Abstract
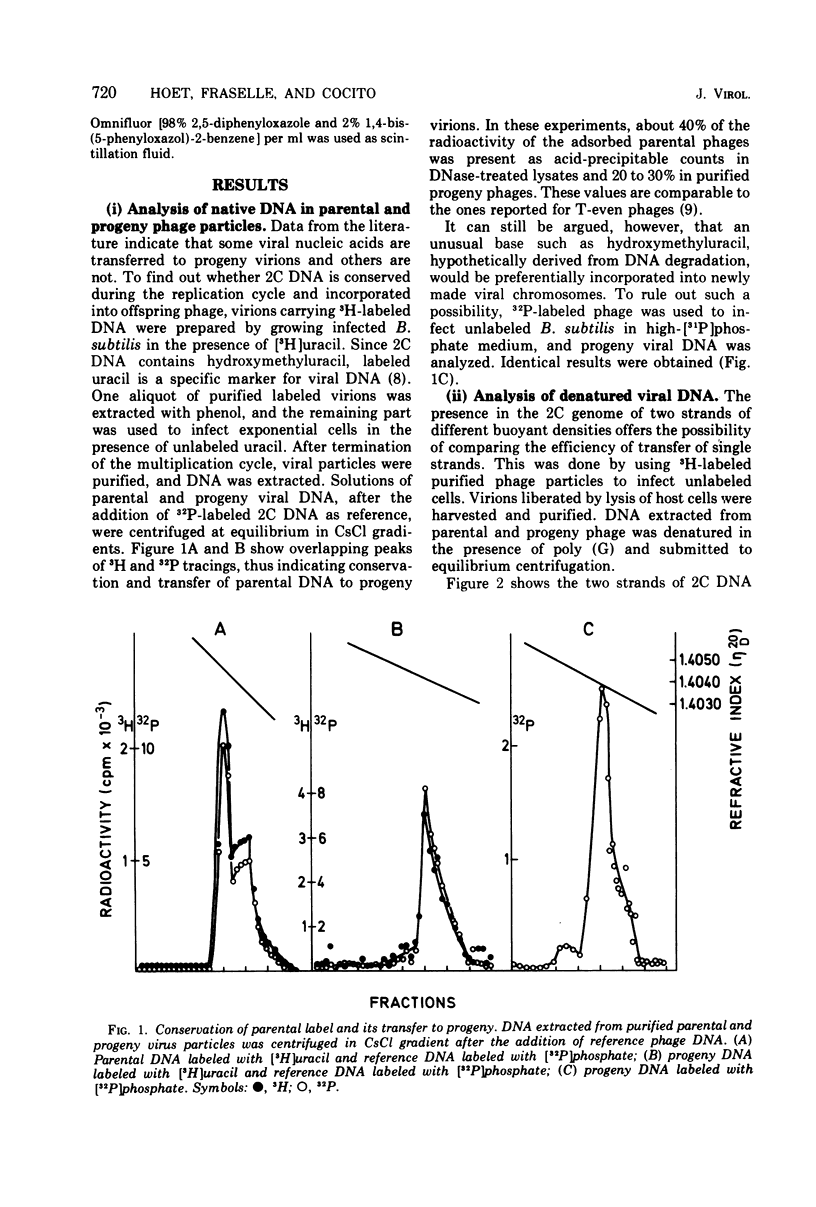
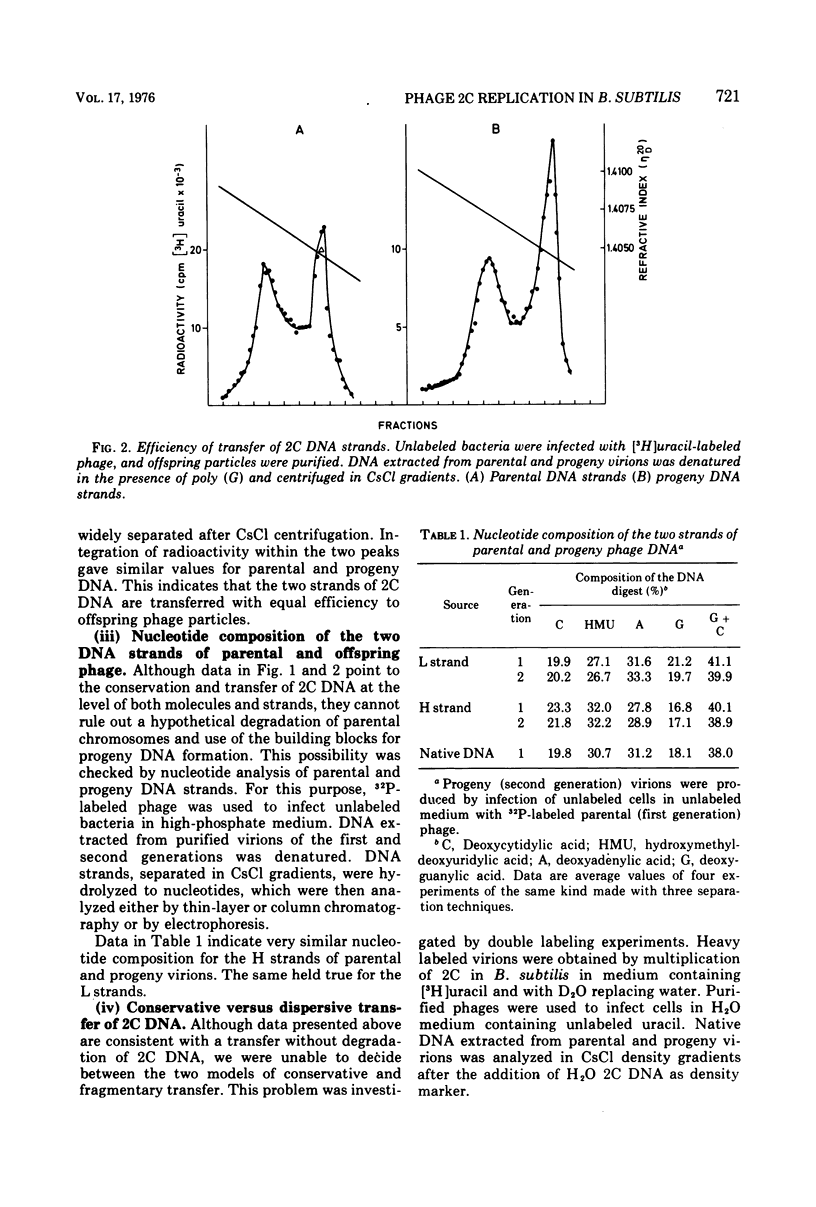
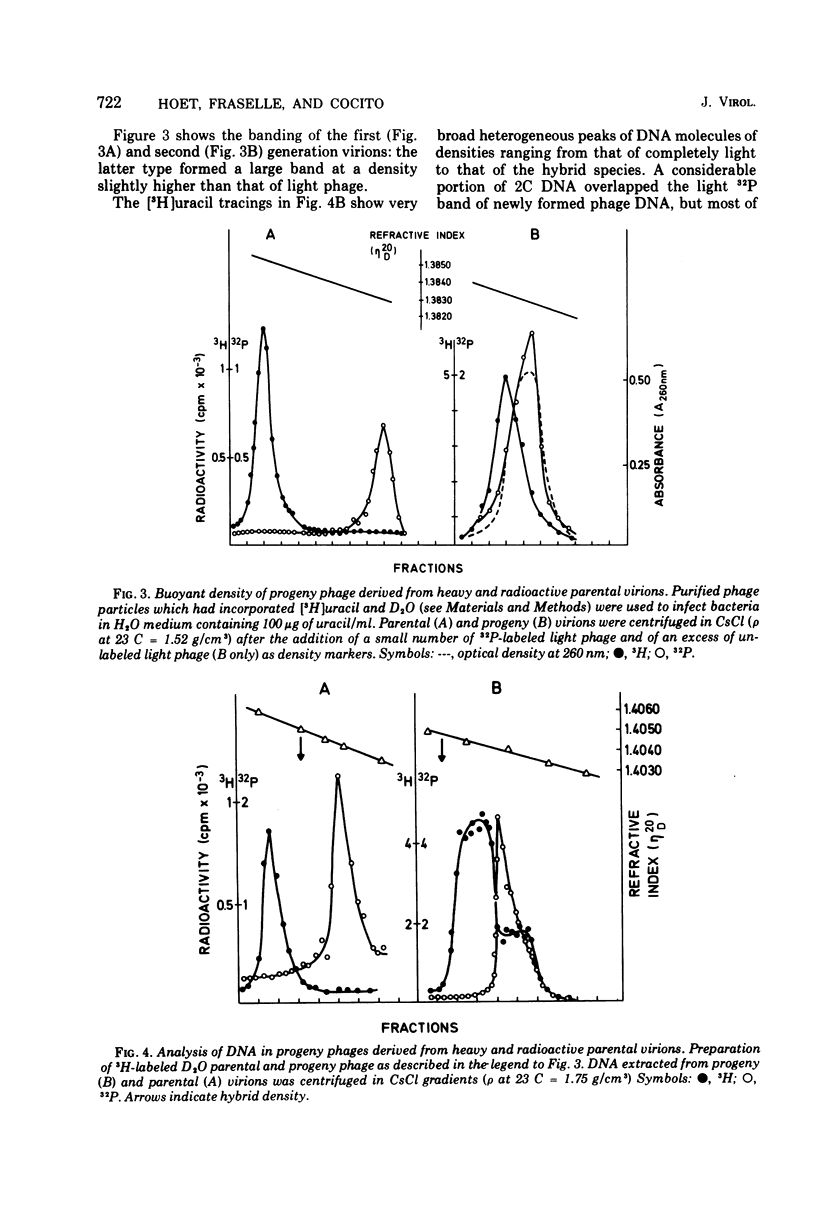
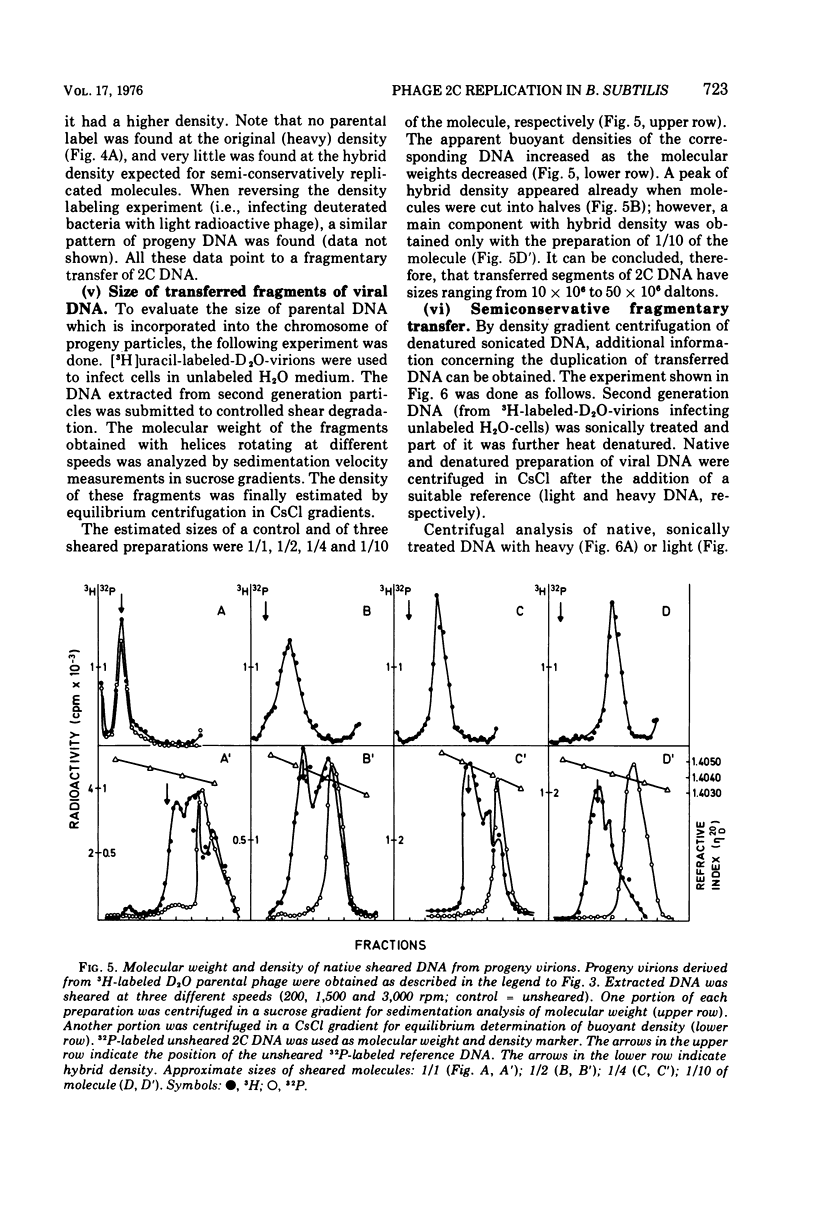
The Bacillus subtilis phage 2C contains one molecule of double-stranded DNA of about 100 x 10(6) daltons in which thymine is replaced by hydroxymethyluracil; the two strands have different buoyant densities. Parental DNA, labeled with either [3H]uracil of [32P]phosphate, was quite effectively transferred to offspring phage, and the efficiency of transfer was the same for the two strands. Labeled nucleotide compositions of the H and L strands from parental and progeny virions were very close. These data exclude a degradation of the infecting DNA and reutilization of nucleotides. Upon infection of light unlabeled cells with heavy radioactive viruses, no DNA with either heavy or hybrid density was extracted from offspring phage. Instead, an heterogeneous population of DNA molecules of densities ranging from that of almost hybrid to that of fully light species was obtained. Shear degradation of such progeny DNA to fragments of decreasing molecular weight produced a progressive shift to the density of hybrid molecules. Denaturation of sheared DNA segments caused the appearance of labeled and heavy single-stranded segments. These findings indicate that 2C DNA replicates semiconservatively and then undergoes extensive genetic recombination with newly formed viral DNA molecules within the vegatative pool, thus mimicking a dispersive transfer of the infecting viral genome. The pieces of transferred parental DNA have an average size of 10 x 10(6) daltons.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURISICCHIO S., DORE E., FRONTALI C., GAETA F., TOSCHI G. CHROMATOGRAPHIC PURIFICATION OF ONE OF THE COMPLEMENTARY STRANDS OF DNA OF PHAGE A. Biochim Biophys Acta. 1964 Mar 23;80:514–516. doi: 10.1016/0926-6550(64)90157-4. [DOI] [PubMed] [Google Scholar]
