Abstract
Digital PCR (dPCR) has been reported to be more precise and sensitive than real-time quantitative PCR (qPCR) in a variety of models and applications. However, in the majority of commercially available dPCR platforms, the dynamic range is dependent on the number of partitions analysed and so is typically limited to four orders of magnitude; reduced compared with the typical seven orders achievable by qPCR. Using two different biological models (HIV DNA analysis and KRAS genotyping), we have demonstrated that the RainDrop Digital PCR System (RainDance Technologies) is capable of performing accurate and precise quantification over six orders of magnitude thereby approaching that achievable by qPCR.
Digital PCR (dPCR) is a sensitive, precise and robust method that could enable quantification of a range of novel biomarker measurements [1]. However, the method is not without its disadvantages that include cost, technical complexity and a reduced dynamic range when compared with real-time quantitative PCR (qPCR).
For dPCR, quantification is typically performed by determining the proportion of positive partitions in the reaction and applying a Poisson correction to account for the fact that at higher DNA concentrations, a positive partition will be more likely to contain more than one molecule [2]. Alternatively, if the DNA concentration is low enough to ensure single molecule occupancy of each positive partition, the Poisson correction is not necessary and the number of positive partitions alone enables quantification.
With both approaches, the dynamic range is determined by the total number of partitions in the reaction. When considering dynamic range, the RainDrop Digital PCR System (RainDance Technologies) could theoretically compete with qPCR as it can generate up to ten million partitions per reaction, giving a potential upper limit in excess of 100 million molecules per reaction if Poisson correction is applied. However, current the recommendations from RainDance are to use low partition occupancy (<10% positive partitions) which makes Poisson correction unnecessary but lessens the dynamic range.
dPCR accuracy is dependent on a number of physical factors such as the partition volume and, when applying a Poisson correction, the partition volume variation should either be small or factored into the calculation [3], [4]. We hypothesised that the low occupancy recommendation for the RainDance platform could be due to the challenge of maintaining precise volume of the very small ∼5 pL partitions at higher DNA concentrations, as increased volume variation would result in an underestimation of the DNA copy number concentration [3].
To investigate this hypothesis, we performed a series of dynamic range experiments using two target molecules based on HIV DNA analysis and KRAS genotyping (Fig. S1). Both target molecules were dsDNA fragments: a 300 bp fragment containing a region of the LTR-gag junction from the HIV HXB2 reference genome (NCBI Accession K03455.1, bases 451 to 750) and a 186 bp fragment containing the KRAS G12D point mutation (NCBI Accession NG_007524.1, bases 10458 to 10671) (Fig. S1). The target fragments were initially quantified using the Qubit 2.0 fluorimeter with the High Sensitivity DNA assay (ThermoFisher Scientific) and converted to copy number concentration using a standard method [5].
For each target fragment, a seven-point 10-fold calibration curve was volumetrically prepared from ∼50 million to ∼50 copies per 50 μL PCR reaction (approximate λ range of 5 to 0.000005) before storing each dilution as single use 50 μL aliquots at −20 °C (Table 1). To mimic the interfering sequences that are present in samples used for HIV analysis and KRAS genotyping, a constant background of fragmented human gDNA (Cambio; 0.25 ng/μL final concentration), prepared in TE buffer, was added to the dilution series. The fragmentation state was chosen to enable droplet formation (high concentration, high molecular weight gDNA interferes with droplet formation and must be fragmented prior to droplet generation) as well as mimicking the template sizes commonly found in cell free DNA [6]. The dilution series was analysed simultaneously by qPCR (ABI 7900HT) and dPCR (RainDance RainDrop) with single replicates for each dilution and the whole experiment was repeated on five days (Tables 2 & S1, Figs. S2, S3 & S4).
Table 1.
Template Dilutions Workflow.
| Nominal copies/reaction | [Plasmid] (c/μL) | Plasmid vol (μL) | Diluent vol (μL) | Total vol (μL) |
|---|---|---|---|---|
| N/A | 1.00E + 08 | 200.00 | ||
| N/A | 1.00E + 07 | 20.0 | 180.0 | 200.0 |
| 50000000 | 2.50E + 06 | 100.0 | 300.0 | 400.0 |
| 5000000 | 2.50E + 05 | 40.0 | 360.0 | 400.0 |
| 500000 | 2.50E + 04 | 40.0 | 360.0 | 400.0 |
| 50000 | 2.50E + 03 | 40.0 | 360.0 | 400.0 |
| 5000 | 2.50E + 02 | 40.0 | 360.0 | 400.0 |
| 500 | 2.50E + 01 | 40.0 | 360.0 | 400.0 |
| 50 | 2.50E + 00 | 40.0 | 360.0 | 400.0 |
| 0 | 0.00E + 00 | 0.0 | 400.0 | 400.0 |
Each dilution was prepared volumetrically from a master stock of 1 × 108 copies/μL. The dilutions were stored in single use 50 μL aliquots at 20 °C for the duration of the study (1 month). For each dPCR and qPCR experiment, 20 μL was added to the 50 μL reaction.
