Abstract
Social stress, including bullying during adolescence, is a risk factor for common psychopathologies such as depression. To investigate the neural mechanisms associated with juvenile social stress-induced mood-related endophenotypes, we examined the behavioral, morphological, and biochemical effects of the social defeat stress model of depression on hippocampal dendritic spines within the CA1 stratum radiatum. Adolescent (postnatal day 35) male C57BL/6 mice were subjected to defeat episodes for 10 consecutive days. Twenty-four h later, separate groups of mice were tested on the social interaction and tail suspension tests.
Hippocampi were then dissected and Western blots were conducted to quantify protein levels for various markers important for synaptic plasticity including protein kinase M zeta (PKMζ), protein kinase C zeta (PKCζ), the dopamine-1 (D1) receptor, tyrosine hydroxylase (TH), and the dopamine transporter (DAT). Furthermore, we examined the presence of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-receptor subunit GluA2 as well as colocalization with the post-synaptic density 95 (PSD95) protein, within different spine subtypes (filopodia, stubby, long-thin, mushroom) using an immunohistochemistry and Golgi-Cox staining technique. The results revealed that social defeat induced a depression-like behavioral profile, as inferred from decreased social interaction levels, increased immobility on the tail suspension test, and decreases in body weight. Whole hippocampal immunoblots revealed decreases in GluA2, with a concomitant increase in DAT and TH levels in the stressed group. Spine morphology analyses further showed that defeated mice displayed a significant decrease in stubby spines, and an increase in long-thin spines within the CA1 stratum radiatum. Further evaluation of GluA2/PSD95 containing-spines demonstrated a decrease of these markers within long-thin and mushroom spine types. Together, these results indicate that juvenile social stress induces GluA2- and dopamine-associated dysregulation in the hippocampus – a neurobiological mechanism potentially underlying the development of mood-related syndromes as a consequence of adolescent bullying.
Keywords: Bullying, CA1, Depression, Dopamine, GluA2, Juvenile, Tail suspension test
1. Introduction
Adolescent bullying has become a major risk factor for several psychiatric illnesses (Nansel et al., 2001), including major depressive disorder (Ttofi, 2015). To gain insight into the neural mechanisms associated with the negative impact of adolescent bullying and the expression of depression-related symptomatology, we used the social defeat stress model of depression (Gottfredson et al., 2015, Kudryavtseva et al., 1991). We selected this preclinical behavioral approach because it can mimic some of the negative emotional and physical aspects of bullying (Bjorkqvist, 2001), resulting in depression-related behavior (Krishnan et al., 2007, Yu et al., 2011). In this paradigm, experimental mice are exposed to social and physical conflict by aggressive members of the same species (see section 2.2 for details), resulting in both physical and emotional stress (Krishnan et al., 2008, Warren et al., 2013). Importantly, social defeat utilizes a naturalistic stressor, which provides strong face and pharmacological validity, in contrast to other experimental approaches that use more artificial forms of stress. For example, social defeat induces both neuroendocrine and behavioral modifications (Keeney et al., 2001), as well as neurobiological alterations across several brain regions that are particularly vulnerable to stress, including the hippocampus (Tse et al., 2014). More specifically, it is reported that the CA1 subregion of the hippocampus displays morphological changes as a consequence of stress exposure (Castaneda et al., 2015, Sebastian et al., 2013a); however, this relationship has yet to be examined during the juvenile stage of development, as a function of social stress specifically. Thus, in order to characterize the effects of social stress on hippocampal spine morphology during adolescence, we examined the expression of synaptic markers within CA1 spines.
To do this, we focused on four distinct spine types (filopodia, stubby, long-thin, and mushroom), which are differentially characterized on the basis of their head and neck ratio (Rochefort and Konnerth, 2012, Spiga et al., 2011) and vary in their synaptic capability. For instance, while filopodia and stubby have smaller spine heads than long-thin and mushroom, they respond more quickly to changes in synaptic activity (Bourne and Harris, 2007, Rochefort and Konnerth, 2012), whereas mushroom spines are more efficient for synaptic transmission. Thus, incorporating a more discrete analysis of specific spine subtypes allows for better characterization of spine morphology, which may otherwise be overlooked when examining total spine changes. Given their role in the development of these spine types, we determined the expression of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-receptor subunit GluA2, the dopamine transporter (DAT), protein kinase C zeta (PKCζ), and protein kinase M zeta (PKMζ).
PKMζ, a specific autonomously active form of the atypical isozyme PKCζ (Hernandez et al., 2003, Zhou et al., 1994), has been shown to function in concert with GluA2 during synaptic plasticity (Ling et al., 2002, Yao et al., 2008). As the trafficking of the GluA2 receptor subunit increases in the synapse during plasticity, clusters of PKMζ/GluA2/PSD95 proteins develop (Shao et al., 2012), preventing AMPA receptors from undergoing endocytosis. Stabilization of AMPA receptors within the synaptic membrane is important for increasing mushroom spine heads (Sebastian et al., 2013a), which in turn facilitates synaptic plasticity. Additionally, the GluA2 subunit is the rate-limiting factor for calcium influx after activation (Isaac et al., 2007), and thus plays an important role in modulating synaptic activity as well (Schmidt et al., 2010). GluA2 is highly expressed in the hippocampus, and is expressed in the form of two heterodimers, GluA1/GluA2 and GluA2/GluA3 (Wenthold et al., 1996), which are important for learning (Joels and Lamprecht, 2010) and long-term memory processes (Braren et al., 2014, Henley and Wilkinson, 2013, Migues et al., 2010, Sebastian et al., 2013b). Of increasing interest is that the GluA2 subunit has been shown to play a functional role in stress-induced depression (Bai et al., 2003, Bleakman et al., 2007), as traditional antidepressants (i.e., imipramine and fluoxetine) increase phosphorylation of AMPA receptors (Du et al., 2007, Svenningsson et al., 2002). This suggests that AMPA receptor stabilization may underlie the therapeutic efficacy of antidepressants that involve monoaminergic- and glutamatergic-related signaling (Berton and Nestler, 2006, Manji et al., 2001, Skolnick et al., 2009).
Interestingly, the dopamine system is subject to change following social defeat stress. Studies have identified social defeat stress-induced decreases in dopamine in the medial prefrontal cortex (Watt et al., 2009), as well as reduced DAT levels within the striatum (Isovich et al., 2001). Additionally, dopamine receptor distribution is altered after social defeat, as evidenced by increased D1 receptor binding in the caudate putamen and prefrontal cortex (Avgustinovich and Alekseyenko, 2010), enhanced mesocorticolimbic dopamine response (Cabib et al., 2000, Tidey and Miczek, 1996), and increased dopamine neuronal activity in the ventral tegmental area (VTA; Razzoli et al., 2011). However, no studies have investigated the expression of hippocampal dopamine receptors across different spine types, as a function of social defeat exposure during adolescence – the developmental stage when the first incidence of major depression is most often reported (Paus et al., 2008). Thus, we investigated the expression of the D1 receptor, TH (a marker for dopamine), and DAT within this brain region, as a function of juvenile social defeat stress exposure.
Our results show that social defeat induces a depression-like phenotype in adolescent male C57BL/6 mice – a behavioral response that correlates with increases in hippocampal cytosolic dopamine markers (DAT and TH), and decreases in synaptic GluA2 levels. Also, social stress induced changes in spine morphology within the CA1 stratum radiatum (i.e., decreases in stubby along with increases in long-thin spine subtypes). Further analyses within hippocampal CA1 spines indicated that defeat stress reduced the colocalization of GluA2 and PSD95 within long-thin and mushroom spines. Together, these data identify a potential neurobiological mechanism involving hippocampal dopamine- and AMPA receptor-associated deregulation in the expression of mood-related syndromes as a consequence of bullying during the adolescent stage of development.
