Conspectus
Lung cancer is the leading cause of cancer death in the world, and cigarette smoking is its main cause. Oral cavity cancer is another debilitating and often fatal cancer closely linked to tobacco product use. While great strides have been made in decreasing tobacco use in the United States and some other countries, there are still an estimated 1 billion men and 250 million women in the world who are cigarette smokers and there are hundreds of millions of smokeless tobacco users, all at risk for cancer. Worldwide, lung cancer kills about 3 people per minute. This account focuses on metabolites and biomarkers of two powerful tobacco-specific nitrosamine carcinogens – 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N′-nitrosonornicotine (NNN) - considered to be among the main causes of lung cancer and oral cavity cancer in people who use tobacco products. Three properties of NNK and NNN are critical for successful biomarker studies: they are present in all tobacco products, they are tobacco-specific and are not found in any other product, and they are strong carcinogens. NNK and NNN are converted in humans to urinary metabolites that can be quantified by mass spectrometry as biomarkers of exposure to these carcinogens. They are also metabolized to diazonium ions and related electrophiles that react with DNA to form addition products that can be detected and quantified by mass spectrometry. These urinary metabolites and DNA addition products can serve as biomarkers of exposure and metabolic activation, respectively. The biomarkers of exposure, in particular the urinary NNK metabolites NNAL and its glucuronides, have been extensively applied to document tobacco-specific lung carcinogen uptake in smokers and non-smokers exposed to secondhand tobacco smoke. Highly sensitive mass spectrometric methods have been developed for quantitative analysis of these NNK metabolites as well as metabolites of NNN in human urine, blood, and toenails. Urinary and serum NNAL have been related to lung cancer risk, and urinary NNN to esophageal cancer risk, in prospective epidemiology studies. These results are consistent with carcinogenicity studies of NNK, NNAL and NNN in rats, which show that NNK and NNAL induce mainly lung tumors, while NNN causes tumors of the esophagus and oral cavity. Biomarkers of metabolic activation of NNK and NNN applied in human studies include the metabolism of deuterium labelled substrates to distinguish NNK and NNN metabolism from that of nicotine, and the determination of DNA and hemoglobin adducts in tissues, blood, and oral cells from people exposed to tobacco products. As these methods are continually improved in parallel with the ever increasing sensitivity and selectivity of mass spectrometers, development of a comprehensive biomarker panel for identifying tobacco users at high risk for cancer appears to be a realistic goal. Targeting high risk individuals for smoking cessation and cancer surveillance can potentially decrease the risk of developing fatal cancers.
Graphical Abstract
1. Introduction
Lung cancer is the leading cause of cancer death in the world, and cigarette smoking is its main cause. Our approach to prevention of cancers caused by tobacco products is based on an understanding of tobacco carcinogens and the biochemical mechanisms by which they cause cancer. There are more than 70 established carcinogens in cigarette smoke.1 The focus of this article is the carcinogenic “tobacco-specific nitrosamines” N′-nitrosonornicotine (NNN, 3) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, for nicotine-derived nitrosamino ketone,2 2, Scheme 1), widely considered as major causative factors for tobacco-induced cancer and evaluated by the International Agency for Research on Cancer as “carcinogenic to humans.”3 We expect that biomarkers of exposure to, and metabolism of NNN and NNK, will help us to identify individuals who are particularly susceptible to their carcinogenic effects, so that more focused and effective tobacco cessation methods as well as lung and oral cancer surveillance can be initiated at a young age. We know that there are particularly susceptible individuals because, in the case of lung cancer as an example, only 11–24% of lifetime smokers will get lung cancer, and this relatively small percentage is not due to competing causes of death from smoking.4
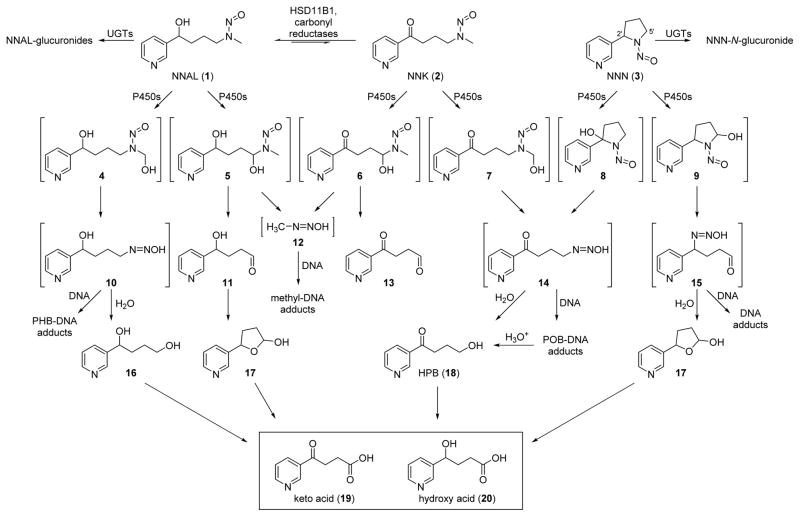
Scheme 1.
Metabolic pathways of NNK, NNN, and NNAL as discussed in the text.
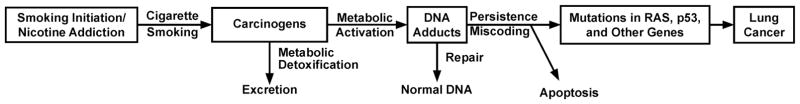
Figure 1 outlines the overall accepted pathway for carcinogenesis by tobacco products.5,6. People start smoking cigarettes as teen-agers and become addicted to nicotine. Nicotine is not a carcinogen, but each puff of each cigarette contains a mixture of carcinogens, including NNN and NNK. NNN and NNK as well as many other carcinogens in cigarette smoke require metabolic activation, generally by cytochrome P450 enzymes, to be converted to electrophilic species that can react with DNA to form DNA adducts, which are critical in the carcinogenic process.5 The general mechanism summarized in Figure 1 is best supported by the vast amount of data available from studies of lung cancer, but is generally applicable to other tobacco-related cancers including cancer of the oral cavity.
Figure 1.
Generally accepted overall mechanism linking carcinogen exposure, DNA adduct formation, mutation induction and lung cancer in smokers.5,6
We will consider biomarkers of human exposure to NNN and NNK, and biomarkers of their metabolic activation. Exposure and metabolic activation are both critical steps in the pathway leading to cancer, as summarized in Figure 1. These biomarkers can test the hypothesis that individuals with both high exposure and high metabolic activation to DNA adducts are at highest risk for cancer.
