Abstract
Objectives
The excitability of primary motor cortex (M1) can be modulated by applying low-frequency repetitive transcranial magnetic stimulation (rTMS) over M1 or premotor cortex (PMC). A comparison of inhibitory effect between the two locations has been reported with inconsistent results. This study compared the response secondary to rTMS applied over M1, PMC and a combined PMC+M1 stimulation approach which first targets stimulation over PMC then M1.
Materials and Methods
Ten healthy participants were recruited for a randomized, cross-over design with a 1-week wash-out between visits. Each visit consisted of a pre-test, an rTMS intervention and a post-test. Outcome measures included short interval intracortical inhibition (SICI), intracortical facilitation (ICF) and cortical silent period (CSP). Participants received one of the three interventions in random order at each visit including: 1-Hz rTMS at 90% of resting motor threshold to: M1 (1200 pulses), PMC (1200 pulses) and PMC+M1 (600 pulses each, 1200 total).
Results
PMC+M1 stimulation resulted in significantly greater inhibition than the other locations for ICF (P = 0.005) and CSP (P < 0.001); for SICI, increased inhibition (group effect) was not observed after any of the three interventions and there was no significant difference between the three interventions.
Conclusion
The results indicate that PMC+M1 stimulation may modulate brain excitability differently from PMC or M1 alone. CSP was the assessment measure most sensitive to changes in inhibition and was able to distinguish between different inhibitory protocols. This work presents a novel procedure that may have positive implications for therapeutic interventions.
Keywords: neuromodulation, repetitive transcranial magnetic stimulation, primary motor cortex, premotor cortex, brain excitability
Introduction
The excitability of the primary motor cortex (M1) can be modulated by repetitive transcranial magnetic stimulation (rTMS) beyond the stimulation period (1). Low-frequency rTMS (0.2Hz to 1Hz) generally results in decreased cortical excitability (2, 3). The degree of inhibition can be assessed by the MEPs induced by different single or paired-pulse transcranial magnetic stimulation (TMS) protocols, e.g. short intra-cortical inhibition (SICI), intra-cortical facilitation (ICF), and cortical silent period (CSP) (4). The ability to non-invasively induce an inhibitory effect to a given brain region makes low-frequency rTMS an area of investigation as a therapeutic tool for patients with neurological disorders that have deficient or excessive cortical inhibition (5–9).
There are two rTMS target sites that are primarily used to inhibit the M1: 1) M1 (10–12) and 2) premotor cortex (PMC) (9, 13, 14). rTMS applied directly over M1 acts on the cortical spinal tract neurons (15) and induces a ‘long term depression (LTD)-like’ inhibition (1). The mechanism of M1 inhibition through rTMS delivered over PMC (indirect inhibition) is not clear. Indeed it is known that due to interconnections between neural areas, inhibition of one site could have secondary effects distant from the area of stimulation (16–18). It is well established that PMC has direct excitatory projections over M1 (19–21); thus, the inhibition of M1 through PMC rTMS is likely due to the down regulation of these excitatory projections (22). The relative - inhibitory effect obtained between these two sites of stimulation has been investigated, but the results are inconsistent. Houdayer et al. compared the single-pulse MEP size, plateau and slope of the stimulus-response curve following 1800 pulses 1-Hz rTMS over M1 or PMC (18). The results demonstrated similar reduction in excitability between the two target sites. Other work found that PMC stimulation induced greater inhibition when measured with CSP, but there was an equal effect in SICI and ICF reduction (22). A different investigation using single-pulse MEP amplitude and positron emission tomography evaluated these two sites of rTMS stimulation and reported that there were no differences in MEP amplitude reduction, but found that each site inhibited different neural networks. The results demonstrated that rTMS over PMC influenced a number of brain regions in the parietal and prefrontal cortices. In contrast, rTMS over M1 influenced a smaller number of brain regions that were confined to the cortical and subcortical motor system (23).
Given the different mechanisms of inhibition between PMC and M1 stimulation, focusing on the excitatory projection from PMC to M1 which may mitigate the inhibitory effect of the rTMS targeted over M1. Also the inhibitory effect induced by targeting rTMS over PMC is an indirect modulation process through the down regulation of this excitatory projection., this experiment explored a combined stimulation approach: targeting rTMS over PMC followed immediately by M1 (PMC+M1). By doing this we speculated that the first bout of stimulation, targeted over PMC, would down regulate the excitatory projections from PMC to M1. Then, the second bout of stimulation, targeted over M1, would further inhibit M1 because with an inhibited/weakened excitation projection from PMC, M1 would be further inhibited. We hypothesized that this PMC+M1 rTMS strategy would inhibit the M1 more effectively than stimulating M1 or PMC alone. Thus, the purpose of this study was: to examine the difference in the modulatory effect between low-frequency rTMS over M1, PMC and a combined PMC+M1 stimulation strategy.
