Summary
Background
Erlotinib is approved for the treatment of all patients with advanced non-small cell lung cancer (NSCLC), but is most active in the treatment of EGFR mutant NSCLC. Cabozantinib, a small molecule tyrosine kinase inhibitor, targets MET, VEGFR, RET, ROS1, and AXL, which are implicated in lung cancer tumorigenesis. We tested the efficacy of cabozantinib and the combination of erlotinib plus cabozantinib, as compared with erlotinib, in patients with EGFR wild-type NSCLC.
Methods
In this three arm, randomised phase 2 study, the primary endpoint was to compare progression-free survival (PFS) of patients treated with cabozantinib versus erlotinib alone, and the combination of erlotinib plus cabozantinib versus erlotinib alone. Patients were eligible if they had received 1–2 previous treatments for advanced non-squamous EGFR wild-type NSCLC. Patients were stratified by performance status and line of therapy, then randomised using permuted blocks within strata to receive open label oral daily dosing of erlotinib (150 mg), cabozantinib (60 mg), or erlotinib (150 mg) and cabozantinib (40 mg). Imaging was performed every 8 weeks. At the time of radiographic progression, there was optional crossover for patients in either single agent arm to receive combination therapy. The comparison between erlotinib and each of the arms was powered (91%) to detect a PFS hazard ratio (HR) of 0.5 (1-sided p-value 0.10-level). Secondary objectives were overall survival (OS), radiographic response by RECIST version 1.1 and description of adverse events by CTCAE version 4.0. This trial is registered with ClinicalTrials.gov, number NCT01708954.
Findings
At complete enrollment, we randomised 125 patients (42 assigned to erlotinib, 40 assigned to cabozantinib, 43 assigned to the combination), of which 111 (89%) were eligible and received treatment per protocol were included in the primary analysis (38, 38, and 35 patients on erlotinib, cabozantinib, and combination, respectively). Compared to erlotinib alone (median 1.8 months), PFS was significantly improved in the cabozantinib arm (4.3 months, HR 0.39, 1-sided p=0.0003, 80% CI 0.27–0.55) and also in the erlotinib plus cabozantinib arm (4.7 months, HR 0.37, 1-sided p=0.0003, 80% CI 0.25–0.53).
The safety analysis population included all patients who received study therapy regardless of eligibility. The most common grade 3 or 4 adverse events were diarrhea (3 [8%] in the erlotinib group vs 3 [8%] in the cabozantinib group vs 11 [28%] in the erlotinib and cabozantinib group), hypertension (none vs 10 [25%] vs 1 [3%]), fatigue (5 [13%] vs 6 [15%] vs 6 [15%]), oral mucositis (none vs 4 [10%] vs 1 [3%]), and thromboembolic event (none vs 3 [8%] vs 2 [5%]). Adverse events that were grade 3 or worse occurred in 13 (33%) patients in the erlotinib group, in 28 (70%) patients in the cabozantinib group, and in 28 (72%) patients in the erlotinib and cabozantinib group. One death of respiratory failure occurred in the cabozantinib group, deemed possibly related to either drug or disease, and one death occurred in the erlotinib plus cabozantinib group from pneumonitis. MET IHC results were available on 86 patients from the primary analysis and 85% were scored as positive (1–3+ membrane or cytoplasm staining with MET4 antibody). There was no association between MET IHC status and PFS when treated with or without cabozantinib.
Interpretation
The ECOG-ACRIN 1512 trial design tested the feasibility of using cabozantinib alone or combined with erlotinib in this patient population with EGFR wild-type NSCLC. Despite its modest sample size, this trial identified signals of clinically meaningful efficacy superior to that of erlotinib alone, and additional toxicity that was generally manageable. Cabozantinib-based regimens are promising for further investigation in this patient population.
Keywords: Non-small cell lung cancer, Erlotinib, Cabozantinib, Epidermal growth factor receptor
Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide, killing more than 1.3 million people annually.(1) In non-squamous non-small cell lung cancer (NSCLC), first-line chemotherapy with a platinum-based doublet for advanced disease has a historical response rate of only approximately 20–30% and a median overall survival of 8–10 months.(2) At the time of progression, second-line chemotherapeutic agents such as docetaxel and pemetrexed confer benefit with response rates of approximately 10% and progression-free survival times of approximately 3 months.(3, 4) Over the last year, immunotherapeutic checkpoint inhibitor antibodies such as nivolumab and pembrolizumab also have been demonstrated to improve outcomes in the second line treatment of NSCLC as compared with docetaxel.(5, 6)
NSCLC adenocarcinomas can be categorized into groups by driver genomic alterations, and an overall survival benefit has been observed in patients that received appropriate targeted therapy based on genomic profiling of their tumors.(7) The most common driver is a mutation in the EGFR gene, present in approximately 15% of NSCLC adenocarcinomas. Erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), is highly active in the treatment of tumours harboring EGFR mutations.(8) However, more than 75% of NSCLC adenocarcinomas have neither an EGFR mutation (described as EGFR wild-type) nor another targetable genomic alteration. In these patients, erlotinib therapy is sometimes used based on a decade-old trial, which demonstrated a 2 month survival benefit for erlotinib as compared with placebo in second and third line treatment of NSCLC.(9) This historical use of erlotinib in wild-type EGFR NSCLC formed the basis for the selection of the erlotinib control arm in this study of EGFR wild-type NSCLC.
Cabozantinib is an orally available TKI that is active against MET and vascular endothelial growth factor receptor-2 (VEGFR2), and also RET, ROS1, AXL, KIT, and TIE-2. MET dysregulation in non-small cell lung cancers by protein overexpression, mutations, and gene amplification can be therapeutically targeted in patients using MET inhibitors (10–12). VEGFR2 is a primary mediator of VEGF-stimulated angiogenesis, and anti-angiogenic strategies have been effective in the treatment of NSCLC. Preclinical studies have demonstrated that MET amplification can be a mechanism of acquired resistance to EGFR inhibitors, and that targeting both MET and EGFR synergistically inhibits proliferation of many cancer cell lines.(13, 14) Cabozantinib was selected for this study in EGFR wild-type NSCLC because MET protein is expressed in approximately 50% of these tumors, and anti-angiogenic therapy appears effective even in wild-type disease. (12, 15, 16) A single arm phase II study of cabozantinib had previously demonstrated that cabozantinib was active as a single agent in the treatment of NSCLC, with a response rate of 10%, disease control rate of 40% and progression-free survival of 4.2 months.(17) Another phase I/II trial showed that the combination of erlotinib and cabozantinib could safely be administered together.(18)
When this study was conceptualized, testing of tumors for EGFR mutations to predict sensitivity to erlotinib was the standard of care in the United States, but patients with advanced EGFR wild-type NSCLC refractory to chemotherapy often still received erlotinib in the second and third line setting. We conducted this trial to directly compare the efficacy of erlotinib with cabozantinib, and to compare erlotinib with cabozantinib plus erlotinib, in patients with previously treated EGFR wild-type advanced NSCLC. The primary objective was to determine whether single agent cabozantinib or combination therapy including cabozantinib extends progression-free survival (PFS) when compared to single agent erlotinib for this patient population. Secondary objectives were estimation of overall survival, best objective response, and toxicity. A retrospective analysis was planned to determine the association of MET expression by immunohistochemistry with outcomes.
