Abstract
Background
It is known that miRNAs play various roles in malignant tumors. This study is designed to investigate whether miR-125b levels can be used to predict the clinical response of patients with osteosarcoma (OS) to cisplatin-based chemotherapy.
Methods
From January 2010 to July 2015, 82 patients with resectable OS and 56 patients with unresectable OS were enrolled. Blood samples were collected and quantitative real-time PCR was applied to determine miR-125b expression. Clinical data was collected through medical records, and patients were treated according to National Comprehensive Cancer Network guidelines on OS.
Results
Our study found that patients with low miR-125b expression had shorter disease-free survival (p<0.001) in the OS group, which was verified by Kaplan-Meier analysis and univariate and multivariate Cox analyses (p<0.001). For patients with unresectable OS, low miR-125b expression was found to be associated with advanced tumor stages (p=0.006). No complete remission was observed, and there were 13 patients with partial remission, 21 with stable disease, and 22 with disease progression. Negative correlation was found between miR-125b expression and response to chemotherapy (p<0.001, r=−0.606). Furthermore, ROC analysis indicated that miR-125b at the cut point of 0.61 yielded an area under the ROC curve of 0.793 (p<0.001, 95% CI: 0.664–0.890) in distinguishing chemotherapy-resistant OS from chemotherapy-sensitive OS, with sensitivity and specificity at 76.9% and 79.1%, respectively. Kaplan-Meier analysis and univariate and multivariate Cox analyses showed that patients with low miR-125b expression suffered shorter overall survival (p=0.014, p=0.024, and p=0.049, respectively).
Conclusion
Down-regulation of circulating miR-125b might have the potential to predict cisplatin-based chemotherapy resistance and poor prognosis in OS.
Keywords: Osteosarcoma, Cisplatin, Chemotherapy, miR-125b, Prognosis
1. Introduction
Osteosarcoma (OS) is the most frequently diagnosed malignant bone tumor in children and adolescents, and about half of the cases have lesions localized in the distal femur and proximal tibia [1]. The incidence of OS is estimated to be about three to five cases/million/year, accounting for about 5% of childhood malignancies and about 9% of malignancy-related deaths in children [2], [3]. OS has a high propensity to metastasize, especially to the lung [4]. The long-term survival rate of OS patients was less than 20% after surgical resection alone prior to the availability of neoadjuvant and adjuvant chemotherapy in the 1980s [5]. The 5-year survival rate improved to 60–70% after the development and use of multi-agent chemotherapy regimens [6]. However, the improvement of survival has not changed significantly for the past 30 years since chemotherapy was developed, and chemoresistance has become a troublesome obstacle during management of OS. There is an urgent need to elucidate the molecular mechanisms of chemotherapy resistance and find reliable biomarkers of its development. This would greatly help identify more effective biological-based therapies and optimize the treatment strategies.
Post-transcriptional regulation by microRNAs (miRNAs) has been identified as an important mechanism underlying oncogenesis, invasiveness, proliferation, and migration of malignant tumors [7], [8]. Growing evidence indicates that various miRNAs (including miR-92a, miR-99b, miR-132, miR-193a-5p, miR-422a, and miR-125b) are involved in the development of resistance to chemotherapy [5], [9], [10]. It is thought that miR-125b can act as both an oncogene and a tumor suppressor, depending on the cellular context [11], [12], [13]. A previous study indicated that miR-125b was significantly reduced in OS tissues, and that it suppresses proliferation and migration of OS cells through down-regulation of STAT3 [14]. Another study showed that miR-125b increases the sensitivity of OS cell lines to cisplatin by targeting Bcl-2 [10]. However, evidence from clinical practice is lacking.
Therefore, this study was designed to explore the possible use of miR-125b levels in patients to predict the response of OS to cisplatin-based chemotherapy.
2. Patients and methods
2.1. Ethical considerations
The study protocol was approved by the medical ethics committee of the Second Clinical Hospital of Lanzhou University. Informed consent was obtained from all adult participants prior to the start of the study. For children under 18 years of age, informed consent was obtained from their legal guardian.
