Abstract
Background
Atrial fibrillation (AF) and renal dysfunction are two common comorbidities in patients with chronic heart failure with reduced ejection fraction (HFrEF). This study evaluated the effect of permanent AF on renal function in HFrEF and investigated the associations of atrial fibrillation, neutrophil gelatinase-associated lipocalin (NGAL), and neutrophil-to-lymphocyte ratio (NLR) with adverse clinical outcome.
Material/Methods
Serum NGAL levels measured by ELISA and NLR were compared between patients with sinus rhythm (HFrEF-SR, n=68), with permanent AF (HFrEF-AF, n=62), and a healthy control group (n=50).
Results
Mean eGFR levels were significantly lower, and NLR and NGAL levels were significantly higher in the HFrEF patients than in the control patients but the difference between HFrEF-SR and HFrEF-AF was not statistically significant (NGAL: 95 ng/mL in HFrEF-SR, 113 ng/mL in HFrEF-AF and 84 ng/mL in the control group; p<0.001). Independent associates of baseline eGFR were age, hemoglobin, NLR, triiodothyronine, and pulmonary artery systolic pressure. In a mean 16 months follow-up, adverse clinical outcome defined as progression of kidney dysfunction and composite of all-cause mortality and re-hospitalization were not different between HFrEF-SR and HFrEF-AF patients. Although NGAL was associated with clinical endpoints in the univariate analysis, Cox regression analysis showed that independent predictors of increased events were the presence of signs right heart failure, C-reactive protein, NLR, triiodothyronine, and hemoglobin. In ROC analysis, a NLR >3 had a 68% sensitivity and 75% specificity to predict progression of kidney disease (AUC=0.72, 95% CI 0.58–0.85, p=0.001).
Conclusions
Presence of AF in patients with HFrEF was not an independent contributor of adverse clinical outcome (i.e., all-cause death, re-hospitalization) or progression of renal dysfunction. Renal dysfunction in HFrEF was associated with both NLR and NGAL levels, but systemic inflammation reflected by NLR seemed to be a more important determinant of progression of kidney dysfunction.
MeSH Keywords: Atrial Fibrillation, Cardio-Renal Syndrome, Heart Failure, Lipocalins
Background
Heart failure is often accompanied by co-morbid conditions that complicate management and patient outcomes. One frequent comorbidity is renal dysfunction, which is observed in up to 56% of chronic heart failure patients and is a strong contributor to poor outcome [1–3]. Another common comorbidity is atrial fibrillation (AF), which has an incidence rate of 5.4% per year [4]. Development AF is associated with impaired cardiac output, which may result in decreased renal perfusion. Impairment of renal perfusion is a well-known risk factor for prerenal kidney injury, which may eventually lead to intrinsic kidney injury.
Neutrophil gelatinase-associated lipocalin (NGAL), also known as lipocalin-2, is a small 25-kDa protein of the lipocalin family [5]. It is expressed in neutrophils, and in the kidney, prostate and epithelia of the respiratory and alimentary tracts; it is filtered in the glomeruli and partially reabsorbed by the megalin receptors in the brush border of proximal tubule. NGAL is accepted as a biomarker for acute kidney injury, however, several studies have also shown that NGAL increases in patients with chronic heart failure [6,7]. Increased immune system activation and renal dysfunction in heart failure leads to an increase in the NGAL mRNA expression and protein concentration. High serum NGAL values in acute and chronic heart failure patients are associated with worse functional capacity, progression of kidney disease and higher short- and long-term mortality [8,9].
Decreased cardiac output and renal perfusion in chronic heart failure patients with permanent AF may lead to chronic kidney injury and accelerate development of kidney dysfunction. The aim of this study was to evaluate NGAL levels in heart failure patients with permanent AF and sinus rhythm, and to investigate the association of NGAL, inflammatory markers, AF, and progression of renal dysfunction in clinical follow-up.
