Abstract
Emerging evidence suggests a positive association of diet and obesity with depression. Researchers have examined several diet-mood hypotheses, including investigating the extent to which carbohydrates may impact mood. There is limited research on how glycemic load, a characteristic of carbohydrates, impacts mood in healthy adults. Eighty-two healthy weight and overweight/obese, but otherwise healthy, adults enrolled in a randomized, crossover controlled feeding study testing low- compared to high- glycemic load diets. All participants completed self-report mood and energy level questionnaires during each arm of the intervention. Diets were isocaloric and were matched by macronutrient content as a percent of total energy. Mood was assessed with the Profile of Mood States (POMS) subscales; tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment, total mood disturbance (TMD), and negative affect (NA) in addition to the Center for Epidemiological Studies – Depression (CES-D) scale at baseline and end of both 28-day feeding periods. Linear mixed models tested the intervention effect on mood, controlling for baseline POMS and CES-D scores, diet type, diet sequence, feeding period, sex, and percent body fat classification. The consumption of the high-glycemic load diet resulted in a 38% higher score for depressive symptoms on the CES-D (P = 0.002) compared to the low-glycemic load diet as well as 55% higher score for TMD (P = 0.05), and 26% higher score for fatigue/inertia (P = 0.04). In subgroup analyses, the overweight/obese participants had 40% higher scores on the CES-D scale compared to healthy weight participants (P = 0.05). In conclusion, a high-glycemic load diet was associated with higher depression symptoms, total mood disturbance, and fatigue compared to a low-glycemic load diet especially in overweight/obese, but otherwise healthy, adults.
Keywords: Depression, Controlled trial, Diet, Glycemic Index, Humans, Obesity
Introduction
Mood disorders, such as anxiety and depression, are the most common mental illnesses in the United States (Kessler, Chiu, Demler, Merikangas, & Walters, 2005). Several studies have examined possible relationships between nutritional intake and mood. Nutrition-related associations with mood include sub-optimal intake of specific nutrients (e.g., omega-3 fatty acids or vitamin C), various diet patterns (e.g., Mediterranean vs. Western), and increased or decreased consumption of carbohydrates (Akbaraly, et al., 2009; Beezhold, Johnston, & Daigle, 2010; Gilbody, Lightfoot, & Sheldon, 2007; Hu, 2002; Jacka, et al., 2011; Jacka, et al., 2010; Kennedy, et al., 2010; Kuczmarski, et al., 2010; Murakami & Sasaki, 2010; Nanri, et al., 2010; Sanchez-Villegas, Delgado-Rodriguez, et al., 2009; Sanchez-Villegas, Doreste, et al., 2009; Sanchez-Villegas, Toledo, et al., 2011; Sanchez-Villegas, Verberne, et al., 2011; Simopoulos, 2011; Smith MA, 2011). No conclusive evidence points to either positive or negative associations of depression and individual nutrients. However, dietary pattern research has repeatedly shown depressive symptoms are positively associated with poor quality diet patterns (“Western”, high in processed grain products, low in fruits, vegetables, and lean protein) and inversely associated with higher quality diet patterns (Mediterranean, traditional (minimally processed), Healthy Eating Index, high in fruits, vegetables, whole foods, and lean protein, low in processed foods) (Akbaraly, et al., 2009; Jacka, et al., 2011; Jacka, et al., 2010; Kuczmarski, et al., 2010; Lucas, et al., 2014; Sanchez-Villegas, Delgado-Rodriguez, et al., 2009).
Several factors may connect carbohydrate quality to neurological function and mood including: the recognized role of glucose as the primary source of fuel for the brain, the influence of a high-carbohydrate meal on increasing serum tryptophan concentrations and subsequent serotonin synthesis, and reported carbohydrate craving among people who also experienced depression, seasonal affective disorder, and premenstrual syndrome (Benton & Nabb, 2003; Christensen, 1997; Christensen & Pettijohn, 2001; Lieberman, Wurtman, & Chew, 1986; Wurtman, 1993). Importantly, dietary glycemic load (GL), which is a measure of blood glucose response to food influenced by carbohydrate type and quality, affects both blood glucose supply and glucose tolerance and these in turn have subsequent effects on brain function (Benton & Nabb, 2003). A few studies have examined the potential effects of varied amounts of carbohydrate in weight-loss diets on mood states (Brinkworth, Buckley, Noakes, Clifton, & Wilson, 2009; Cheatham, et al., 2009; D'Anci, Watts, Kanarek, & Taylor, 2009; Halyburton, et al., 2007). However, there is very little research on the influence of high glycemic load (HGL) and low glycemic load (LGL) diet patterns on mood among healthy individuals. This lack of evidence motivated this study.
GL diet patterns, low to moderate GL in particular, have been used to stabilize fluctuation of blood glucose or to improve glycemic control (Brand-Miller, Wolever, Foster-Powell, & Colagiuri, 2003; Foster-Powell, Holt, & Brand-Miller, 2002; Thomas & Elliott, 2009). Gradual release of glucose to the bloodstream and lower ensuing insulin release from foods with a low glycemic index minimizes glycemic variation, whereas foods with a high glycemic index have a tendency to cause spikes in blood glucose concentrations and insulin response (Thomas & Elliott, 2009). Glycemic variation and fluctuation of insulin levels in people with diabetes may lead to oxidative stress and production of pro-inflammatory cytokines (Kiecolt-Glaser, 2010). This physiological response may be related to mood as there is emerging evidence supporting a possible role of inflammatory processes in the development of depression (Shelton & Miller, 2010; Taylor & Macqueen, 2010). While the effects of glycemic variation have been extensively examined in people with type 1 and type 2 diabetes, less is known about the effects of glycemic variation in healthy people.