2. Materials and methods
2.1. Animals
A total of 55 male, postnatal day (PD) 35, C57BL/6 mice were obtained from the Department of Psychology mouse breeding colony at California State University San Bernardino (CSUSB). Since the social defeat model of depression (i.e., resident/intruder paradigm) involves conflict stress (i.e., physical threat) from a more dominant resident counterpart (Golden et al., 2011, Kudryavtseva et al., 1991), we purchased CD1 male retired breeders from Charles River Laboratories to be used as aggressors for this investigation (Parmigiani et al., 1999). Prior to social defeat stress exposure, CD1 aggressors were single housed, and C57BL/6 mice were housed with littermates in groups of 3–4, in standard polypropylene cages containing wood shavings. Mice were maintained in a colony room with a 12 h light/dark cycle (lights on at 7:00 h), and with access to food and water ad libitum. This study was carried out in accordance with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals developed by the Public Health Service Policy on Humane Care and Use of Laboratory Animals, as well as the Institutional Animal Care and Use Committee (IACUC) at CSUSB.
2.2. Social defeat stress and experimental design
The adolescent social defeat stress paradigm was performed as previously described (Iñiguez et al., 2014b). To do this, CD1 retired breeders with reliable attack latencies (≤30 s on three consecutive screening tests) were housed in cages containing perforated Plexiglas separators, which divide the cage into two separate compartments (for specific details on all aspects of the social defeat paradigm see Golden et al., 2011). For each stress session (10 min per day), defeated mice were placed into the same compartment as the CD1 aggressor. Following each 10 min session, the defeated mice were housed for 24 h in the compartment adjacent to their respective CD1 aggressor. This procedure ensured that defeated mice were exposed to a novel CD1 aggressor each day, for 10 consecutive days (PD35-44). In the event that the CD1 aggressor exhibited repeated forceful attacks (i.e., continuous biting even after the experimental mouse displayed submissive posturing), the defeat bout was immediately terminated (Golden et al., 2011, Iñiguez et al., 2014a). Conversely, if the aggressor did not display a consistent attack towards the experimental C57BL/6 mouse, the aggressor was removed and replaced by a novel CD1 mouse. Non-stressed (control) mice were handled daily and housed in similar cages, one on each side of the perforated Plexiglas separator. Immediately after the last stress episode (i.e., PD44), all C57BL/6 mice were single housed. Twenty-four h later (PD45; see Fig. 1(a)), separate groups of experimental mice were tested in either the social interaction or the tail suspension test. This approach was taken to avoid possible testing carry-over effects (see Table 1 for experimental groups). Behavioral testing was conducted between 10:00 and 14:00 h. Animals were euthanized (live decapitation) 40 min after behavioral assessment or transcardially perfused (see below for details on brain tissue collection).
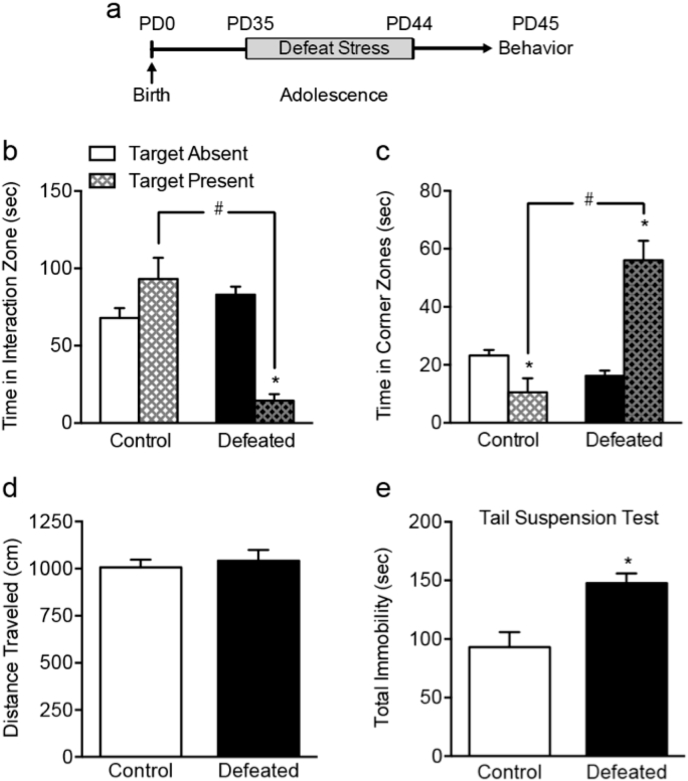
Fig. 1.
Social defeat stress induces a depression-like behavioral response in adolescent C57BL/6 male mice. (a) Timeline of the experimental procedures. Adolescent (postnatal day [PD]) 35 mice were exposed to 10 days of social defeat stress (i.e., PD35-44). Twenty-four h later (PD45), mice were tested on either the social interaction or tail suspension test. (b) Defeated mice spent less time in the interaction zone in the presence, versus the absence, of a social target (∗p < 0.05, within group comparison), which was significantly less than that of control mice during the target present condition (#p < 0.05, between group comparison). (c) This reduction of social behavior was evident when assessing time in the corner zones, in which defeated mice spent significantly more time in the corners regardless of whether the social target was present (#p < 0.05, between group comparison) or absent (∗p < 0.05, within group comparison). (d) No differences in total distance traveled between control and defeated mice were observed during the first 2.5 min of the social interaction test (target absent condition). (e) Defeated mice spent more time (sec) immobile in the tail suspension test, when compared to control mice (∗p < 0.05). Data are presented as mean + SEM.
Table 1.
Experimental groups.
| Group | Subjects | Defeat age | Interval | Procedure | Data |
|---|---|---|---|---|---|
| 1 | Control n = 10 | PD35-44 | 24 h | Social interaction | Fig. 1(b)–(d) |
| Defeat n = 10 | |||||
| 2 | Control n = 6 | PD35-44 | 24 h | Tail suspension test | Fig. 1(e) |
| Defeat n = 9 | |||||
| 3 | Control n = 4 | PD35-44 | 24 h | Social interaction/western blot | Fig. 3 |
| Defeat n = 7 | |||||
| 4 | Control n = 4 | PD35-44 | 24 h | Social interaction/immunohistochemistry | Fig. 4, Fig. 5 |
| Defeat n = 5 |
PD, postnatal day.
2.3. Social interaction test
The social interaction test is used to assess social behavior (Berton et al., 2006). This is a two-step procedure conducted under red light conditions (Krishnan et al., 2008). In the first 2.5 min session, the experimental C57BL/6 mouse is allowed to freely explore an open field arena (40 cm length × 40 cm width × 40 cm height). Along one side of the arena is a circular (9 cm diameter) wire cage (Stoelting Co., Wood Dale, IL) that remains empty during the first trial (target absent condition). The C57BL/6 mouse is then removed from the testing arena for 30 s (into a separate holding cage), and a novel CD1 male mouse is placed into the wire cage. In the second 2.5 min trial (target present condition), the experimental C57BL/6 mouse is reintroduced into this arena now containing a social target (unfamiliar CD1 mouse) within the circular wire cage. Time (sec) spent in the interaction zone (8 cm wide corridor surrounding the circular wire cage) in the presence of the social target, as well as the time (sec) spent in the corners (10 × 10 cm) of the testing arena (Iñiguez et al., 2014b), served as dependent variables. Additionally, we recorded the distance traveled (cm) during the first 2.5 min of the social interaction test to examine whether basal locomotor activity was influenced by social stress exposure (Table 1, group 1). Behavioral outcomes were scored via an automated video tracking system (Noldus, Asheville, NC).
2.4. Tail suspension test
The tail suspension test is a behavioral procedure in which rodents are placed in an inescapable stressful condition, where mice are hung by their tail for 6 min (Steru et al., 1985). Initially, mice engage in escape-directed behaviors but eventually adopt a posture of immobility – however, antidepressant treatment can significantly increase their escape-directed behaviors, an effect that has been correlated with pharmacological antidepressant efficacy in humans (Cryan et al., 2005). Conversely, an animal that spends more time immobile is considered to be more sensitive to the effects of inescapable stress (Iñiguez et al., 2010). The total time (sec) spent immobile during the last 5 min of the test was the dependent variable. Observers that were blind to the experimental conditions scored behavioral outcomes (Table 1, group 2).