Methods
Study design and participants
We conducted this multicenter, randomised phase II trial within the ECOG-ACRIN Cancer Research Group; accrual by institution is listed in appendix (page 9). Complete eligibility criteria are listed in the appendix (page 1). Briefly, patients were included who had metastatic or recurrent non-squamous NSCLC which had progressed following first line platinum-doublet chemotherapy, and optionally progressed following a second-line chemotherapy regimen. Patients were not allowed to have prior erlotinib or MET TKI therapy. Testing for EGFR TKI sensitizing mutations - at minimum, exon 19 deletions and L858R point mutations - was performed by local sites prior to screening for the trial, and patients with these or other known EGFR TKI sensitizing mutations were excluded. Submission of paraffin embedded tissue was required for retrospective MET testing by immunohistochemistry. Patients were required be >= 18 years old and have measurable disease by RECIST 1.1 criteria, and patients were allowed to have previously treated and stable brain metastases. Other eligibility criteria included ECOG performance status of 0–2, adequate bone marrow, renal, hepatic, and cardiac function, and no hemoptysis, tumor invasion of large vessels or organs, or recent surgery, chest irradiation, or major thrombotic events. The institutional review boards at each participating institution approved the study protocol and amendments. All patients in the trial provided written informed consent. The study complied with the Declaration of Helsinki and was done in accordance with Good Clinical Practice guidelines.
Randomisation and masking
The three treatment arms were open-label erlotinib monotherapy, cabozantinib monotherapy, and the combination of erlotinib and cabozantinib. Randomisation (1:1:1) to these arms was determined using permuted blocks within strata with dynamic balancing institutions. Randomisation was stratified by number of prior therapies (1 vs. 2) and ECOG performance status (0 vs. 1 vs. 2). Neither patients nor investigators were blinded to assigned treatment.
Procedures
Following assignment to treatment, the first dose of study drug was administered within 5 working days. Erlotinib was prescribed as standard-of-care therapy by the treating physician to patients on the erlotinib arms at a dose of 150 mg orally daily. Cabozantinib-s-malate was distributed from CTEP via the local research pharmacy and administered at a dose of 60 mg orally daily in the monotherapy arm, and 40 mg orally daily in the combination arm. Toxicity was graded according to the National Cancer Institute common toxicity terminology criteria for adverse events (NCI-CTCAE) version 4.0. Dose reduction levels for intolerable grade 2, grade 3, and grade 4 drug-related events were as follows: erlotinib: 100 mg, 50 mg; cabozantinib 40 mg, 20 mg. A maximum of 2 dose reductions or 28 day drug hold to recover from toxicity was allowed, or patients were removed from the study. Management guidelines were provided in the protocol for diarrhea, rash, and other anticipated toxicities; some toxicities allowed continuation of dose after hold, some required dose reduction, and some required permanent discontinuation.
A cycle of therapy was defined as 4 weeks. Monitoring tests for safety (complete blood count, comprehensive metabolic panel, magnesium, phosphorus, thyroid function testing, electrocardiogram) was performed every 2–4 weeks. Radiographic tumour assessment was performed at baseline and every 2 cycles (8 weeks) according to RECIST 1.1 criteria by site investigators without central image review.(19) There was no limit to length of therapy as long as patients had radiographically controlled disease and managed toxicity. At the time of radiographic progression, patients in the erlotinib or cabozantinib single agent therapy groups were allowed to crossover to combination treatment with erlotinib plus cabozantinib or discontinue treatment.
MET testing was performed in the Center for Molecular Oncologic Pathology at the Dana Farber Cancer Institute/Brigham and Women’s Hospital. The laboratory was blinded as to study arm. Total MET IHC testing was performed on the Leica Bond III automated immunostainer using the Bond Refine Detection system on 4-μm sections of FFPE(formalin fixed, paraffin embedded) specimens with the rabbit polyclonal c-Met clone CVD13 (ThermoFisher Scientific, Waltham, MA, USA) and both membranous and cytoplasmic staining were scored from 0–3+ intensity, and percentage positivity, respectively.
Outcomes
The primary endpoint was progression-free survival (PFS), defined as the time from randomization to documented disease progression or death from any cause, whichever occurs first. Patients who had not experienced an event of interest by the time of analysis were censored at the date they are last known to be alive and progression-free. Overall survival was defined as the time from randomization to death from any cause, and patients who were thought to be alive at the time of final analysis were censored at the last date of contact. Best objective response was evaluated via RECIST1.1 criteria. Toxicity was determined using CTCAE v4.0 criteria. The MET outcome analysis was a pre-planned exploratory endpoint.
Statistical analysis
The primary comparison was designed to accrue and randomise 105 eligible and treated patients 1:1:1, for a total accrual of 35 patients to each of the 3 arms. After adjusting for an ineligibility rate of 10%, the total estimated sample size for randomisation was 117 patients. Using an overall one-sided 0.10 level log rank test for each comparison, this study had 91% power to detect a PFS hazard ratio of 0.50, which corresponds to an improvement in the median PFS from 2.4 months on the control arm to 4.8 months on either experimental arm. The number of PFS events needed to achieve this power for each comparison was 58 events under the alternative hypothesis. Each of the two primary comparisons of PFS used a log rank test stratified on the randomisation stratification factors with a one-sided type I error rate of 10%. PFS was defined as the time from randomisation to documented disease progression or death from any cause, whichever occurred first. Patients who had not experienced an event of interest by the time of analysis were censored at the date of the last radiographic disease assessment.
The primary endpoint was assessed in the per protocol population, which was defined as all patients who were eligible, randomly assigned, and received at least one dose of treatment. Patients were radiographically assessable if there was RECIST 1.1 measurable disease and all sites were evaluated within 4 weeks of starting therapy and a minimum of 8 weeks after starting therapy. The safety analysis population included all patients who received study therapy regardless of eligibility. MET IHC outcome analysis included the primary analysis population with tissue and MET result available. Overall survival was defined as the time from randomisation to death from any cause, and patients who were known to be alive at the time of final analysis were censored at the last date of contact. PFS and OS distributions were estimated using the Kaplan-Meier method, and Cox proportional hazards models were used to estimate the treatment hazard ratios. Response rates and toxicity were compared using Fisher’s exact tests. This study was monitored by the ECOG-ACRIN Data Safety Monitoring Committee (DSMC) with one planned interim analysis for futility of PFS at roughly 50% information using the methodology of Freidlin, Korn, and Gray; at that time, if either point estimate of the PFS HR was consistent with detriment (HR > 1.0), the DSMC may have considered terminating the respective comparison early for overall lack of treatment difference.(20) The study was followed to full information, and at that time the DSMC recommended that the results be released and that patients still receiving erlotinib only be offered one of the other treatments. The software used to conduct the analyses was R version 2.10.0. This trial is registered with ClinicalTrials.gov, number NCT01708954.