The purpose of the present study was to measure the subjective mood and energy levels of healthy participants in a randomized crossover, controlled dietary intervention testing effects of HGL and LGL experimental diets. A secondary objective was to investigate whether the associations of the HGL and LGL diets with mood varied by participant body weight (healthy vs. overweight/obese). We hypothesized that there would be an overall difference in subjective mood and energy levels between the HGL and LGL diets, specifically the HGL diet would be associated with poor mood compared to the LGL experimental diet. A secondary hypothesis was that the observed differences would vary by participants’ body fat classification, specifically with the diet-effect contrast being stronger in participants with higher body weight.
Methods
Study participants
Participants in the Carbohydrates and Related Biomarkers (CARB) Study were healthy, free living, nonsmoking men and women aged 18–45 years, recruited from the Seattle area (Neuhouser, et al., 2012). Efforts were made to enroll equal numbers of women and men, and healthy weight and overweight/obese participants. Enrollment BMI criteria for healthy weight ranged from BMI > 18.5 to < 25.0 kg/m2 and ≥ 28.0 – 40.0 kg/m2 for overweight/obese participants. Extensive exclusion criteria ensured that participants did not have health conditions that could interfere with study results, including diabetes, cardiovascular disease or other disease states requiring treatment medication. Study participants were asked to refrain from taking nutritional supplements during the course of the intervention. Study protocols were approved by the Institutional Review Board and the Clinical Trials Office at Fred Hutchinson Cancer Research Center (FHCRC). The trial was registered at clinicaltrials.gov (NCT00622661) as part of the National Cancer Institute’s Transdisciplinary Research on Energetics and Cancer (U54 CA116847). All study participants gave informed written consent prior to starting the intervention.
Study Diets
The dietary intervention consisted of two 28-day controlled feeding periods in which participants were randomized in a crossover design. Participants resumed their habitual diets during a 28-day washout period between feeding periods. The intervention diets were isocaloric for the two arms, HGL and LGL, with the same target macronutrient composition for both diets (55% energy carbohydrate, 30% energy fat, and 15% energy protein). The GL calculations for this study were based on our previous work (Neuhouser, et al., 2006). In summary, a GL unit is the equivalence of 1g of carbohydrate from white bread or glucose. In the present study the GL = ([glycemic index of individual food×g carbohydrate per serving of food]/100). Diet parameters were set in the primary study protocol as follows: ≥ 250 for the HGL per day and ≤ 125 for the LGL per day, maximizing the contrast between the two diets. Prior to this study, few intervention studies had been conducted to test HGL vs LGL diets on multiple health parameters. Of those studies the GL contrast was not as large. Sloth et al tested HGL vs LGL using an ad libitum design however the mean GL on the “low GL arm” was 93 and the mean on the “high GL arm” was 103 (Sloth, et al., 2004). In addition, we had pilot data to support the current study’s range and contrast of GL as sufficient to show an intervention effect. In the current study, the HGL menu was similar to a “Western” diet pattern with more refined sugars and highly processed grain foods whereas the LGL menu was more in line with a traditional or Mediterranean diet pattern with whole grains, legumes, and minimal processed grain foods and added sugars.
During baseline data collection, participants completed 3-day diet records to estimate habitual intake. Individual participant energy requirements were determined using the Mifflin equation as well as the 3-day diet record (Mifflin, et al., 1990). Participants’ weights were monitored 3 times a week as the CARB study protocol required weight stability over the course of the entire intervention. Each arm of the intervention had a 7-day menu cycle designed using ProNutra® (version 3.2, Viocare, Inc., Princeton, NJ). All foods during both intervention arms were prepared by the Human Nutrition Lab at the Fred Hutchinson Cancer Research Center in a highly standardized manner. Participants received extensive instruction that they were to eat only study foods, which were provided on a daily basis (Monday-Friday at dinner with all week-end food sent home on Friday evenings) during the two 28-day periods. All study food containers were returned at each visit where staff weighed and recorded leftovers, if necessary. Self-administered study food intake check-off forms were also completed on a daily basis. Compliance was excellent as 97% of participants consumed >90.0% of the provided foods. Example of study foods are shown in Table 1. Further details of study development, methods, and procedures were published previously (Neuhouser, et al., 2012).
Table 1.
Intervention menus were designed to maximize the contrast between the two diets, with GL values ≥ 250 for the high glycemic load diet (HGL) per day and ≤ 125 for the low glycemic load diet (LGL) per day. The intervention diets were isocaloric for the two arms, HGL and LGL, with the same target macronutrient composition for both diets (55% energy carbohydrate, 30% energy fat, and 15% energy protein).
