Abstract
Osteosarcoma (OS) is the most common primary malignant tumor of bone and the third most common cancer in childhood and adolescence. However, controversy concerning the ideal combination of chemotherapy agents ensued throughout the last quarter of the 20th century because of conflicting and often nonrandomized data. Collaborative efforts to increase understanding of the biology of osteosarcoma and the use of preclinical models to test novel protein targets will be critical to identify the path toward improving outcomes for patients. We attempted to identify potential protein markers or therapy targets of osteosarcoma and give a glance at tumorigenesis of osteosarcoma. A sensitive and accurate method was employed in comparative proteomic analysis between benign tumor and osteosarcoma. Tumor tissues obtained by open biopsy before induction chemotherapy were investigated With 2D DIGE and MALDI-TOF/TOF MS, 22 differentially expressed proteins were identified after database searching, including 8 up-regulated and 14 down-regulated proteins. We also validated the expression levels of interesting proteins(have higher Ratios(tumor/normal)) by Western blotting assay. Annotating by bioinformatic tools, we found structural and signal transduction associated proteins were in large percentage among altered level proteins. In particular, some low abundant proteins involving translation and transcription, such as EEF2(Elongation Factor 2), LUM Lumican 23 kDa Protein) and GTF2A2(Transcription Initiation Factor Iia Gamma Chain.), were firstly reported by our study comparing to previous observations. Our findings suggest that these differential proteins may be potential biomarkers for diagnosis or molecules for understanding of osteosarcoma tumorigenesis, coming with biologic, preclinical, and clinical trial efforts being described to improve outcomes for patients.
Keywords: Proteomics, Osteosarcoma, Benign Tumor, MALDI-TOF/TOF MS
1. Introduction
Osteosarcoma (OS) is the most common primary malignant bone sarcoma, which usually occurring in children and adolescents [1]. Actually, OS comprises approximately 1/5 of all bone tumors, which is the fifth most common type of cancer in young people [2], [3], [4]. At present, despite modern treatment protocols that combine chemotherapy and surgery, the optimal schedule of therapy is still being investigated because of high recurrence and drug-resistance [5].
Osteosarcoma is pathologically defined by production of osteoid [6], but it is broadly characterized by genetic complexity caused by chromosomal alternations [7]. Perhaps, the most attractive data potentially indicating pathogenesis of osteosarcoma is germ-line genetic alternations. It was reported that germ-line mutations in Rb gene is correlated to OS [8], [9]. In fact, a large number of animal models are developed for osteosarcoma, including P53 knock out mouse model [10], transgenic c-fos mouse model [11] and parathyroid hormone injection mouse model [12]. But it is still unclear which model could accurately recapitulate the human disease.
There is a real need to identify signaling molecules and mechanisms that promote OS progression and metastasis, particularly for the development of novel and more effective treatment strategies.
Since the advent of genomics technology, proteomics provides a powerful tool to discover new biomarkers for early diagnosis [13] . It has been reported that there are vast of genes and proteins expression changes, involved in biochemical pathways, occurred in tumorigenesis [14], [15]. So far, several proteomics researches of osteosarcoma have been established base on serum, cell lines and tissues treated by chemotherapy [16], [17], [18]. According to these studies, the expression of SAA, Fibrinogen, AHA1, SLP-2 and EZR was significantly changed. The most extensively studied prognostic marker is p-glycoprotein encoded by multi-drug resistance (MDR1) gene [19], but its efficacy remains controversial [20]. These proteins may be considered as potential molecular targets for therapy of osteosarcoma. However, there are few reports directly concerning primary bone sarcoma tissues with high-through and sensitive methods. Hence, it needs urgently to uncover robust and specific markers for understanding the biological behavior of osteosarcoma.
In this study, clinical tissues from osteosarcoma were analyzed by 2D DIGE and MALDI-TOF/TOF MS. With sensitive labeling method, some low expression proteins were identified, which play important roles in signal transduction pathways. Many different kinds of structural proteins were also detected firstly in our study comparing to previous research. More importantly, combination of our result and previous proteome data, discovery of specific bio-marker for clinical diagnosis is promising.
2. Materials and methods
2.1. Patients and tissues specimens
Primary tumor samples, including benign tumors and osteosarcomas, were obtained when patients underwent surgery for tumor resection. According to World Health Organization (WHO) histologic classification, 6 patients were diagnosed with an osteoblastic variant of osteosarcoma and two had a chondroblastic variant of osteosarcoma. The benign bone tumors collected included two osteoblastoma, two chondroblastoma, and one giant cell tumor of bone collected from patients ranging from 9 to 41 years old.