Materials and Methods
Participants
Eleven healthy right-handed (24) participants were recruited and ten completed the study (3 males; mean age: 25.2 ± 5.4y). One participant withdrew after one session due to headache. The exclusion criteria were any neurologic conditions, medications with effect on the central nervous system and contraindications to rTMS (25). All participants gave written, informed consent prior to participation according to the Declaration of Helsinki (26). The study was approved by the Clinical and Translational Science Institute and the Institutional Review Board of the University of Minnesota.
Experimental design
A randomized, cross-over, three-session design was used. There was a one-week wash-out period between sessions. Each visit consisted of a pre-test, an rTMS intervention, and a post-test. The pre and post-test consisted of a comprehensive M1 excitability assessment delivered according to previously established protocol (9, 14, 27), including: determination of optimal coil location (TMS hotspot), resting motor threshold (RMT), 1 mV threshold and excitability assessment measures. Excitability assessment measures included SICI, ICF and CSP. The rTMS was delivered to the left hemisphere PMC, M1 or PMC+M1 at each visit. The pre-tests and post-tests were conducted immediately before and after the interventions.
M1 excitability assessment measures were acquired with participants comfortably seated in a semi-reclined chair. A pair of silver-silver chloride electrodes (EL254, BIOPAC System Inc., Aero Camino Goleta, CA) were attached to the skin above the first dorsal interosseous (FDI) muscle of the right hand in a belly-tendon montage. Electromyogram (EMG) signals were amplified by a bi-polar differential EMG amplifier (Y03-2, Motion Lab Systems, Inc., Baton Rouge, LA) with the gain of ×300 and band-pass filter (15–2000Hz), then digitized by an analog-to-digital convertor (NI 9234, National Instruments, Austin, TX) with 24-bit resolution at a sampling rate of 6.4 kHz. All data were collected by a custom LabVIEW program (v2012, National Instruments, Austin, TX) on a laptop computer (Latitude, Dell Co., Ltd, Round Rock, TX) and stored for offline analysis. To fine the TMS hotspot for activating the FDI muscle, a 70-mm figure-of-eight TMS coil connected to a Magstim BiStim2 magnetic stimulator (Magstim Co., Whitland, UK) was used. The coil was positioned with the handle directed posterolaterally 45° to the mid-sagittal line of the head with a posterior-anterior orientation over the approximate location of the hotspot. Single-pulse magnetic stimuli were delivered manually until an optimal MEP was elicited. This location was used to determine the RMT and 1 mV threshold. The hotspot from the initial testing session was recorded in a stereotactic neuronavigation system with a phantom reference (BrainSight 2, Rogue Research Inc. Quebec, Canada) and reused at each subsequent testing session, to ensure stability of stimulus location with all tests and interventions. The RMT was defined as the minimum intensity required to elicit MEP amplitude greater than 50 μV (peak-to-peak) in at least 3 of 5 trials in the resting target muscle (28–30). The 1 mV threshold was defined as the minimum intensity required to elicit MEP amplitude greater than 1 mV (peak-to-peak) in at least 3 of 5 trials in the resting target muscle. For the post-tests, the thresholds were re-tested to ensure comparable relative stimulation intensities which is required by the paired-pulse protocols (31). Adverse effects were assessed after each session.
rTMS interventions
In all interventions, rTMS was delivered by a figure-of-eight coil with a built-in cooling fan (70mm Double Air Film Coil, Magstim Co., Whitland, UK). The coil was connected to Magstim Rapid2 magnetic stimulator (Magstim Co., Whitland, UK). 1200 pulses (20 min) of rTMS were delivered at 1Hz with the intensity of 90% of the RMT to the corresponding site, under neuronavigation guidance. The RMT intensity for the Rapid2 stimulator was re-tested, as the stimulator and coil were different from the Bistim2 used in the pre-tests. For M1 stimulation (1200 pulses), the rTMS pulses were delivered to the M1 hotspot determined during pre-test, under the guidance of the neuronavigation system. For PMC stimulation (1200 pulses), the stimulation site was defined as 2 cm anterior and 1cm medial from the hotspot (9, 13, 32, 33). The handle was directed posterolaterally 45° to the mid-sagittal line of the head with a posterior-anterior orientation over the targeting spot. For PMC+M1 stimulation, 600 pulses were first delivered over PMC site first followed immediately by another 600 pulses delivered to the hotspot (1200 total pulses).