| Food Profile of Treatment Diets | |
|---|---|
| HGL | LGL |
| 2% milk, Shredded wheat, raisins, sugar | 2% milk, All bran, berries, nuts |
| Turkey sandwich on plain white bagel | Turkey sandwich on whole grain rye pumpernickel |
| Broccoli, instant split pea soup, canned apricots |
Carrots, lentil salad, fresh apples |
| Jellybeans, vanilla wafers | Peanut M&M’s |
| Saltines, cheddar, Gatorade | Prunes, dried apricots, cheddar |
| Salmon cakes, instant mashed potatoes, green beans |
Salmon cakes, barley pilaf, green beans |
| Cranberry juice, angel food cake | Apple juice (100% fruit juice), dark chocolate mousse |
Measures of Mood and Depression
The Profile of Mood States (POMS) Brief questionnaire (McNair, Lorr, & Droppleman, 1971) is a standardized, validated and widely used mood assessment tool. It was administered to each participant at baseline prior to starting the intervention and end of week 4 during an evening dining visit of each feeding period. The POMS-Brief consists of 30 adjectives (e.g., tense, angry, weary, efficient, uneasy, etc.). Participants were asked to assess their mood “during the past week, including today” and asked to rate the extent to which they were experiencing each mood adjective using a scale from 0 (“not at all”) – 4 (“extremely”). The POMS-Brief has 6 mood subscales, tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment. Scores for the subscales range from 0 – 20. The POMS-Brief also provides a Total Mood Disturbance (TMD) and Negative Affect (NA) score. The TMD is a composite score of the 6 subscales with vigor reverse scored. The TMD score is used for assessment of a single global estimate of affective state on a scale of −20 – 100. The NA score is a sum of tension-anxiety, depression-dejection, anger-hostility with a range of scores from 0 – 60. It is used to assess only the negative mood states (McNair & Heuchert, 2005). Higher scores indicate more intense perception of the mood type.
The Center for Epidemiological Studies Depression Scale (CES-D) 5-item short form (Bohannon, Maljanian, & Goethe, 2003) was administered to each participant at baseline prior to starting the intervention and at Day 28, the final day of each feeding period. This scale has been widely used in screening for depression and depressive symptoms. The 5-item CES-D, which was developed to reduce participant burden, has shown very good sensitivity (> 0.84), specificity (≥0.80), and high validity (> 0.90) for all identified cut points in identifying patients classified as depressed by the full 20-item scale (Bohannon, et al., 2003). Participants were asked to respond to a list of 5 statements with these instructions, “Below is a list of ways you may have felt or behaved. Please tell me how you have felt during the past week”, using a scale from 0 (“rarely or none of the time, less than 1 day”) to 3 (“most or all of the time, 5–7 days”). Higher scores indicate more reported depressive symptoms on a scale of 0 – 15. The cut point score for clinically significant symptoms for the 5-item CES-D is 5.5 (Bohannon, et al., 2003).
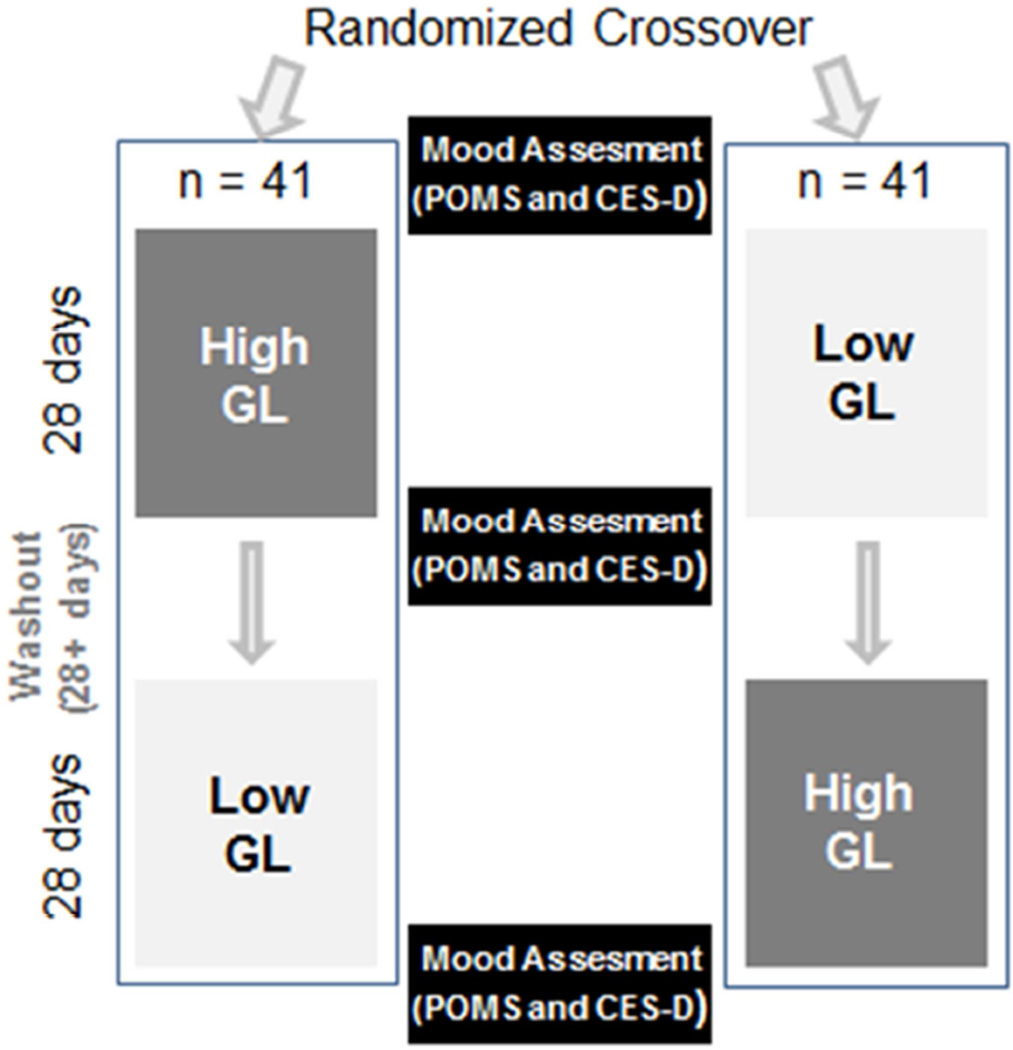
In efforts to capture participants’ best indicator of stable mood based on habitual diet and lifestyle, baseline measures were taken prior to starting the study intervention procedures. While on study, the self-report questionnaires were administered to participants at an evening meal on their final days at the study center. Figure 1 shows study design for the mood assessment measurements. Efforts were taken to provide a neutral, safe, and confidential environment for completing questionnaires. Missing or unanswered data were imputed using the correction formula prepared by the authors of the POMS (0.45% of all gathered data were imputed) (McNair & Heuchert, 2005). The feeding intervention and all mood assessments took place from July 2006 – July 2009 in the Human Nutrition Lab at FHCRC in Seattle, Washington.