2.2. Reagents and apparatus
Cy2, Cy3, and Cy5 were purchased from GE Healthcare. dimethylformamide was purchased from Aldrich. DTT, urea, agarose, glycerol, bromphenol blue, CHAPS, mineral oil, acrylamide, Bis, Tris base, glycine, SDS, iodoacetamide, ammonium persulfate, TEMED, Immobiline DryStrip gels (24 cm, pH 3–10), and Bio-Lyte solutions (pH 3–10) were purchased from Bio-Rad. Thiourea was purchased from Fluka (Buchs, Switzerland). Protease inhibitor mixture was purchased from Roche Applied Science. ACN and methanol were purchased from Fisher. TFA was purchased from Merck. Trypsin (sequencing grade) was purchased from Promega (Madison, WI). All buffers were prepared with Milli-Q water (Millipore, Bedford, MA).
2.3. Protein extraction
The tumor tissues were crushed in a liquid nitrogen cooled mortar and pestle, and then extracted with 300 ml sample buffer containing 7 M urea, 2 M thiourea and 4% CHAPS,. The lysate was separated by centrifugation at top speed for 30 min at 4 °C. The supernatant was collected and stored at −80 °C.
2.4. 2D-DIGE and image analysis
Protein extraction concentration was determined by BCA method and pH was adjusted to 8.5 by 50 mM NaOH. After that, the concentration was made to 5 mg/ml by lysis buffer. Equal amounts of proteins from the 6 pairs of samples were pooled together as the internal standard. Benign and osteosarcoma tissue were randomly labeled with Cy3 or Cy5, whereas internal standards were labeled with Cy2 using 400 pmol of fluorochrome/50 g of protein. Labeling was performed for 30 min on ice in the dark.
Reactions were then stopped by the addition of 1 μl of lysine (10 mM) for 10 min on ice in the dark. Fifty-microgram Cy3- and Cy5-labeled samples from each group were combined before mixing with 50 μg of Cy2-labeled internal standard. Then an equal volume of 2 μl sample buffer (7 M urea, 2 M thiourea, 4% CHAPS, 1% Bio-Lyte, pH 3–10, 20 mg/ml DTT) was added to the sample, and the total volume was made up to 450 μl with rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 0.5% Bio-Lyte, 10 mg/ml DTT).
Samples were actively rehydrated into 24-cm pH 3–10 IPG strips (Bio-Rad) at 17 °C for 7 h using a Protean IEF cell (Bio-Rad). Isoelectric focusing was performed for a total of 80 kV-h, involving in desalting (250 V in 30 min), boosting voltage (held at 1000 V for 1 h, ramped to 10,000 V in 5 h) and holding (10,000 V for 70 kV-h). The IPG strips were equilibrated in equilibration buffer (6 M urea, 2% SDS, 50 mM Tris-Cl, pH 8.8, 30% glycerol) supplemented with 0.5% DTT for 15 min at room temperature followed by 4.5% iodoacetamide in equilibration buffer for another 15-min incubation at room temperature.
IPG strips were placed on the top of 12% homogeneous polyacrylamide gels that had been precast with low fluorescence glass plates using an Ettan DALT twelve gel caster. The second dimension SDS-PAGE was carried out using the Protean Plus system (Bio-Rad). After 2DE, gels were scanned on the Typhoon 9410 scanner with Ettan DALT gel alignment guides using excitation/emission wavelengths specific for Cy2 (488/520 nm), Cy3 (532/580 nm), and Cy5 (633/670 nm). The intensity was adjusted to ensure that the maximum volume of each image was within 60,000–90,000.
