Abstract
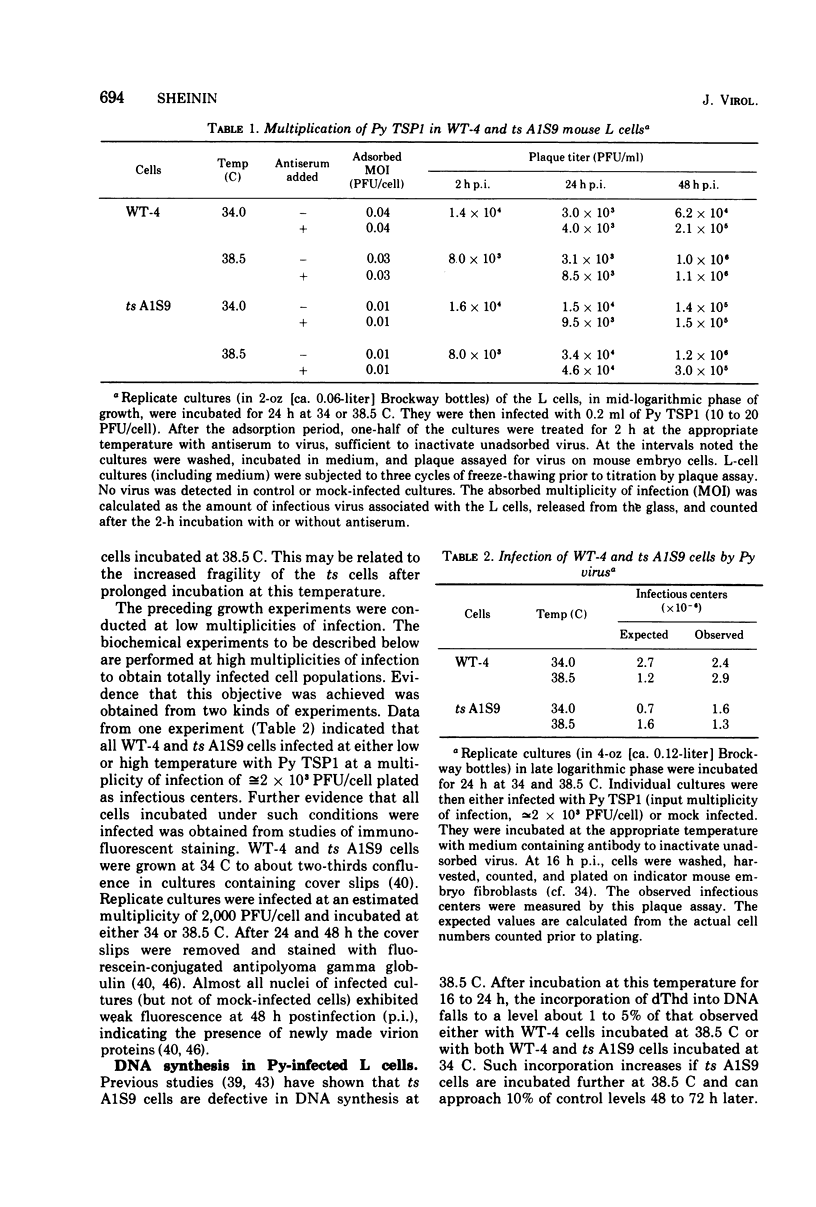
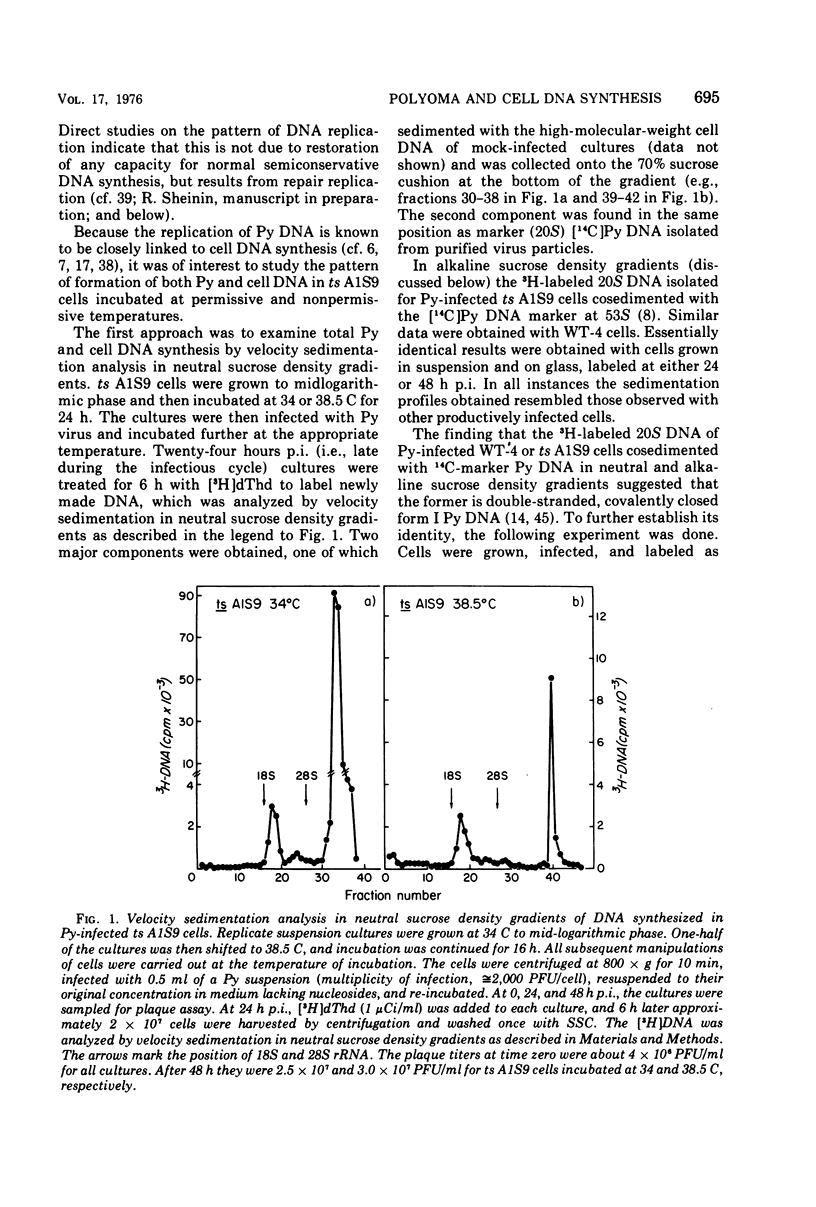
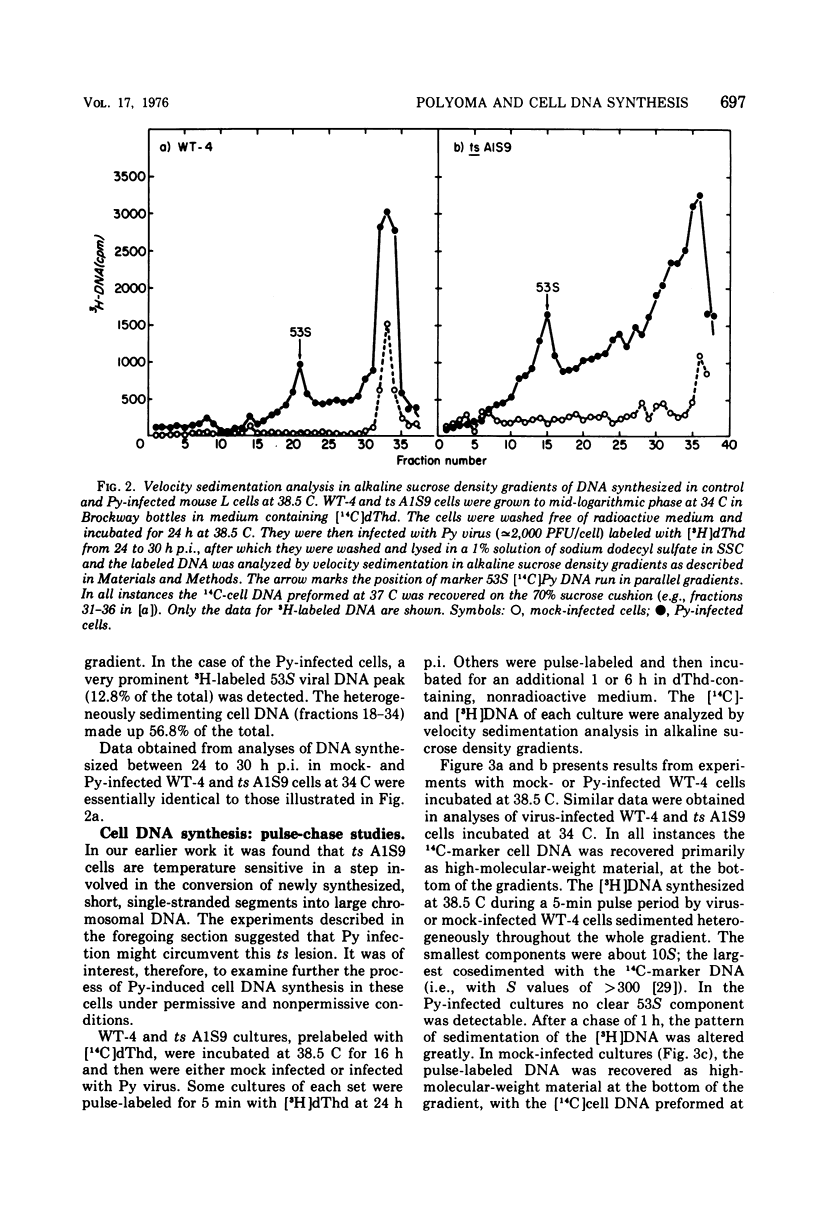
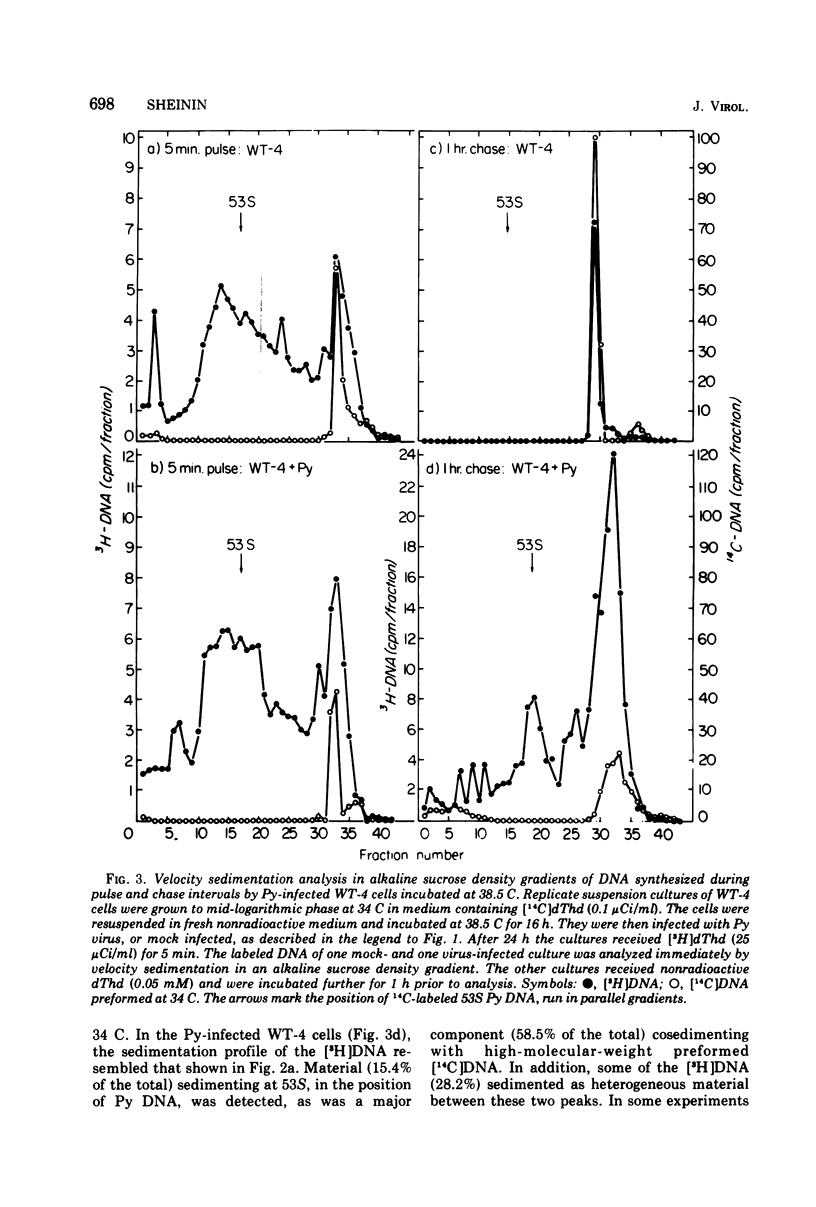
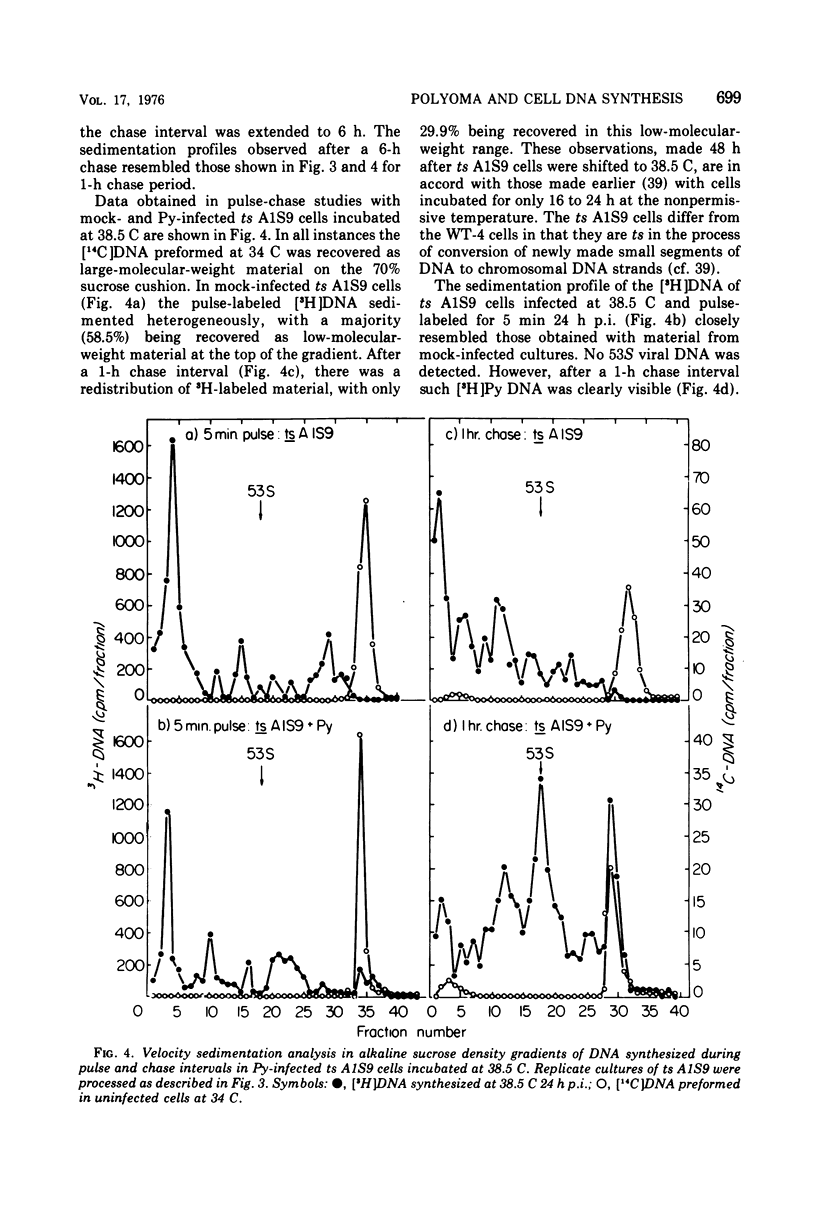
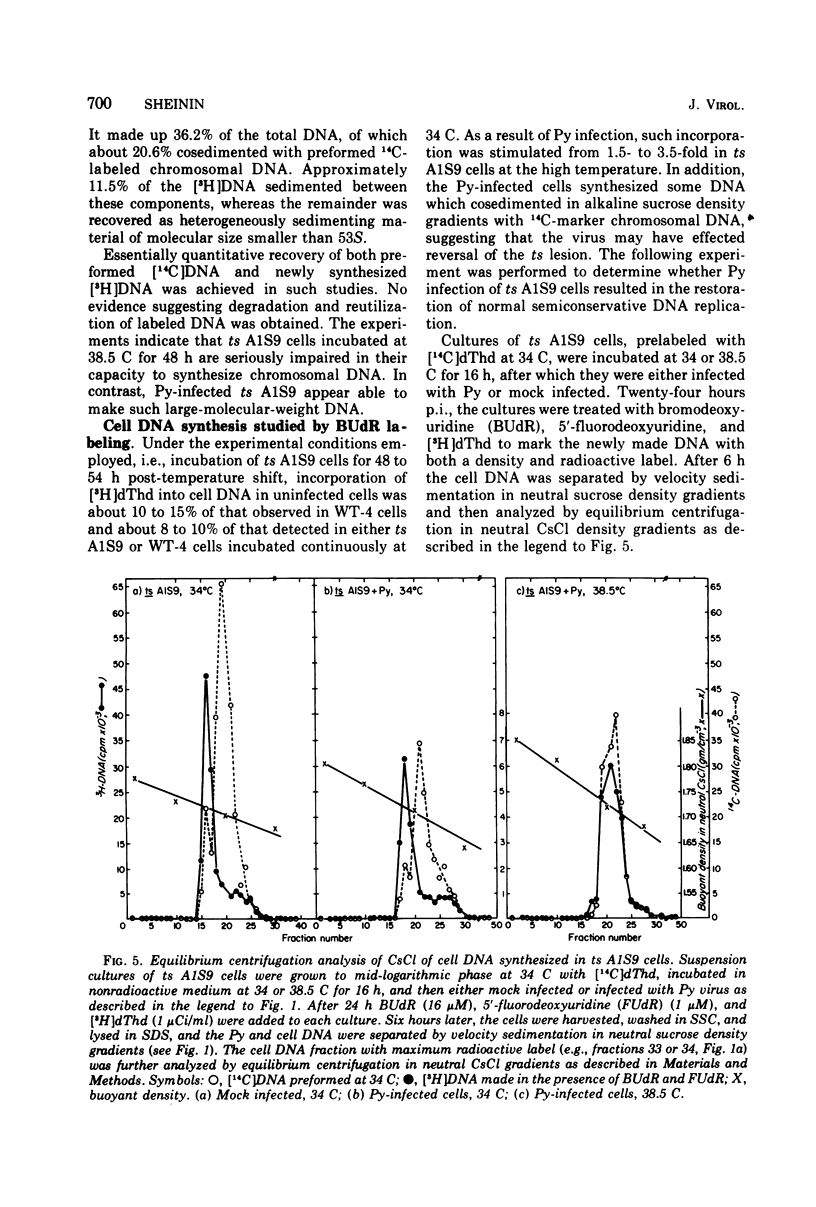
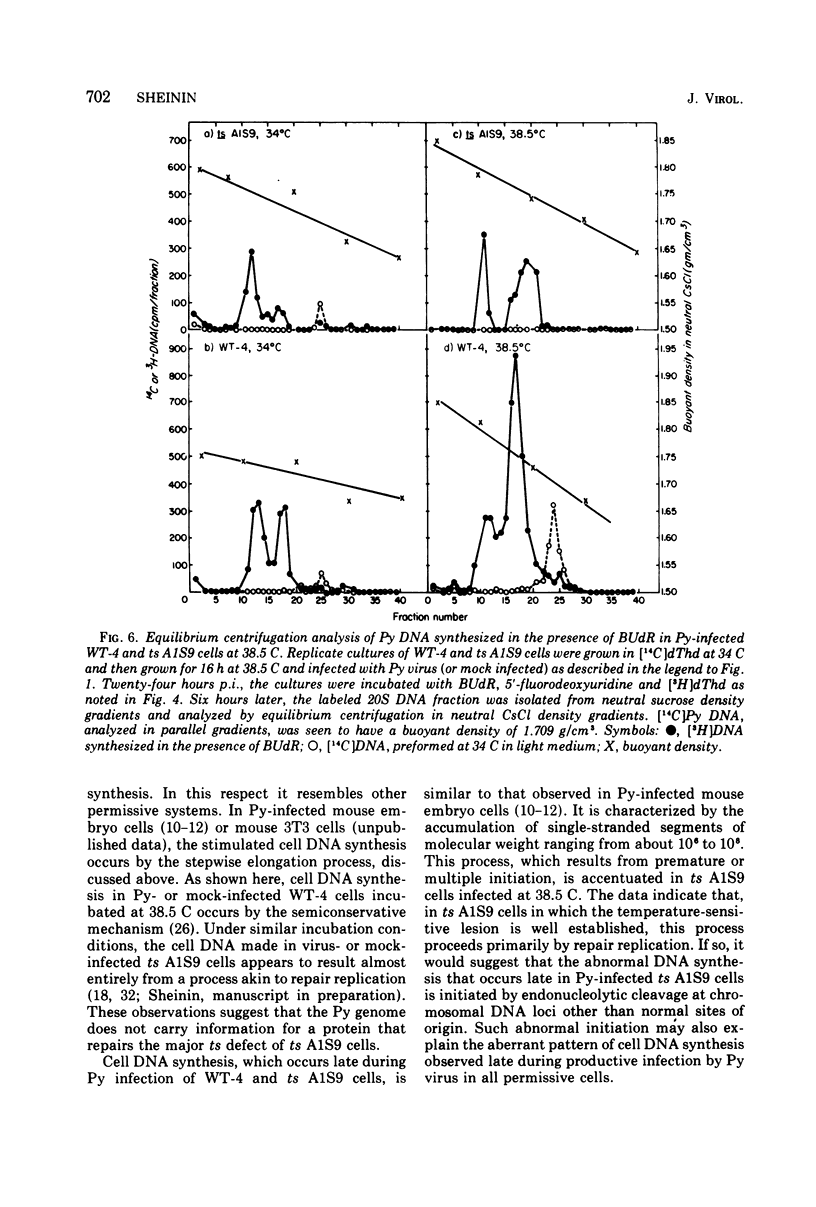
Polyoma (Py) virus multiplies, at 34 and 38.5 C, in wild-type (WT-4) and in ts A1S9 mouse L cells, which are temperature sensitive for growth and for DNA replication (R. Sheinin, 1976; L. H. Thompson et al., 1970). De novo synthesis of double-stranded, fully covalently closed Py DNA has been shown to proceed by semiconservative replication in WT-4 and ts A1S9 cells at the permissive and nonpermissive temperatures. Cell DNA is made late during infection, by both cell types and at both temperatures. Semiconservative replication of cell DNA proceeds in Py-infected WT-4 cells incubated at 34 or at 38.5 C and in Py-infected ts A1S9 cells incubated at 34 C. In virus-infected ts A1S9 cells incubated at 38.5 C, cell DNA synthesis appears to proceed almost entirely by a process analogous to repair replication. The inability of ts A1S9 cells to