2.5. Tissue fractions
Forty min after the social interaction test (Table 1, group 3), bilateral hippocampi were microdissected on dry ice, and stored at −80 °C until processed. The tissue was prepared into two fractions, cytosolic and synaptic (Braren et al., 2014). Hippocampi were thawed from frozen and homogenized in 200 μl buffer containing TEE (Tris 50 mM; EDTA 1 mM; EGTA 1 mM), SigmaFast protease inhibitor cocktail (Sigma Aldrich) diluted to contain AEBSF (2 mM), Phosphoramidon (1 μM), Bestatin (130 μM), E-64 (14 μM), Leupeptin (1 μM), Aprotinin (0.2 μM), and Pepstatin A (10 μM). Homogenates were centrifuged at 3000 g (5 min at 4 °C), to remove unhomogenized tissue. The resulting supernatant was centrifuged at 100,000 g for 30 min. After ultracentrifugation, the supernatant was collected and stored as the cytosolic fraction. The remaining pellet was resuspended in 100 μl of homogenizing TEE buffer containing 0.001% Triton X-100, incubated on ice for 1 h and then centrifuged at 100,000 g for 1 h at 4 °C. The resulting pellet was resuspended in 50 μl of TEE buffer and stored as the synaptic fraction (Braren et al., 2014). The Pierce bicinchoninic acid assay (BCA; Thermo Scientific, Rockford, IL) was used to determine protein concentration for each sample. Samples were reduced with 4× Laemmli sample buffer equivalent to 25% of the total volume of the sample and then boiled and stored frozen at −80 °C.
2.6. Western blotting
Whole hippocampal samples (20 μg) were loaded onto a Tris/Gly 4–20% mini gel to resolve glyceraldehyde-3-phophate dehydrogenase (GAPDH, 37 kDa), PKMζ (55 kDa), PKCζ (70 kDa), GluA2 (102 kDa), TH (58 kDa) DAT (50 kDa) and D1 (48 kDa). Every gel contained 3–4 lanes loaded with the same control sample, all brain sample (ABS). ABS was used to standardize protein signals between gels. Gels were transferred to nitrocellulose membranes in the IBlot® Dry Blotting System (Life Technologies; Carlsbad, CA) for 9 min. Nitrocellulose membranes were then incubated in blocking solution containing 5% sucrose in Tris Buffered Saline with Tween-20 (TBST; 0.1% Tween-20 in TBS) for 30 min at room temperature (Iñiguez et al., 2012). Samples were incubated with the following primary antibodies overnight: GluA2 (monoclonal; anti-mouse 1:2000; Chemicon, Temecula, CA), D1 (polyclonal; anti-rabbit 1:1000, AbCam, Cambridge, MA), DAT (polyclonal; anti-rabbit 1:1000, Santa Cruz Biotechnology; Santa Cruz, CA), TH (polyclonal; anti-rabbit 1:2000; EMD Millipore, Billerica, MA), PKMζ/PKCζ (polyclonal; anti-rabbit 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), and GAPDH: (1:2000, Chemicon, Temecula, CA). Membranes were washed in TBST for 20 min and probed with horseradish peroxidase conjugated secondary antibody. Membranes were incubated with enhanced chemiluminescence substrate and exposed on CL-XPosure film (Thermo Scientific; Rockford, IL). Films were scanned for quantification with NIH Image J.
2.7. Golgi-immunohistochemistry (Golgi-IHC)
Golgi-IHC experiments were performed as previously reported (Pinto et al., 2012, Sebastian et al., 2013a, Spiga et al., 2011). Specifically, 40 min after the social interaction test (i.e., PD45), animals were perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde and post-fixed overnight in 4% paraformaldehyde (Table 1, group 4). The following day, brains were washed in 0.4 M Sorensonon's phosphate buffer prior to being incubated in Golgi-Cox solution for 2 days. The Golgi-Cox solution consisted of 5% potassium chromate, 5% potassium dichromate, and 5% mercuric chloride. Following 2 days of incubation, the brains were transferred to a fresh Golgi-Cox Solution for an additional 14 days. Brains were transferred to a 30% sucrose solution for 2 days for cryoprotection. Brains were then snap frozen and cut serially into 100 μm coronal sections. In order to develop the Golgi stain, three brain sections per animal containing the septal hippocampus (∼1.8–2 mm posterior to Bregma) were washed in deionized water for 1 min, placed in 50% NH4OH for 30 min (Pinto et al., 2012, Sebastian et al., 2013a), and placed in fixer solution (Kodak; Rochester, NY) for an additional 30 min. For the immunohistochemical staining, sections were washed in PBS for 10 min three times and placed in a blocker solution containing 5% normal goat serum, 5% bovine serum albumin, and 0.5% Triton X-100 in PBS. The following day sections were incubated in primary antibodies selective for GluA2 (monoclonal; mouse) and PSD95 (polyclonal; rabbit) (1:1000 in PBS, EMD Millipore; Billercia, MA) for 48 h at 4 °C. Following incubation in primary antibodies, sections were incubated in secondary antibodies (1:1000 in PBS) for 2 h at room temperature. Sections were then washed in PBS three times for 10 min and mounted onto slides and cover slipped with ProLong Gold antifade reagent (Life Technologies; Grand Island, NY). Fluorescent-labeled antibodies were matched to laser excitation wavelengths (anti-rabbit 488 nm, anti-mouse 594 nm), and to visualize Golgi-filled dendrites, a 514 nm laser reflected the branches. Images were taken with a Leica SP2 confocal microscope in a 1024 × 1024 format at 12 bits to achieve 0.146 voxels per μm, and each scan line was averaged twice. Confocal images of dendritic branches emanating from the secondary dendrite in the apical tree were selected from pyramidal cells located in stratum radiatum of CA1. One to three neurons per section were imaged. This amounted to 30 branches per experimental condition (Pinto et al., 2012, Sebastian et al., 2013a). Z-stacks (step size of 0.122 μm) were acquired using preset laser and gain settings.
2.8. IMARIS spine analysis
Following imaging, IMARIS 7.5 filament tracer was used to reconstruct each dendrite in 3D. Using customized algorithms, spines were classified as either filopodia, stubby, long-thin, or mushroom (Sebastian et al., 2013a). In order to quantify the presence of GluA2 and PSD95 positive voxels within the spine, channels were made corresponding to each protein of interest. The number of voxels for GluA2, PSD95, and colocalized voxels for GluA2/PSD95 from the dendritic spine alone were subtracted from the total amount of voxels colocalized within the branch.
2.9. Statistical analyses
Behavioral data was analyzed using ANOVA techniques, with stress (control vs. defeat; between variable), presence of social target (absent vs. present; repeated measure), and day of defeat (10 days; repeated measure) as sources of variance, followed by Tukey post hoc tests. Student's t-tests were used for analyses implicating two-group comparisons (tail suspension test, Western blots, and spine-type density analyses). Golgi-IHC data were analyzed using ANOVA techniques, with spine type (filopodia, stubby, long-thin, mushroom; between variable) and stress (control vs. defeated; between variable) as sources of variance, followed by planned post hoc comparisons (independent t-test). Spine density was normalized by branch length, and spine IHC data were normalized by number of spines on each branch (data was averaged across animals so that each animal was only represented once per dependent measure). Data are presented as mean + SEM. Statistical significance was defined as p < 0.05.