2.2. Patients and samples
Patients with OS who presented to our department between January 2010 and July 2015 were screened for enrollment. Exclusion criteria were previous malignant tumors in another organ or system; hematological disorders; end-stage patients not qualified for chemotherapy; patients with no pathological data; and any patients unwilling to participate. Patients were treated according to National Comprehensive Cancer Network practice guidelines for OS, and any treatment decisions were not affected by participation in the study. Patients with resectable OS received cisplatin-based neoadjuvant and adjuvant chemotherapy and surgery, while cisplatin-based aggressive chemotherapy was given to OS patients with unresectable lesions.
The demographic and clinical characteristics of the study participants were obtained from their medical records on admission, and follow-up of patients receiving surgery was performed by the combination of outpatient visits, letters, and telephone calls. The patients had follow-up visits with physical examinations and radiography every 3 months, as well as computed tomography scans or magnetic resonance imaging when necessary. Disease-free survival was calculated from the date of surgery until an event for each patient. For patients with unresectable OS tumors, the response to chemotherapy was evaluated by the Response Evaluation Criteria in Solid Tumors (RECIST). In this study, chemotherapy sensitivity was defined as complete remission or partial remission, whereas stable disease and disease progression were taken as signs of chemotherapy resistance [15]. The patients were also followed for overall survival after therapy, which was calculated from the date of the beginning of chemotherapy in our department, until the date of death.
2.3. Sample collection, RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
In each participant, a 10 mL peripheral venous blood was collected in EDTA anticoagulation tubes before any therapy was begun. The blood samples were centrifuged at 3,000 rpm for 10 min within 20 min after collection. In order to completely remove the cellular debris, the supernatant was separated and further processed by 15 min of high-speed centrifugation at 12,000 x g. The final plasma was then stored in RNase-free tubes (Axygen, Union, CA) at −80 °C for further analysis.
Total RNA was extracted from specimens using a mirVana™ PARIS™ kit (Applied Biosystems, USA) according to manufacturer's protocol. A NanoDrop™ 1000 Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA) was used to determine the concentration of extracted RNAs. Taqman® MicroRNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA) were then utilized to perform the reverse transcription reactions. A 5 μl reaction system comprising 0.5 μl of different primers, 0.063 μl of 20 units/μl RNase inhibitor, 0.33 μl of 50 units/μl Multiscribe reverse transcriptase, 0.05 μl of 100 mM dNTPs, 0.5 μl of 10× reverse transcription buffer, the RNA sample, and RNase-free water was incubated at 30 °C for 10 min, followed by 30 min of incubation at 50 °C, at 95 °C for 5 min, and then held at 4 °C.
A Bio-Rad IQ5 (Bio-Rad Laboratories Inc.) thermocycler was applied for the qPCR reaction. A 10 μl qPCR reaction solution with 5 μl of TaqMan 2× Perfect Master Mix, 2 μl of cDNA solution, 0.25 μl of specific primers, and 2.75 μl of RNase-free water was used. U6 snRNA (Ambion, AM30303) was used as a reference miR. The qPCR primers were miR-125b: sense, 5′-GCUCCCUGAGACCCUAAC-3′, and antisense, 5′-CAGTGCAGGGTCCGAGGT-3′; U6: sense, 5′-CTCGCTTCGGCAGCACATATACT-3′ and antisense, 5′-ACGCTTCACGAATTTGCGTGTC-3′. The PCR amplification was performed as follows: an initial denaturation at 95 °C was carried out for 2 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The reaction was terminated by incubation at 95 °C for 15 s, and the products held at 4 °C. The expression level of miR-125b was calculated using the ΔΔCT method, and each reaction was repeated in triplicate to avoid bias.
2.4. Statistical analysis
MedCalc for Windows, version 13.0 (MedCalc Software, Ostend, Belgium) and SPSS version 16.0 (SPSS, Chicago, IL, USA) were used for statistical analyses. The Kolmogorov-Smirnov test was applied to test the normality of miR-125b expression in both groups, and one-way analysis of variance testing, Student's t-test and Spearman correlation analysis were flexibly used as appropriate. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the efficacy of miR-125b in distinguishing chemotherapy resistance in the OS group with unresectable lesions. The association between miR-125b expression and survival was assessed by the log-rank test and Cox proportional hazard regression analysis, and age, gender, tumor location, tumor stage, histologic grade, metastasis status, and miR-125b expression level were entered into the multivariate analysis. The mean value of miR-125b expression level was set as the cut-off point to differentiate patients with high or low miR-125b expression in the resectable and unresectable groups. A level of p<0.05 was considered statistically significant.