Table 2.
Experimental set up.
| Platform | qPCR | dPCR |
|---|---|---|
| Mastermix | TaqMan Genotyping mastermix (ThermoFisherScientific) | TaqMan Genotyping mastermix (ThermoFisherScientific) |
| Other reagents | N/A | RainDrop Stabilizer (2 μL per 50 μL rxn) |
| KRAS G12D/WT duplex assay [9] | 900 nM KRAS Forward: 5′-AGGCCTGCTGAAAATGACTGAATAT-3′ |
|
| 900 nM KRAS Reverse: 5′-GCTGTATCGTCAAGGCACTCTT-3′ |
||
| 250 nM KRAS WT Probe: 5′-[VIC]TTGGAGCTGGTGGCGT[NFQ/MGB]-3′ |
||
| 250 nM KRAS G12D Probe: 5′-[FAM]TGGAGCTGATGGCGT[NFQ/MGB]-3′ |
||
| HIV LTR-gag/PDH duplex assay | 900 nM LTR-gag Forward: 5'-GCCTCAATAAAGCTTGCCTTGA-3' | |
| 900 nM LTR-gag Reverse: 5’-GGCGCCACTGCTAGAGATTTT-3’ |
||
| 200 nM LTR-gag Probe: 5’-[FAM]TGTGACTCTGGTAACTAGAGATCCCTCAGAC[BHQ1]-3’ |
||
| 900 nM PDH Forward: 5′-TGAAAGTTATACAAAATTGAGGTCACTGTT-3′ |
||
| 900 nM PDH Revers: 5′-TCCACAGCCCTCGACTAACC-3′ |
||
| 200 nM PDH Probe: 5′-[VIC]CCCCCAGATACACTTAAGGGA[MGB]-3′ |
||
| Oligonucleotide purification method | HPLC | |
| Sample volume | 20 μL | 22.5 μL |
| Total reaction volume prepared | 50 μL | 55 μL |
| Consumable | 96-well plate | RainDrop source chip |
| Reaction volume loaded | 50 μL | 50 μL |
| Partition volume | N/A | 5 pL |
| Partition number | N/A | Up to 10 million |
| Instrumentation | ABI 7900HT (ThermoFisherScientific) | Droplets generated: RainDrop Source Instrument (RainDance). Thermal cycling: DNA Engine Tetrad (Bio-rad). Droplets read: RainDrop Sense instrument (Instrument Control Software v2.1.3.11157) (RainDance) |
| KRAS Cycling Parameters | 95 °C for 15 min, followed by 45 cycles of 94 °C for 60 s and 64 °C for 60 s | 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 64 °C for 60 s, then 98 °C for 10 min, 12 °C for 15 min, and 4 °C hold |
| HIV/PDH Cycling Parameters | 95 °C for 15 min, followed by 45 cycles of 94 °C for 60 s and 60 °C for 60 s | 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 60 s, then 98 °C for 10 min, 12 °C for 10 min, and 4 °C hold |
| Analysis software | SDS v2.4 (ThermoFisherScientific) | RainDrop Analyst II software (1.0.0.520) |
| Analysis parameters | Auto baseline setting, thresholds set manually and applied to all samples within an experiment | Droplets classified independently using polygonal gates, which were then universally applied across all samples within an experiment |
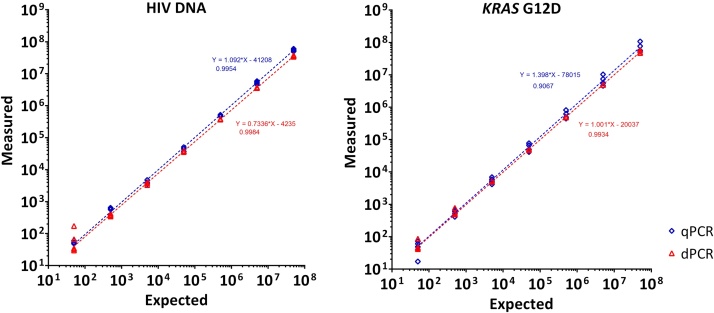
Quantification by qPCR was performed and the slope and intercept of the calibration curve was calculated from the dilution series. The copy number concentration for each dilution point was re-calculated from the slope; a good linear dynamic range was observed over six orders of magnitude for both target fragments (Fig. 1 and Table S2). Quantification by dPCR was performed by applying the Poisson correction to the proportion of positive partitions in each reaction. Comparable linear dynamic ranges were observed between both targets and platform (Fig. 1) demonstrating firstly, that the partition volume precision is high in the RainDrop Digital PCR System and secondly, that the Poisson correction is suitable for this instrument with high occupancy partitions.