Role of the funding sources
The sponsor of this trial was ECOG-ACRIN, a United States grant-funded multidisciplinary, membership-based scientific organization which was formed by the merger of the Eastern Cooperative Oncology Group (ECOG) and the American College of Radiology Imaging Network (ACRIN). ECOG-ACRIN was responsible for approving study design, development, coordinating enrollment, data collection, data management, audits, a pre-planned interim futility analysis, and the final data analysis. ECOG-ACRIN participated in the interpretation of data together with the other co-authors, and reviewed the report. SD had full access to the data, and JWN reviewed and certified the data. Bio-specimens were collected, processed and made available for correlative studies by the ECOG-ACRIN Pathology Coordinating Office and Reference Laboratory. Exelixis supplied cabozantinib for this trial through a cooperative research and development agreement (CRADA) with the National Cancer Institute Cancer Therapy Evaluation Program. The corresponding author had the final responsibility to submit for publication.
Results
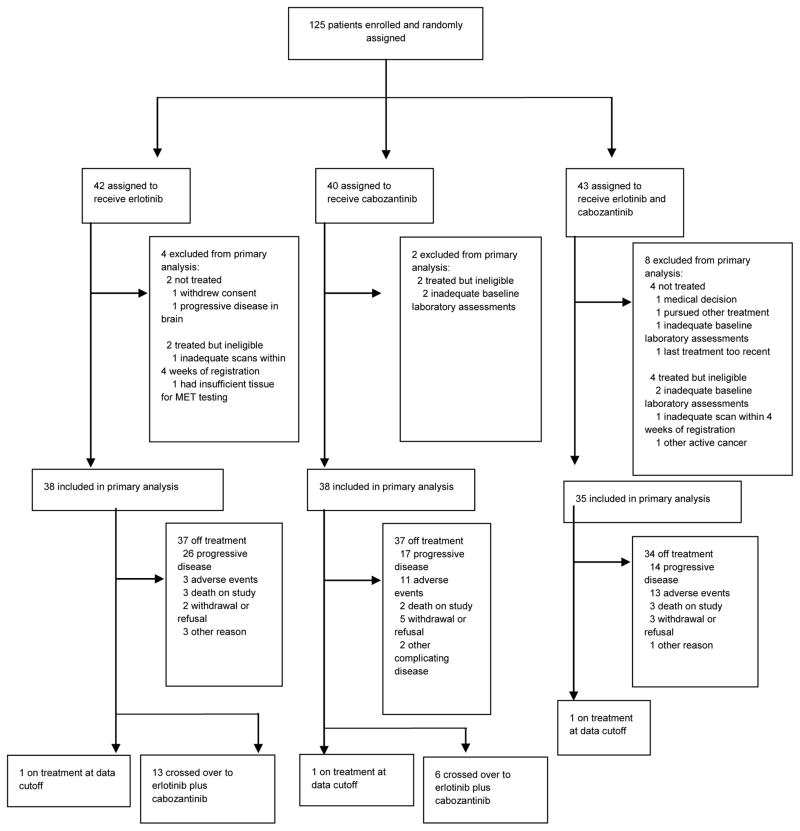
Between February 7, 2013 and July 1, 2014, we completed enrollment of 125 patients and randomly assigned them to erlotinib (n=42), cabozantinib (n=40), or erlotinib plus cabozantinib (n=43). Fourteen (11%) of 125 patients never started assigned therapy or were deemed ineligible, leaving 111 (89%) patients in the primary analysis (Figure 1). At the time of data cutoff for this analysis, August 31, 2015, 33 (30%) patients in the primary analysis population were alive. The median follow-up was 17.0 months (15.4 months for erlotinib, 23.4 months for cabozantinib, and 14.9 months for erlotinib plus cabozantinib, with interquartile range for all groups of 12.7 – 23.1 months). Patient demographics and disease characteristics were generally balanced (table 1) with the exception of ethnicity, history of brain metastases, mediastinal metastases (p=0.03), and prior immunotherapy.
Figure 1.
Trial profile
Table 1.
Baseline characteristics
| Variable | Category | Erlotinib | Cabozantinib (60mg) | Erlotinib + Cabozantinib (40mg) | Total |
|---|---|---|---|---|---|
| Total | 38 | 38 | 35 | 111 | |
| Sex | Female | 20 (53) | 24 (63) | 17 (49) | 61 (55) |
| Male | 18 (47) | 14 (37) | 18 (51) | 50 (45) | |
| Age | Mean (Std Dev) | 66.3 (9.8) | 65.9 (10.1) | 63.5 (9.0) | 65.3 (9.6) |
| PS | 0 | 9 (24) | 9 (24) | 8 (23) | 26 (23) |
| 1 | 24 (63) | 25 (66) | 23 (66) | 72 (65) | |
| 2 | 5 (13) | 4 (11) | 4 (11) | 13 (12) | |
| Weight Loss | <5% | 30 (79) | 29 (76) | 27 (77) | 86 (77) |
| >= 20% | 0 (0) | 1 (3) | 0 (0) | 1 (1) | |
| 10 to <20% | 1 (3) | 3 (8) | 5 (14) | 9 (8) | |
| 5 to <10% | 7 (18) | 5 (13) | 3 (9) | 15 (14) | |
| Ethnicity | Hispanic/Latino | 0 (0) | 0 (0) | 2 (6) | 2 (2) |
| Not Hispanic/Latino | 38 (100) | 38 (100) | 32 (91) | 108 (97) | |
| Not Reported | 0 (0) | 0 (0) | 1 (3) | 1 (1) | |
| Race | American Indian | 2 (5) | 1 (3) | 0 (0) | 3 (3) |
| Asian | 2 (5) | 0 (0) | 0 (0) | 2 (2) | |
| Black | 2 (5) | 3 (8) | 2 (6) | 7 (6) | |
| Native Hawaiian | 0 (0) | 1 (3) | 0 (0) | 1 (1) | |
| White | 32 (84) | 33 (87) | 31 (89) | 96 (86) | |
| Not Reported | 0 (0) | 0 (0) | 2 (6) | 2 (2) | |
| Smoking status | Current | 8 (21) | 9 (24) | 8 (23) | 25 (23) |
| Former | 25 (66) | 23 (61) | 21 (60) | 69 (62) | |
| Never | 5 (13) | 6 (16) | 6 (17) | 17 (15) | |
| Stage | IV M1a | 8 (21) | 6 (16) | 5 (14) | 19 (17) |
| IV M1b | 21 (55) | 18 (47) | 20 (57) | 59 (53) | |
| Recurrent | 9 (24) | 14 (37) | 10 (29) | 33 (30) | |
| Histology | Adenocarcinoma | 35 (92) | 36 (95) | 32 (91) | 103 (93) |
| Combined/mixed | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Large cell | 1 (3) | 2 (5) | 0 (0) | 3 (3) | |
| NSCLC NOS | 2 (5) | 0 (0) | 2 (6) | 4 (4) | |
| Other | 0 (0) | 0 (0) | 1 (3) | 1 (1) | |
| Multi-agent systemic chemotherapy | 36 (95) | 38 (100) | 34 (97) | 108 (97) | |
| Single agent systemic chemotherapy | 23 (61) | 17 (45) | 13 (37) | 53 (48) | |
| Immunotherapy | 2 (5) | 2 (5) | 8 (23) | 12 (11) | |
| Radiation | 20 (53) | 22 (58) | 23 (66) | 65 (59) | |
| Surgery | 8 (21) | 17 (45) | 11 (31) | 36 (32) | |
| Maintenance chemotherapy | None | 9 (24) | 15 (39) | 15 (43) | 39 (35) |