3. Results
3.1. Patients’ characteristics
From January 2010 to July 2015, 176 patients who presented with OS to our orthopedic department were screened. Of these, 138 (78%) were enrolled as participants in the study, 82 with resectable OS and 56 with unresectable OS. The median follow-up time in the resectable OS group is 23.1±10.9 months, while it was 13.9±6.6 months in the unresectable OS group. The details of patients’ clinical characteristics are presented in Table 1.
Table 1.
Pathological and clinical parameters of the enrolled participants.
| Characteristics | Resectable OS (n=82) | Unresectable OS (n=56) |
|---|---|---|
| Age (years) | 22.8±9.7 | 22.9±9.4 |
| Gender | ||
| Male | 52 | 21 |
| Female | 30 | 35 |
| Tumor location | ||
| Tibia/femur | 58 | 45 |
| Elsewhere | 24 | 11 |
| Tumor stage | ||
| T1 | 49 | 17 |
| T2 | 26 | 32 |
| T3 | 7 | 7 |
| Histologic grade | ||
| G1 | 25 | 17 |
| G2 | 26 | 18 |
| G3 | 31 | 21 |
| Number of metastatic sites | ||
| 0 | 69 | 10 |
| 1 | 9 | 21 |
| ≥2 | 4 | 25 |
| Metastatic location | ||
| Lung | 7 | 18 |
| Other sites | 2 | 6 |
| Lung and other sites | 4 | 22 |
| Previous surgery | 9 | 44 |
| Previous chemotherapy | 12 | 32 |
| Previous radiotherapy | 7 | 15 |
| miR-125b in plasma | 0.97±0.55 | 0.50±0.25 |
Note: OS, osteosarcoma.
3.2. miR-125b expression profile in the resectable OS group
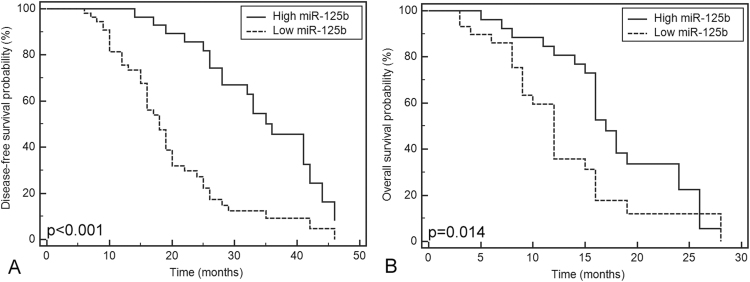
The miR-125b expression level in the resectable OS group was normally distributed with a mean of 0.97±0.55 (p>0.05, Table 2), and no significant correlation was found between miR-125b expression and patients’ age, gender, tumor location, tumor grade, histologic grade, number of metastases, or location (Table 2). The samples with miR-125b expression less than 0.97 were assigned to the low-expression group (n=28), while samples with expression more than the mean value were taken as high miR-125b expression (n=54). Kaplan-Meier analysis indicated that patients with low miR-125b expression suffered shorter disease-free survival (p<0.01, Fig. 1(A)), which was verified by the univariate and multivariate Cox proportional hazard regression analysis (p<0.001, Table 3). The multivariate Cox proportional hazard regression analysis also showed that patients with low tumor stage (T1) experienced longer disease-free survival than those with high tumor stage (T2+T3) (p=0.036, Table 3), which was not found by univariate Cox proportional hazard regression analysis (p=0.447, Table 3).
Table 2.