2.5. Data analysis
All experiments were carried out in triplicate; Analysis of 2D DIGE was done using DeCyder 5.02 software (GE Healthcare) according to the manufacturer’s suggestion. Briefly, the DeCyder biological variation analysis module was used to detect spots (the estimated number of spots was 2500) and simultaneously match all 49 protein spot maps from 3 gels. All matches were also confirmed by manual. Different density of protein spots among benign tumor and osteosarcoma were marked. Only spots that variation was more than 2.0 were selected for identification. Spot picking and in-gel Digestion was carried out with preparative gels. Two-dimensional electrophoresis was performed as described under “2D DIGE and Imaging” except that the IPG strips were loaded with 500–1000 μg of protein, and gels were stained with Coomassie Brilliant Blue. Protein spots of interest were excised and destained with 25 mM ammonium bicarbonate in 50% ACN. Gels were then dried completely by centrifugal lyophilization. In-gel digestion was performed with 0.01 μg/μl trypsin (Promega) in 25 mM ammonium bicarbonate for 15 h at 37 °C. The supernatants were collected, and the tryptic peptides were extracted from the gel sequentially with 5% TFA at 40 °C for 1 h and with 2.5% TFA, 50% ACN at 30 °C for 1 h. The extracts were pooled and dried completely by centrifugal lyophilization.
2.6. Protein identification by MALDI-TOF/TOF MS
Peptide mixtures were dissolved in 0.5% TFA, and 1 μl of peptide solution was mixed with 1 μl of matrix (4-hydroxy-α-cyanocinnamic acid in 30% ACN, 0.1% TFA) before spotting on the target plate. PMF and sequence analysis were carried out on a MALDI-TOF-TOF MS (4800 Proteomics Analyzer, Applied Biosystems). Peptide mass maps were acquired in positive reflection mode, averaging 1500 laser shots per MALDI-TOF spectrum and 3000 shots per TOF/TOF spectrum (the resolution was 20,000). The 4800 calibration mixtures (Applied Biosystems) were used to calibrate the spectrum to a mass tolerance within 0.1 Da. Parent mass peaks with a mass range of 600–4000 Da and minimum signal to noise ratio of 15 were picked out for tandem TOF/TOF analysis. Combined mass and mass/mass spectra were used to interrogate human sequences IPI human database using the MASCOT database search algorithms (version 1.9). In Searching parameter, modification was set as carbamidomethylation, oxidation, and a maximum of one missed trypsin cleavage was permitted. Tolerance of precursor and fragment ions was both 0.2 Da. All of the automatic data analysis and database searching were fulfilled by the GPS ExplorerTM software (version 3.6, Applied Biosystems). Contaminant proteins, such as keratin (from skin or hair), were excluded manually. The confident identification had a statistically significant (p≤0.05) protein score (based on combined mass and mass/mass spectra) and best ion score (based on mass/mass spectra). Redundancy of proteins that appeared in the database under different names and accession numbers was eliminated. If more than one protein was identified in one spot, the single protein member with the highest protein score (top rank) was singled out from the protein family. The molecular weight and pI values of most proteins were consistent with the gel regions from which the spots were excised.
2.7. Western blot assay
Proteins from the benign tumors and osteosarcoma were separated on 12% polyacrylamide gels and transferred to PVDF membranes (Amersham Biosciences). These blots were incubated for 2 h at room temperature in Tris-buffered-saline with Tween (20 mM Tris-Cl, 140 mM NaCl, pH 7.5, 0.05% Tween 20) containing 5% skim milk. Primary antibodies used were anti-glial fibrillary acidic protein monoclonal antibody (diluted 1:200, Promega), anti-pigment epithelium-derived factor (diluted 1:500 Sigma), anti-heat shock cognate 71 kDa protein (diluted 1:1000 Sigma) and anti-Lamin A/C (diluted 1:500 Santa Cruze). Blots were incubated with primary antibodies for 2 h at room temperature. After washing three times in Tris-buffered-saline with Tween, blots were incubated with horseradish peroxidase-conjugated secondary antibody (diluted 1:10,000, Santa Cruz Biotechnology) for 1 h at room temperature. Immunoreactive complexes were visualized using ECL reagents (Santa Cruz Biotechnology).
3. Results
3.1. Comparison of differentially expressed proteins between osteosarcoma and benign tumor
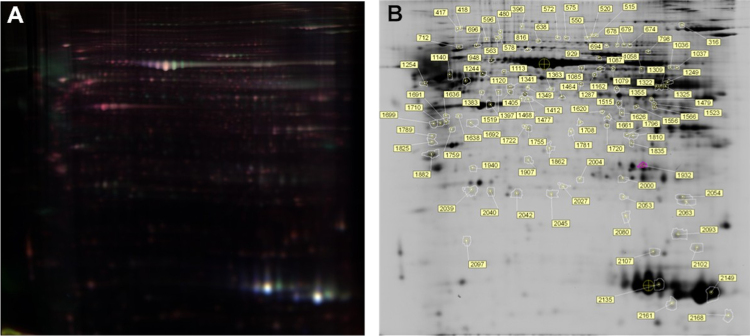
After labeling and electrophoresis, the Cy2, Cy3, and Cy5 channels of each gel were imaged separately, and the images were analyzed using DeCyder 5.02 software. Among 1600 matched protein spots, 11 were significantly up-regulated and 15 were down-regulated in comparison between benign tumors and osteosarcoma ratio of which was set to 2.0 (spots shown in Fig. 1).