| Continuation | 23 (61) | 17 (45) | 14 (40) | 54 (49) | |
| Switch | 6 (16) | 6 (16) | 6 (17) | 18 (16) | |
| Second line chemotherapy received | 15 (39) | 15 (39) | 14 (40) | 44 (40) | |
| EGFR status | Wild-type | 37 (97) | 37 (97) | 33 (94) | 107 (96) |
| Inconclusive | 1 (3) | 0 (0) | 2 (6) | 3 (3) | |
| Not done | 0 (0) | 1 (3) | 0 (0) | 1 (1) | |
| KRAS status | Positive | 4 (11) | 7 (18) | 3 (9) | 14 (13) |
| Wild-type | 7 (18) | 11 (29) | 5 (14) | 23 (21) | |
| Inconclusive | 1 (3) | 0 (0) | 2 (6) | 3 (3) | |
| Not done | 26 (68) | 20 (53) | 25 (71) | 71 (64) | |
| Brain metastasis, history | 3 (8) | 13 (34) | 9 (26) | 25 (23) | |
| Brain metastasis, treatment | Gam. knife/Radiosx. | 2 (67) | 7 (54) | 4 (44) | 13 (52) |
| Surgery | 0 (0) | 0 (0) | 1 (11) | 1 (4) | |
| WBRT | 1 (33) | 6 (46) | 4 (44) | 11 (44) | |
| Mediastinal metastasis | 11 (29) | 22 (58) | 17 (49) | 50 (45) | |
| Pleura metastasis | 5 (13) | 3 (8) | 4 (11) | 12 (11) | |
| Liver metastasis | 10 (26) | 10 (26) | 9 (26) | 29 (26) | |
| Adrenal metastasis | 5 (13) | 6 (16) | 7 (20) | 18 (16) | |
| Bone metastasis | 13 (34) | 10 (26) | 14 (40) | 37 (33) | |
| Pleural effusion | 9 (24) | 9 (24) | 7 (20) | 25 (23) |
Exposure to therapy was assessed for each group. The median number of cycles received by treatment group were: 2 cycles for erlotinib (range: 1–10); 3 cycles for cabozantinib (range: 1–17); and 2 cycles for erlotinib plus cabozantinib (range: 1–15). Planned or unplanned dose modifications were experienced by 29 (76%) of 38 eligible and treated patients in the erlotinib group; 36 (95%) of 38 in the cabozantinib group; and 34 (97%) of 35 in the erlotinib plus cabozantinib group. The data collected did not capture the reason for dose modification, although the protocol only permitted dose modification due to adverse events, not at investigator’s discretion, The average daily dose of erlotinib was 140.2 mg of erlotinib for the erlotinib group and 125.5 mg of erlotinib for the erlotinib plus cabozantinib group. The average daily dose of cabozantinib was 52.6 mg for the cabozantinib group and 31.7 mg for the erlotinib plus cabozantinib group.
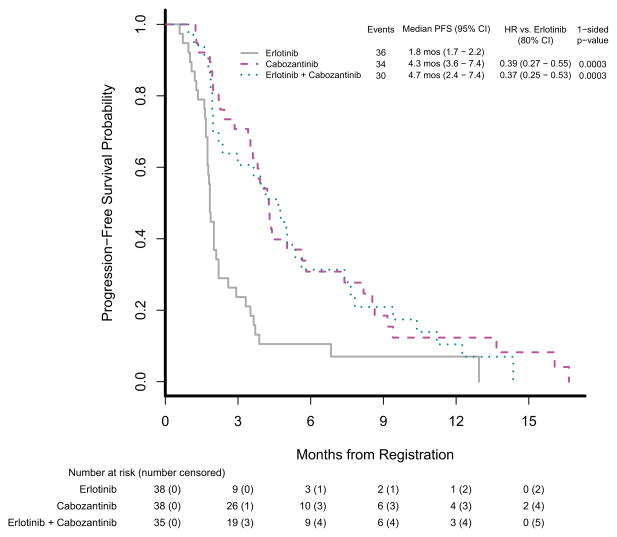
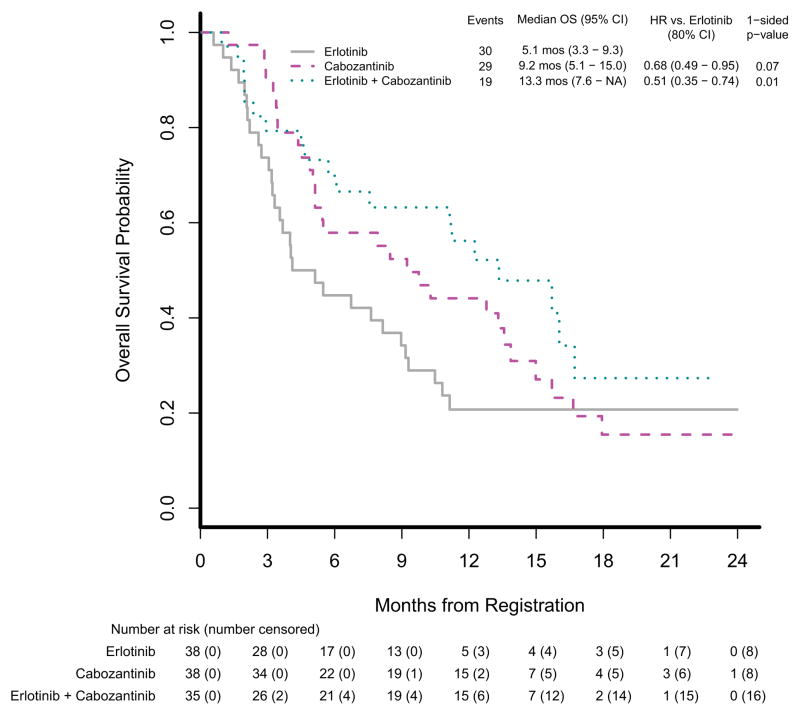
Table 2 summarizes the efficacy results. Progression-free survival was statistically significantly better in the cabozantinib group than in the erlotinib group (HR=0.39, 80% CI [0.27–0.55], 1-sided p=0.0003); it was also better in the cabozantinib plus erlotinib group than in the erlotinib group (HR=0.37, 80% CI [0.25–0.53], 1-sided p=0.0003). Multivariable Cox models were fitted to adjust for imbalanced baseline variables and prognostic factors, and results were consistent with the unadjusted model. The estimated median PFS and corresponding 95% CI on each treatment arm was 1.8 months (1.7–2.2 months) on erlotinib, 4.3 months (3.6–7.4 months) on cabozantinib, and 4.7 months (2.4–7.4 months) on erlotinib plus cabozantinib. Figure 2A displays PFS by treatment arm. Overall survival was also better in the cabozantinib group than in the erlotinib group (HR=0.68, 80% CI [0.49–0.95], 1-sided p=0.07); it was statistically significantly better in the cabozantinib plus erlotinib group than in the erlotinib group (HR=0.51, 80% CI [0.35–0.74], 1-sided p=0.01). The estimated median OS and corresponding 95% CI on each treatment arm was 5.1 months (3.3–9.3 months) on erlotinib, 9.2 months (5.1–15.0 months) on cabozantinib and 13.3 months (7.6-NA months) on erlotinib plus cabozantinib. Figure 2B displays overall survival by treatment arm. Response rate was measured using RECIST 1.1 criteria, and objective responses did not differ significantly between the groups (Table 2). There was one partial response (PR) in the erlotinib group with a 48% reduction in tumor, four PRs in the cabozantinib group with median reduction of 36% (range 30–53%), and one PR in the erlotinib plus cabozantinib group with a 33% reduction in tumor. A total of 19 (17%) of 111 patients from the monotherapy arms crossed over to start combination therapy: 13 (34%) of 38 crossed over from erlotinib, and 6 (16%) of 38 crossed over from cabozantinib. No radiographic responses (complete response or PR) were observed in patients who crossed over to combination chemotherapy.