Comparison of miR-125b expression among patients’ characteristics.
| Characteristics | miR-125b in resectable OS | p | miR-125b in unresectable OS | p |
|---|---|---|---|---|
| Age (years) | 0.956 | 0.658 | ||
| <25 | 0.97±0.51 | 0.49±0.24 | ||
| ≥25 | 0.97±0.62 | 0.52±0.28 | ||
| Gender | 0.094 | 0.774 | ||
| Male | 0.90±0.50 | 0.49±0.25 | ||
| Female | 1.10±0.62 | 0.51±0.25 | ||
| Tumor location | 0.635 | 0.436 | ||
| Tibia/femur | 0.95±0.54 | 0.48±0.23 | ||
| Elsewhere | 1.02±0.57 | 0.55±0.32 | ||
| Tumor stage | 0.588 | 0.006 | ||
| T1 | 1.02±0.54 | 0.60±0.29 | ||
| T2 | 0.88±0.53 | 0.56±0.25 | ||
| T3 | 0.99±0.67 | 0.34±0.15 | ||
| Histologic grade | 0.067 | 0.139 | ||
| G1 | 1.08±0.59 | 0.43±0.21 | ||
| G2 | 0.77±0.35 | 0.59±0.23 | ||
| G3 | 1.05±0.61 | 0.47±0.28 | ||
| Number of metastatic sites | 0.160 | 0.789 | ||
| 0 | 0.99±0.55 | 0.47±0.25 | ||
| 1 | 1.02±0.55 | 0.48±0.25 | ||
| ≥2 | 0.46±0.16 | 0.52±0.25 | ||
| Metastatic location | 0.224 | 0.652 | ||
| Lung | 1.02±0.56 | 0.50±0.26 | ||
| Other sites | 1.03±0.74 | 0.42±0.19 | ||
| Lung and other sites | 0.46±0.16 | 0.53±0.26 | ||
| RECIST | p<0.001 | |||
| Complete response | ||||
| Partial response | 0.69±0.24 | |||
| Stable disease | 0.55±0.17 | |||
| Progressive disease | 0.32±0.21 |
Note: OS, osteosarcoma; RECIST, Response Evaluation Criteria in Solid Tumors.
Fig. 1.
Correlation between circulating miR-125b expression and disease-free survival time in the resectable osteosarcoma (OS) group (A) and overall survival time in the unresectable OS group (B).
Table 3.
Cox proportional regression analysis for assessing the correlation of miR-125b expression in plasma with the disease-free survival of resectable osteosarcoma.
| Covariate | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (years) | 0.209 | 0.243 | ||||
| <25 | 1.000 | 1.000 | ||||
| ≥25 | 0.714 | 0.423–1.208 | 0.717 | 0.410–1.254 | ||
| Gender | 0.660 | 0.525 | ||||
| Female | 1.000 | 1.000 | ||||
| Male | 0.889 | 0.528–1.499 | 1.206 | 0.676–2.152 | ||
| Tumor location | 0.792 | 0.339 | ||||
| Tibia/femur | 1.000 | 1.000 | ||||
| Elsewhere | 1.075 | 0.628–1.839 | 0.746 | 0.410–1.359 | ||
| Tumor stage | 0.447 | 0.036 | ||||
| T1 | 1.000 | 1.000 | ||||
| T2+T3 | 1.223 | 0.728–2.054 | 1.902 | 1.042–3.472 | ||
| Histologic grade | 0.449 | 0.769 | ||||
| G1 | 1.000 | 1.000 | ||||
| G2+G3 | 1.221 | 0.728–2.047 | 1.083 | 0.635–1.846 | ||
| Metastasis | 0.484 | 0.550 | ||||
| No | 1.000 | 1.000 | ||||
| Yes | 0.785 | 0.398–1.548 | 0.807 | 0.400–1.626 | ||
| miR-125b expression | <0.001 | <0.001 | ||||
| Low | 1.000 | 1.000 | ||||
| High | 0.299 | 0.171–0.523 | 0.230 | 0.124–0.426 | ||
3.3. miR-125b expression profile in the unresectable OS group
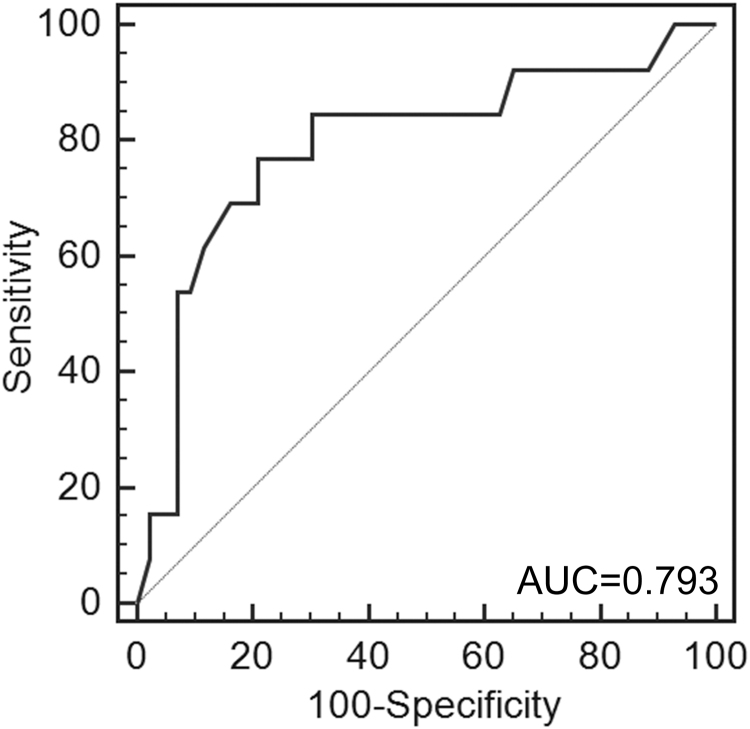