2. Properties of tobacco-specific nitrosamines
Three properties of NNN and NNK are highly attractive with respect to evaluating human exposure and cancer risk. First, they are present in all tobacco products. Second, they are tobacco-specific, and are not found in any other product (although there is evidence that NNN can form endogenously in some users of nicotine replacement products).3,7 Third, they are strong carcinogens. NNK causes lung tumors, mainly adenoma and adenocarcinoma, in all species tested, and independent of the route of administration.8 NNN induces tumors of the oral cavity, esophagus, and nasal cavities in rats, and respiratory tract tumors in mice, Syrian golden hamsters, and mink.8 The “organoselective” carcinogenicity of NNN and NNK, due in part to highly efficient cytochrome P450-catalyzed metabolic activation in their target tissues,8,9 is a well-established characteristic of nitrosamines, many of which are strong carcinogens affecting various specific target organs in laboratory animals.
3. Biomarkers of exposure
3.1. Urinary NNAL and its glucuronides
When NNK is introduced into any biological system, from simple cell culture systems to a human, a portion of it is rapidly converted by carbonyl reductases and HSD11B1 to NNAL (1, Scheme 1).10 We first observed NNAL in our initial metabolism studies of NNK in the F-344 rat.11 We extracted the urine of these rats with organic solvents and analyzed the extracts by TLC and HPLC. As Ruth Sciame, Andre Castonguay, and I stood by the bench staring at the TLC plate and considering the available spectral data, it suddenly dawned on me: it’s the alcohol! Then Andre said “NNAL!” (a logical extension of our trivial NNK nomenclature2), and the name stuck.
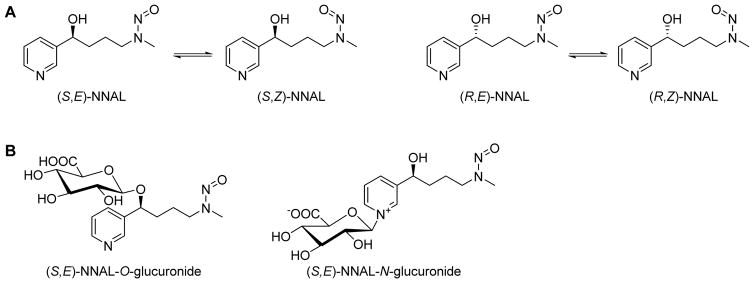
NNAL has a chiral center at the carbinol carbon, thus exists in the enantiomeric forms (S)-NNAL and (R)-NNAL (Figure 2A). It also exists as separable rotamers, a characteristic of all nitrosamines due to the partial double bond character of the N-N=O bond, and thus hindered rotation. These (E)- and (Z)-forms are also illustrated in Figure 2A.
Figure 2.
Representative structures of NNAL and NNAL-glucuronide isomers.
The Chung group was the first to identify an NNAL-O-glucuronide as a urinary metabolite of NNK in mice and rats (Figure 2B).12 We observed both diastereomers of NNAL-O-glucuronide in the urine of patas monkeys treated with a low dose of NNK; the glucuronides accounted for 15–25% of the urinary metabolites of NNK suggesting that they might be useful dose monitors in humans exposed to NNK.13 Later, it became evident that NNAL-N-glucuronide (Figure 2B) was also formed and was present in the urine of smokers.14,15 NNK itself is not generally detected in the urine of laboratory animals treated with the compound, or in smokers’ urine.
The study in patas monkeys was critical because it encouraged us to analyze human urine for NNAL and NNAL-O-glucuronide.16 This was a considerable analytical challenge since the daily dose of NNK experienced by a smoker in the early 1990s was estimated to range from about 4 – 23 nmol/day.16 Even in the unlikely event that all of this were converted to NNAL, the amount in urine would not exceed about 3 ppb. Based on these considerations and the technology available at the time, our original method for the separate quantitation of NNAL and NNAL-glucuronides in smokers’ urine used 100 ml urine to which [3H]NNAL was added as the internal standard. The analysis was carried out one urine sample at a time using multiple partitioning steps, HPLC purification, silylation, and quantitation by gas chromatography-nitrosamine selective detection by the “Thermal Energy Analyzer”, a highly reliable but not overly sensitive instrument for the selective determination of nitrosamines. The identity of NNAL in smokers’ urine was confirmed by GC-MS.16 In the years since, our group and others have constantly refined and validated the methods which have continually evolved to a state in which they are now applicable in relatively large studies.17 As an example, our current high throughput method for the individual analysis of NNAL, NNAL-O-glucuronide, and NNAL-N-glucuronide uses only 0.18 ml urine, [13C6]NNAL as an internal standard, 96-well based solid-phase extraction, and detection and quantitation by LC-MS/MS with a detection limit of about 100 fmol/ml urine.18 Using this method, we recently reported data for 2641 smokers.18 Multiple laboratories have contributed to the substantial advances in analysis of NNAL and its glucuronides in human urine, the results of which are generally expressed per mg creatinine or per ml of urine. Improvements in LC-MS/MS technology which now permit detection limits of less than 0.5 fmol of NNAL on column have been critical in allowing analysis of relatively large numbers of samples. Another advance has been the use of molecularly imprinted polymers for enhanced sample purification.17
3.11 Stereochemistry of urinary NNAL and NNAL-glucuronides
The rotamers of NNAL illustrated in Figure 2A provide a useful confirmation for the identification of NNAL in urine samples. Under some LC-MS conditions, the rotamers are partially or completely separated, thus supplying additional confirmation for the peak(s) assigned as NNAL.15,18 As shown in Figure 2B for (S,E)-NNAL, there are two possible glucuronides, an O-glucuronide and an N-glucuronide for each rotamer shown in Figure 2A. Standards were available for 6 of the 8 possible isomers, and their LC separation has been reported, along with the identification of each isomer of the N-glucuronide in the urine of Sudanese toombak users, who are exposed to exceptionally high amounts of NNK.15 The (R)- and (S)-enantiomers of NNAL from the fraction of urine containing both the O- and N-glucuronides have also been individually characterized by chiral stationary phase chromatography. In both smokers and smokeless tobacco users, (S)-NNAL and its glucuronides predominated in urine with (S):(R)-ratios ranging from 1.23 to 2.94. Seven days after cessation of smoking or smokeless tobacco use, these (S):(R)-ratios increased to 7.8 – 11.2 indicating selective retention of (S)-NNAL in the body, as the major enantiomer involved in the slow elimination of NNAL discussed below.