Material and Methods
From January 2013 to December 2013, consecutive patients with stable chronic systolic heart failure (ejection fraction (EF) ≤45%) and NYHA class II–IV symptoms were included in the study prospectively. The exclusion criteria were acute decompensated heart failure, recent myocardial infarction (in the last 6 months), abdominal or thoracic surgery within the previous 3 months, hypertrophic or restrictive cardiomyopathy, clinically significant heart valve disease, myocarditis, uncontrolled hypertension, active infection, pregnancy, history of malignancy, connective tissue diseases, active or chronic inflammatory or autoimmune disease, and renal dysfunction requiring dialysis. Patients were divided into two groups according to their heart rhythms as heart failure with sinus rhythm (HFrEF-SR) and heart failure with permanent atrial fibrillation (HFrEF-AF). Fifty healthy volunteers (22 male, 28 female, aged 57±11 years) who were free of hypertension, diabetes or any cardiovascular disease were included in the control group for comparison of NGAL and biochemical parameters.
All patients underwent conventional transthoracic echocardiography using Vivid-7, GE Vingmed ultrasound with a broadband probe. Chamber diameters and ventricular function were assessed according to current guidelines. Routine biochemistry analysis included creatinine, uric acid, lipid profile, NT-proBNP, high sensitive C-reactive protein, triiodothyronine, and thyroid stimulating hormone levels. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Whole blood counts were determined using an automated blood cell counter. Neutrophil-to-lymphocyte ratio (NLR) was calculated as the ratio of neutrophils to lymphocytes obtained from the same blood sample.
For measurement of NGAL levels, venous blood samples were taken the same day as the biochemical analysis. Serum samples were processed and immediately frozen at −80°C until analyzed. NGAL was measured with Biovendor Human Lipocalin-2/NGAL ELISA kit (BioVendor Laboratorni medicina a.s., Brno, Czech Republic).
Endpoints of the study were: 1) composite of all-cause death and re-hospitalization and 2) progression of renal dysfunction, defined as a decline in eGFR category, accompanied by a decrease in eGFR ≥25% from baseline [10]. Patient follow-up was by outpatient visit or by phone contact if the patient was unable to attend an outpatient visit. The last creatinine value taken in-hospital and obtained while the patient was in a stable condition was used in the analysis for follow-up of kidney functions.
The protocol was approved by the institutional review board of the Kocaeli University, Faculty of Medicine, and adhered to the standards for clinical research in the Declaration of Helsinki.
Statistical analysis
All analyses were performed using the SPSS 13.0 statistical software package. All variables were tested for normality using the Shapiro-Wilk test. Continuous variables were presented as median and 25th–75th percentiles; and categorical variables were given as percentages. The two patient groups and the control group were compared by Kruskal-Wallis test. For the comparison of patients with HFrEF-SR and HFrEF-AF, continuous variables were evaluated by Mann Whitney U test. The chi-square test was used for the comparison of categorical variables. Correlates of NGAL were assessed with Spearman rank correlation test. Clinical, biochemical, and echocardiographic variables with possible relationship to baseline eGFR were tested with stepwise linear regression analysis adjusted for age, heart rhythm, diabetes, signs of right heart failure, ejection fraction, pulmonary artery systolic pressure, systolic blood pressure, hemoglobin, NT-proBNP, hs-CRP, triiodothyronine, albumin, NLR, and NGAL levels. Possible associations to each endpoint were evaluated by multivariable Cox regression analyses with backward elimination adjusting for age, heart rhythm, signs of right heart failure, ejection fraction, pulmonary artery systolic pressure, systolic blood pressure, hemoglobin, baseline eGFR, NT-proBNP, hs-CRP, triiodothyronine, albumin, NLR, and NGAL levels. Receiver operating curve (ROC) analysis was performed to assess best cutoff value to predict progression of kidney disease. The Kaplan-Meier method was used to demonstrate the timing of events during follow-up, and statistical evaluation was carried out using the log-rank test. A p value <0.05 was accepted as statistically significant.