The miR-125b expression level in the unresectable OS group was also normally distributed (p>0.05), and averaged 0.50±0.25. No significant correlation was found between miR-125b expression and patients’ age, gender, tumor location, histologic grade, number of metastasis, or metastatic location (Table 2). Low miR-125b expression was found to be associated with advanced tumor stages (p=0.006). No complete remission was observed, but there were 13 patients with partial remission, 21 with stable disease, and 22 with disease progression in the study. There was a significant difference of miR-125b expression with different tumor responses to chemotherapy (p<0.001). Spearman correlation analysis showed a negative correlation between miR-125b expression and tumor response to chemotherapy (p<0.001, r=−0.606). Furthermore, ROC analysis indicated that miR-125b at the cut-off point of 0.61 yielded an area under the ROC curve of 0.793 (p<0.001, 95% confidence interval (CI): 0.664–0.890) in distinguishing chemotherapy-resistant OS from chemotherapy-sensitive OS, the sensitivity and specificity of which were 76.9% and 79.1%, respectively (Fig. 2). Kaplan-Meier analysis showed that the study participants with low miR-125b expression (miR-125b<0.50) had shorter overall survival (p=0.014, Fig. 1(B)), which was consistent with the univariate and multivariate Cox proportional hazard regression analysis (p=0.024 and p=0.049, respectively, Table 4).
Fig. 2.
Receiver operating characteristic (ROC) analysis to determine the efficiency of circulating miR-125b in separating patients with a poor chemotherapy response.
Table 4.
Cox proportional regression analysis for assessing the correlation of miR-125b expression in plasma with the overall survival of unresectable osteosarcoma.
| Covariate | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age(years) | 0.708 | 0.379 | ||||
| <25 | 1.000 | 1.000 | ||||
| ≥25 | 0.994 | 0.965–1.025 | 0.698 | 0.313–1.555 | ||
| Gender | 0.509 | 0.285 | ||||
| Female | 1.000 | 1.000 | ||||
| Male | 1.234 | 0.661–2.301 | 0.693 | 0.354–1.358 | ||
| Tumor location | 0.755 | 0.664 | ||||
| Tibia/femur | 1.000 | 1.000 | ||||
| Elsewhere | 0.884 | 0.409–1.912 | 0.826 | 0.349–1.958 | ||
| Tumor stage | 0.122 | 0.374 | ||||
| T1 | 1.000 | 1.000 | ||||
| T2+T3 | 0.608 | 0.324–1.142 | 1.443 | 0.643–3.239 | ||
| Histologic grade | 0.229 | 0.198 | ||||
| G1 | 1.000 | 1.000 | ||||
| G2+G3 | 0.672 | 0.351–1.285 | 1.558 | 0.793–3.058 | ||
| Metastasis | 0.861 | 0.807 | ||||
| ≤1 | 1.000 | 1.000 | ||||
| ≥2 | 1.055 | 0.577–1.929 | 0.921 | 0.476–1.784 | ||
| miR-125b expression | 0.024 | 0.049 | ||||
| Low | 1.000 | 1.000 | ||||
| High | 0.496 | 0.269–0.913 | 0.485 | 0.235–0.998 | ||
4. Discussion
Accumulating knowledge of the molecular pathogenesis of malignant tumors has provided new insights for disease characterization and therapy; these insights may be applied to selected patients for more rational treatment decisions and better clinical outcomes. In recent years, miRNAs, classified as oncogenes or tumor suppressor genes, have been widely studied and have the potential to serve as classification criteria. In the present study, we found that OS patients with low miR-125b expression had shorter disease-free survival in the resectable OS group and shorter overall survival in the unresectable OS group. Additionally, we found a negative correlation between miR-125b expression and tumor response to chemotherapy.
