Abstract
Background
The aim of this study was to investigate the accuracy of contrast-enhanced ultrasound (CEUS) enhancement patterns in the assessment of thyroid nodules.
Material/Methods
A total of 158 patients with suspected thyroid cancer underwent conventional ultrasound (US) and CEUS examinations. The contrast enhancement patterns of the lesions, including the peripheries of the lesions, were assessed by CEUS scans. The relationship between the size of the lesions and the degree of enhancement was also studied. US- and/or CEUS-guided biopsy was used to obtain specimens for histopathological diagnosis.
Results
The final data included 148 patients with 157 lesions. Seventy-five patients had 82 malignant lesions and 73 patients had 75 benign lesions. Peripheral ring enhancement was seen in 40 lesions. The differences of enhancement patterns and peripheral rings between benign and malignant nodules were significant (p=0.000, 0.000). The diagnostic sensitivity, specificity, and accuracy for malignant were 88%, 65.33%, and 88.32%, respectively, for CEUS, whereas they were 98.33%, 42.67%, and 71.97%, respectively, for TC by conventional US. The misdiagnosis rate by conventional US was 57.33% and 34.67% by CEUS (p=0.005). With regard to the size of lesions, a significant difference was found between low-enhancement, iso-enhancement, high-enhancement, iso-enhancement with no-enhancement area and no-enhancement (p=0.000).
Conclusions
In patients with suspicious US characteristics, CEUS had high specificity and contributed to establishing the diagnosis. Therefore, CEUS could avoid unnecessary biopsy.
MeSH Keywords: Contrast Media; Thyroid Neoplasms; Ultrasonography, Doppler
Background
Carcinoma of the thyroid gland is the most common endocrine malignancy, accounting in the USA for example for 95% of endocrine tumors [1]. Over the last 3 decades, its incidence is reported to have risen worldwide [2]. In the USA, the incidence of well-differentiated thyroid cancer (TC) increased from 4.9 per 100 000 in 1975 to 14.3 per 100 000 in 2009 [3]. In China, the standardized incidence of TC has increased from 1.6 per 100 000 in 1995 to 9.9 per 100 000 in 2010 [4].
The behavior of TC varies considerably. Some are asymptomatic and do not progress over many years. Others are invasive early on, giving rise to clinical symptoms and metastasizing to lymph nodes. Accurate and timely diagnosis is essential to optimize management. Ultrasound (US) is the first-choice imaging modality for the diagnosis of TC. The diagnostic sensitivity, specificity, and accuracy rate are 92%, 72.9%, and 62~78%, respectively, for TC [5–7]. However, the sonograms of some lesions were overlapped between the benign and malignant as a result of the various ultrasonic appearances among different lesions. Therefore, biopsy was relied on to get the final diagnosis, and is considered the diagnostic criterion standard [8, 9], but biopsy is invasive and has some risk for patients. Previous studies showed that 48–75% of patients who undergo biopsy are subsequently found to have benign lesions [10,11], the overall dissatisfaction rate for FNA is between 10–20% [12], and 2% of samples obtained by CNB are inadequate for histological diagnosis [8]. How to reduce the number of unnecessary biopsies is an area of current research.
Angiogenesis is important in tumor growth and proliferation. Contrast-enhanced ultrasound (CEUS) scanning can detect differences in the distribution of blood and in the hemodynamics between tumors and surrounding tissues. It has found widespread application in many organs, such as the liver, gall bladder, and kidney. Its use in assessing diseases of the thyroid gland has been the subject of a number of reports, although its precise role remains uncertain [13–16]. ACE/AME/ETA (American Association of Clinical Endocrinologists/Associazione Medici Endocrinology/European Thyroid Association) guidelines in 2010 state that contrast agents provide only ancillary data for the diagnosis of malignant thyroid nodules and offer only a modest improvement over the information obtained with traditional color Doppler or power Doppler examinations, and that the use of US contrast agents should be restricted to defining the size of necrotic areas in lesions after US-guided ablation procedures [17]. The 2013 NCCN guidelines did not discuss the role of CEUS scanning in diagnosing thyroid lesions [18]. Chinese TC diagnosis and therapy guidelines in 2012 state that the diagnostic value of CEUS scanning should be explored in future studies [19]. Different diagnosis methods may cause controversial results.