Results
A total of 130 patients who met inclusion and exclusion criteria were included in the study between February 1, 2013 and December 31, 2013. Clinical characteristics of the study groups are presented in Table 1. The patients with HFrEF-AF were older, and had more ischemic etiology and signs of right heart failure. Drug usage was similar between the two groups, except for a higher frequency of digoxin and warfarin usage in patients with HFrEF-AF
Table 1.
Baseline characteristics of the HFrEF patients with atrial fibrillation (AF) and sinus rhythm (SR).
| HFrEF-AF (n=62) | HFrEF-SR (n=68) | P | |
|---|---|---|---|
| Age (years) | 69 (63–79) | 61 (53–70) | <0.001 |
| Male | 37 (60%) | 37 (54%) | 0.545 |
| Body mass index (kg/m2) | 27 (24–29) | 26 (23–28) | 0.227 |
| Ischemic etiology | 35 (56%) | 35 (51%) | <0.001 |
| Hypertension | 45 (73%) | 54 (79%) | <0.001 |
| Diabetes mellitus | 17 (27%) | 25 (37%) | 0.005 |
| NYHA Class | 2.7 (2.0–3.0) | 2.7 (2.0–3.0) | 0.866 |
| Signs of right heart failure* | 41 (66%) | 30 (44%) | <0.001 |
| Ejection fraction (%) | 30 (20–40) | 25 (20–33) | 0.145 |
| Left ventricular end-diastolic diameter (mm) | 57 (50–67) | 57 (53–64) | 0.688 |
| Pulmonary artery systolic pressure (mmHg) | 45 (30–50) | 35 (25–50) | 0.034 |
| Medications | |||
| ACE-I/ARB | 31 (50%) | 45 (66%) | 0.062 |
| Beta-blockers | 49 (%9%) | 53 (78%) | 0.880 |
| Spironolactone | 12 (19%) | 9 (13%) | 0.344 |
| Digoxin | 16 (26%) | 5 (7%) | 0.004 |
| Loop diuretics | 49 (79%) | 56 (82%) | 0.631 |
| Warfarin | 45 (73%) | 6 (9%) | <0.001 |
Presence of jugulary venous distention/abdominojugular reflux and/or peripheral edema.
Biochemical characteristics of the study groups and control group (n=50) are listed in Table 2. Both groups of HFrEF patients had lower eGFR, hemoglobin, albumin, total cholesterol, triglycerides, and triiodothyronine, and had higher uric acid, hs-CRP, NT-proBNP, and NGAL levels compared to controls. In a comparison of HFrEF patients according to their baseline rhythm, no significant difference was detected in serum creatinine and eGFR levels despite a statistically significant older age of AF patients. NGAL levels were slightly but not statistically higher in patients with HFrEF-AF.
Table 2.
Biochemical characteristics of the HFrEF patients with atrial fibrillation (AF) and sinus rhythm (SR) and controls.
| HFrEF-AF (n=62) | HFrEF-SR (n=68) | Controls (n=50) | P1 | P2 | |
|---|---|---|---|---|---|
| Creatinine (mg/dl) | 1.1 (0.83–1.32) | 1.06 (0.79–1.39) | 0.78 (0.68–0.85) | <0.001 | 0.694 |
| eGFR (ml/min) | 53 (43–81) | 66 (41–92) | 101 (53–98) | <0.001 | 0.328 |
| Uric acid (mg/dl) | 7.8 (6.4–9.5) | 6.6 (4.8–8.8) | 5.6 (4.6–6.1) | <0.001 | 0.014 |