Figure 1.
Study design of mood assessments. All study procedures were intended to be completed within 4 months. Participants resumed habitual diet during the washout period.
Statistical Analysis
The statistical analysis was designed to test the effects of the HGL and LGL intervention diets on subjective mood and energy. Descriptive statistics were used to characterize the study participants. Mood outcome scores (POMS tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, confusion-bewilderment, total mood disturbance, and negative affect, and CES-D) were assessed for outliers and normality. POMS subscale and CES-D scores at the end of Week 4, the last days of each feeding period, were the dependent variables in the present analysis. Participants were enrolled into the study based on measured BMI (healthy weight = 18.5 – < 25.0 kg/m2 and overweight/obese = 28.0 – 40.0 kg/m2), but initial exploration of the body composition assessed by duel-energy X-ray absorptiometry (DXA) scan data indicated that some participants were misclassified as lean with a lower BMI when their percent body fat was considered high. The overweight/obese or high body fat group was defined as percent body fat > 32% for females and >25% for males and low body fat or healthy adiposity group was below 32% for women and 25% for men (Neuhouser, et al., 2012).
A mixed-effects linear model was used to evaluate the effects of the HGL and LGL diets, where body fat classification, sex, diet, diet sequence, and feeding period were fixed effects and participant was a random effect. The mixed-effects analysis appropriately allows use of all available data points in one model for this randomized, crossover design with repeated measures as seen in other similar study designs (Kral, Bannon, & Moore, 2016; Mills, et al., 2009; Sabate, Haddad, Tanzman, Jambazian, & Rajaram, 2003). Age, race, and education were considered as possible confounders, but these variable were neither statistically significant nor influential on parameter estimates and therefore not included so as to present the most parsimonious models. Least squared means contrasting diet effect on POMS subscale and CES-D scores were reported. All data analyses were performed with SAS 9.3 (SAS Institute, Cary, NC USA). Statistical significance was set at a two-tailed, 0.05 level of probability.
Results
Table 2 provides information on participant characteristics. Based on participant BMI at enrollment, there was an equal distribution of men and women among healthy weight and overweight/obese classification. However, the DXA scan data determined there were 53 participants (31 female, 22 male) with higher percent body fat in the overweight/obese group and 29 participants (10 female, 19 male) had lower percent body fat in the healthy weight group. The overweight/obese participants were on average, older than the healthy weight group. Over half of the participants were racial and ethnic minorities, although the distribution among the body fat classification varied and was not equal, the majority of overweight/obese group were non-Hispanic white individuals.
Table 2.
Participant characteristics based on percent body fat classification determined by duel-energy X-ray absorptiometry (DXA) scan. The overweight/obese or high body fat group was defined as percent body fat > 32% for females and >25% for males and low body fat or healthy weight group was below 32% for women and 25% for men.
| Characteristics | Overweight/Obese N = 53 |
Healthy weight N = 29 |
|---|---|---|
| Female N (%) | 31 (58) | 10 (34) |
| Age, yr1 | 31.3 (8.4) | 26.4 (6.4) |
| % Body fat1 | 39.6 (8.3) | 21.2 (6.9) |
| Female1 | 45.7 (7.5) | 25.2 (6.7) |
| Male1 | 30.4 (9.6) | 9.2 (7.6) |
| Race/Ethnicity N (%) | ||
| Non-Hispanic white | 26 (49.1) | 10 (34.5) |
| Asian/Pacific Islander/Native American |
4 (7.5) | 4 (13.8) |
| Black | 10 (18.9) | 7 (24.1) |
| Hispanic | 13 (24.5) | 8 (27.6) |
mean(SD)
Results from the mixed model analyses comparing the HGL diet to the LGL diet are displayed in Table 3 as adjusted mean differences for the relevant POMS subscales and CES-D. The POMS subscale tension-anxiety, depression-dejection and anger-hostility were accounted for in the negative affect scores and were therefore not analyzed individually in the mixed linear model. The models controlled for baseline POMS subscale and CES-D scores, sex, percent body fat, feeding period, diet type, and diet order for all participants. Vigor-activity was significantly lower on the HGL diet (P = 0.01), whereas fatigue-inertia was significantly higher on the HGL diet (P = 0.04). The total mood disturbance was higher on the HGL diet (P = 0.05) and the negative affect had no associations with diet. There was a significant effect of diet on CES-D score, with higher depressive symptom scores positively associated with the HGL diet (P = 0.002) compared to the LGL diet.
Table 3.