miR-125b is a highly conserved miRNA among different species, which includes the miR-99/miR100 and the let7 family members [16]. It has been reported that miR-125b is involved in control of cell proliferation, cell cycling, and inflammation [17], [18], [19]. The role of miR-125b in malignant tumors is controversial: it has been reported to be overexpressed in prostate cancer and hematological malignances and to act as oncogene [11], [13], and is also reported to be down-regulated in breast, ovarian, and thyroid carcinomas, as well as hepatocellular cancer, and is assumed to be a tumor suppressor. [12], [18], [20]. In OS, miR-125b was down-regulated in human osteosarcoma tissues compared with the adjacent tissues [10], [14]. Down-regulation of miR-125b was reported to be associated with advanced TNM stage, metastasis, and higher tumor size, and it was said to be acting as a tumor suppressor [21]. In the present study, a significant correlation was found only between miR-125b expression and tumor stage in the unresectable OS group. Various factors may contribute to this difference, such as race, sample category, sample size, detection techniques, and experimental reagents. One important factor may be that the prior study analyzed miR-125b expression in tissue, while we analyzed miR-125b in plasma. The circulating miR-125b could be easily affected by multiple internal environmental factors.
The key issue in this study is the role of miR-125b in predicting OS patients’ response to cisplatin-based chemotherapy. For the resectable OS group, it was hard to evaluate patients’ response to chemotherapy directly. Therefore, we applied disease-free survival to indirectly assess this, and found that OS patients with low miR-125b expression suffered shorter disease-free survival. We also found that patients with low miR-125b expression experienced shorter overall survival in the unresectable OS group. A previous study analyzing resected OS tissue found that the overall survival time was significantly shorter in patients with low miR-125b expression compared with those with high miR-125b expression [21]. In the present study, RECIST was utilized to assess patients’ response to chemotherapy in the unresectable OS group, and a negative correlation was found between miR-125b expression and the tumor response to cisplatin-based chemotherapy. In vitro, similar results were reported previously by Wang et al., who found that stable overexpression of miR-125b in OS cell lines U2OS and MG-63 inhibited cell proliferation, migration, and invasion, and miR-125b increased the sensitivity of OS cell lines to cisplatin [10].
The mechanisms for development of chemotherapy resistance are complicated, including decreased intracellular drug accumulation, drug inactivation, enhanced DNA repair, perturbations in signal transduction pathways, apoptosis, cell cycle-related gene expression turbulence, autophagy-related chemoresistance, and miRNA dysregulation [1], [5]. Furthermore, miRNAs levels have been shown to be associated with the development of chemoresistance in OS; specifically, miR-34a, miR-92a, miR-99b, miR-132, miR-193a-5p, miR-422a, miR-140, miR-215, miR-15a, and miR-16–1 have been identified [9], [22], [23], [24], [25]. Wang et al. showed that Bcl-2 was inversely associated with miR-125b in OS tissues, and that miR-125b could bind to the 3′ untranslated region of Bcl-2 and therapy reduced its expression [10]. The combined treatment of miR-125b and cisplatin significantly induced cell apoptosis, while overexpressed Bcl-2 partially reduced the effect induced by miR-125b plus cisplatin treatment. Additionally, they found that the activity of caspase 3, a key factor in cell apoptosis, was significantly improved with treatment by miR-125b plus cisplatin compared with miR-125b or cisplatin treatment alone. In contrast, up-regulation of Bcl-2 attenuated the activation of caspase-3 induced by miR-125b plus cisplatin treatment. These results showed that miR-125b improved OS cells’ sensitivity to cisplatin treatment by targeting Bcl-2 in OS cells [10]. miR-125b could also promote apoptosis by diminishing the expression of Mcl-1, Bcl-w, and IL-6R [26]. In contrast, miR-125b could play an anti-apoptotic role through suppressing Bcl-2 antagonist killer 1 (Bak1) [27]. Moreover, it is reported that miR-125b could suppress the proliferation and migration of OS cells through down-regulation of STAT3 [14]. Determining the exact mechanism involved in miR-125b regulation of OS response to cisplatin-based chemotherapy will require further investigation.