3.12 Distributions of NNAL and NNAL-Glucuronides in urine
Most studies that have investigated free and glucuronidated NNAL separately have determined free NNAL by simple extraction of the urine sample, and the sum of free NNAL, NNAL-O-glucuronide, and NNAL-N-glucuronide (e.g., total NNAL) by β-glucuronidase treatment of the urine sample followed by extraction. The glucuronides are then determined by subtraction of the amount of free NNAL from that of total NNAL. We have quantified the glucuronides individually by a combination of simple extraction (for free NNAL), base treatment and extraction (for free NNAL plus NNAL-N-glucuronide), and enzyme hydrolysis and extraction (for total NNAL). NNAL-N-glucuronide was then determined by subtracting free NNAL from the sum of free NNAL and NNAL-N-glucuronide. In the largest study of this type reported to date, of 2641 smokers, free NNAL, NNAL-N-glucuronide, and NNAL-O-glucuronide represented 31 ± 11%, 22 ± 14%, and 48 ± 15% of total NNAL, respectively.18
3.13. Some applications of the urinary NNAL biomarker
The three essential characteristics of NNK – universal presence in tobacco products, tobacco-specificity, and powerful lung carcinogenicity – are critical in supporting the use of its metabolite NNAL as a biomarker. A fourth characteristic, the slow release of NNAL from the human body, with an elimination half-life of 40–45 days, is also favorable in this respect.19,20 Consistently, levels of “total NNAL” (the sum of NNAL and its glucuronides) in urine correlate with levels of the nicotine metabolite cotinine, or with a panel of nicotine metabolites referred to as “total nicotine equivalents.”21–23 This correlation results from the fact that both NNK and nicotine are tobacco-specific compounds with somewhat similar structural and physical characteristics. However, there are two important differences that favor the use of NNAL as a carcinogen exposure biomarker: 1) NNK and NNAL are lung carcinogens while nicotine and cotinine are not; 2) the elimination half-life of NNAL is far longer than that of cotinine (1–2 days).
Perhaps the most impactful studies carried out with the NNAL biomarker have documented the uptake of the lung carcinogen NNK by non-smokers exposed to secondhand tobacco smoke. When our first study on this topic was published in 1993,24 the clean air policies which are now taken for granted and are nearly universal in this country and others, were just beginning to be enacted, and it was still not widely accepted that secondhand smoke was a cause of lung cancer. We concluded that “nonsmokers exposed to sidestream cigarette smoke take up and metabolize a lung carcinogen, which provides experimental support for the proposal that environmental tobacco smoke can cause lung cancer”.24 Such cautious language would not be necessary today25. Another key study of this type demonstrated the presence of NNAL and its glucuronides in the urine of women living with smokers, substantiating the epidemiologic evidence that these women were at increased risk for lung cancer compared to women who did not live with smokers. Some of the highest levels of total NNAL have been found in the urine of children, particularly young children of mothers who smoke.26–29 Our initial observations on children were confirmed in the Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey.30 In some young children who do not smoke, levels of total NNAL approach those found in smokers. This is still a critical problem.
We have collaborated with epidemiologists to test the hypothesis that total NNAL is a risk biomarker for lung cancer. In these prospective studies, urine samples are collected from smokers who are healthy, then the samples are stored frozen for many years, until sufficient numbers of lung cancer cases are observed. The samples are then retrieved from the freezers as are samples from smokers who did not develop cancer, but otherwise had similar characteristics as those who did. Two studies of this type, one carried out in Shanghai (with 18,244 male participants) and the other in Singapore (with 63,257 male and female Chinese participants), both currently led by epidemiologist Jian-Min Yuan of the University of Pittsburgh, demonstrated a significant relationship between total NNAL in urine and lung cancer risk in smokers, after adjustment for number of years of smoking and number of cigarettes per day.31,32 While these studies demonstrate that total NNAL is a risk biomarker for lung cancer, receiver operating characteristic curves indicate that it has only moderate predictive ability. Further studies are needed in which total NNAL is perhaps combined with biomarkers of metabolic activation, to establish a mathematical model that is truly predictive for lung cancer.
3.2 NNAL in plasma or serum
Analysis of total NNAL in plasma or serum has been performed less frequently than analysis of urine, but is important for potential use in epidemiologic studies which often save plasma or serum. Two reliable LC-MS/MS methods have been reported.33,34 An important application of the analysis of NNAL in serum was in samples collected from smokers at the beginning of the prospective Prostate, Lung, Colorectal, and Ovarian Screening Trial, which followed approximately 25,000 smokers. Similar to the results of the Shanghai and Singapore trials noted above, levels of total NNAL in serum samples from smokers in this study were significantly associated with lung cancer risk, after adjustment for other factors such as duration of smoking.35
3.3 NNAL in toenails
Toenails are collected in some epidemiologic studies and have certain advantages over urine or blood. They grow slowly and potentially reflect exposure over a longer period. They are also relatively easy to collect and store, and the analyte may be quite stable in the keratinic matrix. NNAL has been analyzed in toenails by LC-MS/MS; levels correlated with those of nicotine and cotinine measured in the same samples, and with urinary total NNAL.36,37 Thus, toenails could represent a unique resource for further investigation of the relationship of NNK to lung cancer in cigarette smokers.
3.4 Urinary NNN and NNN-N-glucuronide
NNN is extensively metabolized in laboratory animals by α-hydroxylation as shown in Scheme 1 and by other pathways with relatively small amounts of unchanged NNN being excreted in the urine.8,38 Therefore, we did not seriously pursue analysis of human urine for NNN itself until it became evident from our studies of NNK and NNAL metabolism that pyridine-N-glucuronidation might be a significant pathway.14,15 Indeed, analysis of the urine of smokers and smokeless tobacco users did demonstrate the presence of NNN and NNN-N-glucuronide in similar amounts.39 These were quantified along with the related tobacco-specific nitrosamines N′-nitrosoanabasine (NAB) and N′-nitrosoanatabine (NAT) and their glucuronides. NAB and NAT are considered less important than NNN and NNK because NAB is only weakly carcinogenic and occurs in relatively low quantities in tobacco products, and NAT while present in significant amounts has not shown any carcinogenic activity.8 Several groups have reported methods for the quantitation of total NNN, NNK, NAB, and NAT in urine.40–42 Levels of total NNN in smokers range from 0.023 – 0.12 pmol/ml urine. These levels are typically about 30 times lower than those of total NNAL in urine. Since NNN levels in tobacco products almost always exceed those of NNK,43,44 the relatively low amount of total NNN in urine is clearly a reflection of its extensive metabolism.
Since NNN causes cancer of the esophagus in rats, we investigated its possible relationship to esophageal cancer in smokers, again using samples from the prospective Shanghai cohort study.45 We found a significant and strong relationship of urinary total NNN to esophageal cancer risk in this study, but no relationship to lung cancer risk while urinary total NNAL was significantly related to lung cancer risk but not to esophageal cancer risk.46 These results demonstrate considerable coherence between rat target tissues for the carcinogenicity of NNN (esophagus) or NNK (lung) and observations in this epidemiologic study.