| Hs-CRP (mg/dl) | 0.94 (0.23–2.72) | 0.87 (0.31–3.39) | 0.27 (0.08–0.54) | <0.001 | 0.460 |
| Haemoglobin (g/dl) | 13.0 (11.1–14.5) | 12.2 (11.2–13.7) | 13.7 (12.8–14.6) | 0.003 | 0.214 |
| Neutrophil to lymphocyte ratio | 2.94 (2.24–4.32) | 2.56 (1.80–3.56) | 1.82 (1.45–2.49) | <0.001 | 0.067 |
| Albumin (mg/dl) | 3.6 (3.2–3.9) | 3.6 (3.31–4.09) | 4.4 (4.1–4.5) | <0.001 | 0.575 |
| Total cholesterol (mg/dl) | 142 (117–174) | 167 (132–195) | 197 (177–222) | <0.001 | 0.017 |
| Triglycerides (mg/dl) | 94 (80–130) | 132 (88–169) | 133 (90–187) | 0.004 | 0.007 |
| T3 (mIU/L) | 2.65 (2.40–2.95) | 2.76 (2.44–3.08) | 3.18 (3.00–3.48) | <0.001 | 0.182 |
| TSH (mIU/L) | 1.21 (0.50–2.54) | 1.16 (0.73–2.05) | 1.17 (0.70–1.67) | 0.619 | 0.955 |
| NT-Pro BNP (pg/ml) | 1910 (900–4030) | 1040 (291–4300) | 61 (25–105) | <0.001 | 0.102 |
| NGAL (ng/ml) | 113 (88–148) | 95 (75–143) | 84 (53–98) | <0.001 | 0.090 |
eGFR – estimated glomerular filtration rate; hs-CRP – high sensitive C-reactive protein; NGAL – Neutrophyil gelatinase-associated lipocalin; T3 – triiodothyronine; TSH – thyroid stimulating hormone; P1 – significance of group differences in Kruskal-Wallis test; P2 – significance of difference between HFrEF-AF and HFrEF-SR in Mann-Whitney U test.
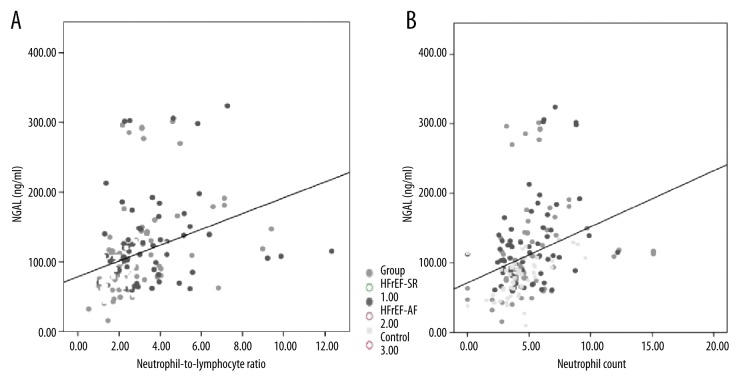
Clinical, echocardiographic, and biochemical correlates of NGAL were evaluated for all three groups and for HFrEF patients separately. None of the clinical and echocardiographic variables were significantly correlated with NGAL levels, but in HFrEF patients, NGAL levels showed a mild correlation with the presence of signs of right heart failure (r=0.20, p=0.025). In all three groups, neutrophil count and NLR were the two parameters that showed significant correlations with NGAL levels (Figure 1A, 1B). When groups were analyzed separately, controls and HFrEF-SR patients showed higher correlations between NGAL and inflammatory parameters. For controls the rates were: r=0.33, p=0.022 for hs-CRP; r=0.59, p<0.001 for neutrophil count; r=0.39, p=0.006 for NLR; and r=−0.32, p=0.025 for albumin. For HFrEF-SR the rates were: r=0.45, p<0.001 for hs-CRP; r=0.42, p<0.001 for neutrophil count; r=0.42, p<0.001 for NLR; and r=−0.28, p=0.021 for albumin. Whereas for HFrEF-AF patients the correlations seemed to be absent or weaker: r=0.12, p=0.355 for hs-CRP; r=0.33, p=0.009 for neutrophil count; r=0.22, p=0.096 for NLR; and r=−0.03, p=0.833 for albumin. Creatinine and eGFR had significant correlations with NGAL in only the HFrEF-SR patients (r=0.39, p=0.001 and r=−0.352, p=0.003, respectively), but not in controls and HFrEF-AF patients.
Figure 1.
Relation of serum NGAL to (A) neutrophil count (×109) and (B) neutrophil-to-lymphocyte ratio.