3. Results
3.1. Social interaction
The effects of social defeat stress on adolescent social behavior are shown in Fig. 1(b)–(d). A two-way ANOVA, with stress (control vs. defeat) and presence of social target (absent vs. present) as independent variables, indicated that the time (sec) spent in the interaction zone was influenced by stress exposure (main effect: F1,36 = 14.42, p < 0.05), the presence of the social target (main effect: F1,36 = 6.93, p < 0.05), as well as their interaction (F1,36 = 32.44, p < 0.05). Specifically, Fig. 1(b) displays how defeated mice (n = 10) spent less time in the interaction zone in the presence of the target (target absent vs. present, p < 0.05), or when compared to non-stressed controls (between group comparison, p < 0.05). Fig. 1(c) demonstrates how this stress-induced avoidance-like phenotype is also evident when assessing the time spent in the corner zones (stress × target interaction: F1,36 = 36.29, p < 0.01). Not surprisingly, non-defeated controls (n = 10) spent significantly less time in the corner zones in the presence, versus the absence, of the social target (within group comparison, p < 0.05). Conversely, defeated mice spent greater time in the corners in the presence of the target (within group comparison, p < 0.05), as well as when compared to non-stressed controls (between group comparison, p < 0.05). When assessing distance traveled (cm) during the first 2.5 min interaction trial (i.e., target absent condition), no differences were evident as a function of defeat stress (t18 = 0.51, p > 0.05), thus, indicating that adolescent social stress exposure did not influence general locomotor activity or exploratory behavior (Fig. 1(d)).
3.2. Tail suspension test
Fig. 1(e) shows how social defeat stress increases sensitivity to behavioral despair measures, as inferred by the tail suspension test, in adolescent male C57BL/6 mice. Here, when compared to non-stressed controls (n = 6), defeated mice (n = 9) spent significantly more time (sec) in the immobile position (t13 = 3.75, p < 0.01).
3.3. Body weight
Fig. 2 shows the effects of adolescent social defeat stress on body weight in male C57BL/6 mice. Body weight was recorded prior to the initiation of each defeat episode (PD35-44), as well as before behavioral testing (PD45). A mixed-design repeated measures ANOVA indicated that body weight (g) changed as a function of stress exposure (between group main effect: (F1,53 = 7157.0, p < 0.0001)), day of defeat episode (repeated measure main effect: F10,530 = 156.5, p < 0.0001), and a stress by day of defeat interaction (F10,530 = 20.4, p < 0.0001). Post hoc analyses further revealed that when compared to control mice (n = 24), defeated mice (n = 31) displayed lower body weight as of the fourth day (i.e., PD38) of stress exposure (p < 0.05, respectively). Twenty-four h after the last defeat episode (i.e., PD45), adolescent mice exposed to defeat stress weighted significantly less than control mice (t53 = 4.1, p < 0.001).
Fig. 2.
Effects of social defeat stress on body weight in adolescent male C57BL/6 mice. Social defeat (postnatal day 35–44; gray area) reduced overall body weight across days of stress, starting on day 4 of stress exposure (postnatal day 38), when compared to non-stressed controls (n = 24). Body weight remained significantly lower in the defeated group (n = 31) 24 h after the last day of stress exposure (postnatal day 45). Arrow indicates day of behavioral testing and brain tissue collection. *Significantly different when compared to controls (p < 0.05). Data are presented in grams (mean ± SEM).
3.4. Western immunoblot analysis
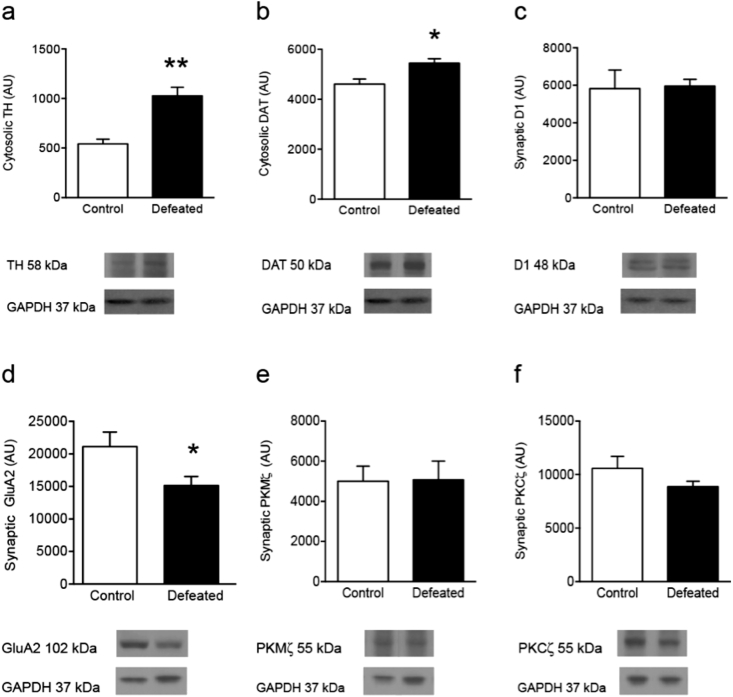
Fig. 3 shows the protein expression differences between social defeat (n = 7) and control (n = 4) conditions for TH, DAT, D1, GluA2, PKMζ, and PKCζ, in adolescent C57BL/6 mice. Social defeat stress increased cytosolic TH (t9 = 3.49, p < 0.01) and DAT (t9 = 2.67, p < 0.05) in the adolescent hippocampus of male C57BL/6 mice, when compared to controls (Fig. 3(a) and (b)). No differences in synaptic D1 levels were observed between the groups (p > 0.05, Fig. 3(c)). The protein expression for the AMPA receptor subunit GluA2 (Fig. 3(d)) was significantly decreased as a function of social defeat stress (t9 = 2.39, p < 0.05). Lastly, there were no differences in synaptic PKMζ or PKCζ levels (p > 0.05, respectively) as a function of social defeat stress (Fig. 3(e) and (f)).
Fig. 3.
Effects of social defeat stress on hippocampal dopamine- and GluA2-related function in adolescent male C57BL/6 mice. (a) Social defeat stress increased cytosolic tyrosine hydroxylase (TH), when compared to controls (∗∗p < 0.01). (b) Similarly, social defeat stress increased cytosolic dopamine transporter (DAT) levels, when compared to controls (∗p < 0.05). (c) No differences in synaptic dopamine-1 receptors (D1) were observed between the groups (p > 0.05). (d) Conversely, social defeat stress reduced synaptic GluA2 when compared to controls (∗p < 0.05). (e–f) No differences in synaptic PKMζ or PKCζ were evident following social defeat stress (p > 0.05). Arbitrary units (AU). Data are presented as ratio of total protein normalized to GAPDH (mean + SEM).
3.5. Spine morphology analysis
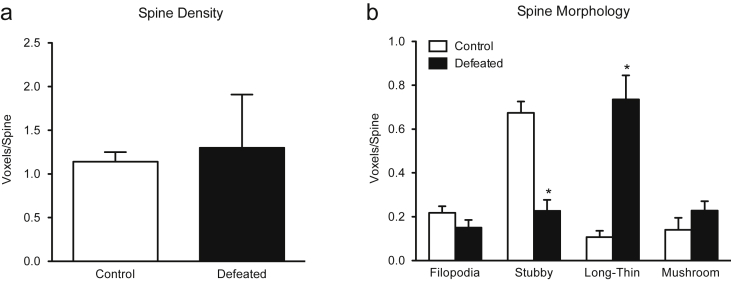
Fig. 4 shows the effects of social defeat stress on spine density and morphology (filopodia, stubby, long-thin, and mushroom) within the CA1 region of the adolescent hippocampus, in male C57BL/6 mice. Fig. 4(a) shows that there was no difference in overall spine density (voxels/spine) as a function of stress exposure between the groups (p > 0.05). Conversely, when examining spine density across spine morphology (Fig. 4(b)), a significant decrease in stubby spines (t28 = 2.90, p < 0.001) with a concomitant increase in long-thin spines (t28 = 5.72, p < 0.001) was observed. Lastly, social stress did not influence the total number of filopodia (p > 0.05), or mushroom (p > 0.05) spine types between the groups.
Fig. 4.
Effects of social defeat stress on spine density and morphology within the CA1 of the adolescent hippocampus in male C57BL/6 mice. (a) Social defeat did not alter overall spine density as a function of stress (p > 0.05). (b) Conversely, when assessing spine morphology, social defeat significantly decreased stubby spines (p < 0.01), and increased long-thin spines (p < 0.01), while having no effect on filopodia (p > 0.05) or mushroom spines (p > 0.05). Data are presented as voxels per micron (mean + SEM).