Another application of the total NNN assay concerns its possible endogenous formation in people using nicotine replacement products as tobacco cessation aids. We have found evidence for NNN formation in some individuals during use of oral nicotine replacement products.7 This is believed to occur by endogenous nitrosation of nornicotine, a metabolite of nicotine which is easily converted to NNN by reaction with salivary nitrite, or with nitrite under the favorable conditions of gastric pH.47
3.5 NNN in toenails
Similar to NNAL, nicotine, and cotinine, NNN can be quantified in human toenails by LC-MS/MS, and its levels were significantly correlated with these analytes.48 Interestingly, levels of NNN in toenails averaged 2.8 times as great as those of NNAL, while the levels of total NNN in urine are typically only about 3% of those of total NNAL. Apparently, NNN accumulates rather easily in toenails.
4. Biomarkers of metabolic activation
Biomarkers of exposure to tobacco carcinogens such as urinary NNAL and NNN are validated and well established. It is now possible to routinely quantify a panel of urinary carcinogen and toxicant biomarkers of exposure encompassing representatives of virtually every biologically active class of compounds in tobacco products, enabling the design and performance of large studies.49 This aspect of the science is mature. But for biomarkers of metabolic activation, comprising the next major step in carcinogenesis as shown in Figure 1, the field is still developing, as these measurements are more challenging due to their specialized nature and generally low analyte levels.
4.1. Urinary [pyridine-D4]4-hydroxy-4-(3-pyridyl)butanoic acid ([pyridine-D4]hydroxy acid)
The major pathways of metabolic activation of NNK, NNN, and NNAL are catalyzed by cytochrome P450 enzymes as illustrated in Scheme 1, resulting in a series of unstable α-hydroxynitrosamines 4–9, which rapidly decompose to diazohydroxides 10, 12, 14, and 15. Human P450s 2A13 and 2A6 are particularly important, and some data indicate that variants in these enzymes may be associated with cancer risk, due to altered metabolism of NNK as well as nicotine.50,51 The diazohydroxides react with DNA to form methyl-DNA adducts, pyridylhydroxybutyl (PHB)-DNA adducts, pyridyloxobutyl (POB)-DNA adducts, and other NNN-DNA adducts. Aldehydes 11 and 13, lactol 17, and formaldehyde are also formed in these reactions. The main reaction of the diazohydroxides is with H20, producing a series of hydroxylated metabolites 16 – 18, that are all further oxidized to give the major ultimate urinary metabolites – keto acid 19 and hydroxy acid 20. Levels of these acids in urine are therefore potential biomarkers of metabolic activation of NNK and NNN. There is one problem with this approach: keto acid and hydroxy acid are also minor metabolites of nicotine. Levels of nicotine in tobacco products generally are about 5,000 – 10,000 times as great as those of NNN and NNK, so the vast majority of the keto acid and hydroxy acid measured in urine samples from people who used tobacco products will have originated from nicotine, not from NNN and NNK. Our solution to this problem is the use of tobacco products containing [pyridine-D4]NNN or [pyridine-D4]NNK. Since it would not be ethical to expose humans to additional amounts of these strong carcinogens, our approach has been to add [pyridine-D4]NNK to the tobacco of cigarettes that have a relatively low natural NNK content, such that the total amount of [pyridine-D4]NNK plus natural NNK in the smoke of these cigarettes does not exceed that in the brand normally used by the subjects in the study. This approach has been reviewed and approved by the U.S. Food and Drug Administration and by the University of Minnesota Institutional Review Board. A similar approach is envisioned for [pyridine-D4]NNN. Analysis of the urine of these subjects for [pyridine-D4]hydroxy acid (after NaBH4 treatment to convert [pyridine-D4]keto acid to [pyridine-D4]hydroxy acid) provides a measure of individual metabolic activation of [pyridine-D4]NNK.
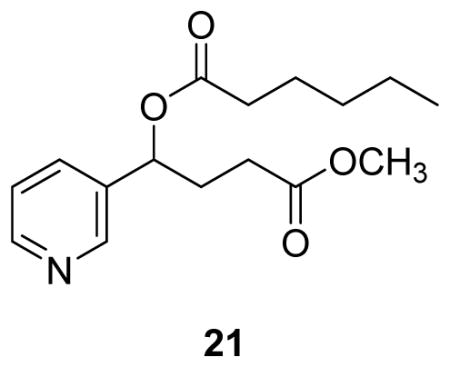
The analytical chemistry for determination of [pyridine-D4]hydroxy acid in urine is challenging due to its relatively low levels in urine and high polarity. Two derivatization steps – esterification with acidic methanol and conversion of the hydroxyl group to a hexanoate ester, producing 21 – are required, along with associated purification and enrichment steps prior to analysis by LC-MS/MS. The current validated method, while complex, is nevertheless applicable in studies of moderate size. In one recent study, the method was applied for the analysis of [pyridine-D4]hydroxy acid in the urine of 87 smokers who smoked cigarettes containing [pyridine-D4]NNK, at least 10 per day for at least one week.52 Levels of [pyridine-D4]hydroxy acid averaged 130 fmol/mL urine, about one third of the amount of [pyridine-D4]NNAL in the same subjects. The distribution of [pyridine-D4]hydroxy acid levels in these subjects is illustrated in Figure 3. When this approach becomes more routine, one could envision large studies statistically encompassing both an established dose monitor (such as total NNAL in urine) and an indicator of metabolic activation such as [pyridine-D4]hydroxy acid. Those individuals who have the highest levels of total NNAL, and in addition are extensive metabolizers of [pyridine-D4]NNK to [pyridine-D4]hydroxy acid should presumably be at highest risk for the carcinogenic effects of NNK.
Figure 3.