Adjusted mean scores of Profile of Mood States (POMS) subscale and Center for Epidemiological Studies Depression Scale (CES-D) for all participants observed on both high-glycemic load (HGL) diet and a low-glycemic load (LGL) diet in a crossover design study, (N = 82). The models were adjusted for baseline POMS and CES-D scores, diet type, sex, body fat classification, diet order, and feeding period. Reported as least square means with standard error, LSmean (SE).
| HGL | LGL | Difference between diet types |
P value | |
|---|---|---|---|---|
| LSmean (SE) | LSmean (SE) | LSmean (SE) | ||
| POMS subscales | ||||
| Vigor/Activity | 8.43 (0.43) | 9.65 (0.43) | −1.22 (0.48) | 0.01 |
| Fatigue/Inertia | 5.33 (0.44) | 4.24 (0.44) | 1.09 (0.53) | 0.04 |
| Total Mood Disturbance | 10.06 (1.62) | 6.50 (1.63) | 3.56 (1.81) | 0.05 |
| Negative Affect | 9.07 (0.92) | 8.53 (0.93) | 0.54 (1.04) | 0.61 |
| CES-D | 2.80 (0.21) | 2.03 (0.22) | 0.78 (0.24) | 0.002 |
Table 4 displays results from the mixed model analyses for relevant POMS subscale and the CES-D in models stratified by body weight classification comparing the HGL and LGL. As above, the models controlled for baseline POMS subscale and CES-D scores, sex, feeding period, diet type, and diet order. Overall, the overweight/obese group reported higher symptom scores than the healthy weight group on both HGL and LGL diets. The most salient difference for the overweight/obese participant group was an increase in the CES-D scores on the HGL (P = 0.02). The healthy weight participant group saw the greatest magnitude of difference by nearly 2 points on the POMS subscale vigor/activity (P = 0.001) on the LGL diet. All other POMS subscales did not differ significantly for either body weight group, however the trend followed the overall participant analyses that reflected a positive association of worse mood symptoms and the HGL diet.
Table 4.
Adjusted mean scores of Profile of Mood States (POMS) subscale and Center for Epidemiological Studies Depression Scale (CES-D) stratified by Healthy (N = 29) or Overweight/obese (N = 53) participants observed on both high-glycemic load (HGL) diet and a low-glycemic load (LGL) diet in a crossover design study. The models were adjusted for baseline POMS and CES-D scores, diet type, sex, body fat classification (based on DXA), diet order, and feeding period. Reported as least square means with standard error, LSmean (SE).
| Overweight/Obese | Healthy Weight | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HGL | LGL | Difference between diet types |
P value | HGL | LGL | Difference between diet types |
P value | ||||
|
POMS subscales |
LSmean ±SE | LSmean ±SE | LSmean ±SE |
POMS subscales |
LSmean ±SE | LSmean ±SE | LSmean ±SE | ||||
| Vigor/ Activity |
8.07(0.56) | 8.88(0.57) | −0.80(0.65) | 0.22 | Vigor/ Activity |
9.03(0.60) | 10.99(0.59) | −1.96(0.70) | 0.001 | ||
| Fatique/ Inertia |
5.64(0.57) | 4.51(0.59) | 1.13(0.72) | 0.13 | Fatique/ Inertia |
4.77(0.64) | 3.75(0.63) | 1.02(0.75) | 0.19 | ||
| Total Mood Disturbance |
11.70(2.13) | 8.67(2.17) | 3.03(2.36) | 0.21 | Total Mood Disturbance |
7.10(2.12) | 2.68(2.09) | 4.42(2.81) | 0.13 | ||
| Negative Affect |
9.88(1.27) | 9.47(1.30) | 0.41(1.41) | 0.77 | Negative Affect |
7.60(1.10) | 6.84(1.07) | 0.76(1.49) | 0.62 | ||
| CES-D | 3.17(0.29) | 2.41(0.29) | 0.76(0.31) | 0.02 | CES-D | 2.13 (0.30) | 1.33(0.29) | 0.79(0.41) | 0.07 | ||
Table 5 displays results from the mixed model analyses for relevant POMS subscale and the CES-D in models considering the unique contribution of percent body fat, comparing the healthy weight group to the overweight/obese group. As above, the models controlled for baseline POMS subscale and CES-D scores, sex, feeding period, diet type, and diet order. When comparing the adjusted means differences between body fat groups within the context of diet types, the POMS subscale in the overweight/obese group’s adjusted means tended to be higher for total mood disturbance (P = 0.08) and negative affect (P = 0.09) than the healthy weight participants. The adjusted means scores of CES-D in the overweight/obese group had higher scores (P = 0.05) compared to the healthy weight group.
Table 5.
Adjusted mean scores of Profile of Mood States (POMS) subscale and Center for Epidemiological Studies Depression Scale (CES-D) stratified by body fat classification observed in both high-glycemic load (HGL) and low-glycemic load (LGL) intervention diets in a crossover design study. The models were adjusted for baseline POMS and CES-D scores, diet type, sex, body fat classification, diet order, and feeding period. Reported as least square means with standard error, LSmean (SE).