Table 2.
Efficacy endpoints
| Erlotinib (n=38) | Cabozantinib (n=38) | Erlotinib plus Cabozantinib (n=35) | |
|---|---|---|---|
| Progression-free survival | |||
| Deaths or disease progression | 36 (95%) | 34 (89%) | 30 (86%) |
| Median progression-free survival, months (95% CI) | 1.8 (1.7–2.2) | 4.3 (3.6–7.4) | 4.7 (2.4–7.4) |
| Overall survival | |||
| Deaths | 30 (79%) | 29 (76%) | 19 (54%) |
| Median overall survival, months (95% CI) | 5.1 (3.3–9.3) | 9.2 (5.1–15.0) | 13.3 (7.6-NR) |
| Best overall response | |||
| Complete response | 0 | 0 | 0 |
| Partial response | 1 (3%) | 4 (11%) | 1 (3%) |
| Stable disease | 6 (16%) | 19 (50%) | 16 (46%) |
| Progressive disease | 25 (66%) | 9 (24%) | 8 (23%) |
| Not evaluable/not assessed | 6 (16%) | 6 (16%) | 10 (29%) |
Data are n (%) unless otherwise indicated. NR = not reached
Figure 2.
Kaplan-Meier estimates of progression-free survival and overall survival
(A) Progression-free survival and (B) overall survival (OS) in the treatment per protocol population. HR=hazard ratio.
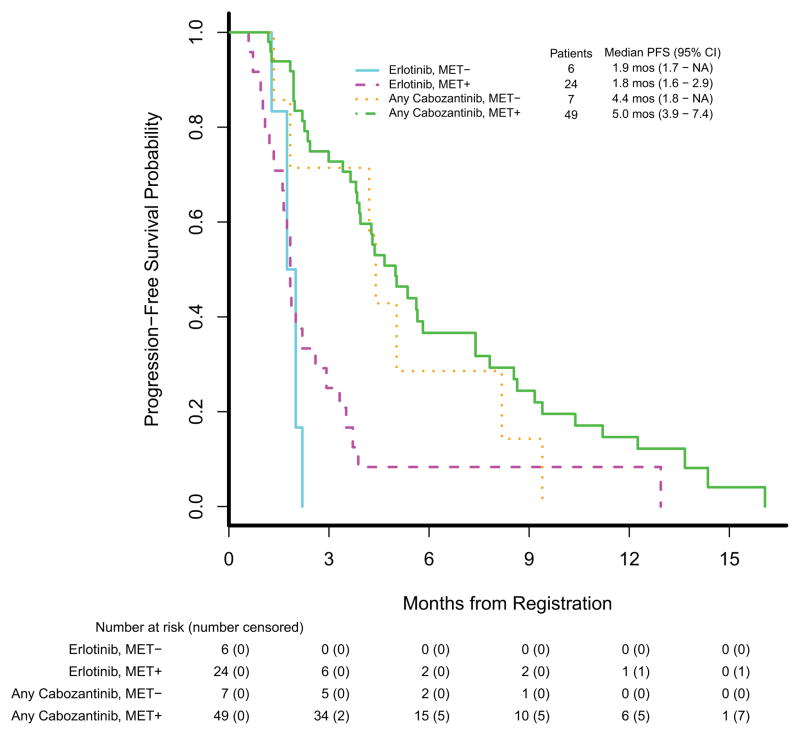
Tissue samples were collected on all patients at baseline for central MET IHC testing. Membranous and cytoplasmic staining were individually scored, and positivity was declared if MET was expressed in either the membrane or cytoplasm. A total of 107 independent patient samples were tested. Twelve samples were excluded from the analysis due to no sufficient tumor tissue available for scoring. From the 95 remaining samples, 86 came from the primary analysis population of eligible and treated patients. The overall of MET positivity in tissue samples was 73 (85%) of 86; by group it was 24 (80%) of 30 on erlotinib, 26 (81%) of 32 on cabozantinib, and 23 (96%) of 24 on erlotinib plus cabozantinib. Per protocol, we combined the cabozantinib treated groups for this analysis. MET status was not a significant predictor of PFS in a model also adjusted for whether or not a patient received cabozantinib: the estimated PFS HR for MET positivity was 0.65 (2-sided p=0.19). Progression-free survival by MET status is displayed in figure 3. The median PFS among MET-negative patients randomised to erlotinib was 1.9 months (95% CI: 1.7 months - NR); for MET-negative patients who received any cabozantinib it was 4.4 months (95% CI: 1.8 months - NR). The median PFS among MET-positive patients randomised to erlotinib was 1.8 months (95% CI: 1.6–2.9 months); for MET-positive patients who received any cabozantinib it was 5.0 months (95% CI: 3.9–7.4 months). Testing of additional MET positive cutpoints (cytoplasmic, membranous, or either) did not demonstrate that these were a significant predictor of PFS either (data not shown).
Figure 3.
Kaplan-Meier estimates of progression-free survival (PFS) by MET IHC status (positive vs. negative) and cabozantinib exposure (any or none).