produce large-molecular-weight chromosomal DNA strands, at 38.5 C, by the normal mechanism is not overcome by Py infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDUZZI P., MORGAN H. R. Growth of SE polyoma virus in primary and established cell cultures. Proc Soc Exp Biol Med. 1960 May;104:23–25. doi: 10.3181/00379727-104-25713. [DOI] [PubMed] [Google Scholar]
- BARSKI G., CORNEFERT F. Response of different mouse cell strains to polyoma infection in vitro. Latency and self-inhibition effect in infected cultures. J Natl Cancer Inst. 1962 Apr;28:823–843. [PubMed] [Google Scholar]
- Blackstein M. E., Stanners C. P., Farmilo A. J. Heterogeneity of polyoma virus DNA: isolation and characterization of non-infectious small supercoiled molecules. J Mol Biol. 1969 Jun 14;42(2):301–313. doi: 10.1016/0022-2836(69)90045-x. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. Is a specific protein responsible for the supercoiling of polyoma DNA? Nature. 1972 Jan 14;235(5333):105–107. doi: 10.1038/235105a0. [DOI] [PubMed] [Google Scholar]
- Branton P. E., Cheevers W. P., Sheinin R. The effect of cycloheximide on DNA synthesis in cells productively-infected with polyoma virus. Virology. 1970 Dec;42(4):979–992. doi: 10.1016/0042-6822(70)90346-6. [DOI] [PubMed] [Google Scholar]
- Branton P. E., Sheinin R. Control of DNA synthesis in cells infected with polyoma virus. Virology. 1968 Dec;36(4):652–661. doi: 10.1016/0042-6822(68)90196-7. [DOI] [PubMed] [Google Scholar]
- Branton P. E., Sheinin R. Studies on the replication of polyoma DNA: physicochemical properties of viral DNA synthesized when protein synthesis is inhibited. Can J Biochem. 1973 Mar;51(3):305–317. doi: 10.1139/o73-036. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Branton P. E., Sheinin R. Characterization of abnormal DNA formed in polyoma virus-infected cells. Virology. 1970 Mar;40(3):768–772. doi: 10.1016/0042-6822(70)90227-8. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Branton P. E., Sheinin R. Formation of cellular deoxyribonucleic acid during productive polyoma virus infection. J Virol. 1970 Nov;6(5):573–582. doi: 10.1128/jvi.6.5.573-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., Kowalski J., Yu K. K. Synthesis of high-molecular-weight cellular DNA in productive polyoma virus infection. J Mol Biol. 1972 Mar 28;65(2):347–364. doi: 10.1016/0022-2836(72)90286-0. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P. Protein and messenger RNA requirements for superhelicity of polyoma virus DNA. Nat New Biol. 1973 Apr 18;242(120):202–204. doi: 10.1038/newbio242202a0. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Sheinin R. RNA synthesis in polyoma virus-infected cells. I. Pattern of formation of polyribosome-associated messenger RNA during productive infection. Can J Biochem. 1970 Oct;48(10):1104–1112. doi: 10.1139/o70-174. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. The state of the DNA of polyoma virus and SV40 in transformed cells. Cold Spring Harb Symp Quant Biol. 1968;33:777–783. doi: 10.1101/sqb.1968.033.01.089. [DOI] [PubMed] [Google Scholar]
- Echkart W. Genetics of DNA tumor viruses. Annu Rev Genet. 1974;8:301–317. doi: 10.1146/annurev.ge.08.120174.001505. [DOI] [PubMed] [Google Scholar]
- Edenberg H., Hanawalt P. Size of repair patches in the DNA of ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1972 Jul 20;272(3):361–372. doi: 10.1016/0005-2787(72)90389-9. [DOI] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Fried M. Characterization of a temperature-sensitive mutant of polyoma virus. Virology. 1970 Mar;40(3):605–617. doi: 10.1016/0042-6822(70)90205-9. [DOI] [PubMed] [Google Scholar]
- Friedmann T. Genetic economy of polyoma virus: capsid proteins are cleavage products of same viral gene. Proc Natl Acad Sci U S A. 1974 Feb;71(2):257–259. doi: 10.1073/pnas.71.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel K. Virus tumor antigens: specific fingerprints? Cancer Res. 1966 Sep;26(9):2018–2024. [PubMed] [Google Scholar]
- Hirt B. Evidence for semiconservative replication of circular polyoma DNA. Proc Natl Acad Sci U S A. 1966 Apr;55(4):997–1004. doi: 10.1073/pnas.55.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Horwitz H. Discontinuous DNA synthesis in mammalian cells. Cold Spring Harb Symp Quant Biol. 1974;38:233–238. doi: 10.1101/sqb.1974.038.01.026. [DOI] [PubMed] [Google Scholar]
- KIT S. Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J Mol Biol. 1961 Dec;3:711–716. doi: 10.1016/s0022-2836(61)80075-2. [DOI] [PubMed] [Google Scholar]