| Overweight/ Obese (N = 53) |
Healthy Weight (N = 29) |
Difference between participant groups |
P value | |
|---|---|---|---|---|
| LSmean (SE) | LSmean (SE) | LSmean (SE) | ||
| POMS subscales | ||||
| Vigor/Activity | 8.62 (0.45) | 9.76 (0.61) | 1.13 (0.77) | 0.15 |
| Fatigue/Inertia | 5.09 (0.44) | 4.24 (0.59) | −0.85 (0.75) | 0.26 |
| Total Mood Disturbance | 10.16 (1.71) | 4.98 (2.29) | −5.18 (2.90) | 0.08 |
| Negative Affect | 9.81 (0.97) | 7.01 (1.30) | −2.80 (1.65) | 0.09 |
| CES-D | 2.68 (0.22) | 1.92 (0.31) | −0.76 (0.38) | 0.05 |
Discussion
To our knowledge, this is the first randomized controlled feeding trial to uniquely test HGL and LGL diets on standardized measures of mood among healthy weight and overweight/obese, but otherwise healthy, adults. The primary finding was that compared to the LGL diet, the HGL diet was associated with significantly higher scores on the POMS subscale for total mood disturbance and for fatigue/inertia and measures of depressive symptoms on the CES-D. In contrast, the LGL diet was associated with more favorable scores for POMS vigor/activity, but no differences in CES-D scores. Although the magnitude of the difference did not result in clinical differences in depression in the present study sample of healthy, euthymic participants, it is important to note the significant differences over the course of 4 weeks on the two divergent GL diets. The multivariate adjusted scores for the POMS subscale, total mood disturbance, was 3.5 points higher on the HGL diet than the LGL diet suggesting worse global mood state in the HGL diet. The multivariate adjusted scores for CES-D was nearly 1 point greater for the HGL diet than the LGL diet, indicating that participants were reporting greater depressive symptoms on the HGL diet. Because depression is one component of the POMS negative affect subscale it often correlates with CES-D scores. However, unlike the CES-D scores in the present study, we did not see any differences in the POMS negative affect subscale scores. This may be due to the general healthy emotional state of participants who would have lower scores for tension-anxiety, and anger-hostility which would dilute the association between POMS- depression and the overall negative affect composite score.
In addition to the above findings, the overweight/obese group appeared more susceptible to depressive symptoms as reported over the course of the intervention. The multivariate adjusted scores of POMS fatigue/inertia, total mood disturbance, negative affect, and CES-D were higher for the overweight/obese participants on the HGL diet. Together these findings bring attention to the potential interaction of two major health problems, depression and obesity.
These findings are important because the age-adjusted prevalence of overweight and obesity among American adults combined is about 68.8%, and 35.7% are obese (Flegal, Carroll, Kit, & Ogden, 2012). The 2008 Behavioral Risk Factor Surveillance System report listed prevalence of current depression at 9.1% of the US adult population and a 400% increase in antidepressant use between 1988–1994 and 2005–2008 (Center for Disease Control & Prevention, 2011; Pratt, Brody, & Gu, 2011). Results from two large population-based studies from Canada and the US support associations between obesity and mood disorders (Gadalla, 2009; Zhao, et al., 2011). The Canadian study revealed higher odds of obesity among people with anxiety or mood disorder (OR = 1.48, 95% CI 1.29 – 1.69) compared to those without (Gadalla, 2009). The 2005–2006 National Health and Nutrition Examination Survey (NHANES) reported that overweight and obese individuals with abdominal obesity were 2.3 times more likely to experience moderate to severe depression or twice as likely to experience major depression compared to obese and overweight individuals without abdominal obesity (Zhao, et al., 2011).
Emerging evidence is linking obesity and depression through a hypothesis that dysregulation of neuroendocrine processes and subsequent rise in inflammatory cytokines play a role in both disease states (Luppino, et al., 2010; Shelton & Miller, 2010; Taylor & Macqueen, 2010). Adipose tissue, predominately abdominal adipose tissue, secretes a myriad of adipokines (e.g., leptin, adiponectin, and resistin) and inflammatory cytokines (e.g., interleukin-6 and tumor necrosis factor α) that may play a role in biological mechanisms of depression (Luppino, et al., 2010; Shelton & Miller, 2010; Taylor & Macqueen, 2010). The results of this study indicate that HGL diet patterns may play a role in the interplay of these diseases.
The present study provides insight on the extent to which carbohydrate characteristics, such as GL diet patterns in a controlled intake intervention in healthy, non-clinically depressed adults, may impact mood. Our results are similar to those of a weight-loss study, that tested the effects of high-carbohydrate/HGL and low-carbohydrate/LGL diets on mood and cognition over six months. The investigators reported a significant diet by time interaction with a rise in POMS subscale depression score (P = 0.009) on the high-carbohydrate, high-glycemic diet after controlling for hunger and weight loss in the analysis (Cheatham, et al., 2009). The authors of the study concluded that participants randomized into the high-carbohydrate, high-glycemic diet report a negative change in mood compared to the participants on the low-carbohydrate, low-glycemic diet. Our present findings are consistent with this previous research, as participants reported more negative mood on the HGL diet. The present study may be considered slightly more robust since the design was a rigorously controlled feeding trial.
The present findings are consistent with several epidemiological studies that have shown associations between worse mood and “Western” and processed foods diet patterns that include higher content of processed food items. Authors of these diet pattern and depressive symptom studies suggest that increased systemic inflammation and oxidative processes resulting from the western and processed foods diet patterns are major contributors to poor mood and depression symptoms (Akbaraly, et al., 2009; Jacka, et al., 2010; Kiecolt-Glaser, 2010; Shelton & Miller, 2010). For example, a recent analysis of the Nurses’ Health Study linked an inflammatory diet pattern with higher risk for broader defined depression, RR 1.29 (95% CI, 1.18, 1.41; P-trend < .001) (Lucas, et al., 2014). Kiecolt-Glaser points out evidence that rapid rise in post-prandial blood sugar, a fundamental characteristic of HGL diets, can increase production of pro-inflammatory cytokines as well as free radicals (Kiecolt-Glaser, 2010). An analysis of glycemic index scores of self-reported diet patterns from the Women’s Health Initiative Observational Study showed a positive association with increased odds of incident depression, OR 1.22 (95% CI, 1.09,137; P-trend = 0.0032)(Gangwisch, et al., 2015).