The enhancement patterns of thyroid nodules in previous studies were classified into low-enhancement, iso-enhancement, and high-enhancement [12,13], or homogeneous, heterogeneous, ring-enhancing, and no-enhancement [20], but no combination of them was reported in detail. In fact, variable thyroid nodules were observed during CEUS scanning, and it may be that every each scan shows specific pathological changes. The peripheral ring of the lesion has been reported in previous papers, and ring enhancement was considered predictive of benign lesions. However, several kinds of peripheral rings were found in our clinical works, besides the common regular high-enhancement ring (e.g., irregular high-enhancement ring and no-enhancement ring). We decided to analyze the contrast enhancement patterns of the lesions and its peripheries on the basis of pervious research to assess the diagnostic value of CEUS in thyroid nodules.
Material and Methods
Subjects
The study was approved by the local Ethics Committee and those participating gave written informed consent. It was undertaken between August 2014 and February 2015. Patients were eligible for inclusion if they were scheduled for thyroid biopsy as a consequence of having a palpable thyroid nodule or an abnormal ultrasound scan of the thyroid gland showing at least 1 of the following characteristics: hypoechogenicity, calcification, irregular or microlobulated margin, intranodular vascularity, and taller than wide [21,22]. Patients were also included if they had a suspicious family history. Patients were excluded if they were known to be allergic to sulfur hexafluoride microbubbles (SonoVue) or had a coagulation disorder.
A total of 159 patients were identified, but 11 were excluded because no definite diagnosis was available. The mean ±SD age of the remaining 148 patients was 45.4±10.5 years (range, 16–71 years). Between them, they had 157 thyroid lesions. The mean lesion size was 1.2±0.9 cm (range, 0.3–5.7 cm).
Ultrasound imaging
A Sequoia 512 ultrasound machine (Acuson, Sequoia 512 Encompass, Siemens, USA) and a 15L8w probe (8–14 MHz) were used to image all patients. This has the ability to perform contrast pulse sequence (CPS) imaging. The probe’s frequency was set at 7.0 MHz when used for CEUS. The contrast agent used was 59 mg dry powder SonoVue (Bracco S.p.A Inc., Milan, Italy) made up in 5 ml of normal saline. This has a low mechanical index (0.20–0.23) and was administered intravenously at the elbow. The dose used was 2.4 ml.
Patients were scanned in the supine position with their neck extended. First, a conventional US examination was performed. The size, position, boundaries, internal structure, and blood supply of lesions were assessed. The ultrasound machine was then switched to CEUS mode and the resulting images displayed on the monitor alongside the conventional gray-scale images. The real-time microbubble perfusion within lesions and surrounding tissues were observed for a minimum of 2 minutes and recorded on the ultrasound machine’s internal hard drive.
Image interpretation and analysis
The recorded CEUS images were reviewed and the degree of enhancement of the thyroid solid lesions was classified as: no-enhancement, low-enhancement, iso-enhancement, and high-enhancement. When enhancement was present, it was further assessed for the degree of homogeneity, producing the following lesion enhancement categories: (1) homogeneous low-enhancement, (2) heterogeneous low-enhancement, (3) homogeneous iso-enhancement, (4) heterogeneous iso-enhancement, (5) homogeneous high-enhancement, and (6) heterogeneous high-enhancement.
The peripheral rings of nodules were divided into: (1) regular high-enhancement ring, (2) irregular high-enhancement ring, (3) regular no-enhancement ring, and (4) irregular no-enhancement ring. Regular rings were round or oval, and their thickness was uniform. Conversely, irregular rings were misshapen and their thickness was non-uniform.
Thyroid biopsies
Following CEUS, a conventional biopsy of the thyroid lesion was performed on all patients, using an 18-gauge biopsy needle (Biopty; Bard, Covington, GA, USA) powered by an automatic biopsy device. The puncture position and direction were determined and monitored by ultrasound and non-enhancing areas on CEUS were avoided. The biopsy specimens were examined by a single specialized thyroid pathologist with more than 15 years of experience. The ultrasonographer, the reviewer of the recorded CEUS images, and the pathologist were all blinded to patient history and to each other’s findings.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software package, Version 11.5 for Windows (SPSS Inc., Chicago, IL, USA). The differences between lesion enhancement categories and the diagnostic value of conventional US and CEUS were analyzed by Pearson’s chi-squared test (χ2). The relationship between the size of lesions and the lesion enhancement category was analyzed by F test. p<0.05 was considered statistically significant.