Excitability assessment measures
The SICI, ICF and CSP were used because they are well established and widely adopted in TMS-related studies and represent different mechanisms of intracortical excitability (31). This measurement combination provides a comprehensive assessment of the overall excitability of the motor cortex which helps distinguish the three interventions in different inhibitory mechanism aspects.
Short interval intracortical inhibition and Intracortical facilitation
SICI and ICF measures are responses elicited secondary to paired-pulse stimulation. SICI is a measure of the inhibition reflective of intracortical activation of gamma-aminobutyric acid-A (GABAA) receptors (34, 35). ICF is a measure of facilitation, which is mediated by N-methyl-D-aspartate receptors (NMDA) and GABAA (36, 37). These two paired-pulse protocols contrast how the three interventions modulate the inhibitory processes that are mediated by GABAA and NMDA.
In these excitability assessment measures, the first stimulus is the conditioning pulse with an intensity of 80% of RMT. This intensity was determined according to the well-established protocols (4). The second is the testing pulse (intensity: 1mV threshold) delivered after 3ms for SICI and 13ms for ICF (31). Ten trials of each paired-pulse and single pulse (intensity: 1mV threshold) measure were collected in random sequence to eliminate the order effect. There was an approximate 5-second interval between two consecutive trials.
Cortical silent period
The CSP is a measure of inhibition reflective of GABAB mediated processes (38, 39), which may reveal the difference between the three interventions in modulating the inhibitory process that are mediated by GABAB. CSP is defined as the duration of the EMG quiescence that occurs during voluntary contraction secondary to a superimposed pulse (28). Procedurally, the participants contracted their right index finger against a fixed barrier and the resultant EMG intensity was calculated in real time and displayed on a screen placed in front of the participant. Three trials of the participant’s maximum voluntary isometric contraction were recorded and 20% of the peak EMG intensity was displayed on the screen as a target line. During the test, participants performed an isometric contraction of the FDI and maintained the EMG intensity to the target line until instructed to relax. Single-pulse stimulation with the intensity of 1mV threshold was applied approximately 3 s after contraction initiation and participants were instructed to relax 2–3 s after stimulation. Ten CSP trials were obtained with a minimum 10-second rest interval between each trial to prevent fatigue.
Data Processing
All assessment trials collected were used without filtering or screening of data. The size of 1 mV single-pulse, SICI and ICF trials was defined as the peak-to-peak amplitude of the MEP. For both pre- and post-tests, the ratio between individual paired-pulse responses (SICI and ICF) and the averaged single pulse responses were calculated (ie, paired-pulse MEP/average 1 mV single-pulse MEP). Thus, a value less than 1 indicates an inhibited response and a value greater than 1 indicates a facilitated response. For CSP, the EMG data were first rectified, and a 10-ms moving standard deviation (SD) calculation was applied to slide through the rectified EMG curve to generate an SD curve of the signal. The average value of this SD curve during the pre-stimulus period (−100ms to −5ms) was calculated. This value was used to define the offset of the CSP when the signal returns to pre-stimulus level. The stimulus delivery defined the onset of the CSP. We used moving SD instead of moving average to diminish error caused by baseline shifting that can occur secondary to the large superimposed MEP. This is mainly a movement artifact caused by the muscle contraction after being activated by TMS. To minimize the variability of the excitability measures and to eliminate the baseline difference to make the data comparable across subject and between groups, the normalized individual change scores of SICI, ICF and CSP were calculated using a linear transformation defined as: Normalized change score = (Y − X̄pre)/X̄pre. Where, Y is the individual SICI, ICF or CSP values of the post-test; X̄pre is the mean value of the individual SICI, ICF or CSP values of the pre-test before each intervention.
Statistical Analysis
First, a Shapiro-Wilk W test was conducted on the normalized change score of all excitability assessment measures to determine normality. Non-parametric statistical analysis were used due to the non-normality of the data distribution. To determine the difference between interventions, Kruskal-Wallis one-way analysis of variance was performed on normalized change score data with Steel-Dwass-Critchlow-Fligner pairwise ranking for all post hoc tests as appropriate; to determine the difference within intervention, between pre and post-tests for each rTMS intervention, the Wilcoxon signed-rank test was conducted based on the raw values of each measure. To evaluate the intervention order effect, Kruskal-Wallis one-way analysis of variance was performed on normalized change score data. The significance level of all statistical tests was p < 0.05.