Fig. 1.
the comparison of 2D-DIGE of normal and osteosarcomas tissue.
3.2. Analysis of differential proteome by mass spectrometry
In total, 25 differently expressed spots were excised from gels and analyzed for protein identification with the MALDI-TOF/TOF mass spectrometer. After searching database, 8 up-regulated proteins were identified, including fibrinogen beta chain (FGB), glial fibrillary acidic protein (GFAP), hemoglobin subunit alpha (HBA1), hemoglobin subunit beta (HBB), pigment epithelium derived factor (SERPINF1), ATP synthase subunit alpha (ATP5A1), LUM 23 kDa protein (LUM) and hemoglobin alpha-2 (HBA2)(Table 1). While, 14 down-regulated proteins were detected, namely heat shock cognate 71 kDa protein (HSPA8), lamin A/C (LMNA), moesin (MOES), vimentin (VIM), L-lactate dehydrogenase B (LDHB), tropomyosin 3 (TPM3), elongation factor 2 (EEF2), neutral alpha-glucosidase Ab (GANAB), transcription initiation factor IIA (GTF2A2), 60 kDa heat shock protein (HSPD1), Tu translation elongation factor (TUFM), type II keratin (KRT1), enolase 1 (ENO1) and actin, cytoplasmic 1 (ACTB) (Table 1).
Table 1.
Differential proteins identified by 2D-DIGE.
| Up-regulated proteins | |||
|---|---|---|---|
| IPIAccession | Gene_name | Ratio(tumor/normal) | Definition |
| IPI00440493 | ATP5A1 | 1.52 | ATP synthase subunit alpha, Mitochondrial precursor. |
| IPI00298497 | FGB | 2.17 | Fibrinogen beta chain precursor. |
| IPI00025363 | GFAP | 2.24 | Isoform 1 of glial fibrillary acidic protein, Astrocyte . |
| IPI00410714 | HBA1 | 2.63 | Hemoglobin subunit alpha. |
| IPI00853068 | HBA2 | 1.61 | Hemoglobin Alpha-2. |
| IPI00654755 | HBB | 3.09 | Hemoglobin subunit beta. |
| IPI00794403 | LUM | 1.53 | LUM 23 kDa protein. |
| IPI00006114 | SERPINF1 | 3.58 | Pigment epithelium-derived factor precursor. |
| Down-regulated proteins | |||
| IPIAccession | Gene_name | Ratio(tumor/normal) | Definition |
| IPI00894365 | ACTB | −1.53 | cDNA FLJ52842, highly similar to actin, Cytoplasmic 1. |
| IPI00186290 | EEF2 | −1.88 | Elongation factor 2. |
| IPI00465248 | ENO1 | −1.57 | Enolase 1. |
| IPI00011454 | GANAB | −1.75 | Isoform 2 Of neutral alpha-glucosidase Ab precursor. |
| IPI00004353 | GTF2A2 | −1.7 | Transcription initiation factor lia gamma chain. |
| IPI00003865 | HSPA8 | −2.37 | Isoform 1 of heat shock cognate 71 kDa protein. |
| IPI00784154 | HSP60 | −1.68 | 60 kDa heat shock protein, Mitochondrial precursor. |
| IPI00220327 | KRT1 | −1.57 | Keratin, Type II cytoskeletal 1. |
| IPI00789173 | LDHB | −2.07 | LDHB_HUMAN L-lactate dehydrogenase B chain |
| IPI00514204 | LMNA | −2.3 | Lamin A/C |
| IPI00872814 | MOES | −2.13 | Moesin |
| IPI00479185 | TPM3 | −1.95 | Tropomyosin 3 Isoform 4 |
| IPI00027107 | TUFM | −1.58 | Tu translation elongation factor, Mitochondrial |
| IPI00418471 | VIM | −2.12 | Vimentin |
As for up-regulated proteins, some spots appeared to be detected as the same protein. For example, spots 542 and 631 were SERPINF1, while spots 1054 and 1154 corresponded to ATP5A1 (Fig. 1).