Although the results of the present study are encouraging, several limitations need to be acknowledged. First, the sample size of this study was relatively small, and confirming data from larger groups of patients are needed. Second, all participants were drawn from a limited geographic area from a single center, and so whether the results apply to the whole population is open to question. Third, previous treatments were excluded from the statistical analysis. Fourth, the conclusions of the study are derived from the clinical data; evidence from laboratory studies and the mechanism by which miR-125b affects OS response to cisplatin-based chemotherapy are lacking.
5. Conclusion
This study presents evidence that miR-125b levels have the potential to be a valid biomarker to predict the effect of cisplatin-based chemotherapy and prognosis in OS. The limitation of the study is that it was derived from a retrospective analysis, and the positive and negative predictive values of miR-125b testing need to be evaluated prospectively with predefined cut-off levels and in a variety of clinical settings and centers before we can be certain of its efficacy.
References
- 1.Chou A.J., Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev. Anticancer Ther. 2006;6(7):1075–1085. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G., Jaffe N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Bruland O.S., Bauer H., Alvegaard T., Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat. Res. 2009;152:309–318. doi: 10.1007/978-1-4419-0284-9_16. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat. Res. 2009;152:239–262. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- 5.He H., Ni J., Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review) Oncol. Lett. 2014;7(5):1352–1362. doi: 10.3892/ol.2014.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eilber F.R., Rosen G. Adjuvant chemotherapy for osteosarcoma. Semin. Oncol. 1989;16(4):312–322. [PubMed] [Google Scholar]
- 7.Yuxia M., Zhennan T., Wei Z. Circulating miR-125b is a novel biomarker for screening non-small-cell lung cancer and predicts poor prognosis. J. Cancer Res. Clin. Oncol. 2012;138(12):2045–2050. doi: 10.1007/s00432-012-1285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croce C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gougelet A., Pissaloux D., Besse A., Perez J., Duc A., Dutour A., Blay J.Y., Alberti L. Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int. J. Cancer J. Int. Cancer. 2011;129(3):680–690. doi: 10.1002/ijc.25715. [DOI] [PubMed] [Google Scholar]
- 10.Wang F., Yu D., Liu Z., Wang R., Xu Y., Cui H., Zhao T. MiR-125b Functions as a Tumor Suppressor and Enhances Chemosensitivity to Cisplatin in Osteosarcoma. Technol. Cancer Res. Treat. 2016 doi: 10.1177/1533034615618849. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri A.A., So A.Y., Mehta A., Minisandram A., Sinha N., Jonsson V.D., Rao D.S., O’Connell R.M., Baltimore D. Oncomir miR-125b regulates hematopoiesis by targeting the gene Lin28A. Proc. Natl. Acad. Sci. U. S. A. 2012;109(11):4233–4238. doi: 10.1073/pnas.1200677109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao A., Zeng Q., Xie X., Zhou J., Yue W., Li Y., Pei X. MicroRNA-125b induces cancer cell apoptosis through suppression of Bcl-2 expression. J. Genetics Genomics =Yi chuan xue bao. 2012;39(1):29–35. doi: 10.1016/j.jgg.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Pang Y., Young C.Y., Yuan H. MicroRNAs and prostate cancer. Acta Biochim. Biophys. Sin. 2010;42(6):363–369. doi: 10.1093/abbs/gmq038. [DOI] [PubMed] [Google Scholar]