3.6. Spine immunohistochemistry analysis
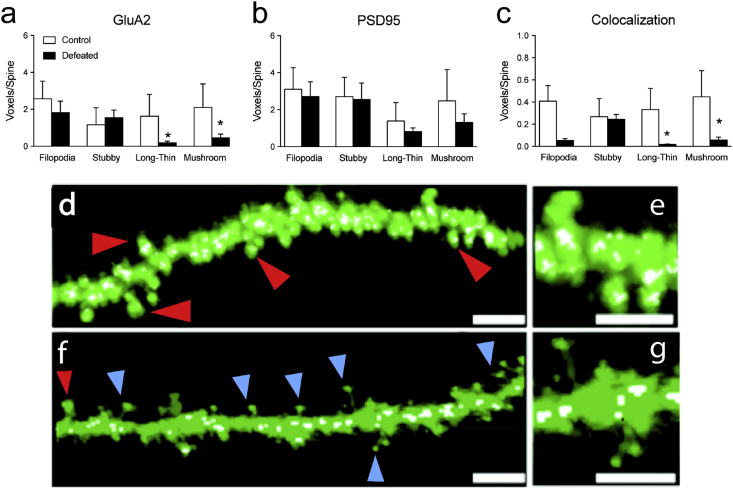
Fig. 5 shows the effects of social defeat stress on the expression of GluA2, PSD95, and their colocalization, as a function of spine-type within the adolescent CA1 region of the hippocampus. A 2-way ANOVA with stress (control vs. defeat) and spine-type (filopodia, stubby, long-thin, mushroom) as sources of variance indicated that the number of spines expressing GluA2 (main effect: F1,28 = 6.66, p < 0.01), as well as the colocalization of GluA2 and PSD95 (main effect: F1,28 = 17.43, p < 0.001) varied as a function of stress exposure (control, n = 4; defeated, n = 5). Planned comparisons further indicated that the defeated mice displayed decreases of GluA2 (Fig. 5(a)) as well as the colocalization of GluA2 and PSD-95 (Fig. 5(c)) within long-thin and mushroom spine subtypes, when compared to non-stressed control mice (p < 0.05, respectively).
Fig. 5.
Effects of social stress on GluA2, PSD95, and their colocalization across spine types in the CA1 region of the hippocampus in adolescent C57BL/6 male mice. Social defeat stress decreased (a) GluA2 expression within long-thin and mushroom spines (*p < 0.05). (b) No changes in PSD95 were observed across spine types (p > 0.05) as a function of stress exposure. Conversely, (c) the number of spines expressing the colocalization of GluA2 and PSD95 was reduced in long-thin and mushroom spines (*p < 0.05). Representative images of a dendritic branch from a control (d–e) and socially defeated animal (f–g). Scale bar = 5 μm for d and f; 2.5 μm for e and g. Red arrows indicate stubby spines. Blue arrows indicate long-thin spines. White voxels represent GluA2/PSD95 colocalization. Data are represented as mean voxels per spine (mean + SEM). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our data show that social defeat stress induces a depression-like behavioral phenotype in adolescent male C57BL/6 mice, as inferred from decreased social behavior in the social interaction test (Fig. 1(b) and (c)), increased time spent immobile in the tail suspension test (Fig. 1(e)), and decreases in body weight (Fig. 2). As such, this behavioral profile indicates that social stress (i.e., bullying), during adolescence, mimics some of the core symptoms of depression (social avoidance, despair, and weight fluctuation). Consequently, by including the tail suspension test as a despair measure, our experimental approach provides additional face validity for the social defeat model (Chaudhury et al., 2015) in juvenile mice (Iñiguez et al., 2014b).
Preclinical, clinical, and postmortem studies suggest that various hippocampal-signaling molecules implicated in the remodeling of neuronal processes (Duric et al., 2013), including glutamate and dopamine receptors, play a critical role in the etiology of depression (Hashimoto, 2011, Leggio et al., 2013). Therefore, we selected to examine how juvenile social defeat stress influences receptors associated with dopamine and glutamate signaling (D1 and GluA2) and the expression of distinct spine types within the hippocampus. This approach was taken given that the hippocampus is a brain region that is undergoing volumetric changes during adolescence (Andersen and Teicher, 2008, Meyer et al., 1978), the stage of development when the first incidence of depression is most often reported (Paus et al., 2008).
4.1. Adolescent social defeat decreases stubby spines and increases long-thin spines in CA1
Our results show that social defeat stress alters the morphology (Fig. 4(b)), but not the density of dendritic spines (Fig. 4(a)), 24 h post last defeat, in adolescent male C57BL/6 mice. This is distinct from other reports, using adult rodents, where they have identified significant decreases in overall spine densities within the hippocampus after social defeat stress (Jiang et al., 2015). Specifically, our data demonstrate that CA1 dendrites of adolescent defeated mice exhibit a decrease in spines lacking a neck (stubby) and an increase in spine with a long neck (long-thin). It is possible that these spine-type specific changes (Fig. 4(b)), as a function of social defeat stress, are age-dependent, given that the adolescent hippocampus is undergoing substantial overproduction and pruning of synapses during this time (Andersen and Teicher, 2008). Indeed, it has been demonstrated that other forms of stress can dysregulate the signaling molecules that influence the developmental pattern of dendritic spines in the hippocampus (Bath et al., 2013). An increase in long-thin spines with a concomitant decrease in stubby spines identifies a shift in spine stability as a consequence of social defeat stress. Large spines form stronger, longer lasting synapses, while small spines are generally transient, forming weaker synapses (Kasai et al., 2003, Sebastian et al., 2013a). We hypothesize that this dynamic switch between stubby and long-thin spines may underlie the expression of the depression-related behavior observed in the current study. This interpretation is consistent with our behavioral data showing increased avoidance and despair-like responses after juvenile exposure to stress (Fig. 1(b)–(e)) – a behavioral phenotype that is regulated in a bidirectional manner by stress and antidepressants in a hippocampus-circuit-dependent manner (Bagot et al., 2015, Duman and Aghajanian, 2012, Snyder et al., 2011).
Due to the lack of a restrictive neck on stubby spines, a loss of these spine types should negatively influence the excitability of its parent dendrite. Two-photon microscopy studies on CA1 pyramidal neurons have shown that the length of the spine neck restricts the amount of calcium influx into the dendritic shaft (Noguchi et al., 2005, Takasaki and Sabatini, 2014), thereby reducing the spread of calcium within the dendrite following stimulation, which is necessary for action potential generation. The idea that social defeat is altering the excitability of the hippocampus by altering spine morphology is further supported by studies showing that the length of the spine neck is negatively correlated with membrane potentials in the dendritic shaft, as well excitatory postsynaptic potentials generated within the spine head (Araya et al., 2006). The potential decrease in hippocampal excitability after social defeat stress may be an underlying mechanism for the endophenotypes associated with adolescent depression. Interestingly, this process (i.e., decrease of stubby spines along with increases in long-thin spines) may be a neurobiological factor of the juvenile social defeat model (i.e., social avoidance, despair, and weight fluctuation). Thus, this paradigm may be useful when examining additional features of depression that are hippocampal-dependent, such as memory impairment that may result from stress-induced hypoexcitability. Indeed, recent reports demonstrate that adult patients suffering from depression who perform poorly on various memory-related tests display decreased blood oxygen-level dependent (BOLD) activity within this brain region (Milne et al., 2012). However, whether these observations would extend to the adolescent human population remains to be investigated. To date, only long-term effects on memory performance have been examined at the preclinical level with the use of the juvenile social defeat model (Novick et al., 2013), and thus, caution should be practiced when extending our results to the clinical population.