Results
No serious adverse effects were reported, one participant experienced headache from the rTMS and withdrew from the study. The overall mean and SD threshold values before and after rTMS interventions (group effect) were: RMT: 42.93 ± 5.91% (pre), 44.57 ± 6.61% (post) and 1 mV: 54.90 ± 7.38% (pre), 55.70 ± 8.50% (post) of the stimulator output. The RMT values tested by the rTMS coil were: 50.27 ± 7.85%. No intervention order effect was observed (For SICI, χ2 = 5.5394; DF = 2; P =0.0627; for ICF, χ2 = 1.9450; DF = 2; P =0.3781; and for CSP, χ2 = 4.2091; DF = 2; P = 0.1219).
Differences in the inhibitory effect between interventions
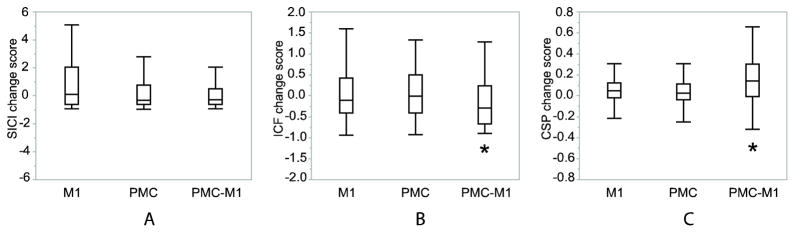
In the comparison between interventions per excitability assessment measure, in CSP, the PMC+M1 stimulation increased the duration of silent period to a greater extent than the other two interventions (χ2 = 20.9292; DF = 2; P < 0.001), indicating more inhibition. For ICF, the PMC+M1 stimulation change score was significantly lower than the other two (χ2 = 10.5510; DF = 2; P = 0.005), indicating a reduced facilitatory response. For SICI, there was no significant difference between the three interventions, although there was a trend of reduced inhibition after M1 stimulation (χ2 = 5.1135; DF = 2; P = 0.078). These results indicate that PMC+M1 stimulation 1) was the only intervention that decreased ICF (indicating a reduced facilitatory effect), and 2) induced greater inhibitory effect in CSP compared to the other two interventions (Figure 1).
Figure 1.
Normalized change score group results for each stimulation location with boxplots indicating median, 25th and 75th percentiles and 1.5 IQRs (interquartile distance, the difference between the 25th and 75th percentiles). A: No difference in any intervention with SICI. B: PMC+M1 stimulation decreased ICF which is different from the other two interventions *: p<0.05. C: PMC+M1 stimulation increased the CSP duration to a greater extent than the other two interventions *: p<0.05. SICI change score: normalized change score of short interval intracortical inhibition. ICF change score: normalized change score of intracortical facilitation. CSP change score: normalized change score of cortical silent period. Normalized change score = (Y − X̄pre)/X̄pre. Where, Y is the individual SICI, ICF or CSP values of the post-test; X̄pre is the mean value of the individual SICI, ICF or CSP values of the pre-test before each intervention. M1: M1 stimulation. PMC: PMC stimulation. PMC+M1: PMC+M1 stimulation.
Differences within intervention
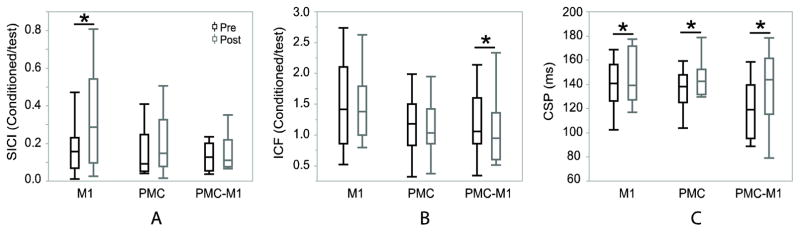
In the pre and post-test comparison within intervention, M1 stimulation significantly increased CSP and SICI values (Figure 2), meaning GABAB-mediated intracortical inhibition was increased according to increased CSP values, whereas, GABAA-mediated intracortical inhibition was not significantly modulated based on SICI results. No significant difference was shown in ICF. After PMC stimulation, CSP was significantly increased, indicating increased inhibition. No significant difference was shown in ICF or SICI. After PMC+M1 stimulation, CSP was significantly increased and ICF value was significantly decreased, indicating increased inhibition according to CSP, and reduced facilitation according to ICF. No significant difference was shown in SICI. The statistical results are listed in Table 1.