In the current study, the LGL diet was associated with significantly higher scores for vigor/activity and significant reductions in fatigue/inertia compared to the HGL diet suggesting improved perceptions of subjective energy during the LGL diet phase. The LGL diet also had a lower total mood disturbance adjusted mean score than the HGL diet, suggesting overall less mood disturbance and better global mood scores at the end of the LGL diet period. These results are consistent with studies of the traditional or whole food patterns diets, which have shown either no change in mood state or slight increase in positive mood. These results may be due to the LGL diet providing minimized post-prandial glycemic variation and therefore lessened pro-inflammatory or free radical production.
There are several strengths of this study. The randomized, controlled, crossover feeding design is the gold-standard for testing diet intervention effects on outcome measures. This rigorous study of HGL and LGL diets for 4 weeks each provided a unique opportunity to capture these diet pattern effects on mood. Another strength of the diet design is that the macronutrient distribution was the same for both the HGL and LGL diets. The participants were healthy and euthymic at study entry. Their composite POMS scores fell in line or below the normative adult samples (McNair, et al., 1971). This is important as diet effects may be greater in a sample of depressed individuals compared to the present sample of non-depressed participants. The distinction between percent body fat classification and weight stability maintenance over the course of the interventions is another strength of this study compared to the few other glycemic intervention studies where mood and depression changes may have been mediated by weight loss or gain.
Study limitations should be mentioned. As noted above, compliance to the study protocol was excellent. Extensive efforts were made to encourage diet adherence and report non-study food consumption. However, because the participants were free-living and not under constant observation, there may have been some participant non-compliance that we were not able to measure or detect. Another limitation is that subjective mood and energy levels are potentially influenced by a wide variety of factors that may not have been accounted for in the analysis. The consumption of strictly a HGL or LGL diet pattern may not be generalizable as a mixed diet is more realistic. The study participants may not represent the general population given the stringent inclusion and exclusion criteria. Although sex was not a factor in the mood assessment analysis, initial study enrollment based on BMI was equally distributed for men and women in the overweight/obese and healthy weight groups. However, there were more women in the overweight/obese group as determined by the DXA scan results. Finally, due to sample size, we were not able to statistically test whether the diet response was different for the overweight and obese participants versus the healthy weight participants. Future, larger studies could be powered to statistically test this interaction.
In conclusion, a HGL experimental diet resulted in higher fatigue, total mood disturbance and depression symptoms than a LGL experimental diet. Additionally, the overweight and obese but otherwise healthy participants, reported higher scores on poor mood assessments compared to the healthy weight participants. Given the current rates of obesity and depression, these study results are important for the consideration of public health care practitioners and policy makers when examining diet patterns not only of people who want to maintain healthy weight and mood, but particularly overweight and obese individuals.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants as well as Yvonne Schwarz, Lisa Levy, and Anna Klimova for their involvement in study implementation, proof reading, document formatting, and statistical analysis guidance. This work was supported by NIH/NCI grant: U54CA116847. This trial was registered at clinicaltrials.gov: NCT00622661.
Funding Source: This work was supported by NIH/NCI grant U54CA116847 and Fred Hutchinson Cancer Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kara L. Breymeyer, Email: kbreymey@fredhutch.org.
Johanna W. Lampe, Email: jlampe@fredhutch.org.
Bonnie A. McGregor, Email: mcgregor@fredhutch.org.
Marian L. Neuhouser, Email: mneuhous@fredhutch.org.
References
- Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195:408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beezhold BL, Johnston CS, Daigle DR. Vegetarian diets are associated with healthy mood states: a cross-sectional study in seventh day adventist adults. Nutr J. 2010;9:26. doi: 10.1186/1475-2891-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Nabb S. Carbohydrate, memory, and mood. Nutr Rev. 2003;61:S61–S67. doi: 10.1301/nr.2003.may.S61-S67. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Maljanian R, Goethe J. Screening for depression in clinical practice: reliability and validity of a five-item subset of the CES-Depression. Percept Mot Skills. 2003;97:855–861. doi: 10.2466/pms.2003.97.3.855. [DOI] [PubMed] [Google Scholar]
- Brand-Miller J, Wolever TMS, Foster-Powell K, Colagiuri S. The New Glucose Revolution. New York, NY: Marlowe & Company; 2003. [Google Scholar]
- Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med. 2009;169:1873–1880. doi: 10.1001/archinternmed.2009.329. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control & Prevention. Morbidity and Mortality Weekly Report. Vol. 2011. Center for Disease Control & Prevention; 2011. Mental Illness Surveillance Among Adults in the United States. [Google Scholar]
- Cheatham RA, Roberts SB, Das SK, Gilhooly CH, Golden JK, Hyatt R, Lerner D, Saltzman E, Lieberman HR. Long-term effects of provided low and high glycemic load low energy diets on mood and cognition. Physiol Behav. 2009;98:374–379. doi: 10.1016/j.physbeh.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L. The effect of carbohydrates on affect. Nutrition. 1997;13:503–514. doi: 10.1016/s0899-9007(97)00003-8. [DOI] [PubMed] [Google Scholar]
- Christensen L, Pettijohn L. Mood and carbohydrate cravings. Appetite. 2001;36:137–145. doi: 10.1006/appe.2001.0390. [DOI] [PubMed] [Google Scholar]