4.2. Adolescent social defeat stress increases corticosterone: a mechanism for spine remodeling
It is plausible that there is a role for corticosterone in the observed juvenile stress-induced spine remodeling. Results from our previous work show that adolescent social defeat stress increases blood serum corticosterone (Iñiguez et al., 2014b), which we hypothesize may play a role in spine remodeling. It has been demonstrated that corticotropin-releasing hormone (CRH) receptor 1 (CRHR1) is located on dendritic spines of pyramidal neurons (Chen et al., 2004, Van Pett et al., 2000). Acute psychological stress induces release of hippocampal CRH activating the CRHR1 (Chen et al., 2006, Refojo et al., 2005), and leads to a rapid reduction in dendritic spines (Chen et al., 2008). Not surprisingly, memory deficits induced by social defeat stress are reversed in a CRHR1-dependent manner (Wang et al., 2011). As such, these findings point to the possibility that a sustained elevation of endogenous CRH during social defeat stress may play a role in spine remodeling, and thus, underlie the increases in sensitivity to behavioral despair measures (tail suspension test), as well as the decreases in social behavior observed in adolescent mice. However, whether this proposed CRH-mediated increase in sensitivity to mood-related behaviors, after social defeat stress, is specific to the juvenile stage of development requires future detailed investigation – given that the deleterious effects of stress on hippocampal dependent behaviors are not always similar across age (Barha et al., 2011, Eiland and Romeo, 2013, McCormick and Green, 2013).
4.3. Tyrosine hydroxylase (TH) and dopamine transporter (DAT) increase after social defeat exposure during adolescence
Our results demonstrate that the levels of TH and DAT were both significantly elevated after social defeat indicating elevated dopamine activity within the adolescent hippocampus following stress. This is likely the case, as it has been shown that increased levels of VTA-dopamine are mediated through the activation of the corticotropin releasing factor receptor-2 in the prefrontal cortex (Holly et al., 2015), which in turn, could activate projections to CA1 (Goldman-Rakic et al., 1984). Dopamine has been shown to decrease low frequency signals while enhancing high frequency signals in CA1 specifically (Ito and Schuman, 2007). This may suggest that dopamine modulates the interaction between cortical activity following stress exposure, and hippocampal frequency-dependent synaptic plasticity. Indeed, a recent study has shown that decreases in dopamine D1 receptors within the prefrontal cortex are associated with increased social avoidance after social defeat stress (Huang et al., 2016). Not surprisingly, because the hippocampus is important for learning and memory processes, this study also showed that social stress led to impaired recognition memory, in a somewhat similar fashion as in D1 knockout mice (El-Ghundi et al., 1999). Thus, the cortical-related dopamine frequency signals to the hippocampus may be important for the expression of cognitive deficits that are associated with stress-induced illnesses like depression (Pittenger and Duman, 2008). It is possible that the increases in TH and DAT (independent of D1 receptor expression; Fig. 3(a)–(c)) observed within the hippocampus in the current study may be mediated by altered cortical frequency signals induced by social stress, thus leading to increased depressive-like outcomes.
4.4. GluA2 and its colocalization with PSD95 is decreased in long-thin and mushroom spines after adolescent social defeat stress
A decrease in GluA2 expression within long-thin and mushroom dendritic spines after social defeat stress is consistent with reports that identify altered MAPk signaling after chronic social defeat (Iio et al., 2011, Iñiguez et al., 2010). MAPk is an upstream marker of GluA2 trafficking, suggesting that the downstream expression involving the synaptic trafficking of GluA2 would also be decreased. Given that the GluA2 subunit is responsible for mediating the majority of excitatory neural transmission, the decrease in this subunit may reduce the efficacy of synaptic transmission within various spine types. This finding is corroborated by the western blot findings identifying an overall decrease in GluA2 levels within a synaptic fraction (Fig. 3(d)). Other studies support these data, showing decreases in AMPA receptor number and function in CA1 after chronic unpredictable stress (Kallarackal et al., 2013). Low levels of AMPA receptors containing GluA1 have also been associated with increased vulnerability to depression-like behavior (Schmidt et al., 2010). Reductions in GluA2 levels, specifically, have been shown to also decrease spine densities in a synaptic scaffolding molecule (S-SCAM)-dependent manner (Danielson et al., 2012). Here, we extend these findings by showing alterations across specific spine types (long-thin and mushroom) in adolescent male mice (Fig. 5(a)).
Elevated corticosterone induced by adolescent social defeat stress (Iñiguez et al., 2014b) may be a mechanism by which we observe reductions in GluA2 containing spines. Although corticosterone does not affect the level of AMPA receptor subunit mRNA (Liu et al., 2006), it has been shown to decrease synaptic AMPA receptor trafficking and mobility (Martin et al., 2009). However, the reduced levels of GluA2 did not influence the synaptic levels of either PKMζ or PKCζ, suggesting that the social defeat effects are restricted to receptor trafficking.
Future studies will be needed to determine whether these trafficking mechanisms are affected by the ability of the subunit to interact with the cytoskeletal architecture of the spine. Indeed, previous reports examining post-mortem tissue (dorsolateral prefrontal cortex) from patients with depression have shown a dysregulation in the phosphorylation of spectrin, clathrin, and synapsin (Martins-de-Souza et al., 2012) – all which are involved in the expression of transmembrane protein, cell morphology, and synaptic transmission. Clathrin in particular has been shown to be involved in NMDA receptor dependent internalization of GluA2 (Anggono and Huganir, 2012), which may underlie the observed decrease in GluA2 levels.
It remains to be determined whether the levels of cytosolic PKMζ and/or PKCζ are altered, which could be a contributing factor in reduced synaptic levels of GluA2 (Yao et al., 2008). Thus, lower levels of GluA2 containing spines suggest that it may also disrupt dopamine function. Dopamine bursting activity is independent of baseline firing rates and, as such, can produce transient periods of high frequency activity that require glutamatergic input (Grace and Bunney, 1984). The number of dopamine neurons active at a given time is largely regulated by the hippocampus (Floresco et al., 2001, Lodge and Grace, 2011), indicating that GluA2 and dopamine activity are intimately linked during hippocampal function potentially modulating depressive-like behavior (Bagot et al., 2015).
4.5. Concluding summary
Our results highlight a role for dopamine and AMPA receptors, within the hippocampus, in the mediation of juvenile social defeat-induced depression-like behavior. The observed hippocampal reduction in GluA2 expression within long-thin and mushroom spines, along with a concomitant increase in TH and DAT represents potential dysfunction associated with mood-related illnesses, such as depression (Korte et al., 2015, Lodge and Grace, 2011). Antidepressants have been found to rescue AMPA dysfunction in chronically stressed animals (Kallarackal et al., 2013) and further increase AMPA phosphorylation and surface expression (Martinez-Turrillas et al., 2002, Svenningsson et al., 2002). Specifically, fluoxetine, a selective reuptake inhibitor, increases AMPA-induced currents in pyramidal cells via activation of D1 receptors in the prefrontal cortex (Bjorkholm et al., 2015) and reverses behavioral signs of depression by increasing them (Kobayashi et al., 2012). Future studies will be needed to delineate whether the alterations observed in hippocampal spine morphology may be restored within the CA1 via dopamine and glutamate-dependent antidepressant mechanisms. Collectively, these data provide novel insight into the potential neurobiological factors that underlie the expression of stress-induced depression symptomology in the juvenile population, as a result of social stressors like bullying.
Author contributions
SDI and PAS designed the research and wrote the manuscript. LMR, JBA, FJF-R, MAH, and SJN conducted the behavioral experiments. AA and DM performed the immunohistochemistry and confocal imaging. RMZ conducted the western blots. SDI, AA, RMZ, and PAS conducted the statistical analyses. All authors edited the manuscript prior to submission.
Conflict of interest
The authors report no financial interests or potential conflicts of interest.
Acknowledgments
This project was supported by the RCMI grant number RR003037 from the National Center for Research Resources (NCRR, to Hunter College), NIH 5R24DA012136-13 (to PAS), PSC CUNY grant number 68872-0046 (to PAS), and a grant from the National Institute of General Medical Sciences (NIH-SC2GM109811, to SDI).