- 14.Liu L.H., Li H., Li J.P., Zhong H., Zhang H.C., Chen J., Xiao T. miR-125b suppresses the proliferation and migration of osteosarcoma cells through down-regulation of STAT3. Biochem. Biophys. Res. Commun. 2011;416(1-2):31–38. doi: 10.1016/j.bbrc.2011.10.117. [DOI] [PubMed] [Google Scholar]
- 15.E.A. Eisenhauer, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1), 45(2) (2009) 228-47. [DOI] [PubMed]
- 16.Ribeiro J., Sousa H. MicroRNAs as biomarkers of cervical cancer development: a literature review on miR-125b and miR-34a. Mol. Biol. Rep. 2014;41(3):1525–1531. doi: 10.1007/s11033-013-2998-0. [DOI] [PubMed] [Google Scholar]
- 17.Jia H.Y., Wang Y.X., Yan W.T., Li H.Y., Tian Y.Z., Wang S.M., Zhao H.L. MicroRNA-125b functions as a tumor suppressor in hepatocellular carcinoma cells. Int. J. Mol. Sci. 2012;13(7):8762–8774. doi: 10.3390/ijms13078762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Yan L.X., Wu Q.N., Du Z.M., Chen J., Liao D.Z., Huang M.Y., Hou J.H., Wu Q.L., Zeng M.S., Huang W.L., Zeng Y.X., Shao J.Y. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71(10):203552–203562. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]
- 19.Tili E., Michaille J.J., Costinean S., Croce C.M. MicroRNAs, the immune system and rheumatic disease. Nat. Clin. Pract. Rheumatol. 2008;4(10):534–541. doi: 10.1038/ncprheum0885. [DOI] [PubMed] [Google Scholar]
- 20.Visone R., Pallante P., Vecchione A., Cirombella R., Ferracin M., Ferraro A., Volinia S., Coluzzi S., Leone V., Borbone E., Liu C.G., Petrocca F., Troncone G., Calin G.A., Scarpa A., Colato C., Tallini G., Santoro M., Croce C.M., Fusco A. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene. 2007;26(54):7590–7595. doi: 10.1038/sj.onc.1210564. [DOI] [PubMed] [Google Scholar]
- 21.Karbasy S.H., Taheriazam A., Mirghasemi A., Sedaghati F., Shakeri M., Yahaghi E., Bahador R. Upregulation of miR-300 and downregulation of miR-125b act as potential predictor biomarkers in progression, metastasis, and poor prognosis of osteosarcoma. Tumour Biol.: J. Int. Soc. Oncodev. Biol. Med. 2015 doi: 10.1007/s13277-015-4000-3. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani F., Ferracin M., Manara M.C., Ventura S., Del Monaco V., Ferrari S., Alberghini M., Grilli A., Knuutila S., Schaefer K.L., Mattia G., Negrini M., Picci P., Serra M., Scotlandi K. miR-34a predicts survival of Ewing’s sarcoma patients and directly influences cell chemo-sensitivity and malignancy. J. Pathol. 2012;226(5):796–805. doi: 10.1002/path.3007. [DOI] [PubMed] [Google Scholar]
- 23.Song B., Wang Y., Xi Y., Kudo K., Bruheim S., Botchkina G.I., Gavin E., Wan Y., Formentini A., Kornmann M., Fodstad O., Ju J. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28(46):4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song B., Wang Y., Titmus M.A., Botchkina G., Formentini A., Kornmann M., Ju J. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol. Cancer. 2010;9:96. doi: 10.1186/1476-4598-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai C.K., Zhao G.Y., Tian L.Y., Liu L., Yan K., Ma Y.L., Ji Z.W., Li X.X., Han K., Gao J., Qiu X.C., Fan Q.Y., Yang T.T., Ma B.A. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol. Rep. 2012;28(5):1764–1770. doi: 10.3892/or.2012.1995. [DOI] [PubMed] [Google Scholar]
- 26.Gong J., Zhang J.P., Li B., Zeng C., You K., Chen M.X., Yuan Y., Zhuang S.M. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2013;32(25):3071–3079. doi: 10.1038/onc.2012.318. [DOI] [PubMed] [Google Scholar]
- 27.Zhou M., Liu Z., Zhao Y., Ding Y., Liu H., Xi Y., Xiong W., Li G., Lu J., Fodstad O., Riker A.I., Tan M. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J. Biol. Chem. 2010;285(28):21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]