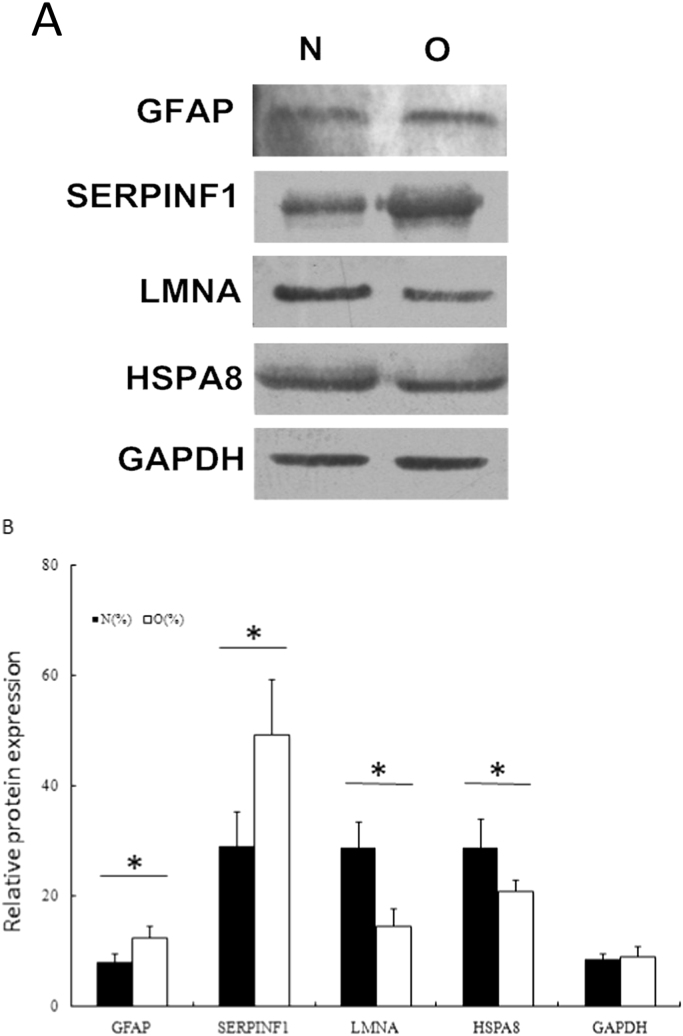
3.3. Western blotting assay
Two up-regulated proteins of interest, GFAP and SERPINF1, as well as two down-regulated proteins, LMNA and HSPA8 were selected for further validation by Western blotting assay. According to Fig. 2, the changes of these proteins between benign tumors and osteosarcoma were consistent with proteomic data.
Fig. 2.
Validation of differentially expressed proteins by Western blotting assay.
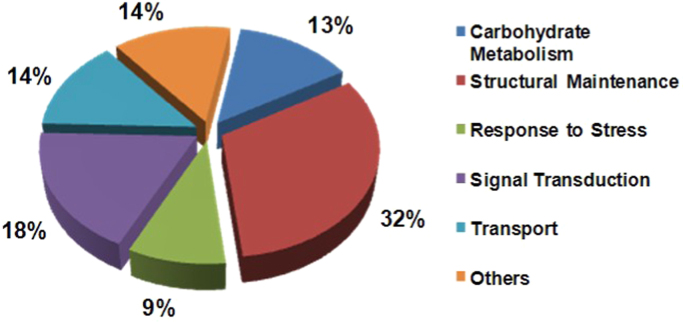
3.4. Function analysis of differentially expressed proteins
To investigate the function of differentially expressed proteins, Gene Ontology was employed for annotating the results. Consequently, some functional factors were classified, including carbohydrate metabolism, structural maintenance, stress response, signal transduction, transport and so on (Fig. 3). Among these categories, more than 1/3 proteins were related to cellular structure and proteins involving in signal transduction were also in large percentage.
Fig. 3.
Function distribution of differential protein between normal and tumor tissue.
4. Discussion
One of the major challenges in treatment of osteosarcoma is lack of validated prognostic markers. By standard clinical treatment, the recurrent rate of patients received surgical operation and chemotherapy is near about 1/3 [21]. In post genome, proteomics serves as powerful tools in screening marker and targets, especially employed in cancer research. In this study, we identified 14 up-regulated proteins and 8 down-regulated proteins in osteosarcoma, including metabolic proteins, cytoskeletal proteins, and signal transmitting proteins.