References
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Anggono V., Huganir R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22(3):461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R., Jiang J., Eisenthal K.B., Yuste R. The spine neck filters membrane potentials. Proc. Natl. Acad. Sci. U. S. A. 2006;103(47):17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgustinovich D.F., Alekseyenko O.V. [3H]SCH 23390 binding in various brain regions of C57BL/6J mice with repeated experience of victory or social defeat in agonistic interactions. Physiol. Res. 2010;59(3):455–458. doi: 10.33549/physiolres.931779. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., Parise E.M., Pena C.J., Zhang H.X., Maze I., Chaudhury D., Persaud B., Cachope R., Bolanos-Guzman C.A., Cheer J.F. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F., Bergeron M., Nelson D.L. Chronic AMPA receptor potentiator (LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology. 2003;44(8):1013–1021. doi: 10.1016/s0028-3908(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Barha C.K., Brummelte S., Lieblich S.E., Galea L.A. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21(11):1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- Bath K.G., Schilit A., Lee F.S. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Berton O., McClung C.A., Dileone R.J., Krishnan V., Renthal W., Russo S.J., Graham D., Tsankova N.M., Bolaños C.A., Rios M. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Berton O., Nestler E.J. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7(2):137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bjorkholm C., Jardemark K., Schilstrom B., Svensson T.H. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur. Neuropsychopharmacol. 2015;25(10):1842–1847. doi: 10.1016/j.euroneuro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol. Behav. 2001;73(3):435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Bleakman D., Alt A., Witkin J.M. AMPA receptors in the therapeutic management of depression. CNS Neurol. Disord. Drug Targets. 2007;6(2):117–126. doi: 10.2174/187152707780363258. [DOI] [PubMed] [Google Scholar]
- Bourne J., Harris K.M. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17(3):381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Braren S.H., Drapala D., Tulloch I.K., Serrano P.A. Methamphetamine-induced short-term increase and long-term decrease in spatial working memory affects protein Kinase M zeta (PKMzeta), dopamine, and glutamate receptors. Front. Behav. Neurosci. 2014;8:438. doi: 10.3389/fnbeh.2014.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S., D'Amato F.R., Puglisi-Allegra S., Maestripieri D. Behavioral and mesocorticolimbic dopamine responses to non aggressive social interactions depend on previous social experiences and on the opponent's sex. Behav. Brain Res. 2000;112(1–2):13–22. doi: 10.1016/s0166-4328(00)00157-1. [DOI] [PubMed] [Google Scholar]
- Castaneda P., Munoz M., Garcia-Rojo G., Ulloa J.L., Bravo J.A., Marquez R., Garcia-Perez M.A., Arancibia D., Araneda K., Rojas P.S. Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J. Neurosci. Res. 2015;93(10):1476–1491. doi: 10.1002/jnr.23602. [DOI] [PubMed] [Google Scholar]
- Chaudhury D., Liu H., Han M.H. Neuronal correlates of depression. Cell Mol. Life Sci. 2015;72(24):4825–4848. doi: 10.1007/s00018-015-2044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Brunson K.L., Adelmann G., Bender R.A., Frotscher M., Baram T.Z. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126(3):533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Dube C.M., Rice C.J., Baram T.Z. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. 2008;28(11):2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Fenoglio K.A., Dube C.M., Grigoriadis D.E., Baram T.Z. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol. Psychiatry. 2006;11(11):992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav Rev. 2005;29(4–5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Danielson E., Zhang N., Metallo J., Kaleka K., Shin S.M., Gerges N., Lee S.H. S-SCAM/MAGI-2 is an essential synaptic scaffolding molecule for the GluA2-containing maintenance pool of AMPA receptors. J. Neurosci. 2012;32(20):6967–6980. doi: 10.1523/JNEUROSCI.0025-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Suzuki K., Wei Y., Wang Y., Blumenthal R., Chen Z., Falke C., Zarate C.A., Jr., Manji H.K. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32(4):793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V., Banasr M., Stockmeier C.A., Simen A.A., Newton S.S., Overholser J.C., Jurjus G.J., Dieter L., Duman R.S. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 2013;16(1):69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L., Romeo R.D. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M., Fletcher P.J., Drago J., Sibley D.R., O'Dowd B.F., George S.R. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur. J. Pharmacol. 1999;383(2):95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Floresco S.B., Todd C.L., Grace A.A. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J. Neurosci. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Selemon L.D., Schwartz M.L. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gottfredson N.C., Foshee V.A., Ennett S.T., Haberstick B., Smolen A. Genetic heterogeneity in adolescents' depressive symptoms in response to victimization. J. Clin. Child. Adolesc. Psychol. 2015;44(5):762–774. doi: 10.1080/15374416.2014.910787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A.A., Bunney B.S. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. The role of glutamate on the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(7):1558–1568. doi: 10.1016/j.pnpbp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Henley J.M., Wilkinson K.A. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin. Neurosci. 2013;15(1):11–27. doi: 10.31887/DCNS.2013.15.1/jhenley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A.I., Blace N., Crary J.F., Serrano P.A., Leitges M., Libien J.M., Weinstein G., Tcherapanov A., Sacktor T.C. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J. Biol. Chem. 2003;278(41):40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Holly E.N., DeBold J.F., Miczek K.A. Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacol. Berl. 2015;232(24):4469–4479. doi: 10.1007/s00213-015-4082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.B., Zhao T., Gao X.L., Zhang H.X., Xu Y.M., Li H., Lv L.X. Effect of chronic social defeat stress on behaviors and dopamine receptor in adult mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;66:73–79. doi: 10.1016/j.pnpbp.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Iio W., Matsukawa N., Tsukahara T., Kohari D., Toyoda A. Effects of chronic social defeat stress on MAP kinase cascade. Neurosci. Lett. 2011;504(3):281–284. doi: 10.1016/j.neulet.2011.09.047. [DOI] [PubMed] [Google Scholar]
- Iñiguez S.D., Alcantara L.F., Warren B.L., Riggs L.M., Parise E.M., Vialou V., Wright K.N., Dayrit G., Nieto S.J., Wilkinson M.B. Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J. Neurosci. 2014;34(3):1007–1021. doi: 10.1523/JNEUROSCI.5725-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez S.D., Charntikov S., Baella S.A., Herbert M.S., Bolaños-Guzmán C.A., Crawford C.A. Post-training cocaine exposure facilitates spatial memory consolidation in c57bl/6 mice. Hippocampus. 2012;22(4):802–813. doi: 10.1002/hipo.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez S.D., Riggs L.M., Nieto S.J., Dayrit G., Zamora N.N., Shawhan K.L., Cruz B., Warren B.L. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17(3):247–255. doi: 10.3109/10253890.2014.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez S.D., Vialou V., Warren B.L., Cao J.L., Alcantara L.F., Davis L.C., Manojlovic Z., Neve R.L., Russo S.J., Han M.H. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J. Neurosci. 2010;30(22):7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J.T., Ashby M.C., McBain C.J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54(6):859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Isovich E., Engelmann M., Landgraf R., Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. Eur. J. Neurosci. 2001;13(6):1254–1256. doi: 10.1046/j.0953-816x.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- Ito H.T., Schuman E.M. Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front. Neural Circuits. 2007;1:1. doi: 10.3389/neuro.04.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Wang F., Yang S., Fang P., Deng Z.F., Xiao J.L., Hu Z.L., Chen J.G. SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF-TrkB pathway. Int. J. Neuropsychopharmacol. 2015;18(6) doi: 10.1093/ijnp/pyu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels G., Lamprecht R. Interaction between N-ethylmaleimide-sensitive factor and GluR2 is essential for fear memory formation in lateral amygdala. J. Neurosci. 2010;30(47):15981–15986. doi: 10.1523/JNEUROSCI.1872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallarackal A.J., Kvarta M.D., Cammarata E., Jaberi L., Cai X., Bailey A.M., Thompson S.M. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J. Neurosci. 2013;33(40):15669–15674. doi: 10.1523/JNEUROSCI.2588-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Matsuzaki M., Noguchi J., Yasumatsu N., Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Keeney A.J., Hogg S., Marsden C.A. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol. Behav. 