Adverse events
Selected adverse events of interest are presented in Table 3, and all treatment-related adverse events are presented in the appendix (page 3). The most common grade 3 or 4 adverse events were diarrhea (3 [8%] in the erlotinib group vs 3 [8%] in the cabozantinib group vs 11 [28%] in the erlotinib and cabozantinib group), hypertension (none vs 10 [25%] vs 1 [3%]), fatigue (5 [13%] vs 6 [15%] vs 6 [15%]), oral mucositis (none vs 4 [10%] vs 1 [3%]), and thromboembolic event (none vs 3 [8%] vs 2 [5%]). Hypertension was significantly higher in the cabozantinib group compared with the erlotinib group (2-sided p=0.001), as was diarrhea in the erlotinib plus cabozantinib group compared with the erlotinib group (2-sided p=0.02). Adverse events of grade 3 or worse occurred in 13 (33%) patients in the erlotinib group, and were significantly higher in the cabozantinib group (28 patients [70%], 2-sided p=0.001), and in the erlotinib and cabozantinib group (28 patients [72%], 2-sided p=0.002). In the erlotinib group, 3 patients discontinued treatment for adverse events, compared with 11 patients in the cabozantinib group, and 13 patients in the erlotinib and cabozantinib group. Deaths on or within 30 days of last dose of treatment included 7 (17%) in the erlotinib group, 3 (8%) in the cabozantinib group, and 7 (16%) in the cabozantinib plus erlotinib group, and are presented in the appendix (page 8). All were deemed unlikely or unrelated to treatment, except for two: one death due to respiratory failure in the cabozantinib group, deemed possibly related to either drug or disease, and one death in the erlotinib plus cabozantinib group from drug pneumonitis due to either agent or the combination.
Table 3.
Adverse events of interest
| Erlotinib (n=40) | Cabozantinib (n=40) | Erlotinib + Cabozantinib (n=39) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gr 1–2 | Gr 3 | Gr 4 | Gr 5 | Gr 1–2 | Gr 3 | Gr 4 | Gr 5 | Gr 1–2 | Gr 3 | Gr 4 | Gr 5 | |
| Diarrhea | 21 (53%) | 3 (8%) | 0 | 0 | 20 (50%) | 3 (8%) | 0 | 0 | 25 (64%) | 11 (28%) | 0 | 0 |
| Acneiform rash | 22 (55%) | 1 (3%) | 0 | 0 | 6 (15%) | 1 (3%) | 0 | 0 | 23 (59%) | 2 (5%) | 0 | 0 |
| Fatigue | 18 (45%) | 5 (13%) | 0 | 0 | 22 (55%) | 6 (15%) | 0 | 0 | 27 (69%) | 6 (15%) | 0 | 0 |
| Anorexia | 10 (25%) | 2 (5%) | 0 | 0 | 15 (38%) | 1 (3%) | 0 | 0 | 17 (44%) | 3 (8%) | 0 | 0 |
| Nausea | 8 (20%) | 1 (3%) | 0 | 0 | 18 (45%) | 2 (5%) | 0 | 0 | 17 (44%) | 1 (3%) | 0 | 0 |
| Oral mucositis | 2 (5%) | 0 | 0 | 0 | 13 (33%) | 4 (10%) | 0 | 0 | 8 (21%) | 1 (3%) | 0 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 3 (8%) | 0 | 0 | 0 | 6 (15%) | 1 (3%) | 0 | 0 | 6 (15%) | 0 | 0 | |
| Hypothyroidism | 0 | 0 | 0 | 10 (25%) | 0 | 0 | 0 | 2 (5%) | 0 | 0 | 0 | |
| Aspartate aminotransferase increased | 8 (20%) | 0 | 0 | 0 | 26 (65%) | 0 | 0 | 0 | 17 (44%) | 0 | 0 | 0 |
| Hypertension | 4 (10%) | 0 | 0 | 0 | 8 (20%) | 10 (25%) | 0 | 0 | 17 (44%) | 1 (3%) | 0 | 0 |
| Thromboembolic event | 2 (5%) | 0 | 0 | 0 | 1 (3%) | 3 (8%) | 0 | 0 | 0 | 1 (3%) | 1 (3%) | 0 |
| Intracranial hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 1(3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3%) |
| Respiratory failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 0 | 0 | 0 |
| Worst degree toxicity | 23 (58%) | 13 (33%) | 0 | 0 | 12 (30%) | 26 (65%) | 1 (3%) | 1 (3%) | 11 (28%) | 24 (62%) | 3 (8%) | 1 (3%) |
Data are n (%). The table shows selected adverse events of interest possibly related to study treatment. All treatment-related adverse events are presented in the appendix (page 3).
Discussion
Our findings show that cabozantinib treatment alone, or cabozantinib plus erlotinib, was associated with a statistically significant improvement in progression-free survival when compared to erlotinib alone in patients with EGFR wild-type NSCLC who progressed after prior therapy. This treatment effect was supported by a corresponding improvement in overall survival, albeit with an increase in toxicity.
The results for the control arm were consistent with other trials using erlotinib in EGFR wild-type patients. During the conduct of this study, other trials were reported that used erlotinib as a control arm in EGFR wild-type NSCLC, in comparison to second line single agent chemotherapy. In the Italian TAILOR trial, 222 patients were randomised to erlotinib or docetaxel.(21) Median overall survival was 8.2 months with docetaxel, compared with 5.4 months with erlotinib (adjusted hazard ratio (HR) 0.73, 95% CI 0.53–1.00; p=0.05), and median progression-free survival (PFS) was 2.9 months with docetaxel versus 2.4 months with erlotinib (adjusted HR 0.71, 95% CI 0.53–0.95; p=0.02). In the Japanese DELTA trial, 301 patients were randomly assigned to erlotinib or docetaxel. (22) In a subset analysis of 199 patients with EGFR wild-type tumors, OS for erlotinib versus docetaxel was 9.0 v 10.1 months (HR, 0.98; 95% CI, 0.69 to 1.39; P = 0.91), and PFS for erlotinib versus docetaxel was 1.3 versus 2.9 months (HR, 1.45; 95% CI, 1.09 to 1.94; P = 0.01). The phase 3 TITAN study randomised 424 patients to erlotinib versus docetaxel or pemetrexed chemotherapy.(23) No differences in OS or PFS were identified between the groups, even for the EGFR wild-type subgroup, although EGFR mutation status was indeterminate or missing on more than half of patients. Overall, these studies consistently observe modest efficacy of erlotinib in EGFR wild-type NSCLC, and suggest inhibiting other non-EGFR signaling pathways is a rational treatment strategy in this subgroup of patients. Our observed median PFS of 1.8 months was similar to the 1.3, 1.4, and 2.4 months observed on the DELTA, TITAN, and TAILOR trials, respectively. Our observed median OS of 5.1 months was similar to the 5.3 and 5.4 months observed on the TITAN and TAILOR trials, but less than the 9.0 months observed in the DELTA trial. In addition, the PFS on the cabozantinib monotherapy arm of 4.3 months was quite similar to the 4.2 months previously observed in the previously conducted single arm phase II study of cabozantinib, and OS on this study was not reported. Therefore, the favorable efficacy outcomes observed in both experimental arms in our study are both clinically and statistically significant.