Figure 2.
Within intervention pre- and post-test responses of all interventions with boxplots indicating median, 25th and 75th percentiles and 1.5 IQRs (interquartile distance, the difference between the 25th and 75th percentiles). A: SICI was significantly increased after M1 stimulation (z = 2.0415, P = 0.041). B: ICF was significantly decreased after PMC stimulation (z = −2.4055, P = 0.016). C: CSP was increased after all of the 3 interventions (PMC: z = 2.7403, P = 0.006; M1: z = 4.1477, P < 0.001; PMC+M1: z = 5.9765, P < 0.001). *: Significantly different between pre and post-test (p<0.05). SICI: ratio between short interval intracortical inhibition (SICI) and 1 mV single-pulse. ICF: ratio between intracortical facilitation (ICF) and 1 mV single-pulse. CSP: cortical silent period in millisecond. M1: M1 stimulation. PMC: PMC stimulation. PMC+M1: PMC+M1 stimulation.
Table 1.
Within intervention Wilcoxon test result
| Measure | M1 | PMC | PMC+M1 | |||
|---|---|---|---|---|---|---|
| Z | P | Z | P | Z | P | |
| CSP | 4.1477 | <0.001* | 2.7403 | 0.006* | 5.9765 | <0.001* |
| SICI | 2.0415 | 0.041* | 0.4606 | 0.645 | 0.8124 | 0.417 |
| ICF | 0.3702 | 0.711 | 1.2840 | 0.199 | −2.4055 | 0.016* |
Significantly different between pre and post-test (p<0.05).
SICI: short interval intracortical inhibition. ICF: intracortical facilitation. CSP: cortical silent period. M1: M1 stimulation. PMC: PMC stimulation. PMC+M1: PMC+M1 stimulation.
Discussion
The purpose of the present study was to examine the efficacy of low-frequency rTMS targeted over M1, PMC or a combined PMC+M1 approach to inhibit the excitability of M1. To the best of our knowledge, this is the first study to evaluate the performance of a combined PMC+M1 low-frequency rTMS intervention. Findings were that PMC+M1 stimulation demonstrated the greatest inhibitory effect as measured by CSP, compared to an equal dose of M1 or PMC stimulation alone. PMC+M1 stimulation was the only intervention that induced an inhibitory effect as measured by ICF. However, SICI was not significantly modulated following any of the stimulation protocols. CSP measured inhibitory effects after all three interventions and was sensitive to a greater inhibitory effect with the PMC+M1 stimulation. Thus, CSP was the assessment measure with the most sensitivity to measure changes in excitability regardless of the form of intervention and was able to differentiate between the different inhibitory protocols.
Inhibitory efficacy of interventions
Our results support the hypothesis that PMC+M1 stimulation most effectively inhibits M1 excitability compared to PMC or M1 stimulation alone, as measured by CSP and ICF response. When comparing the effects of rTMS targeted over M1 or PMC alone, there appears to be no clear difference between the two sites. These results are consistent with previous studies (18, 23).
The mechanistic bases of these findings are beyond the scope of this paper, but we speculate as to potential explanations. There are excitatory projections between PMC and M1 (19–21) that may contribute to the inhibitory response observed following the combined PMC+M1 and PMC only stimulation protocols. In PMC stimulation alone, the excitatory projections to M1 are likely inhibited, producing less excitatory input to M1 and a resultant down-regulated excitatory state. In contrast, during M1 stimulation alone, M1 is directly inhibited by the rTMS. However, M1 is still receiving excitatory projections from PMC, which may mitigate some of the inhibitory effects due to rTMS. Furthermore, recent evidence suggests this excitatory projection may be facilitated as a compensatory effect when M1 is inhibited (40). During the combined PMC+M1 stimulation, PMC excitatory projections may be initially weakened by rTMS targeted over PMC. As neuromoduatory effects have been shown to extend beyond the period of stimulation (41), it is possible that this first bout of rTMS over PMC may produce an optimal environment for M1 to be further inhibited as there would be absent or weakened excitatory influence from PMC. As a result, a secondary bout of rTMS directly over M1 would be optimized as both input to and output from the M1 region is inhibited.