In stepwise linear regression analysis, significant independent correlates of baseline eGFR were age (p<0.001), hemoglobin (B=3.27, p<0.001) and NLR (B=−2.29, p=0.009). When age was removed from the model (as it is a parameter in calculation of eGFR), pulmonary artery systolic pressure (B=−0.27, p=0.043) and triiodothyronine (B=7.17, p=0.045) appeared as the next independent correlates of eGFR. Neither rhythm nor NGAL was an independent determinant of eGFR, despite having significant correlation in Spearman analysis.
Patients were followed for a mean duration of 16 months (range 1–39 months). During that period, nine patients were lost to follow-up. The remaining 121 patients were followed by outpatient visits or phone contact. Follow-up eGFR measurements were available in 85 patients (70%). Death occurred in 26 of 121 patients (22%), and 63 patients (52%) were re-hospitalized. Progression of kidney disease was detected in 28 of 85 patients (33%).
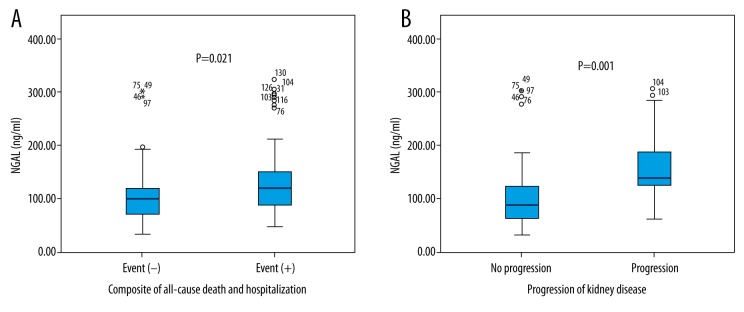
Incidence of endpoints were similar between HFrEF-SR patients and HFrEF-AF patients (composite of all-cause mortality and hospitalization: 32/63 (51%) versus 36/58 (62.1%) respectively, p=0.271; and progression of kidney disease: 17/48 (35%) versus 11/37 (30%), p=0.646, respectively). NGAL level was significantly higher in patients with both endpoints (Figure 2A, 2B).
Figure 2.
NGAL levels in patients with and without study endpoints: (A) Composite of all-cause motrality; (B) Progression of kidney disease.
In Cox regression analysis, significant associations of the composite of all-cause mortality were signs of right heart failure and hs-CRP (Table 3). Independent predictors for progression of kidney disease were hemoglobin, triiodothyronine, hs-CRP, and NLR. Presence of AF was not associated with death, re-hospitalization, or progression of kidney dysfunction. Also, the relation of NGAL levels to the study endpoints lost their significance in multivariate analysis.
Table 3.
Correlates of study endpoints in Cox regression analysis.
| B | Exp(B) | 95% CI for Exp(B) | p | |
|---|---|---|---|---|
| Composite of all-cause mortality and hospitalization | ||||
| Signs of RHF | −0.867 | 0.420 | 0.230–0.766 | 0.002 |
| Hs-CRP | 0.075 | 1.078 | 1.010–1.150 | 0.032 |
| Progression of kidney disease | ||||
| NLR | 0.308 | 1.361 | 1.102–1.680 | 0.003 |
| Hs-CRP | 0.239 | 1.270 | 1.081–1.493 | 0.004 |
| Triiodothyronine | 1.170 | 3.220 | 1.291–8.031 | 0.009 |
| Hemoglobin | −0.245 | 0.783 | 0.620–0.988 | 0.046 |
Hs-CRP – high sensitive C-reactive protein; NLR – neutrophil-to-lymphocyte ratio; RHF – right heart failure.
As NLR was an independent determinant for both baseline eGFR and progression of kidney disease, a ROC curve analysis was performed in order to assess the best cutoff value for predicting progression of kidney disease. A NLR >3 had a sensitivity of 68% and a specificity of 75% to predict progression of kidney disease (AUC=0.72, 95% CI 0.58–0.85, p=0.001). Survival without progression in kidney disease was significantly higher in patients with a NLR <3 (p<0.001 in log-rank analysis; Figure 3).