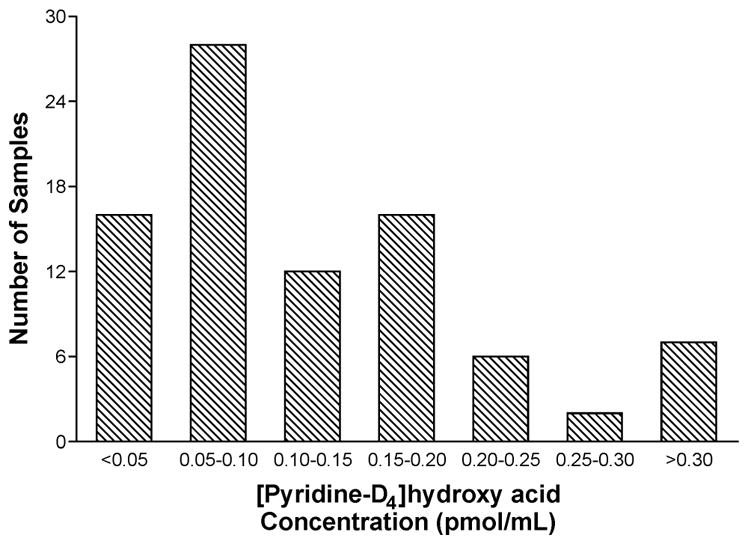
Histogram demonstrating the distribution of [pyridine-D4]hydroxy acid levels in 87 urine samples from study subjects who smoked cigarettes containing [pyridine-D4]NNK for 1 week.52
4.2. 4-Hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing hemoglobin adducts
Inspired by the work of Ehrenberg, Tornqvist, Tannenbaum, and others on hemoglobin adducts of carcinogens and their metabolically activated forms, as presented in a series of energizing meetings sponsored by the International Agency for Research on Cancer in the 1980’s and 1990’s, we began research on hemoglobin adducts of NNK and NNN.53–55 Advantages of hemoglobin adducts as dosimeters include the ready availability of relatively large quantities of hemoglobin, the lack of adduct repair, and the relatively long lifetime, 120 days, of the red blood cell in humans, which would potentially allow accumulation of adducts. Our main hypothesis, confirmed in studies of laboratory animals treated with NNK or NNN, was that the pyridyloxobutyl diazohydroxide 14 (Scheme 1) would react with globin to produce adducts which could be quantified as an indicator of metabolic activation of both NNN and NNK.56 The adducts were identified as esters of aspartate and glutamate in globin.57 Mild base treatment of globin from laboratory animals exposed to NNN or NNK hydrolyzed these ester adducts releasing HPB (18, Figure 1) which was quantified by derivatization and GC-MS, and expressed per weight of hemoglobin or globin.58 We have previously reviewed this research in some detail.8,59 In summary, dose-response studies and experiments with inhibitors of NNK metabolism in rats clearly demonstrated that HPB-releasing hemoglobin adducts were excellent dosimeters of NNK exposure plus metabolic activation. However, the results of studies in humans were less clear. Levels of HPB-releasing hemoglobin adducts in tobacco users were considerably lower than hemoglobin adducts of other carcinogens such as 4-aminobiphenyl, even though the levels of NNN and NNK in cigarette smoke are at least 50 times greater than those of 4-aminobiphenyl.59 While elevated levels of HPB-releasing hemoglobin adducts were found in smokeless tobacco users compared to non-users, the differences between smokers and non-smokers were less pronounced and levels of the adducts were frequently near background.59
4.3. HPB-releasing DNA adducts
Diazohydroxide 14 (Scheme 1) reacts with DNA to form pyridyloxobutyl adducts at the O6- and 7-positions of deoxyguanosine, the O2-position of thymidine, the O2-position of cytosine, and the phosphate oxygens.60,61 Acid treatment of this DNA releases HPB (18,Scheme 1), which has been used as a biomarker of DNA adduct formation by NNN and NNK.8 Multiple studies in laboratory animals conclusively demonstrate the relationship of HPB-releasing DNA adducts to NNN and NNK dose as well as the effects of modifiers of NNK metabolic activation. These studies have been reviewed.8 In 1991, we demonstrated the presence of HPB-releasing DNA adducts in human peripheral lung and tracheobronchial tissue collected at autopsy.62 Adduct levels were higher in smokers than non-smokers, which was expected based on the formation of HPB-releasing DNA adducts from tobacco-specific compounds. This finding was confirmed in a subsequent study of lung tissue obtained at surgery,63 but another investigation of lung tissue, esophagus, and cardia from tumor-free sudden death victims did not show a significant difference in levels of HPB-releasing DNA adducts between smokers and non-smokers, who were identified by cotinine levels in blood or urine.64 A cogent explanation for this latter result has not emerged.
Exfoliated oral mucosa cells appear to have considerable promise for the non-invasive quantitation of tobacco-specific nitrosamine-DNA adducts. We have developed a highly sensitive and specific LC-MS/MS method for HPB-releasing DNA adducts obtained from DNA collected using a mouthwash or from buccal brushings.65 Relatively high levels of HPB-releasing DNA adducts were observed, with means of 12 pmol/mg DNA in oral cells collected by mouthwash and 45 pmol/mg DNA in DNA from cells obtained from buccal mucosa brushings. These amounts are up to 1000 times greater than those found in human lung and esophagus, and are comparable to those reported in tissues of rats treated with NNN or NNK.66 The relatively high levels of HPB-releasing DNA adducts found in oral cells compared to other tissues could be a reflection of the fact that the oral mucosa is the first site of contact of tobacco smoke in cigarette smokers. It is also possible that there are other compounds in tobacco smoke, so far unidentified, that could produce HPB-releasing DNA adducts.
5. Prospects
Our goal is to use biomarkers of exposure to and metabolic activation of tobacco-specific nitrosamines, together with corresponding biomarkers of other tobacco carcinogens and toxicants to develop an algorithm to identify those tobacco users who are at high risk for cancer, so that effective cessation and surveillance methods can be initiated, leading ultimately to prevention of cancer. We stated essentially the same goal in a review published just over 20 years ago;59 without doubt we are much closer to that goal today. At that time it would have been unimaginable to quantify NNAL and its glucuronides as well as multiple other tobacco carcinogen and toxicant biomarkers by mass spectrometric analysis of 1–2 ml samples of urine. Indeed, those methods are now routinely applied. Progress has been more difficult in defining differences in metabolic activation. The use of deuterated compounds as probes and the detection of DNA adducts in oral cells are approaches which both appear to have great promise. The DNA adduct detection methods in oral cells can likely be expanded to quantify individual pyridyloxobutyl-DNA adducts as the sensitivity and selectivity of mass spectrometers continues to increase. The highest risk individuals should be those who have high levels of exposure, as measured by urinary metabolites, and high levels of metabolic activation as determined by deuterated metabolite profiles or oral cell DNA adducts. We hope to identify these potentially susceptible individuals by analysis of a urine sample and a simple oral swab.
One of my fellow graduate students, a few decades ago, liked to say: “Someday you will be able to go into a doctor’s office and cough into a mass spectrometer to see if anything is wrong.” It seems we are approaching that day.
Acknowledgments
Our studies on tobacco-specific nitrosamines are supported by Grant No. CA-81301 from the U.S. National Cancer Institute. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, funded in part by Cancer Center Support grant CA-77598. We thank Bob Carlson for editorial assistance.
Biographies
Stephen S. Hecht received his B.S. in chemistry from Duke University and Ph.D. in organic chemistry from the Massachusetts Institute of Technology, where he also did postdoctoral work in mass spectrometry in the laboratory of Professor Klaus Biemann. He began his research on mechanisms and prevention of tobacco induced cancer at the American Health Foundation in 1973. He served as Director of Research there from 1987–1996. He moved to the University of Minnesota as Wallin Professor of Cancer Prevention in 1996. His laboratory focuses on chemical mechanisms of carcinogenesis by compounds present in tobacco products and is widely recognized as a leader in this research area.