Differences between measures in revealing inhibitory effect
According to the intervention comparison (Figure 1), SICI, ICF and CSP respond to rTMS differently both within and between subjects. CSP was the most sensitive excitability assessment measure, in inhibition and distinguishing between different interventions. For SICI, no inhibitory effect was observed after low-frequency rTMS, regardless of stimulation location, which was contrary to our expectation. However, inconsistent SICI responses after 1 Hz rTMS have been reported throughout the literature. For example, SICI has been found decreased (42), unchanged (43, 44) and increased (45, 46) after low frequency rTMS. However, our SICI results after M1 intervention showed reduced intracortical inhibition response which is consistent with the result reported by Modugno and colleagues in 2002 (45). According to a comprehensive review on the effect of rTMS, the inhibitory effect measured by SICI remains inconclusive (47). Given that the SICI values did not demonstrate an inhibitory effect and there was no difference across interventions, we cannot conclude superiority of one inhibitory protocol as measured by this test. In other words, it is possible that if SICI can be modulated by tested protocols, there may be difference in the modulation effect. For ICF, variable results following rTMS have also been reported (48–54). This measure did demonstrate a between group difference, however, with only the PMC+M1 protocol demonstrating inhibition.
The differences between outcome excitability assessment measures (SICI, ICF and CSP) in response to low-frequency rTMS may be due to the different cortical inhibitory mechanisms measured and the sensitivity of each outcome, which has been reported to be interrelated(55). The LTD-like effect induced by low-frequency rTMS with the present parameters might affect these three mechanisms differentially (40, 45), suggesting that CSP is the most sensitive to the modulations performed, but other modulations may affect other measures differently. One consideration regarding the paired-pulse assessments is that the ISIs and conditioning pulse intensity of SICI and ICF were not optimized for individual participants. Although 80% of RMT was reported as the optimal conditioning intensity for SICI and ICF (56), this optimal value may be differentially modulated based upon in the specific rTMS parameters. As demonstrated by previous studies, both ISI and conditioning intensity significantly affect the responses of SICI and ICF (40, 45, 56–60). Following low frequency rTMS, some subjects may respond with inhibited responses while others are facilitated to the same test (52). In effect, inter-subject variability may dampen statistical power. In our protocol, paired-pulse tests were performed uniformly across all participants, as is widely used as an accepted procedure (31). It is possible, however, that individual stimulus response curves should be used, whereby optimal intensity and ISI are determined at pretest for each individual (4). This may provide more sensitivity information since multiple ISIs will be covered. In contrast, CSP measurement is associated with less vulnerability to parameter selection, resulting in less variability between and within subjects (49, 50, 52). This may be why CSP was the most sensitive to changes in inhibition and distinguishing between different interventions.
A reduced ICF response suggests a weakened intracortical facilitatory process secondary to the rTMS. As for SICI, a lack of significant difference in SICI responses indicates that the GABAA mediated processes were not strongly affected, or the method we chose to measure SICI was not optimal as discussed above. For CSP, a significantly reduced response may indicate the GABAB mediated inhibitory process was modulated by all of the interventions with the PMC+M1 demonstrating the strongest effect. The contrast between ICF, SICI and CSP results indicate that low-frequency rTMS may selectively modulate GABAB but not GABAA.
Differences between pre and post-tests within interventions
It is noteworthy that after M1 stimulation alone, on average, SICI values increased. This could indicate a reduced inhibitory effect. There were no significant change in SICI responses, after PMC alone and PMC+M1 stimulations (Figure 2). These mixed SICI responses are consistent with the results reported in previous studies (47, 52). The potential reasons for these mixed responses may be: 1) 1Hz rTMS may not be strictly inhibitory across subjects (53) and could be variable depending on stimulation location. Additionally, the inhibitory effect reflected by each measure may be dependent on the stimulation intensity of rTMS (47). 2) SICI is already an inhibited response compared to 1 mV single-pulse or CSP measures, thus due to a floor effect, it may be harder to inhibit SICI in some participants.
While our findings demonstrated group effects, it is noteworthy that the responses were variable when examined within single participants. The source of this variability should be examined in future work, as it remains an issue (61–63). In addition to the considerations mentioned above, other sources of the variability within SICI and ICF presented in this work may be related to the RMT and 1mV threshold determination. Although our protocol has been used extensively (28–30), current recommendations suggest that the definition should require 5 out of 10 trials (64). This point is open to debate however, as evidence also suggests that, there were no significant differences between the 3 out of 6 and the 5 out of 10 methods (65).
Future work and limitations
These findings present a unique method to further optimize inhibitory effects of low-frequency rTMS in the motor cortex to potentially increase efficacy of inhibitory interventions. Limitations of the study include a relatively small sample size that may have hampered the ability to demonstrate effect in SICI. There was high variability within and between interventions. Overall, the results are encouraging. Future work should focus on a systematic investigation of the PMC+M1 protocol with different stimulation distributions between PMC and M1 and the potential causes of variability. Such investigations may reveal the optimal site distribution and dose to promote inhibition.