Results
Pathological results
Eighty-two lesions (from 75 patients) were malignant: papillary thyroid carcinoma (81 lesions), and medullary thyroid carcinoma (1 lesion). Seventy-five lesions (from 73 patients) were benign: nodular goiter (43 lesions), follicular thyroid adenoma (15 lesions), Hashimoto thyroiditis (HT) (10 lesions), chronic inflammation (3 lesions), subacute thyroiditis (2 lesions), oncocytic thyroid adenoma (1 lesion), and postoperative stitches (1 lesion).
Internal enhancement patterns of thyroid lesions
There were 137 solid lesions, and their contrast enhancement patterns are shown in Table 1. Examples of these are shown in Figures 1–4. For each enhancement pattern, the difference between the benign and malignant lesions was significant (χ2=42.1334; p=0.000). Solid lesions that were malignant were found to have the following enhancement patterns on CEUS scanning: 70.37% (57/81) low-enhancement (including 75.44% heterogeneous patterns and 24.56% homogeneous patterns), 22.22% (18/81) iso-enhancement (including 66.67% heterogeneous patterns and 33.33% homogeneous patterns), and 7.41% (6/81) heterogeneous high-enhancement. Eighteen of 20 heterogeneous iso-enhancement lesions displayed iso-enhancement with focal low-enhancement regions; the low-enhancement area comprised <50% lesion, and 12 (66.67%, 12/18) lesions were malignant.
Table 1.
The CEUS appearances of benign and malignant thyroid solid lesions.
| Enhancement patterns | Low-enhancement | Iso-enhancement | High-enhancement | |
|---|---|---|---|---|
| Benign | Homogeneous | 5 | 11 | 7 |
| Heterogeneous | 15 | 8 | 10 | |
| Malignant | Homogeneous | 14 | 6 | 0 |
| Heterogeneous | 43 | 12 | 6 | |
| Total | 137 | 77 | 37 | 23 |
Figure 1.
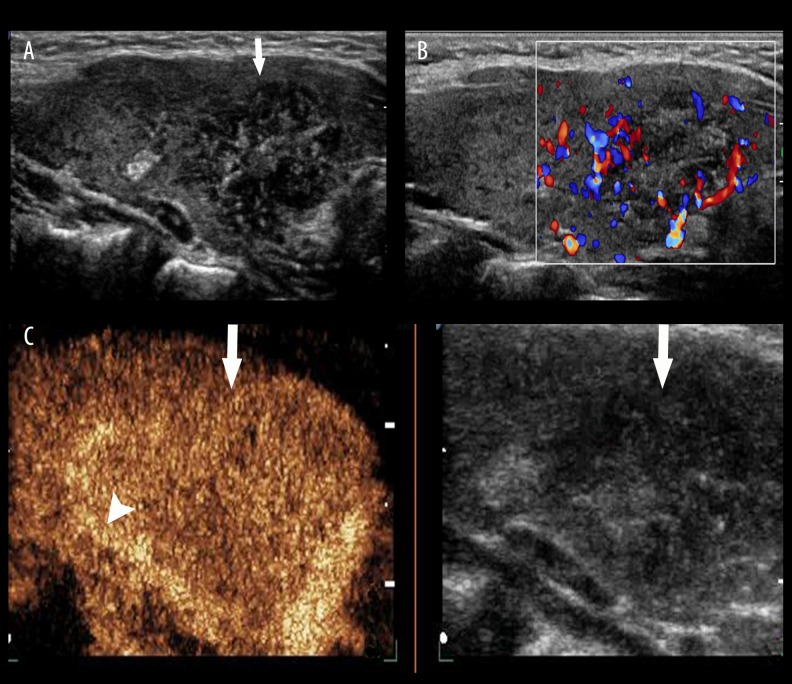
Thyroid papillary carcinoma. (A) A hypoechoic lesion (arrow) with unclear boundaries and irregular shape was found in the right lobe of the thyroid gland by conventional US scanning. (B) A tiny blood signal (arrow) was detected by color Doppler flow imaging. (C) Heterogeneous low-enhancement was seen by CEUS. No ring was seen in its peripheral area (arrow).