4.1. Structural proteins in osteosarcoma
In organisms, structural proteins are widely distributed and play a role in celluar maintenance. We indentified structural proteins, including GFAP, KRT1, LMNA, TPM3, VIM and MOES. GFAP is a member of intermediary filament protein family, which is an important component of astrocytes and a known diagnostic marker of glial differentiation. By Q-PCR and immunohistochemistry, GFAP was found to be highly expressed in chondrosarcomas and chondroblastic osteosarcomas [22]. It was implicated a potential marker for tumors with cartilaginous differentiation. As for moesin, it is a member of the ERM (ezrin, radixin, moesin) protein family and connects major cytoskeletal structures to the plasma membrane. It has been proved that ERM is activated by phosphorylation in OS [23] and ezrin, another member of ERM, is believed to involve in matastatsis in osteosarcoma [24]. It is suggested that ezrin and, potentially, other members of the ERM (ezrin-radixin-moesin) family have key roles in the coordination of signals and cellular complexes that are required for the successful metastasis of these and other malignancies [24]. Vimentin is class-III intermediate filament, and its expression is elevated when oxidative phosphorylation is reduced in osteosarcoma [25].
4.2. Proteins involving in signal transduction
According to GO annotation, 4 genes were related to signal transduction, EEF2, FGB, LUM and TUFM. Fibrinogen is a pleiotropic blood protein that regulates coagulation, inflammation and tissue repair. In microglia, fibrinogen mediates activation of Akt and Rho via the CD11b/CD18 integrin receptor, while in neurons fibrinogen induces phosphorylation of epidermal growth factor (EGF) receptor via the alphavbeta3 integrin [26]. Lumican is a member of small eucine-rich proteoglycan gene family and is known to play a role in the assembly and regulation of collagen fibres [27]. In human osteosarcoma cell lines, it was demonstrated that lumican had a novel out-in signaling circuit that inhibited endogenous TGF-β2 activity, resulting in downstream effector modulation including pSmad 2, integrin β1 and pFAK to regulate osteosarcoma adhesion [28]. Another study of lumican showed its potential correlation with the differentiation and progression of osteosarcoma [29]. EEF2 is a component of the mRNA surveillance SURF complex and catalyzes the GTP-dependent ribosomal translocation step during translation elongation. It was reported that EEF1A1, an isoform of EEF, was over expressed in osteosarcoma and showed chemo-sensitization toward MTX by treatment with siRNA [30]. In our result, EEF2 had an opposite expression regulation pattern to EEF1A1. As for TUFM, there is no report of its relationship with osteosarcoma.
4.3. Heat shock proteins down-regulated in osteosarcomas
Heat shock proteins (HSPs) are a kind of molecular chaperones and protect cells against injuries form stress. HSPA8 and HSP60 were identified in our experiment, and both of these two proteins were down regulated. By previous study on clinical samples, HSP27, HSP60, and HSP70 were determined and found HSP27 and HSP70 may be potential markers to distinguish conventional and low grade central osteosarcoma [31], [32]. HSPA8 may function as an endogenous inhibitory regulator of HSC70 by competing to the co-chaperones. The effect of HSPA8 was firstly reported in ostosarcoma by our research.
4.4. Comparison with other published proteome of osteosarcoma
At present, there are several proteomic studies of osteosarcoma, including serum, tissues and cell lines [18], [19], [20]. To give a survey of biological behavior of OS, we incorporated these datasets and compared it to our result at gene level (Table 1). From the table, the overlap among identifications was small, which suggested proteins were differentially expressed in tissues/fluids. Comparing to published data, several low abundant proteins related to signal transduction and transcription, such as EEF2, LUM and GTF2A2, were uncovered for the first time. These proteins may help to understand the regulation of osteosarcoma.
5. Conclusion
In this study, the proteins differentially expressed in osteosarcoma were analyzed by high sensitive labeling method and mass spectrometry. Although some proteins were accordant with previous reports, but many proteins had been identified and validated in osteosarcoma at first. In particular, the identification of structural and signal transduction associated proteins implied that these proteins play an important role in the process of tumorigenesis and metastasis in osteosarcoma. The identified uniquely expressed proteins comparing to literature may be valuable to elucidate the regulation mechanism of osteosarcoma, as well as provide potential markers or targets for diagnosis and therapy. Of course, the exact effect of these molecules still unclear, so additional work is required to give an answer to the role of these proteins in osteosarcoma genesis.