Acknowledgments
Financial support: Research reported in this publication was supported by the Minnesota Medical Foundation (MMF), Faculty Development Grant 4090-9224-12. And National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. All funding sources supporting this work are acknowledged.
We acknowledge Sara Sokolowski, Karla Wallner, Lindsey Weyer, Thomas Williams and Caitlin Wooldridge for their help with the data collection in this work. Research reported in this publication was supported by the Minnesota Medical Foundation (MMF), Faculty Development Grant 4090-9224-12. And National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114.
Footnotes
Authorship statement: All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the journal of “Neuromodulation: Technology at the Neural Interface”.
Conflict of interest statement: Authors have no conflict of interest to declare.
References
- 1.Hoogendam JM, Ramakers GMJ, Lazzaro VDi. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low frequency transcranial magnetic stimulation. Neurol. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 3.Jäncke L, Steinmetz H, Benilow S, Ziemann U. Slowing fastest finger movements of the dominant hand with low-frequency rTMS of the hand area of the primary motor cortex. Exp Brain Res. 2004;155:196–203. doi: 10.1007/s00221-003-1719-7. [DOI] [PubMed] [Google Scholar]
- 4.Hallett M. Transcranial Magnetic Stimulation: A Primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B. Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson’s disease. J Neurol Sci. 2000;178:91–94. doi: 10.1016/s0022-510x(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 6.Huang YZ, Edwards MJ, Bhatia KP, Rothwell JC. One-Hz repetitive transcranial magnetic stimulation of the premotor cortex alters reciprocal inhibition in DYT1 dystonia. Mov Disord. 2004;19:54–59. doi: 10.1002/mds.10627. [DOI] [PubMed] [Google Scholar]
- 7.Campenhausen S, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, et al. Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol. 2005;15:473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Ridding M, Rothwell J. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 9.Kimberley TJ, Borich MR, Arora S, Siebner HR. Multiple sessions of low-frequency repetitive transcranial magnetic stimulation in focal hand dystonia: Clinical and physiological effects. Restorative Neurol and Neurosci. 2013;31:533–542. doi: 10.3233/RNN-120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, et al. A. Low frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurol. 1999;52:529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- 12.Wu T, Sommer M, Tergau F, Paulus W. Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett. 2000;287:37–40. doi: 10.1016/s0304-3940(00)01132-0. [DOI] [PubMed] [Google Scholar]
- 13.Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain. 2005;128:104–115. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- 14.Borich M, Arora S, Kimberley TJ. Lasting effects of repeated rTMS application in focal hand dystonia. Restor Neurol Neurosci. 2009;27:55–65. doi: 10.3233/RNN-2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol. 1994;481:243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 17.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houdayer E, Degardin A, Cassim F, Bocquillon P, Derambure P, Devanne H. The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp Brain Res. 2008;187:207–217. doi: 10.1007/s00221-008-1294-z. [DOI] [PubMed] [Google Scholar]
- 19.Roland PE, Skinhoj E, Lassen NA, Larsen B. Different cortical areas in man in organization of voluntary movements in extrapersonal space. J Neurophysiol. 1980;43:137–150. doi: 10.1152/jn.1980.43.1.137. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, et al. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Münchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional Connectivity of Human Premotor and Motor Cortex Explored with Repetitive Transcranial Magnetic Stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating Neural Networks With Transcranial Magnetic Stimulation Applied Over the Dorsal Premotor and Primary Motor Cortices. J Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- 24.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 25.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Medical Association. World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Repetitive transcranial magnetic stimulation induced analgesia depends on N-methyl-D-aspartate glutamate receptors. Pain. 2014;155:598–605. doi: 10.1016/j.pain.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 29.Cirillo J, Lavender AP, Ridding MC, Semmler JG. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J Physiol. 2009;587:5831–5842. doi: 10.1113/jphysiol.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kuijk AA, Bakker CD, Hendriks JC, Geurts AC, Stegeman DF, Pasman JW. Definition dependent properties of the cortical silent period in upper-extremity muscles, a methodological study. J Neuroeng Rehabil. 2014;11:1. doi: 10.1186/1743-0003-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossini PM, Rossi S. Transcranial magnetic stimulation-diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- 32.Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- 33.Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- 34.Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 35.Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- 36.Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurol. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 37.Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, et al. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett. 1999;270:137–140. doi: 10.1016/s0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- 38.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physio. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierantozzi M, Marciani MG, Palmieri MG, Brusa L, Galati S, Caramia MD, et al. Effect of Vigabatrin on motor responses to transcranial magnetic stimulation: an effective tool to investigate in vivo GABAergic cortical inhibition in humans. Brain Res. 2004;1028:1–8. doi: 10.1016/j.brainres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt S, Fleischmann R, Bathe-Peters R, Irlbacher K, Brandt SA. Evolution of Premotor Cortical Excitability after Cathodal Inhibition of the Primary Motor Cortex: A Sham-Controlled Serial Navigated TMS Study. PLoS ONE. 2013;8:e57425. doi: 10.1371/journal.pone.0057425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Mima T, Siebner HR, Oga T, Hara H, Satow T, et al. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol. 2003;114:1628–1637. doi: 10.1016/s1388-2457(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 42.Khedr EM, Gilio F, Rothwell J. Effects of low frequency and low intensity repetitive paired-pulse stimulation of the primary motor cortex. Clin Neurophysiol. 2004;115:1259–1263. doi: 10.1016/j.clinph.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 43.Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory eVects on motor cortex excitability. Exp Brain Res. 2010;203:31–38. doi: 10.1007/s00221-010-2206-6. [DOI] [PubMed] [Google Scholar]
- 44.Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R. Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurol. 2013;80:1746–1753. doi: 10.1212/WNL.0b013e3182919029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modugno N, Curra A, Conte A, Inghilleri M, Fofi L, Agostino R, et al. Depressed intracortical inhibition after long trains of subthreshold repetitive magnetic stimuli at low frequency. Clin Neurophysiol. 2003;114:2416–2422. doi: 10.1016/s1388-2457(03)00262-1. [DOI] [PubMed] [Google Scholar]
- 46.Delvendahl I, Jung N, Mainberger F, Mall V. Low-frequency rTMS selectively modulates inhibitory intracortical networks. Neuropediatrics. 2010;41:V1258. [Google Scholar]
- 47.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 48.Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113:101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- 49.Cincotta M, Borgheresi A, Gambetti C, Balestrieri F, Rossi L, Zaccara G, et al. Suprathreshold 0.3 Hz repetitive TMS prolongs the cortical silent period: potential implications for therapeutic trials in epilepsy. Clin Neurophysiol. 2003;114:1827–1833. doi: 10.1016/s1388-2457(03)00181-0. [DOI] [PubMed] [Google Scholar]
- 50.Stinear CM, Byblow WD. Impaired modulation of corticospinal excitability following subthreshold rTMS in focal hand dystonia. Hum Mov Sci. 2004;23:527–538. doi: 10.1016/j.humov.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 51.Brighina F, Giglia G, Scalia S, Francolini M, Palermo A, Fierro B. Facilitatory effects of 1 Hz rTMS in motor cortex of patients affected by migraine with aura. Exp Brain Res. 2005;161:34–38. doi: 10.1007/s00221-004-2042-7. [DOI] [PubMed] [Google Scholar]
- 52.Daskalakis Z, Möller B, Christensen B, Fitzgerald P, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 53.Caparelli EC, Backus W, Telang F, Wang GJ, Maloney T, Goldstein RZ, et al. Is 1 Hz rTMS Always Inhibitory in Healthy Individuals? Open Neuroimag J. 2012;6:69–74. doi: 10.2174/1874440001206010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Lazzaro V, Pilato F, Oliviero A, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–71. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- 55.Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583:971–82. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacKinnon CD, Gilley EA, Weis-McNulty A, Simuni T. Pathways mediating abnormal intracortical inhibition in Parkinson’s disease. Ann Neurol. 2005;58:516–524. doi: 10.1002/ana.20599. [DOI] [PubMed] [Google Scholar]
- 57.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physio. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peurala SH, Muller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition and short-interval intracortical facilitation. Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 61.Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113:376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 62.Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- 63.Du XM, Summerfelt A, Chiappelli J, Holcomb HH, Hong LE. Individualized Brain Inhibition and Excitation Profile in Response to Paired-Pulse TMS. J Mot Behav. 2014;46:39–48. doi: 10.1080/00222895.2013.850401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conforto AB, Z’Graggen WJ, Kohl AS, Rösler KM, Kaelin-Lang A. Impact of coil position and electrophysiological monitoring on determination of motor thresholds to transcranial magnetic stimulation. Clin Neurophysiol. 2004;115:812–819. doi: 10.1016/j.clinph.2003.11.010. [DOI] [PubMed] [Google Scholar]