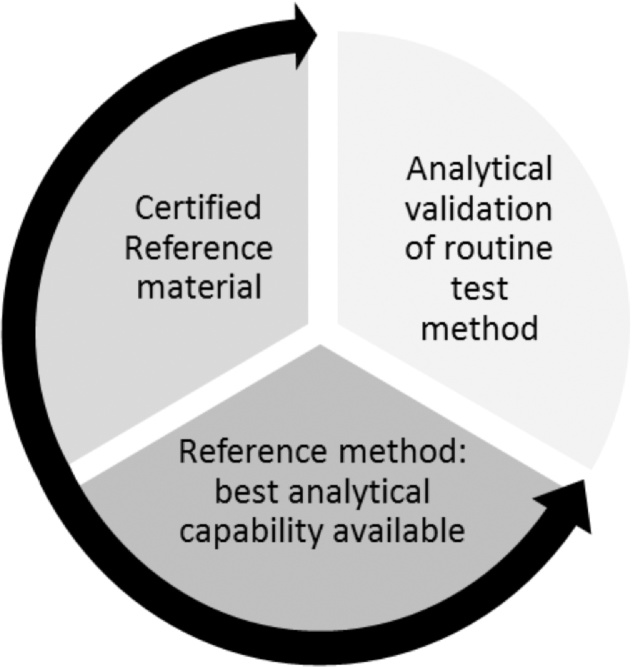
Why are RMs and CRMs important? Reference materials can be used for assessment of a measurement procedure, quality control purposes and to assign values to other materials [21]. Certified reference materials are reference materials characterised by a metrologically valid procedure for use in calibration and method validation providing metrological traceability [21], [24]. A reference method is the starting point of a measurement system. Certified reference materials are needed to establish a valid method for routine testing (Fig. 1).
Fig. 1.
Schematic representation of the use of a reference method and CRM for development and validation of a routine analytical method.
A CRM can provide calibration and an assessment of accuracy due to the defined traceability and measurement uncertainty for such materials. A single material cannot be used for both calibration and validation of results in the same measurement procedure [23]. The use of such materials will allow the laboratory, (a) to gain information about the quality of the reported value; (b) to have a greater level of confidence when making decisions based on the results; (c) to compare results and monitor changes between laboratories over time and (d) to compare the performance of different measurement methods and judge whether a method is fit-for-purpose.
The first example of a DNA CRM certified by dPCR was from National Institute of Standards and Technology (NIST). Standard Reference Material (SRM) 2366 produced at NIST was assigned a certified value to the number of amplifiable copies of human cytomegalovirus (CMV) per microliter (copies per μL) by dPCR using the CP1 assay. The SRM 2366 provides metrological traceability to the SI for laboratory calibrants [14]. Standardisation of calibrants to such CRMs will facilitate traceable and comparable measurement results. Likewise, more recently in 2015, White et al. [15], produced a CRM for the standardisation of BCR-ABL1 measurement of residual disease in chronic myeloid leukaemia. The certified values (copies per μL) for six plasmids (ERM-AD623a-f) were assigned by dPCR measurements [15].
Conclusion
The accuracy of PCR based measurements is vital for reliable and cost-effective diagnosis and treatment of disease. Digital PCR is a powerful quantitative method which has been used as a reference method for certification of nucleic acid CRMs. Although dPCR is an absolute measurement method, factors that could affect the reliability of dPCR data need to be taken into consideration to improve the overall quality of measurement process and to reduce bias and uncertainty. The availability of DNA RMs and CRMs will improve accuracy and confidence in molecular testing.
Conflict of interest
“The authors declare that there are no conflicts of interest.”
Acknowledgements
We thank Dr. Lindsey Mackay and Dr. Leonardo Pinheiro for reviewing the manuscript and providing valuable suggestions.