Contributor Information
Qi gao, Email: youyoyo@126.com.
Xiaoyu Yang, Email: wangguoxiang198354@126.com.
References
- 1.Tang N., Song W.X., Luo J., Haydon R.C., He T.C. Osteosarcoma development and stem cell differentiation. Clin. Orthop. Relat. Res. 2008;466(9):2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L.L. Biology of osteogenic sarcoma. Cancer J. 2005;11(4):294–305. doi: 10.1097/00130404-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Gorlick R., Anderson P., Andrulis I., Arndt C., Beardsley G.P., Bernstein M., Bridge J., Cheung N.K., Dome J.S., Ebb D., Gardner T., Gebhardt M., Grier H., Hansen M., Healey J., Helman L., Hock J., Houghton J., Houghton P., Huvos A., Khanna C., Kieran M., Kleinerman E., Ladanyi M., Lau C., Malkin D., Marina N., Meltzer P., Meyers P., Schofield D., Schwartz C., Smith M.A., Toretsky J., Tsokos M., Wexler L., Wigginton J., Withrow S., Schoenfeldt M., Anderson B. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin. Cancer Res. 2003;9(15):5442–5453. [PubMed] [Google Scholar]
- 4.Helman L.J., Meltzer P. Mechanisms of sarcoma development. Nat. Rev. Cancer. 2003;3(9):685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson W.S., Goorin A.M. Current treatment of osteosarcoma. Cancer Invest. 2001;19(3):292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- 6.Dorfman H.D., Czerniak B. Bone Cancers Cancer. 1995;75(1 Suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Ladanyi M., Gorlick R. The molecular pathology and pharmacology of osteosarcoma. Pediatr. Pathol. Mol. Med. 2000;19:391–413. [Google Scholar]
- 8.Wong F.L., Boice J.D., Abramson D.H. Cancer incidence after retinoblastoma: radiation dose and sarcoma risk. J. Am. Med. Assoc. 1997;278:1262–1267. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 9.Draper G.J., Sanders B.M., Kingston J.E. Second primary neoplasms in patients with retinoblastoma. Br. J. Cancer. 1986;53:661–671. doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacks T., Remington L., Williams B.O. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 11.Grigoriadis A.E., Schellander K., Wang Z.W., Wagner E.R. Osteoblasts are target cells for transformation in c-fos transgenic mice. J. Cell Biol. 1993;122:685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vahle J.L., Sato M., Long G.G. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol. Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 13.Byrum S., Montgomery C.O., Nicholas R.W., Suva L.J. The promise of bone cancer proteomics. Ann. N.Y. Acad. Sci. 2010;1192:222–229. doi: 10.1111/j.1749-6632.2009.05220.x. [DOI] [PubMed] [Google Scholar]
- 14.Nomura D.K., Dix M.M., Cravatt B.F. Activity-based protein profiling for biochemical pathway discovery in cancer. Nat. Rev. Cancer. 2010;10(9):630–638. doi: 10.1038/nrc2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolch W., Pitt A. Functional proteomics to dissect tyrosine kinase signalling pathways in cancer. Nat. Rev. Cancer. 2010;10(9):618–629. doi: 10.1038/nrc2900. [DOI] [PubMed] [Google Scholar]
- 16.Jin S., Shen J.N., Guo Q.C., Zhou J.G., Wang J., Huang G., Zou C.Y., Yin J.Q., Liu S.J., Liu W., Li M.T., Wang L.N. 2-D DIGE and MALDI-TOF-MS analysis of the serum proteome in human osteosarcoma. Proteom. Clin. Appl. 2007;1(3):272–285. doi: 10.1002/prca.200600869. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Zeng B., Ma J., Wan C. Comparative proteomic analysis of osteosarcoma cell and human primary cultured osteoblastic cell. Cancer Invest. 2009;27(3):345–352. doi: 10.1080/07357900802438577. [DOI] [PubMed] [Google Scholar]