Irina Stepanov was born in Chisinau, the capital of the Republic of Moldova, in 1973. She obtained her B.S. and Ph.D. degrees from Moldova State University before joining the Masonic Cancer Center at the University of Minnesota, first as a Postdoctoral Associate and later as a Research Associate. She has developed assays for the analysis of urinary total NNN, toenail biomarkers and oral cell DNA adducts discussed in this article, and played a key role in the effort on the application of deuterium-labeled NNK to understand inter-individual variations in NNK metabolic activation. Dr. Stepanov is currently an Assistant Professor in the Division of Environmental Health Sciences, School of Public Health, University of Minnesota, and is continuing research in the area of tobacco carcinogenesis, with particular emphasis on tobacco regulatory science.
Steven G. Carmella joined the Hecht laboratory after receiving his B.A. in chemistry from Queens College, City University of New York, in 1976. He is an expert in applications of gas chromatography and high performance liquid chromatography to the trace analysis of tobacco-related compounds and their metabolites. He has played a leading role in the development and application of multiple biomarker analyses in the Hecht laboratory, including those for urinary NNAL and NNN, phenanthrene metabolites, mercapturic acids, and hemoglobin adducts of tobacco-specific nitrosamines.
References
- 1.Hecht SS. Research Opportunities Related to Establishing Standards for Tobacco Products Under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaction of nicotine with sodium nitrite produces low yields of the three primary products -, NNN, NNK, and NNA (nicotine-derived nitrosamino aldehyde). The names NNK and NNA were devised to signify the clear relationship of these new nitrosation products to NNN, which was already known. I rejected the alternative to NNK - MNPB - as an unwieldy tongue twister. In summary, NNK is no worse than some names given to various genes.
- 3.International Agency for Research on Cancer Smokeless Tobacco and Tobacco-Specific Nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. IARC; Lyon, FR: 2007. pp. 421–583. [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. IARC; Lyon, FR: 2004. pp. 174–176. [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht SS. Tobacco Smoke Carcinogens and Lung Cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 6.United States Department of Health and Human Services. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. The Health Consequences of Smoking: 50 Years of Progress. [Google Scholar]
- 7.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami DK, Hecht SS. Presence of the Carcinogen N′-Nitrosonornicotine in the Urine of Some Users of Oral Nicotine Replacement Therapy Products. Cancer Res. 2009;69:8236–8240. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht SS. Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 9.Jalas J, Hecht SS, Murphy SE. Cytochrome P450 2A Enzymes As Catalysts of Metabolism of 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone (NNK), a Tobacco-Specific Carcinogen. Chem Res Toxicol. 2005;18:95–110. doi: 10.1021/tx049847p. [DOI] [PubMed] [Google Scholar]
- 10.Breyer-Pfaff U, Martin HJ, Ernst M, Maser E. Enantioselectivity of Carbonyl Reduction of 4-Methylnitrosamino-1-(3-Pyridyl)-1-Butanone by Tissue Fractions From Human and Rat and by Enzymes Isolated From Human Liver. Drug Metab Dispos. 2004;32:915–922. [PubMed] [Google Scholar]
- 11.Hecht SS, Young R, Chen CB. Metabolism in the F344 Rat of 4-(N-Methyl-N-Nitrosamino)-1-(3-Pyridyl)-1-Butanone, a Tobacco Specific Carcinogen. Cancer Res. 1980;40:4144–4150. [PubMed] [Google Scholar]
- 12.Morse MA, Eklind KI, Toussaint M, Amin SG, Chung FL. Characterization of a Glucuronide Metabolite of 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone (NNK) and Its Dose-Dependent Excretion in the Urine of Mice and Rats. Carcinogenesis. 1990;11:1819–1823. doi: 10.1093/carcin/11.10.1819. [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS, Trushin N, Reid-Quinn CA, Burak ES, Jones AB, Southers JL, Gombar CT, Carmella SG, Anderson LM, Rice JM. Metabolism of the Tobacco-Specific Nitrosamine 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone in the Patas Monkey: Pharmacokinetics and Characterization of Glucuronide Metabolites. Carcinogenesis. 1993;14:229–236. doi: 10.1093/carcin/14.2.229. [DOI] [PubMed] [Google Scholar]
- 14.Ren Q, Murphy SE, Zheng Z, Lazarus P. O-Glucuronidation of the Lung Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL) by Human UDP-Glucuronosyltransferases 2B7 and 1A9. Drug Metab Dispos. 2000;28:1352–1360. [PubMed] [Google Scholar]
- 15.Carmella SG, Le K, Upadhyaya P, Hecht SS. Analysis of N- and O-Glucuronides of 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL) in Human Urine. Chem Res Toxicol. 2002;15:545–550. doi: 10.1021/tx015584c. [DOI] [PubMed] [Google Scholar]
- 16.Carmella SG, Akerkar S, Hecht SS. Metabolites of the Tobacco-Specific Nitrosamine 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone in Smokers’ Urine. Cancer Res. 1993;53:721–724. [PubMed] [Google Scholar]
- 17.Shah KA, Karnes HT. A Review of the Analysis of Tobacco-Specific Nitrosamines in Biological Matrices. Crit Rev Toxicol. 2010;40:305–327. doi: 10.3109/10408440903394435. [DOI] [PubMed] [Google Scholar]