- Arwert F., Venema G. Transfection of Bacillus subtilis with bacteriophage H1 DNA: fate of transfecting DNA and transfection enhancement in B. subtilis uur+ and uur- strains. Mol Gen Genet. 1974;128(1):55–72. doi: 10.1007/BF00267294. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCITO C., HERSHEY A. D. Transfer of DNA-glucose from parental to offspring phage T2. Biochim Biophys Acta. 1960 Jan 29;37:543–544. doi: 10.1016/0006-3002(60)90519-9. [DOI] [PubMed] [Google Scholar]
- COCITO C., LADURON P. A MICROANALYTICAL PROCEDURE OF ELECTROPHORESIS FOR SEPARATION OF NUCLEOTIDES. Anal Biochem. 1964 Apr;7:429–438. doi: 10.1016/0003-2697(64)90153-8. [DOI] [PubMed] [Google Scholar]
- Carlson K. Intracellular fate of deoxyribonucleic acid from T7 bacteriophages. J Virol. 1968 Oct;2(10):1230–1233. doi: 10.1128/jvi.2.10.1230-1233.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K., Kozinski A. W. Parent-to-progeny transfer and recombination of T4rII bacteriophage. J Virol. 1970 Sep;6(3):344–352. doi: 10.1128/jvi.6.3.344-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocito C. Metabolism of macromolecules in bacteria treated with virginiamycin. J Gen Microbiol. 1969 Aug;57(2):179–194. doi: 10.1099/00221287-57-2-179. [DOI] [PubMed] [Google Scholar]
- Cocito C. The action of virginiamycin on nucleic acid and protein synthesis in Bacillus subtilis infected with bacteriophage 2C. J Gen Microbiol. 1969 Aug;57(2):195–206. doi: 10.1099/00221287-57-2-195. [DOI] [PubMed] [Google Scholar]
- DAVIS J. E., SINSHEIMER R. L. The replication of bacteriophage MS2. 1. Transfer of parental nucleic acid to progeny phage. J Mol Biol. 1963 Mar;6:203–207. doi: 10.1016/s0022-2836(63)80069-8. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSON R. L., FENWICK M. L., FRANKLIN R. M. REPLICATION OF BACTERIOPHAGE RNA: STUDIES ON THE FATE OF PARENTAL RNA. J Mol Biol. 1964 Dec;10:519–529. doi: 10.1016/s0022-2836(64)80070-x. [DOI] [PubMed] [Google Scholar]