- 18.Guo Q.C., Shen J.N., Jin S., Wang J., Huang G., Zhang L.J., Huang G., Yin J.Q., Zou C.Y., Li M.T. Comparative proteomic analysis of human osteosarcoma and SV40-immortalized normal osteoblastic cell lines. Acta Pharmacol. Sin. 2007;28(6):850–858. doi: 10.1111/j.1745-7254.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 19.Baldini N., Scotlandi K., Barbanti-Brodano G. Expression of p-glycoprotein in highgrade osteosarcomas in relation to clinical outcome. N Engl. J. Med. 1995;333:380–1385. doi: 10.1056/NEJM199511233332103. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz C.L., Gorlick R., Teot L. Multiple drug resistance in osteogenic sarcoma (INT0133) J. Clin. Oncol. 2007;25:2057–2062. doi: 10.1200/JCO.2006.07.7776. [DOI] [PubMed] [Google Scholar]
- 21.Kempf-Bielack B., Bielack S.S., Jürgens H., Branscheid D., Berdel W.E., Exner G.U., Göbel U., Helmke K., Jundt G., Kabisch H., Kevric M., Klingebiel T., Kotz R., Maas R., Schwarz R., Semik M., Treuner J., Zoubek A., Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J. Clin. Oncol. 2005;23(3):559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari S., Briccoli A., Mercuri M., Bertoni F., Picci P., Tienghi A., Del Prever A.B., Fagioli F., Comandone A., Bacci G. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J. Clin. Oncol. 2003;21(4):710–715. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 23.Santos G.C., Carvalho K.C., Falzoni R., Simoes A.C., Rocha R.M., Lopes A., Vassallo J., Reis L.F., Soares F.A., da Cunha I.W. Glial fibrillary acidic protein in tumor types with cartilaginous differentiation. Mod. Pathol. 2009;22(10):1321–1327. doi: 10.1038/modpathol.2009.99. [DOI] [PubMed] [Google Scholar]
- 24.Pignochino Y., Grignani G., Cavalloni G., Motta M., Tapparo M., Bruno S., Bottos A., Gammaitoni L., Migliardi G., Camussi G., Alberghini M., Torchio B., Ferrari S., Bussolino F., Fagioli F., Picci P., Aglietta M. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol. Cancer. 2009;10 doi: 10.1186/1476-4598-8-118. 8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter K.W. Ezrin, a key component in tumor metastasis. Trends Mol. Med. 2004;10(5):201–204. doi: 10.1016/j.molmed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Annunen-Rasila J., Ohlmeier S., Tuokko H., Veijola J., Majamaa K. Proteome and cytoskeleton responses in osteosarcoma cells with reduced OXPHOS activity. Proteomics. 2007;7(13):2189–2200. doi: 10.1002/pmic.200601031. [DOI] [PubMed] [Google Scholar]
- 27.Ryu J.K., Davalos D., Akassoglou K. Fibrinogen signal transduction in the nervous system. J. Thromb. Haemost. 2009;7(Suppl 1):151–154. doi: 10.1111/j.1538-7836.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiga N., Tojyo I., Matsumoto T., Hiraishi Y., Shinohara Y., Fujita S. Expression of lumican in the articular disc of the human temporomandibular joint. Eur. J. Histochem. 2010;54(3):e34. doi: 10.4081/ejh.2010.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikitovic D., Chalkiadaki G., Berdiaki A., Aggelidakis J., Katonis P., Karamanos N.K., Tzanakakis G.N. Lumican regulates osteosarcoma cell adhesion by modulating TGFβ2 activity. Int. J. Biochem. Cell Biol. 2011 doi: 10.1016/j.biocel.2011.03.008. Mar 21. [DOI] [PubMed] [Google Scholar]
- 30.Nikitovic D., Berdiaki A., Zafiropoulos A., Katonis P., Tsatsakis A., Karamanos N.K., Tzanakakis G.N. Lumican expression is positively correlated with the differentiation and negatively with the growth of human osteosarcoma cells. FEBS J. 2008;275(2):350–361. doi: 10.1111/j.1742-4658.2007.06205.x. Epub 2007 Dec 17. [DOI] [PubMed] [Google Scholar]
- 31.Selga E., Oleaga C., Ramírez S., de Almagro M.C., Noé V., Ciudad C.J. Networking of differentially expressed genes in human cancer cells resistant to methotrexate. Genome Med. 2009;1(9):83. doi: 10.1186/gm83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon A., Bacchini P., Bertoni F., Olvi L.G., Santini-Araujo E., Kim Y.W., Park Y.K. Expression of heat shock proteins in osteosarcomas. Pathology. 2010;42(5):421–425. doi: 10.3109/00313025.2010.493866. [DOI] [PubMed] [Google Scholar]