- Lombardi P. S., Balduzzi P., Hare J. D., Morgan H. R. Mechanism of the development of a stable carrier system of L cells with polyoma virus. J Natl Cancer Inst. 1970 Jul;45(1):171–178. [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Molecular weight analysis of mammalian DNA. Biochem Biophys Res Commun. 1972 Jan 31;46(2):898–904. doi: 10.1016/s0006-291x(72)80226-2. [DOI] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A. Studies on the structure and formation of polyoma DNA replicative intermediates. J Mol Biol. 1971 Nov 14;61(3):543–563. doi: 10.1016/0022-2836(71)90064-7. [DOI] [PubMed] [Google Scholar]
- Paulin D., Cuzin F. Polyoma virus T antigen. I. Synthesis of modified heat-labile T angiten in cells transformed with the ts-a mutant. J Virol. 1975 Feb;15(2):393–397. doi: 10.1128/jvi.15.2.393-397.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faurès-Miller A., Errera M. Isolation of replicating DNA segments from Chinese hamster cells by density equilibrium centrifugation. J Mol Biol. 1974 Dec 15;90(3):491–508. doi: 10.1016/0022-2836(74)90230-7. [DOI] [PubMed] [Google Scholar]
- SHEININ R. Procedures for the purification of polyoma T virus. Virology. 1962 Jul;17:426–440. doi: 10.1016/0042-6822(62)90138-1. [DOI] [PubMed] [Google Scholar]
- SHEININ R., QUINN P. A. EFFECT OF POLYOMA VIRUS ON THE REPLICATIVE MECHANISM OF MOUSE EMBRYO CELLS. Virology. 1965 May;26:73–84. doi: 10.1016/0042-6822(65)90027-9. [DOI] [PubMed] [Google Scholar]
- SHEININ R. STUDIES ON THE EFFECT OF 5-FLUORO-2'-DEOXYURIDINE ON POLYAMA T FORMATION IN MOUSE EMBRYO CELLS. Virology. 1964 Mar;22:368–376. doi: 10.1016/0042-6822(64)90027-3. [DOI] [PubMed] [Google Scholar]
- STANNERS C. P. STUDIES ON THE TRANSFORMATION OF HAMSTER EMBRYO CELLS IN CULTURE BY POLYOMA VIRUS. II. SELECTIVE TECHNIQUES FOR THE DETECTION OF TRANSFORMED CELLS. Virology. 1963 Nov;21:464–476. doi: 10.1016/0042-6822(63)90207-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J. Transformation by polyoma virus and simian virus 40. Adv Cancer Res. 1972;16:141–180. [PubMed] [Google Scholar]
- Sheinin R. Deoxyribonucleic acid synthesis in cells replicating polyoma virus. Virology. 1966 Apr;28(4):621–632. doi: 10.1016/0042-6822(66)90247-9. [DOI] [PubMed] [Google Scholar]
- Sheinin R. Preliminary characterization of the temperature-sensitive defect in DNA replication in a mutant mouse L cell. Cell. 1976 Jan;7(1):49–57. doi: 10.1016/0092-8674(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Till J. E., Siminovitch L., Whitmore G. F. Isolation of temperature-sensitive mutants of L-cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):377–384. doi: 10.1073/pnas.66.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türler H. Interactions of Polyoma and Mouse DNAs III. Mechanism of Polyoma Pseudovirion Formation. J Virol. 1975 May;15(5):1158–1167. doi: 10.1128/jvi.15.5.1158-1167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Watson R. Early and late helix-coil transitions in closed circular DNA. The number of superhelical turns in polyoma DNA. J Mol Biol. 1968 Apr 14;33(1):173–197. doi: 10.1016/0022-2836(68)90287-8. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M. G., SHEININ R. Cytological studies of mouse embryo cells infected with polyoma virus, using acridine orange and fluorescent antibody. Virology. 1961 Mar;13:368–370. doi: 10.1016/0042-6822(61)90158-1. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]