Fig. 1.
Dynamic range experiments using qPCR and dPCR to measure HIV DNA and the KRAS G12D single nucleotide variant. Each plot compares measured versus expected copies per 50 μL reaction mix of a 10-fold standard curve performed by qPCR and dPCR. Each standard curve dilution was measured with a single reaction and repeated on five different days.
In previous applications of dPCR, dilution has been necessary to quantify higher copy number samples [7]. Crucially this requires a prior knowledge of the concentration range necessitating some initial analysis of the sample. We have demonstrated here that dPCR is capable of directly quantifying DNA over a six log linear dynamic range thereby approaching the seven logs typically achievable by qPCR. A further benefit is that dPCR is an absolute method as the DNA molecules are being directly counted.
A method that can precisely quantify specific nucleic acid molecules over a large dynamic range has numerous applications, which is one of the main reasons that qPCR is widely used in research and clinical laboratories. While qPCR can be precise, its accuracy is dependent on a calibrator. Quantification of the initial calibrator, its commutability, and the fact that the uncertainty of the calibration is seldom considered, limits the accuracy and reproducibility of qPCR. As dPCR directly counts the number of DNA molecules in a sample it does not need the same level of calibration as qPCR and so is more reproducible [8]. Current dPCR experiments are more complex to perform than qPCR, but the digital readout is much simpler to analyse.
With further development to reduce the technical complexity, dPCR could become the method of choice for research and clinical use. Furthermore the digital readout would also make the method suitable for automation both in routine testing laboratories and ultimately point of care. The data presented here demonstrates that a commercially available dPCR platform can perform quantification over a broad dynamic range approaching that achievable by qPCR in a single reaction.
Acknowledgments
We acknowledge Adam Corner of RainDance Technologies, Inc. for advice and access to the RainDrop instrument. The work described in this paper was funded in part by the UK government Department for Business, Energy & Industrial Strategy (BEIS) and the European Metrology Research Programme (EMRP) joint research project (SIB54) Bio-SITrace (http://biositrace.lgcgroup.com) which is jointly funded by the EMRP participating countries within EURAMET and the European Union.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bdq.2016.10.001.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Day E., Dear P.H., McCaughan F. Digital PCR strategies in the development and analysis of molecular biomarkers for personalized medicine. Methods. 2012 doi: 10.1016/j.ymeth.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Huggett J.F., Foy C.A., Benes V., Emslie K., Garson J.A., Haynes R., Hellemans J., Kubista M., Mueller R.D., Nolan T. The Digital MIQE Guidelines: Minimum Information for Publication of Quantitative Digital PCR Experiments. Clin. Chem. 2013;59:892–902. doi: 10.1373/clinchem.2013.206375. [DOI] [PubMed] [Google Scholar]
- 3.Huggett J.F., Cowen S., Foy C.A. Considerations for digital PCR as an accurate molecular diagnostic tool. Clin. Chem. 2015;61:79–88. doi: 10.1373/clinchem.2014.221366. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs B.K., Goetghebeur E., Clement L. Impact of variance components on reliability of absolute quantification using digital PCR. BMC Bioinf. 2014;15:283. doi: 10.1186/1471-2105-15-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhanasekaran S., Doherty T.M., Kenneth J. Comparison of different standards for real-time PCR-based absolute quantification. J. Immunol. Methods. 2010;354:34–39. doi: 10.1016/j.jim.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Devonshire A.S., Whale A.S., Gutteridge A., Jones G., Cowen S., Foy C.A., Huggett J.F. Towards standardisation of cell-free DNA measurement in plasma: controls for extraction efficiency, fragment size bias and quantification. Anal. Bioanal. Chem. 2014;406:6499–6512. doi: 10.1007/s00216-014-7835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbisier P., Pinheiro L., Mazoua S., Kortekaas A.M., Chung P.Y., Gerganova T., Roebben G., Emons H., Emslie K. DNA copy number concentration measured by digital and droplet digital quantitative PCR using certified reference materials. Anal. Bioanal. Chem. 2015;407:1831–1840. doi: 10.1007/s00216-015-8458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devonshire A.S., Honeyborne I., Gutteridge A., Whale A.S., Nixon G., Wilson P., Jones G., McHugh T.D., Foy C.A., Huggett J.F. Highly reproducible absolute quantification of Mycobacterium tuberculosis complex by digital PCR. Anal. Chem. 2015 doi: 10.1021/ac5041617. [DOI] [PubMed] [Google Scholar]
- 9.Taly V., Pekin D., Benhaim L., Kotsopoulos S.K., Le Corre D., Li X., Atochin I., Link D.R., Griffiths A.D., Pallier K. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin. Chem. 2013;59:1722–1731. doi: 10.1373/clinchem.2013.206359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.