- 18.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High Throughput Liquid and Gas Chromatography-Tandem Mass Spectrometry Assays for Tobacco-Specific Nitrosamine and Polycyclic Aromatic Hydrocarbon Metabolites Associated With Lung Cancer in Smokers. Chem Res Toxicol. 2013;26:1209–1217. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS, Carmella SG, Chen M, Koch JFD, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of Urinary Metabolites of a Tobacco-Specific Lung Carcinogen After Smoking Cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 20.Goniewicz ML, Havel CM, Peng MW, Jacob P, III, Dempsey D, Yu L, Zielinska-Danch W, Koszowski B, Czogala J, Sobczak A, Benowitz NL. Elimination Kinetics of the Tobacco-Specific Biomarker and Lung Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol. Cancer Epidemiol Biomarkers Prev. 2009;18:3421–3425. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecht SS. Human Urinary Carcinogen Metabolites: Biomarkers for Investigating Tobacco and Cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 22.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, Mendes PE. Population Estimates for Biomarkers of Exposure to Cigarette Smoke in Adult U.S. Cigarette Smokers. Nicotine Tob Res. 2009;11:1216–1225. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-Specific Nitrosamine 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL) in Smokers in the United States: NHANES 2007–2008. Biomarkers. 2011;16:112–119. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 24.Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, Hoffmann D. A Tobacco-Specific Lung Carcinogen in the Urine of Men Exposed to Cigarette Smoke. N Engl J Med. 1993;329:1543–1546. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. IARC; Lyon, FR: 2004. pp. 1191–1413. [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas JL, Guo H, Carmella SG, Balbo S, Han S, Davis A, Murphy SE, An LC, Ahluwalia JS, Hecht SS. Metabolites of a Tobacco-Specific Lung Carcinogen in Children Exposed to Secondhand or Thirdhand Tobacco Smoke in Their Homes. Cancer Epidemiol Biomarkers Prev. 2011;20:1213–1221. doi: 10.1158/1055-9965.EPI-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht SS, Ye M, Carmella SG, Fredrickson A, Adgate JL, Greaves IA, Church TR. Metabolites of a Tobacco-Specific Lung Carcinogen in the Urine of Elementary School-Aged Children. Cancer Epidemiol Biomarkers Prev. 2001;10:1109–1116. [PubMed] [Google Scholar]
- 28.Hecht SS, Carmella SG, Le K, Murphy SE, Boettcher AJ, Le C, Koopmeiners J, An L, Hennrikus DJ. 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol and Its Glucuronides in the Urine of Infants Exposed to Environmental Tobacco Smoke. Cancer Epidemiol Biomarkers Prev. 2006;15:988–992. doi: 10.1158/1055-9965.EPI-05-0596. [DOI] [PubMed] [Google Scholar]
- 29.Vogel RI, Carmella SG, Stepanov I, Hatsukami DK, Hecht SS. The Ratio of a Urinary Tobacco-Specific Lung Carcinogen Metabolite to Cotinine Is Significantly Higher in Passive Than in Active Smokers. Biomarkers. 2011;16:491–497. doi: 10.3109/1354750X.2011.598565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei B, Blount BC, Xia B, Wang L. Assessing Exposure to Tobacco-Specific Carcinogen NNK Using Its Urinary Metabolite NNAL Measured in US Population: 2011–2012. J Expo Sci Environ Epidemiol. 2015 doi: 10.1038/jes.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, Han S, Wickham K, Gao YT, Yu MC, Hecht SS. Urinary Levels of Tobacco-Specific Nitrosamine Metabolites in Relation to Lung Cancer Development in Two Prospective Cohorts of Cigarette Smokers. Cancer Res. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan JM, Gao YT, Murphy SE, Carmella SG, Wang R, Zhong Y, Moy KA, Davis AB, Tao L, Chen M, Han S, Nelson HH, Yu MC, Hecht SS. Urinary Levels of Cigarette Smoke Constituent Metabolites Are Prospectively Associated With Lung Cancer Development in Smokers. Cancer Res. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan J, Song Q, Shi H, King M, Junga H, Zhou S, Naidong W. Development, Validation and Transfer of a Hydrophilic Interaction Liquid Chromatography/Tandem Mass Spectrometric Method for the Analysis of the Tobacco-Specific Nitrosamine Metabolite NNAL in Human Plasma at Low Picogram Per Milliliter Concentrations. Rapid Commun Mass Spectrom. 2004;18:2549–2557. doi: 10.1002/rcm.1656. [DOI] [PubMed] [Google Scholar]
- 34.Carmella SG, Han S, Villalta PW, Hecht SS. Analysis of Total 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL) in Smokers’ Blood. Cancer Epidemiol Biomarkers Prev. 2005;14:2669–2672. doi: 10.1158/1055-9965.EPI-05-0129. [DOI] [PubMed] [Google Scholar]
- 35.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le C, Zhang Y, Benoit AR, Carmella SG, Hecht SS. A Prospectively Measured Serum Biomarker for a Tobacco-Specific Carcinogen and Lung Cancer in Smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Mass Spectrometric Quantitation of Nicotine, Cotinine, and 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol, in Human Toenails. Cancer Epidemiol Biomarkers Prev. 2006;15:2378–2383. doi: 10.1158/1055-9965.EPI-06-0265. [DOI] [PubMed] [Google Scholar]
- 37.Stepanov I, Hecht SS, Lindgren B, Jacob P, III, Wilson M, Benowitz NL. Relationship of Human Toenail Nicotine, Cotinine, and 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol to Levels of These Biomarkers in Plasma and Urine. Cancer Epidemiol Biomarkers Prev. 2007;16:1382–1386. doi: 10.1158/1055-9965.EPI-07-0145. [DOI] [PubMed] [Google Scholar]
- 38.Upadhyaya P, Zimmerman CL, Hecht SS. Metabolism and Pharmacokinetics of N′-Nitrosonornicotine in the Patas Monkey. Drug Metab Dispos. 2002;30:1115–1122. doi: 10.1124/dmd.30.10.1115. [DOI] [PubMed] [Google Scholar]
- 39.Stepanov I, Hecht SS. Tobacco-Specific Nitrosamines and Their N-Glucuronides in the Urine of Smokers and Smokeless Tobacco Users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–891. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 40.Urban M, Scherer G, Kavvadias D, Hagedorn HW, Feng S, Serafin R, Kapur S, Muhammad R, Jin Y, Mendes P, Roethig H. Quantitation of N′-Nitrosonornicotine (NNN) in Smokers’ Urine by Liquid Chromatography-Tandem Mass Spectrometry. J Anal Toxicol. 2009;33:260–265. doi: 10.1093/jat/33.5.260. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar M, Liu J, Koval T, Wang J, Feng S, Serafin R, Jin Y, Xie Y, Newland K, Roethig HJ. Evaluation of Biomarkers of Exposure in Adult Cigarette Smokers Using Marlboro Snus. Nicotine Tob Res. 2010;12:105–116. doi: 10.1093/ntr/ntp183. [DOI] [PubMed] [Google Scholar]
- 42.Xia B, Xia Y, Wong J, Nicodemus KJ, Xu M, Lee J, Guillot T, Li J. Quantitative Analysis of Five Tobacco-Specific N-Nitrosamines in Urine by Liquid Chromatography-Atmospheric Pressure Ionization Tandem Mass Spectrometry. Biomed Chromatogr. 2014;28:375–384. doi: 10.1002/bmc.3031. [DOI] [PubMed] [Google Scholar]