Figure 3.
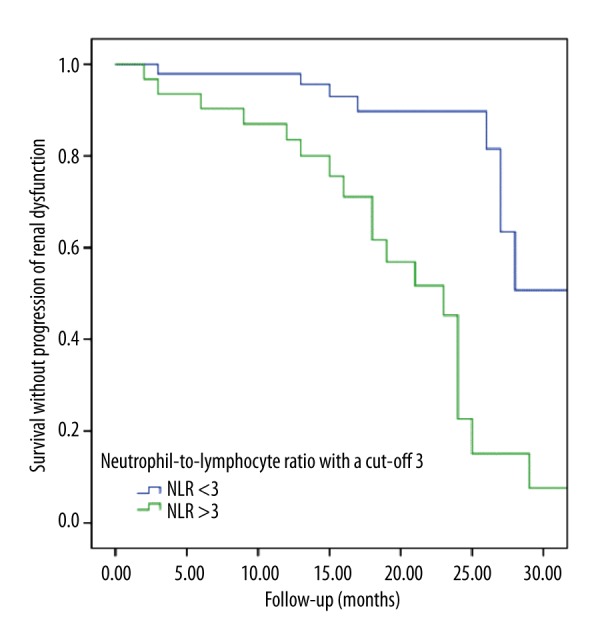
Survival without progression of kidney disease stratified by neutrophil-to-lymphocyte ratio.
Discussion
The results of this study show that presence of permanent AF does not confer a significant effect on renal dysfunction in patients with HFrEF. Both baseline eGFR and progression of kidney dysfunction in the follow-up period were not associated with heart rhythm, but did show significant associations with age, hemoglobin, NLR, pulmonary artery systolic pressure, and triiodothyronine. These findings suggest that renal dysfunction in HFrEF is related to older age, and severity of heart failure and its systemic effects; as anemia, inflammation, right heart failure and low triiodothyronine (euthyroid sick syndrome) are all markers of advanced heart failure.
AF and heart failure are two conditions that often coexist and lead to a vicious circle, where structural, neuro-hormonal, and inflammatory changes that are associated with one condition contributes to the deterioration of the other condition [11]. However, there are conflicting reports of the effect of AF on adverse cardiac outcome in heart failure patients likely due to different study designs. Possible explanations for the lack of a significant effect of AF on clinical outcomes in our study can be attributed to the selection of the study group. HFrEF patients with an ejection fraction between 0.25–0.30 already have low cardiac output and increased inflammatory and neuro-humoral activation. Presence of AF in these patients may not have a significant additional impact on the outcomes, as these patients are already at high risk. The findings of our study confirm the results of previous studies that have not been able to show an independent association between AF and adverse prognosis in patients with systolic heart failure [12,13]. A novel finding of our study was the lack of an accelerated effect on the progression of renal dysfunction. Several studies have evaluated the impact of renal dysfunction on the incidence and management of AF [14,15], but studies about the impact of AF on the progression of renal dysfunction in HFrEF are limited [16]. Our study findings suggest that the presence of permanent AF was only a marker for advanced disease in HFrEF patients, and increased inflammation and thromboembolic events associated with AF did not significantly affect presence and development of renal dysfunction in these patients. Another possible hypothesis is that low-dose warfarin plays a protective role against renal dysfunction in these patients [17], but the number of patients on oral anticoagulants in our study was small making it not possible for further statistical analysis to prove this hypothesis.
Serum NGAL levels were increased in chronic HFrEF patients, and this increase was closely associated with the severity of systemic inflammation rather than the presence of AF. In our univariate analysis, NGAL was one of the predictors for progression of kidney dysfunction, all-cause mortality, and re-hospitalization. However, after multivariate analysis, other markers of systemic inflammation (e.g., hs-CRP and NLR) proved to be more important factors for kidney dysfunction and clinical outcomes, whereas NGAL lost its independent association.