- Echols H. Lysogeny: viral repression and site-specific recombination. Annu Rev Biochem. 1971;40:827–854. doi: 10.1146/annurev.bi.40.070171.004143. [DOI] [PubMed] [Google Scholar]
- Egbert L. N. Isolation of the intact strands of the deoxyribonucleic acid of Tphi3, a bacteriophage for Bacillus stearothermophilus. Biochim Biophys Acta. 1972 Oct 27;281(3):310–318. doi: 10.1016/0005-2787(72)90443-1. [DOI] [PubMed] [Google Scholar]
- Epstein H. T., Mahler I. Mechanisms of enhancement of SP82 transfection. J Virol. 1968 Jul;2(7):710–715. doi: 10.1128/jvi.2.7.710-715.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Green D. M. Gene dislinkage in transfection of SP82G phage DNA. Genetics. 1968 Dec;60(4):673–680. doi: 10.1093/genetics/60.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., BURGI E. Genetic significance of the transfer of nucleic acid from parental to offspring phage. Cold Spring Harb Symp Quant Biol. 1956;21:91–101. doi: 10.1101/sqb.1956.021.01.008. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Buckley P. J., Carlson K. M., Kozinski A. W. Multiple and specific initiation of T4 DNA replication. J Virol. 1973 Jul;12(1):130–148. doi: 10.1128/jvi.12.1.130-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W. Fragmentary transfer of P32-labeled parental DNA to progeny phage. Virology. 1961 Jan;13:124–134. doi: 10.1016/0042-6822(61)90039-3. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. Fragmentary transfer of P32-labeled parental DNA to progeny phage. II. The average size of the transferred parental fragment. Two-cycletransfer. Repair of the polynucleotide chain after fragmentation. Virology. 1963 Jun;20:213–229. doi: 10.1016/0042-6822(63)90109-0. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. REPLICATIVE FRAGMENTATION IN T4 BACTERIOPHAGE DNA. II. BIPARENTAL MOLECULAR RECOMBINATION. Proc Natl Acad Sci U S A. 1964 Aug;52:211–218. doi: 10.1073/pnas.52.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W., UCHIDA H. Phage DNA subunits in the phage precursor pool. J Mol Biol. 1961 Jun;3:267–276. doi: 10.1016/s0022-2836(61)80068-5. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B. Early intracellular events in the replication T4 phage DNA. II. Partially replicated DNA. Proc Natl Acad Sci U S A. 1965 Aug;54(2):634–640. doi: 10.1073/pnas.54.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., James R. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. I. Tertiary structure of early replicative and recombining deoxyribonucleic acid. J Virol. 1967 Aug;1(4):758–770. doi: 10.1128/jvi.1.4.758-770.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Lin T. H. Early intracellular events in the replication of T4 phage DNA. I. Complex formation of replicative DNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):273–278. doi: 10.1073/pnas.54.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W. Unbiased participation of T4 phage DNA strands in replication. Biochem Biophys Res Commun. 1969 Apr 29;35(2):294–299. doi: 10.1016/0006-291x(69)90281-2. [DOI] [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., May E., Granboulan P., Granboulan N., Marmur J. Ultrastructure du bactériophage 2C et propriétés de son DNA. Ann Inst Pasteur (Paris) 1968 Dec;115(6):1029–1046. [PubMed] [Google Scholar]
- Miller R. C., Jr, Kozinski A. W. Early intracellular events in the replication of bacteriophage T4 deoxyribonucleic acid. V. Further studies on the T4 protein-deoxyribonucleic acid complex. J Virol. 1970 Apr;5(4):490–501. doi: 10.1128/jvi.5.4.490-501.