- 43.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and Traditional Smokeless Tobacco: Comparison of Toxicant and Carcinogen Levels. Nicotine Tob Res. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stepanov I, Biener L, Knezevich A, Nyman AL, Bliss R, Jensen J, Hecht SS, Hatsukami DK. Monitoring Tobacco-Specific N-Nitrosamines and Nicotine in Novel Marlboro and Camel Smokeless Tobacco Products: Findings From Round 1 of the New Product Watch. Nicotine Tob Res. 2011;14:274–281. doi: 10.1093/ntr/ntr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary Levels of the Tobacco-Specific Carcinogen N′-Nitrosonornicotine and Its Glucuronide Are Strongly Associated With Esophageal Cancer Risk in Smokers. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepanov I, Sebero E, Wang R, Gao YT, Hecht SS, Yuan JM. Tobacco-Specific N-Nitrosamine Exposures and Cancer Risk in the Shanghai Cohort Study: Remarkable Coherence With Rat Tumor Sites. Int J Cancer. 2014;134:2278–2283. doi: 10.1002/ijc.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knezevich A, Muzic J, Hatsukami DK, Hecht SS, Stepanov I. Nornicotine Nitrosation in Saliva and Its Relation to Endogenous Synthesis of N′-Nitrosonornicotine in Humans. Nicotine Tob Res. 2013;15:591–595. doi: 10.1093/ntr/nts172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepanov I, Hecht SS. Detection and Quantitation of N′-Nitrosonornicotine in Human Toenails by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. Cancer Epidemiol Biomarkers Prev. 2008;17:945–948. doi: 10.1158/1055-9965.EPI-07-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hecht SS, Yuan JM, Hatsukami DK. Applying Tobacco Carcinogen and Toxicant Biomarkers in Product Regulation and Cancer Prevention. Chem Res Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Tan W, Hao B, Miao X, Zhou G, He F, Lin D. Substantial Reduction in Risk of Lung Adenocarcinoma Associated With Genetic Polymorphism in CYP2A13, the Most Active Cytochrome P450 for the Metabolic Activation of Tobacco-Specific Carcinogen NNK. Cancer Res. 2003;63:8057–8061. [PubMed] [Google Scholar]
- 51.Yuan J-Y, Nelson HH, Butler LM, Carmella SG, Wang R, Kuriger-Laber J, Adams-Haduch J, Hecht SS, Gao Y-T, Murphy SE. Genetic Determinants of Cytochrome P450 2A6 and Biomarkers of Tobacco Smoke Exposure in Releation to Risk of Lung Cancer Development in the Shanghai Cohort Study. Int J Cancer. 2015 doi: 10.1002/ijc.29963. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jing M, Wang Y, Upadhyaya P, Jain V, Yuan JM, Hatsukami DK, Hecht SS, Stepanov I. Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry Quantitation of Urinary [Pyridine-D4]4-Hydroxy-4-(3-Pyridyl)Butanoic Acid, a Biomarker of 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone Metabolic Activation in Smokers. Chem Res Toxicol. 2014;27:1547–1555. doi: 10.1021/tx5001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrenberg L, Osterman-Golkar S. Alkylation of Macromolecules for Detecting Mutagenic Agents. Teratogenesis Carcinog Mutagen. 1976;1:105–127. doi: 10.1002/tcm.1770010111. [DOI] [PubMed] [Google Scholar]
- 54.Tornqvist M, Osterman-Golkar S, Kautiainen A, Hensen S, Farmer PB, Ehrenberg L. Tissue Doses of Ethylene Oxide in Cigarette Smokers Determined From Adduct Levels in Hemoglobin. Carcinogenesis. 1986;7:1519–1521. doi: 10.1093/carcin/7.9.1519. [DOI] [PubMed] [Google Scholar]
- 55.Bryant MS, Skipper PL, Tannenbaum SR, Maclure M. Hemoglobin Adducts of 4-Aminobiphenyl in Smokers and Nonsmokers. Cancer Res. 1987;47:602–608. [PubMed] [Google Scholar]
- 56.Carmella SG, Hecht SS. Formation of Hemoglobin Adducts Upon Treatment of F344 Rats With the Tobacco-Specific Nitrosamines 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone and N′-Nitrosonornicotine. Cancer Res. 1987;47:2626–2630. [PubMed] [Google Scholar]
- 57.Carmella SG, Kagan SS, Hecht SS. Evidence That a Hemoglobin Adduct of 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone Is a 4-(3-Pyridyl)-4-Oxobutyl Carboxylic Acid Ester. Chem Res Toxicol. 1992;5:76–80. doi: 10.1021/tx00025a013. [DOI] [PubMed] [Google Scholar]
- 58.Murphy SE, Palomino A, Hecht SS, Hoffmann D. Dose-Response Study of DNA and Hemoglobin Adduct Formation by 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone in F344 Rats. Cancer Res. 1990;50:5446–5452. [PubMed] [Google Scholar]
- 59.Hecht SS, Carmella SG, Foiles PG, Murphy SE. Biomarkers for Human Uptake and Metabolic Activation of Tobacco-Specific Nitrosamines. Cancer Res [Suppl ] 1994;54:1912s–1917s. [PubMed] [Google Scholar]
- 60.Hecht SS. Progress and Challenges in Selected Areas of Tobacco Carcinogenesis. Chem Res Toxicol. 2008;21:160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma B, Villalta PW, Zarth A, Kotandeniya D, Upadhyaya P, Stepanov IS, Hecht SS. Comprehensive High Resolution Mass Spectrometric Analysis of DNA Phosphate Adducts Formed by the Tobacco-Specific Lung Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone (NNK) Chem Res Toxicol. 2015 doi: 10.1021/acs.chemrestox.5b00318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foiles PG, Akerkar SA, Carmella SG, Kagan M, Stoner GD, Resau JH, Hecht SS. Mass Spectrometric Analysis of Tobacco-Specific Nitrosamine-DNA Adducts in Smokers and Nonsmokers. Chem Res Toxicol. 1991;4:364–368. doi: 10.1021/tx00021a017. [DOI] [PubMed] [Google Scholar]
- 63.Hölzle D, Schlöbe D, Tricker AR, Richter E. Mass Spectrometric Analysis of 4-Hydroxy-1-(3-Pyridyl)-1-Butanone-Releasing DNA Adducts in Human Lung. Toxicology. 2007;232:277–285. doi: 10.1016/j.tox.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Schlobe D, Holzle D, Hatz D, Von Meyer L, Tricker AR, Richter E. 4-Hydroxy-1-(3-Pyridyl)-1-Butanone-Releasing DNA Adducts in Lung, Lower Esophagus and Cardia of Sudden Death Victims. Toxicology. 2008;245:154–161. doi: 10.1016/j.tox.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 65.Stepanov I, Muzic J, Le CT, Sebero E, Villalta P, Jensen J, Hatsukami D, Hecht SS. Analysis of 4-Hydroxy-1-(3-Pyridyl)-1-Butanone (HPB)-Releasing DNA Adducts in Human Exfoliated Oral Mucosa Cells by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. Chem Res Toxicol. 2013;26:37–45. doi: 10.1021/tx300282k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao L, Balbo S, Wang M, Upadhyaya P, Khariwala SS, Villalta PW, Hecht SS. Quantitation of Pyridyloxobutyl-DNA Adducts in Tissues of Rats Treated Chronically With (R)- or (S)-N′-Nitrosonornicotine (NNN) in a Carcinogenicity Study. Chem Res Toxicol. 2013;26:1526–1535. doi: 10.1021/tx400235x. [DOI] [PMC free article] [PubMed] [Google Scholar]