As a marker of tubular injury, NGAL has been investigated in several acute and chronic heart failure studies, and has been shown to be an independent predictor of worsening renal function and adverse outcome [18,19]. NGAL levels may be measured from serum, plasma, urine, and stool. Shrestha et al. [20] showed that there was a difference between urine and serum NGAL measurements. Although both markers predict worsening renal function, the former reflects renal distal tubular injury with impaired natriuresis and diuresis, and the later, systemic NGAL levels, demonstrate a stronger association with glomerular filtration function. Only one study has investigated progression of renal dysfunction in chronic heart failure patients as a follow-up study and it showed that urine NGAL and kidney injury molecule-1 (KIM-1) were independent predictors for progression of kidney dysfunction [21]. Our study differs from that study in the measurement of serum instead of urine NGAL levels, and in the evaluation of other inflammatory markers. Therefore, our finding of the lack of an independent association between progression of kidney dysfunction and serum NGAL but a significant association with NLR is a novel finding.
NLR, which is calculated from complete blood count, is an inexpensive, easily accessible marker for systemic inflammation. It combines two markers of inflammation (i.e., high neutrophil and low lymphocyte count), and has proven prognostic utility in predicting adverse outcomes in cardiovascular diseases, including heart failure [22,23]. The utility of NLR as a predictor for worsening renal function has been evaluated in only one observational study of diabetic patients [24]. Results of that study showed that a NLR >1.6 was associated with a 3-fold increase and a NLR >2.36 with a 4-fold increase in worsening renal function in diabetic patients. The average value of NLR in the non-Hispanic white American population is 2.24 (95% CI 2.19–2.28) [25]. Therefore, the cutoff NLR value >3 in our study represents an increased inflammatory state in patients with HFrEF leading to progression of kidney dysfunction.
It is clear that all three conditions (i.e., heart failure, atrial fibrillation, and renal dysfunction) examined in our study are considered states of chronic inflammation that are associated with increase in circulating biomarkers of inflammation [26].Elevations in these biomarkers are related to the functional class of heart failure rather than the decrease in left ventricular ejection fraction. They are bioactive molecules that exert a direct and often overlapping detrimental effect on exposed tissue. Several mediators like tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, and CRP act on the pathophysiology of renal impairment by inducing interstitial infiltration of inflammatory cells, induction of tubular injury, interstitial fibrosis, and loss of local tissue integrity. The stronger association of NLR to adverse outcomes compared to NGAL suggests that NGAL reflects mainly renal inflammation, and that NLR is a more general marker for systemic inflammation and reflects other conditions (e.g., venous congestion, endothelial dysfunction, arterial stiffness) that worsen the clinical outcome.
Limitations
The total number of the patients in our study, and the number of patients in the follow-up period may have limited our ability to show any significant association with AF and renal dysfunction in HFrEF patients. Therefore, a type 1 error cannot be excluded.
Conclusions
The presence of AF in patients with HFrEF does not have a significant additional effect on clinical outcomes (i.e., all-cause mortality, re-hospitalization) or on progression of renal dysfunction. Renal dysfunction in HFrEF was associated with both NLR and NGAL levels, but systemic inflammation reflected by NLR seems to be a more important contributor to progression of kidney dysfunction.
Footnotes
Statement
There is no conflict of interest.