2001;74(1–2):177–184. doi: 10.1016/s0031-9384(01)00541-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Haneda E., Higuchi M., Suhara T., Suzuki H. Chronic fluoxetine selectively upregulates dopamine D(1)-like receptors in the hippocampus. Neuropsychopharmacology. 2012;37(6):1500–1508. doi: 10.1038/npp.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte S.M., Prins J., Krajnc A.M., Hendriksen H., Oosting R.S., Westphal K.G., Korte-Bouws G.A., Olivier B. The many different faces of major depression: it is time for personalized medicine. Eur. J. Pharmacol. 2015;753:88–104. doi: 10.1016/j.ejphar.2014.11.045. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Han M.H., Graham D.L., Berton O., Renthal W., Russo S.J., Laplant Q., Graham A., Lutter M., Lagace D.C. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Han M.H., Mazei-Robison M., Iñiguez S.D., Ables J.L., Vialou V., Berton O., Ghose S., Covington H.E., 3rd, Wiley M.D. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol. Psychiatry. 2008;64(8):691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva N.N., Bakshtanovskaya I.V., Koryakina L.A. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 1991;38(2):315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Leggio G.M., Salomone S., Bucolo C., Platania C., Micale V., Caraci F., Drago F. Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. Eur. J. Pharmacol. 2013;719(1–3):25–33. doi: 10.1016/j.ejphar.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Ling D.S., Benardo L.S., Serrano P.A., Blace N., Kelly M.T., Crary J.F., Sacktor T.C. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat. Neurosci. 2002;5(4):295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Liu H.H., Payne H.R., Wang B., Brady S.T. Gender differences in response of hippocampus to chronic glucocorticoid stress: role of glutamate receptors. J. Neurosci. Res. 2006;83(5):775–786. doi: 10.1002/jnr.20782. [DOI] [PubMed] [Google Scholar]
- Lodge D.J., Grace A.A. Developmental pathology, dopamine, stress and schizophrenia. Int. J. Dev. Neurosci. 2011;29(3):207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H.K., Drevets W.C., Charney D.S. The cellular neurobiology of depression. Nat. Med. 2001;7(5):541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Martin S., Henley J.M., Holman D., Zhou M., Wiegert O., van Spronsen M., Joels M., Hoogenraad C.C., Krugers H.J. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One. 2009;4(3):e4714. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Turrillas R., Frechilla D., Del Rio J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43(8):1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D., Guest P.C., Vanattou-Saifoudine N., Rahmoune H., Bahn S. Phosphoproteomic differences in major depressive disorder postmortem brains indicate effects on synaptic function. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262(8):657–666. doi: 10.1007/s00406-012-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.M., Green M.R. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- Meyer G., Ferres-Torres R., Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 1978;155(1):108–112. doi: 10.1016/0006-8993(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Migues P.V., Hardt O., Wu D.C., Gamache K., Sacktor T.C., Wang Y.T., Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat. Neurosci. 2010;13(5):630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Milne A.M., MacQueen G.M., Hall G.B. Abnormal hippocampal activation in patients with extensive history of major depression: an fMRI study. J. Psychiatry Neurosci. 2012;37(1):28–36. doi: 10.1503/jpn.110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel T.R., Overpeck M., Pilla R.S., Ruan W.J., Simons-Morton B., Scheidt P. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285(16):2094–2100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J., Matsuzaki M., Ellis-Davies G.C., Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46(4):609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A.M., Miiller L.C., Forster G.L., Watt M.J. Adolescent social defeat decreases spatial working memory performance in adulthood. Behav. Brain Funct. 2013;9:39. doi: 10.1186/1744-9081-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani S., Palanza P., Rogers J., Ferrari P.F. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci. Biobehav Rev. 1999;23(7):957–969. doi: 10.1016/s0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L., Mateus-Pinheiro A., Morais M., Bessa J.M., Sousa N. Immuno-Golgi as a tool for analyzing neuronal 3D-dendritic structure in phenotypically characterized neurons. PLoS One. 2012;7(3):e33114. doi: 10.1371/journal.pone.0033114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C., Duman R.S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Andreoli M., Michielin F., Quarta D., Sokal D.M. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav. Brain Res. 2011;218(1):253–257. doi: 10.1016/j.bbr.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Refojo D., Echenique C., Muller M.B., Reul J.M., Deussing J.M., Wurst W., Sillaber I., Paez-Pereda M., Holsboer F., Arzt E. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc. Natl. Acad. Sci. U. S. A. 2005;102(17):6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort N.L., Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13(8):699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.V., Trumbach D., Weber P., Wagner K., Scharf S.H., Liebl C., Datson N., Namendorf C., Gerlach T., Kuhne C. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. 2010;30(50):16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian V., Estil J.B., Chen D., Schrott L.M., Serrano P.A. Acute physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus. PLoS One. 2013;8(10):e79077. doi: 10.1371/journal.pone.0079077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian V., Vergel T., Baig R., Schrott L.M., Serrano P.A. PKMzeta differentially utilized between sexes for remote long-term spatial memory. PLoS One. 2013;8(11):e81121. doi: 10.1371/journal.pone.0081121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C.Y., Sondhi R., van de Nes P.S., Sacktor T.C. PKMzeta is necessary and sufficient for synaptic clustering of PSD-95. Hippocampus. 2012;22(7):1501–1507. doi: 10.1002/hipo.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P., Popik P., Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009;30(11):563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S., Acquas E., Puddu M.C., Mulas G., Lintas A., Diana M. Simultaneous Golgi-Cox and immunofluorescence using confocal microscopy. Brain Struct. Funct. 2011;216(3):171–182. doi: 10.1007/s00429-011-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. Berl. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Svenningsson P., Tzavara E.T., Witkin J.M., Fienberg A.A., Nomikos G.G., Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc. Natl. Acad. Sci. U. S. A. 2002;99(5):3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki K., Sabatini B.L. Super-resolution 2-photon microscopy reveals that the morphology of each dendritic spine correlates with diffusive but not synaptic properties. Front. Neuroanat. 2014;8:29. doi: 10.3389/fnana.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey J.W., Miczek K.A. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721(1–2):140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tse Y.C., Montoya I., Wong A.S., Mathieu A., Lissemore J., Lagace D.C., Wong T.P. A longitudinal study of stress-induced hippocampal volume changes in mice that are susceptible or resilient to chronic social defeat. Hippocampus. 2014;24(9):1120–1128. doi: 10.1002/hipo.22296. [DOI] [PubMed] [Google Scholar]
- Ttofi M.M. Adolescent bullying linked to depression in early adulthood. BMJ. 2015;350:h2694. doi: 10.1136/bmj.h2694. [DOI] [PubMed] [Google Scholar]
- Van Pett K., Viau V., Bittencourt J.C., Chan R.K., Li H.Y., Arias C., Prins G.S., Perrin M., Vale W., Sawchenko P.E. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wang X.D., Chen Y., Wolf M., Wagner K.V., Liebl C., Scharf S.H., Harbich D., Mayer B., Wurst W., Holsboer F. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol. Dis. 2011;42(3):300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren B.L., Vialou V.F., Iñiguez S.D., Alcantara L.F., Wright K.N., Feng J., Kennedy P.J., Laplant Q., Shen L., Nestler E.J. Neurobiological sequelae of witnessing stressful events in adult mice. Biol. Psychiatry. 2013;73(1):7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Burke A.R., Renner K.J., Forster G.L. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav. Neurosci. 2009;123(3):564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold R.J., Petralia R.S., Blahos J., II, Niedzielski A.S. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 1996;16(6):1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Kelly M.T., Sajikumar S., Serrano P., Tian D., Bergold P.J., Frey J.U., Sacktor T.C. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J. Neurosci. 2008;28(31):7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Guo M., Garza J., Rendon S., Sun X.L., Zhang W., Lu X.Y. Cognitive and neural correlates of depression-like behaviour in socially defeated mice: an animal model of depression with cognitive dysfunction. Int. J. Neuropsychopharmacol. 2011;14(3):303–317. doi: 10.1017/S1461145710000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Wooten M.W., Coleman E.S. Regulation of atypical zeta-protein kinase C in cellular signaling. Exp. Cell Res. 1994;214(1):1–11. doi: 10.1006/excr.1994.1227. [DOI] [PubMed] [Google Scholar]