Cabozantinib therapy, or the combination of cabozantinib and erlotinib, was associated with an increased occurrence of grade 3 or worse adverse events compared with erlotinib alone. Many of these adverse events were symptomatic, such as fatigue, nausea, oral mucositis, and palmar-plantar erythrodysesthesia syndrome, all more frequently associated with cabozantinib treatment. The previous phase I/II trial of erlotinib and cabozantinib demonstrated that cabozantinib needed to be reduced to 40 mg daily in combination with erlotinib to limit diarrhea; despite this, patients still received an average of 32 mg of cabozantinib daily on the combination arm. While not statistically imbalanced for this randomised trial, the fatal adverse events of respiratory failure and pneumonitis, and life threatening adverse events of intracranial hemorrhage, thromboembolic event, other skin disorder, and thrombocytopenia were only observed on the cabozantinib arms. This suggests that cabozantinib is potentially less tolerable than erlotinib, though given its more potent clinical benefit this may be a worthwhile tradeoff. The recent FDA approval of cabozantinib 60 mg daily for renal cell carcinoma suggests that it has an acceptable overall safety profile as monotherapy.
Given the potential mechanism of action as a MET inhibitor, it was hypothesized that MET protein expression might be predictive of response to cabozantinib. However, no effect was observed on PFS by MET status in the subset of patients in whom MET IHC testing and response assessment was available. Additionally, cabozantinib is known to inhibit AXL, which may be activated together with other driver tyrosine kinases. While there is no standardized assay for AXL expression, a biomarker may be identified in an ongoing clinical trial of cabozantinib that includes patients with NSCLC that has increased AXL activity (NCT01639508). Cabozantinib also may be exerting its clinical benefit as a VEGFR2 inhibitor. It is known that VEGFR2 inhibition is effective in the second line treatment of NSCLC, as a VEGFR2 monoclonal antibody, ramucirumab, is FDA approved in combination with docetaxel based on a median overall survival of 10.5 months compared with 9.1 months for docetaxel alone (HR 0.86, 95% CI [0.75–0.98]; p=0.023).(15) Additionally, the small molecule VEGFR2 inhibitor nintedanib plus docetaxel is active in patients with adenocarcinoma histology, with a median overall survival of 12.6 months versus 10.3 months for docetaxel alone (HR 0.83 [95% CI 0.70–0.99], p=0.0359), which led to approval by European regulatory agencies (24). However, no broadly validated predictive biomarker of anti-angiogenic therapy has been identified to date.
Limitations of this study include the modest sample size and the lack of detailed molecular driver oncogene characterization. Although effects on overall survival were observed, a larger trial would be needed to confirm these results. However, conducting a larger trial of similar design would be challenging. We believe that erlotinib is no longer a suitable control arm for a confirmatory trial given the mounting evidence that erlotinib is minimally effective in an EGFR wild-type NSCLC population. One potential comparator would be docetaxel, with a median PFS of 3.0 months and median OS of 9.1 months in a recent large randomised trial.(15) Another potential comparison therapy would be nivolumab, which was superior to docetaxel in non-squamous NSCLC for median OS (12.2 months for nivolumab vs 9.4 months for docetaxel) but not median PFS (2.3 months for nivolumab vs 4.2 months for docetaxel). However, with numerical medians of PFS and OS similar to those we observed for cabozantinib, it appears unlikely that cabozantinib monotherapy would be superior to either docetaxel or nivolumab in a randomised trial. Another limitation is that we only collected known KRAS driver oncogene status, and limited tissue exists to pursue further testing which has become a standard of care in the intervening years since the study began. It is possible that potential cabozantinib sensitive molecular drivers such as RET rearrangement, ROS1 rearrangement, and MET amplification or MET exon 14 skipping mutation were imbalanced between the groups, leading to the observed survival benefits of cabozantinib. This is unlikely, because we would predict all of these to total no more than 10% of this study population, and patients with these alterations would be expected to have radiographic responses to targeted therapy. Few such responses were observed on this trial, even in the cabozantinib groups, suggesting that individual patients with particularly sensitive disease were unlikely to be imbalanced across the arms. Therefore, it is unlikely that a small subgroup with particular molecular driver alterations was responsible the observed clinical benefit of cabozantinib, though testing of remaining tissue is of interest.
To our knowledge, ECOG-ACRIN 1512 is the first randomised study to show that cabozantinib, either alone or in combination with erlotinib, improved progression-free survival and overall survival compared with single agent erlotinib in EGFR wild-type NSCLC in the 2nd and 3rd line setting. Despite the increased toxicity profile, this suggests that cabozantinib is worthy of further study in this patient population. ECOG-ACRIN investigators plan to initiate a follow-up study to build on these observations and further delineate a role for cabozantinib in the treatment of advanced non-squamous NSCLC.
Supplementary Material
Research in Context.
Evidence before this study
In developing the study design and protocol, we did a systematic review of the scientific literature. We searched PubMed, with no time restrictions; abstracts of major oncology meetings; and trial websites including ClinicalTrials.gov, for preclinical data and clinical trials assessing chemotherapy in patients with lung cancer, EGFR therapies in these patients, MET inhibitor therapies in these patients, and the combination of these methods. Search terms included “non-small cell lung cancer”, “EGFR”, and “MET”.
Clinical data in support of this trial included a phase 2 study cabozantinib in patients with previously treated NSCLC which showed that it was active in generating objective tumour responses and meaningful time to progression of disease. Additionally a phase 1/2 trial of erlotinib and cabozantinib demonstrated the safety of the combination of these drugs. Based on our review of the literature and discussions with clinicians, researchers, and regulatory bodies, we postulated that combining erlotinib with cabozantinib might improve treatment efficacy in patients with previously treated EGFR wild-type advanced non-squamous non-small cell lung cancer.
Added value of this study
Our study shows significant improvement in progression-free survival in patients who were treated with cabozantinib, or the combination of cabozantinib and erlotinib, as compared with erlotinib alone. There was also a signal of improvement in overall survival observed in these groups. We found no evidence of associate of progression-free survival with MET status as determined by immunohistochemical staining.
Implications of all the available evidence
The ECOG-ACRIN 1512 trial design tested the feasibility of using cabozantinib alone or combined with erlotinib in this patient population with EGFR wild-type NSCLC. Despite its modest sample size, this trial identified signals of clinically meaningful efficacy superior to that of erlotinib alone, and additional toxicity that was generally manageable. Further investigation of cabozantinib in this patient population, potentially in combination with other established therapies, is warranted.
Acknowledgments
Funding: This study was conducted by ECOG-ACRIN with support from the National Cancer Institute of the National Institutes of Health.