- D'Anci KE, Watts KL, Kanarek RB, Taylor HA. Low-carbohydrate weight-loss diets. Effects on cognition and mood. Appetite. 2009;52:96–103. doi: 10.1016/j.appet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Gadalla TM. Association of obesity with mood and anxiety disorders in the adult general population. Chronic Dis Can. 2009;30:29–36. [PubMed] [Google Scholar]
- Gangwisch JE, Hale L, Garcia L, Malaspina D, Opler MG, Payne ME, Rossom RC, Lane D. High glycemic index diet as a risk factor for depression: analyses from the Women's Health Initiative. Am J Clin Nutr. 2015;102:454–463. doi: 10.3945/ajcn.114.103846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Comm Health. 2007;61:631–637. doi: 10.1136/jech.2006.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halyburton AK, Brinkworth GD, Wilson CJ, Noakes M, Buckley JD, Keogh JB, Clifton PM. Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. Am J Clin Nutr. 2007;86:580–587. doi: 10.1093/ajcn/86.3.580. [DOI] [PubMed] [Google Scholar]
- Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Kremer PJ, Berk M, de Silva-Sanigorski AM, Moodie M, Leslie ER, Pasco JA, Swinburn BA. A prospective study of diet quality and mental health in adolescents. PLoS One. 2011;6:e24805. doi: 10.1371/journal.pone.0024805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167:305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Veasey R, Watson A, Dodd F, Jones E, Maggini S, Haskell CF. Effects of high-dose B vitamin complex with vitamin C and minerals on subjective mood and performance in healthy males. Psychopharmacology (Berl) 2010;211:55–68. doi: 10.1007/s00213-010-1870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral TV, Bannon AL, Moore RH. Effects of financial incentives for the purchase of healthy groceries on dietary intake and weight outcomes among older adults: A randomized pilot study. Appetite. 2016;100:110–117. doi: 10.1016/j.appet.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski MF, Cremer Sees A, Hotchkiss L, Cotugna N, Evans MK, Zonderman AB. Higher Healthy Eating Index-2005 scores associated with reduced symptoms of depression in an urban population: findings from the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) study. J Am Diet Assoc. 2010;110:383–389. doi: 10.1016/j.jada.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman HR, Wurtman JJ, Chew B. Changes in mood after carbohydrate consumption among obese individuals. Am J Clin Nutr. 1986;44:772–778. doi: 10.1093/ajcn/44.6.772. [DOI] [PubMed] [Google Scholar]
- Lucas M, Chocano-Bedoya P, Shulze MB, Mirzaei F, O'Reilly EJ, Okereke OI, Hu FB, Willett WC, Ascherio A. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun. 2014;36:46–53. doi: 10.1016/j.bbi.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- McNair DM, Heuchert JWP. Profile of Mood States Technical Update. Tonawanda NY, Toronto Ontario Canada: Multi-Health Systems Inc.; 2005. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials. 2009;10 doi: 10.1186/1745-6215-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Sasaki S. Dietary intake and depressive symptoms: a systematic review of observational studies. Mol Nutr Food Res. 2010;54:471–488. doi: 10.1002/mnfr.200900157. [DOI] [PubMed] [Google Scholar]
- Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N, Sasaki S, Mizoue T. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr. 2010;64:832–839. doi: 10.1038/ejcn.2010.86. [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, Noar K, Song X, Lampe JW. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369–374. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhouser ML, Tinker LF, Thomson C, Caan B, Horn LV, Snetselaar L, Parker LM, Patterson RE, Robinson-O'Brien R, Beresford SA, Shikany JM. Development of a glycemic index database for food frequency questionnaires used in epidemiologic studies. J Nutr. 2006;136:1604–1609. doi: 10.1093/jn/136.6.1604. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q. National Center for Health Statistics Data Brief. Hyattsville, MD: U. S. Department of Health and Human Services; 2011. Antidepressant use in persons aged 12 and over: United States, 2005–2008. [Google Scholar]
- Sabate J, Haddad E, Tanzman JS, Jambazian P, Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. Am J Clin Nutr. 2003;77:1379–1384. doi: 10.1093/ajcn/77.6.1379. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Serra Majem L, Martinez-Gonzalez MA. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66:1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–133. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Toledo E, de Irala J, Ruiz-Canela M, Pla-Vidal J, Martinez-Gonzalez MA. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. 2011:1–9. doi: 10.1017/S1368980011001856. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Verberne L, De Irala J, Ruiz-Canela M, Toledo E, Serra-Majem L, Martinez-Gonzalez MA. Dietary fat intake and the risk of depression: the SUN Project. PLoS One. 2011;6:e16268. doi: 10.1371/journal.pone.0016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- Sloth B, Krog-Mikkelsen I, Flint A, Tetens I, Bjorck I, Vinoy S, Elmstahl H, Astrup A, Lang V, Raben A. No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am J Clin Nutr. 2004;80:337–347. doi: 10.1093/ajcn/80.2.337. [DOI] [PubMed] [Google Scholar]
- Smith MA, BL, Mori TA, Oddy WH. Essential fatty acids and mood: A systematic review of observational studies. Am J Food Nutr. 2011;1:14–27. [Google Scholar]
- Taylor VH, Macqueen GM. The Role of Adipokines in Understanding the Associations between Obesity and Depression. J Obes. 2010 doi: 10.1155/2010/748048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009:CD006296. doi: 10.1002/14651858.CD006296.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman JJ. Depression and weight gain: the serotonin connection. J Affect Disord. 1993;29:183–192. doi: 10.1016/0165-0327(93)90032-f. [DOI] [PubMed] [Google Scholar]
- Zhao G, Ford ES, Li C, Tsai J, Dhingra S, Balluz LS. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: national health and nutrition examination survey 2005–2006. BMC Psychiatry. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.