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Kozinski A. W., Litwin S. Molecular Recombination in T4 Bacteriophage Deoxyribonucleic Acid: III. Formation of Long Single Strands During Recombination. J Virol. 1970 Mar;5(3):368–380. doi: 10.1128/jvi.5.3.368-380.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharrafa E. T., Schachtele C. F., Reilly B. E., Anderson D. L. Complementary Strands of Bacteriophage phi29 Deoxyribonucleic Acid: Preparative Separation and Transcription Studies. J Virol. 1970 Dec;6(6):855–864. doi: 10.1128/jvi.6.6.855-864.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- Rutberg L., Hoch J. A., Spizizen J. Mechanism of transfection with deoxyribonucleic acid from the temperate Bacillus bacteriophage phi-105. J Virol. 1969 Jul;4(1):50–57. doi: 10.1128/jvi.4.1.50-57.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- Shahn E., Kozinski A. Fragmentary transfer of P32 labeled parental DNA to progeny phage. 3. Incorporation of a single parental fragment to the progeny molecule. Virology. 1966 Nov;30(3):455–470. doi: 10.1016/0042-6822(66)90122-x. [DOI] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. The role of recombination in transfection of B. subtilis. Mol Gen Genet. 1971;113(2):174–190. doi: 10.1007/BF00333191. [DOI] [PubMed] [Google Scholar]
- Truffaut N. Isolement et propriétés des chaînes du DNA de bactériophage 2 C. Eur J Biochem. 1970 Apr;13(3):438–446. doi: 10.1111/j.1432-1033.1970.tb00947.x. [DOI] [PubMed] [Google Scholar]
- Truffaut N., Revet B., Soulie M. O. Etude comparative des DNA de phages 2C, SP8*, SP82, phi e, SP01 et SP50. Eur J Biochem. 1970 Aug;15(2):391–400. doi: 10.1111/j.1432-1033.1970.tb01020.x. [DOI] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCTION AND PROPERTIES OF A TEMPERATURE BACTERIOPHAGE FROM BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Jan;89:175–184. doi: 10.1128/jb.89.1.175-184.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]