References
- 1.Griffiths K.R., Burke D.G., Emslie K.R. Quantitative polymerase chain reaction: a framework for improving the quality of results and estimating uncertainty of measurement. Anal. Methods. 2011;3:2201–2211. [Google Scholar]
- 2.Pinheiro L.B. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal. Chem. 2012;84:1003–1011. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sykes P.J. Quantitation of targets for PCR by use of limiting dilution. Biotechniques. 1992;13:444–449. [PubMed] [Google Scholar]
- 4.Vogelstein B., Kinzler K.W. Digital PCR. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9236–9241. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat S. Comparison of methods for accurate quantification of DNA mass concentration with traceability to the international system of units. Anal. Chem. 2010;82:7185–7192. doi: 10.1021/ac100845m. [DOI] [PubMed] [Google Scholar]
- 6.Bhat S. Single molecule detection in nanofluidic digital array enables accurate measurement of DNA copy number. Anal. Bioanal. Chem. 2009;394:457–467. doi: 10.1007/s00216-009-2729-5. [DOI] [PubMed] [Google Scholar]
- 7.Devonshire A.S. Highly reproducible absolute quantification of Mycobacterium tuberculosis complex by digital PCR. Anal. Chem. 2015;87(7):3706–3713. doi: 10.1021/ac5041617. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y., Raith M.R., Griffith J.F. Droplet digital PCR for simultaneous quantification of general and human-associated fecal indicators for water quality assessment. Water Res. 2015;70:337–349. doi: 10.1016/j.watres.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Hayden R.T. Comparison of droplet digital PCR to real-time PCR for quantitative detection of cytomegalovirus. J. Clin. Microbiol. 2013;51:540–546. doi: 10.1128/JCM.02620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White R.A., Quake S.R., Curr K. Digital PCR provides absolute quantitation of viral load for an occult RNA virus. J. Virol. Methods. 2012;179(1):45–50. doi: 10.1016/j.jviromet.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Morisset D. Quantitative analysis of food and feed samples with droplet digital PCR. PLoS One. 2013;8(5):e62583. doi: 10.1371/journal.pone.0062583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurie M.T. Simultaneous digital quantification and fluorescence-based size characterization of massively parallel sequencing libraries. Biotechniques. 2013;55(2):61–67. doi: 10.2144/000114063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbisier P. CCQM-K86/P113.1: relative quantification of genomic DNA fragments extracted from a biological tissue. Metrologia. 2012;49 [Google Scholar]
- 14.Haynes R.J. Standard reference material 2366 for measurement of human cytomegalovirus DNA. J. Mol. Diagn. 2013;15(2):177–185. doi: 10.1016/j.jmoldx.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 15.White H., Corbisier D.L., Hall P., Lin V., Mazoua F., Trapmann S., Aggerholm S. A certified plasmid reference material for the standardisation of BCR-ABL1 mRNA quantification by real-time quantitative PCR. Leukemia. 2015;29(2):369–376. doi: 10.1038/leu.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke D.G. Digital polymerase chain reaction measured pUC19 marker as calibrant for HPLC measurement of DNA quantity. Anal. Chem. 2013;85(3):1657–1664. doi: 10.1021/ac302925f. [DOI] [PubMed] [Google Scholar]
- 17.Corbisier P. DNA copy number concentration measured by digital and droplet digital quantitative PCR using certified reference materials. Anal. Bioanal. Chem. 2015;407(7):1831–1840. doi: 10.1007/s00216-015-8458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong L. Comparison of four digital PCR platforms for accurate quantification of DNA copy number of a certified plasmid DNA reference material. Sci. Rep. 2015;5:13174. doi: 10.1038/srep13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huggett J.F., Cowen S., Foy C.A. Considertaions for digital PCR as an accurate molecular diagnostic tool. Clin. Chem. 2015;61(1):79–88. doi: 10.1373/clinchem.2014.221366. [DOI] [PubMed] [Google Scholar]
- 20.Huggett J.F. The digital MIQE guidelines: minimum information for publication of quantitative digital PCR experiments. Clin. Chem. 2013;59(6):892–902. doi: 10.1373/clinchem.2013.206375. [DOI] [PubMed] [Google Scholar]
- 21.ISO, Guide 30, Reference materials—selected terms and definitions. 2015, Geneva, Switzerland.
- 22.ISO, Guide 35, Reference materials—General and statistical principles for certification. 2006, Geneva, Switzerland.
- 23.I.S.O. Guide 34, General requirements for the competence of reference material producers. 2009, Geneva, Switzerland.
- 24.I.S.O. Guide 80, Guidance for the in-house preparation of quality control materials (QCMs) 2014: Geneva, Switzerland.