Source of support: Departmental sources
References
- 1.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–96. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 2.Dries DL, Exner DV, Domanski MJ, et al. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–89. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation. 2003;107:2920. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 5.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–70. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 6.Yndestad A, Landrø L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30(10):1229–36. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 7.Villacorta H, Santos RA, Marroig MA, et al. Prognostic value of plasma neutrophil gelatinase-associated lipocalin in patients with heart failure. Rev Port Cardiol. 2015;34(7–8):473–78. doi: 10.1016/j.repc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Palazzuoli A, Ruocco G, Pellegrini M, et al. Comparison of neutrophil gelatinase-associated lipocalin versus B-type natriuretic peptide and cystatin C to predict early acute kidney injury and outcome in patients with acute heart failure. Am J Cardiol. 2015;116(1):104–11. doi: 10.1016/j.amjcard.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Ventoulis I, Mantziari L, Mouratoglou SA, et al. NGAL and ST2levels in ambulatory patients with chronic heart failure. Clinical and echocardiographic correlates. Scand Cardiovasc J. 2015;49(4):213–19. doi: 10.3109/14017431.2015.1043141. [DOI] [PubMed] [Google Scholar]
- 10.Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 11.Patel NJ, Patel A, Agnihotri K, et al. Prognostic impact of atrial fibrillation on clinical outcomes of acute coronary syndromes, heart failure and chronic kidney disease. World J Cardiol. 2015;7:397–403. doi: 10.4330/wjc.v7.i7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paolillo S, Agostoni P, Masarone D, et al. Prognostic role of atrial fibrillation in patients affected by chronic heart failure. Data from the MECKI score research group. Eur J Intern Med. 2015;26:515–20. doi: 10.1016/j.ejim.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Crijns HJ, Tjeerdsma G, de Kam PJ, et al. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J. 2000;21:1238–45. doi: 10.1053/euhj.1999.2107. [DOI] [PubMed] [Google Scholar]
- 14.Ananthapanyasut W, Napan S, Rudolph EH, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients withchronic kidney disease. Clin J Am Soc Nephrol. 2010;5:173–81. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roldán V, Marín F, Fernández H, et al. Renal impairment in a “real-life” cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding) Am J Cardiol. 2013;111:1159–64. doi: 10.1016/j.amjcard.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Laible M, Horstmann S, Rizos T, et al. Prevalence of renal dysfunction in ischaemic stroke and transient ischaemic attack patients with or without atrial fibrillation. Eur J Neurol. 2015;22:64–69. doi: 10.1111/ene.12528. [DOI] [PubMed] [Google Scholar]
- 17.Chiu PF, Huang CH, Liou HH, et al. Lower-dose warfarin delays renal progression and prolongs patient survival in patients with stage 3–5 chronic kidney disease and nonvalvular atrial fibrillation: A 12-year follow-up study. Int J Clin Pharmacol Ther. 2014;52:504–8. doi: 10.5414/CP202053. [DOI] [PubMed] [Google Scholar]
- 18.Palazzuoli A, Ruocco G, Beltrami M, et al. Admission plasma neutrophil gelatinase associated lipocalin (NGAL) predicts worsening renal function during hospitalization and post discharge outcome in patients with acute heart failure. Acute Card Care. 2014;16:93–101. doi: 10.3109/17482941.2014.911915. [DOI] [PubMed] [Google Scholar]
- 19.Van Deursen VM, Damman K, Voors AA, et al. Prognostic value of plasma neutrophil gelatinase-associated lipocalin for mortality in patients with heart failure. Circ Heart Fail. 2014;7:35–42. doi: 10.1161/CIRCHEARTFAILURE.113.000242. [DOI] [PubMed] [Google Scholar]
- 20.Shrestha K, Shao Z, Singh D, et al. Relation of systemic and urinary neutrophil gelatinase-associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol. 2012;110:1329–35. doi: 10.1016/j.amjcard.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jungbauer CG, Uecer E, Stadler S, et al. N-acteyl-β-D-glucosaminidase (NAG) and Kidney injury molecule-1 (KIM-1): New predictors for long-term progression of chronic kidney disease in patients with heart failure. Nephrology (Carlton) 2015 doi: 10.1111/nep.12632. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Benites-Zapata VA, Hernandez AV, Nagarajan V, et al. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am J Cardiol. 2015;115:57–61. doi: 10.1016/j.amjcard.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uthamalingam S, Patvardhan EA, Subramanian S, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–38. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Azab B, Daoud J, Naeem FB, et al. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study) Ren Fail. 2012;34:571–76. doi: 10.3109/0886022X.2012.668741. [DOI] [PubMed] [Google Scholar]
- 25.Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361. doi: 10.1371/journal.pone.0112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombo PC, Ganda A, Lin J, et al. Inflammatory activation: Cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17:177–90. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]