We thank the patients, their families and caregivers for participating in the study; all co-investigators and participating sites; Jeanne Sheehan for coordination of data management; Becky Fillingham (ECOG-ACRIN, Boston, MA, USA) for assistance with the biomarker studies; Maria Pitsiouni (Stanford Cancer Institute, Stanford, California, USA) for effort as study liaison; John Wright at CTEP (National Cancer Institute, Bethesda, Maryland, USA) and Arthur DeCillis with Exelixis (South San Francisco, California, USA) for their assistance with various aspects of the study.
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA21115, CA180794, CA23318, CA66636, CA107868, CA180816, CA180844, CA39229, CA180864, CA180867, CA189825, CA37417, CA35103, CA189830, CA45807, CA35113, CA189863, CA35267, CA189971, CA63848, CA098413, and NCI Contract No. HHSN261200800001E. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Author Contributions
JN was the study chair, HW was the co-chair, and SR was the thoracic group chair. JN, HW, and SR developed the concept for the study in collaboration with ECOG-ACRIN and CTEP. SD was responsible for the statistical design, development, monitoring, and analysis. JN, HW, GG, RL, JR, TO, PJS, PDS, MM and SR were investigators at the centres with the highest recruitment. SA, MB, YH and DC supervised the tissue collection, processing, and molecular and pathological examinations. JN wrote the first draft of the manuscript. All the investigators reviewed and approved the final version. A full list of the EOCG-ACRIN participating sites and investigators can be found in the appendix (page 9).
Declaration of Interests
Dr. Neal reports personal fees from Clovis, personal fees from CARET/Physicians Resource, grants and personal fees from Nektar, grants and personal fees from Boehringer Ingelheim, personal fees from ARMO BioSciences, grants from Genentech/Roche, grants from Merck, grants from ArQule, grants from Novartis, outside the submitted work.
Dr. Dahlberg reports a patent PCT/US2014/054821 pending to Dana-Farber Cancer Institute.
Dr. Wakelee reports personal fees from Peregrine, grants and personal fees from Helsinn, personal fees from ACEA, grants and personal fees from Pfizer, grants from BMS, grants from XCovery, grants from Celgene, grants and personal fees from Roche/Genentech, grants from AstraZeneca/MedImmune, personal fees from Lilly, grants from Gilead, grants from Clovis, grants from Pharmacyclics, grants from Exelixis, grants from Novartis, outside the submitted work.
Dr. Carbone reports personal fees from Ariad, personal fees from AstraZeneca, personal fees from Bayer HealthCare, personal fees from Biothera, personal fees from Boehringer Ingelheim, grants and personal fees from Bristol Myers-Squibb, personal fees from Clovis Oncology, personal fees from Genentech/Roche, personal fees from Guardant Health, personal fees from Inivata, personal fees from Janssen Diagnostics, personal fees from Merck, personal fees from Novartis, personal fees from Peregrine Pharmaceuticals, personal fees from Synta Pharmaceuticals, personal fees from Teva Pharmaceuticals, outside the submitted work.
Dr. Lerner reports grant from Acerta Pharma, grant from Amgen, grant from Bayer HealthCare Pharmaceuticals Inc., grant from Celcuity, LLC, grant from Cephalon, Inc., grant from Teva Branded Pharmaceutical Products R&D, Inc., grant from Janssen Research and Development, LLC, grant from Pfizer, grant from Roche/Genentech, Inc., outside the submitted work.
Dr. Ramalingam reports personal fees from Amgen, AstraZeneca, Abbvie, BMS, Lilly, Celgene, Genentech, and Novartis outside the submitted work.
The other authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joel W. Neal, Stanford Cancer Institute, Stanford, California, USA, CA180816.
Suzanne E. Dahlberg, Dana Farber Cancer Institute, Boston, Massachusetts, USA, Standard Grant.
Heather A. Wakelee, Stanford Cancer Institute, Stanford, California, USA, CA180816.
Seena C. Aisner, Rutgers Cancer Institute of New Jersey, Newark, New Jersey, USA, CA107868.
Michaela Bowden, Dana-Farber Cancer Institute, Boston, Massachusetts, USA, CA180867.
Ying Huang, Dana-Farber Cancer Institute, Boston, Massachusetts, USA, CA180867.
David P. Carbone, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio, USA.
Gregory J. Gerstner, Illinois CancerCare-Peoria, Peoria, Illinois, USA, CA189830, CA45807, CA35113.
Rachel E. Lerner, Park Nicollet Clinic, Saint Louis Park, Minnesota, USA, CA189863, CA35267.
Jerome L. Rubin, Community Hospital of the Monterey Peninsula, Monterey, California, USA, CA180816.
Taofeek K. Owonikoko, The Winship Cancer Institute of Emory University, Atlanta, Georgia, USA, CA180864.
Philip J. Stella, Saint Joseph Mercy Hospital, Ann Arbor, Michigan, USA, CA189971, CA63848.
Preston D. Steen, Roger Maris Cancer Center, Fargo, North Dakota, USA, CA189825, CA37417, CA35103.
Ahmed Ali Khalid, Charleston Area Medical Center, Charleston, West Virginia, USA.
Suresh S. Ramalingam, The Winship Cancer Institute of Emory University, Atlanta, Georgia, USA, CA180864.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011 Mar-Apr;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002 Jan 10;346(2):92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004 May 1;22(9):1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000 May;18(10):2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015 Oct 22;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016 Apr 9;387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014 May 21;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The lancet oncology. 2012 Mar;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005 Jul 14;353(2):123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 10.Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015 Aug;5(8):842–9. doi: 10.1158/2159-8290.CD-14-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011 May;6(5):942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 12.Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH, Jr, Blumenschein GR, Jr, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013 Nov 10;31(32):4105–14. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007 May 18;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 14.Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. doi: 10.4103/1477-3163.44372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014 Aug 23;384(9944):665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 16.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 17.Hellerstedt BA, Edelman G, Vogelzang NJ, Kluger HM, Yasenchak CA, Shen X, et al. Activity of cabozantinib (XL184) in metastatic NSCLC: Results from a phase II randomized discontinuation trial (RDT) J Clin Oncol. 2012;30(suppl) Abstr 7514. [Google Scholar]
- 18.Wakelee HA, Gettinger SN, Engelman JA, Janne PA, West HJ, Subramaniam DS, et al. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28:15s. 2010 (suppl; abstr 3017) [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Freidlin B, Korn EL, Gray R. A general inefficacy interim monitoring rule for randomized clinical trials. Clin Trials. 2010 Jun;7(3):197–208. doi: 10.1177/1740774510369019. [DOI] [PubMed] [Google Scholar]
- 21.Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. The lancet oncology. 2013 Sep;14(10):981–8. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M, Nakagawa H, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA) J Clin Oncol. 2014 Jun 20;32(18):1902–8. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 23.Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. The lancet oncology. 2012 Mar;13(3):300–8. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 24.Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. The lancet oncology. 2014 Feb;15(2):143–55. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.