Figure 2.
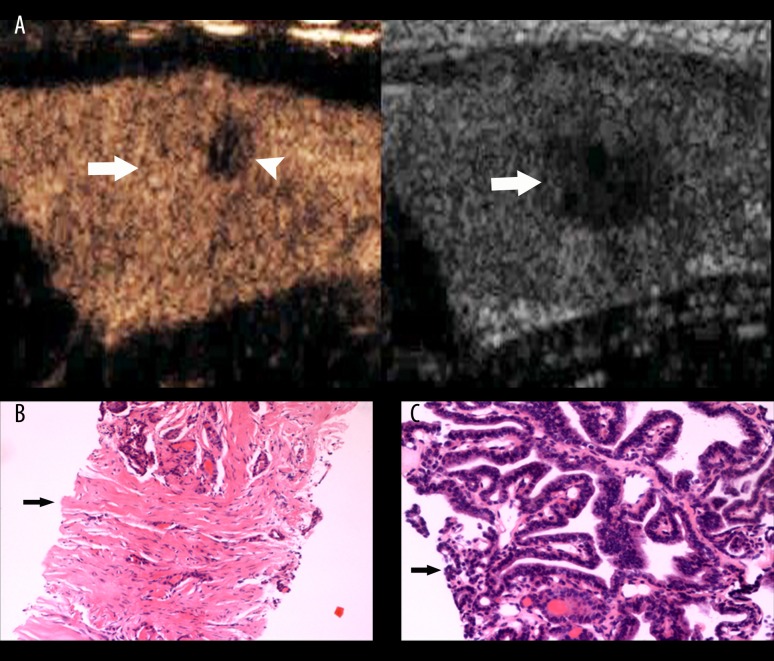
Thyroid papillary carcinoma. (A) A hypoechoic lesion (arrow) with unclear boundary and irregular shape was found in the left lobe of thyroid by conventional US (right image). Iso-enhancement with focal low-enhancement (arrow head) was seen by CEUS. No ring was seen in its peripheral area (left image, arrow). (B) Many fibers, a few blood vessels (arrow), and a few cancerous cells were apparent in a biopsy specimen from an area of low-enhancement. (C) A biopsy specimen from an area of iso-enhancement showed many cancerous cells (arrow).
Figure 3.
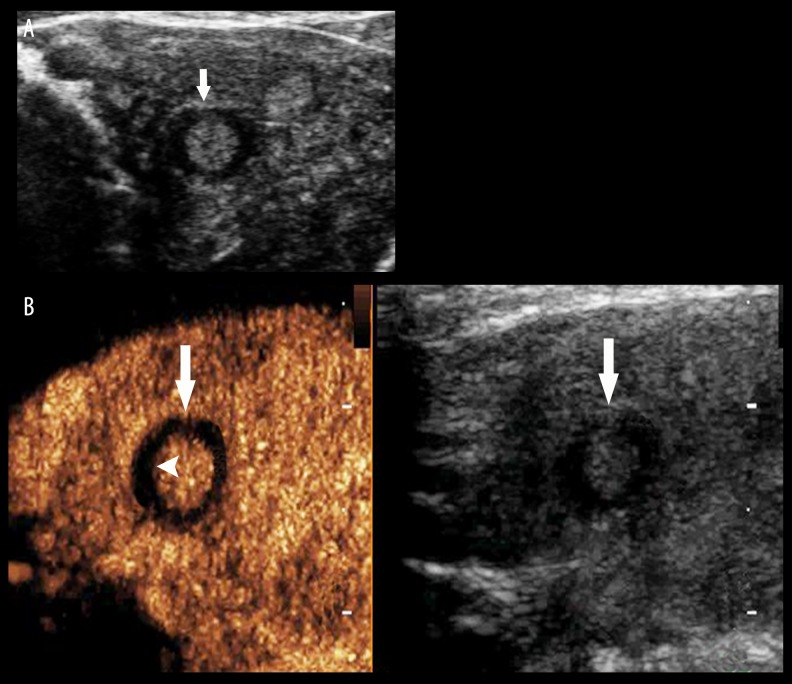
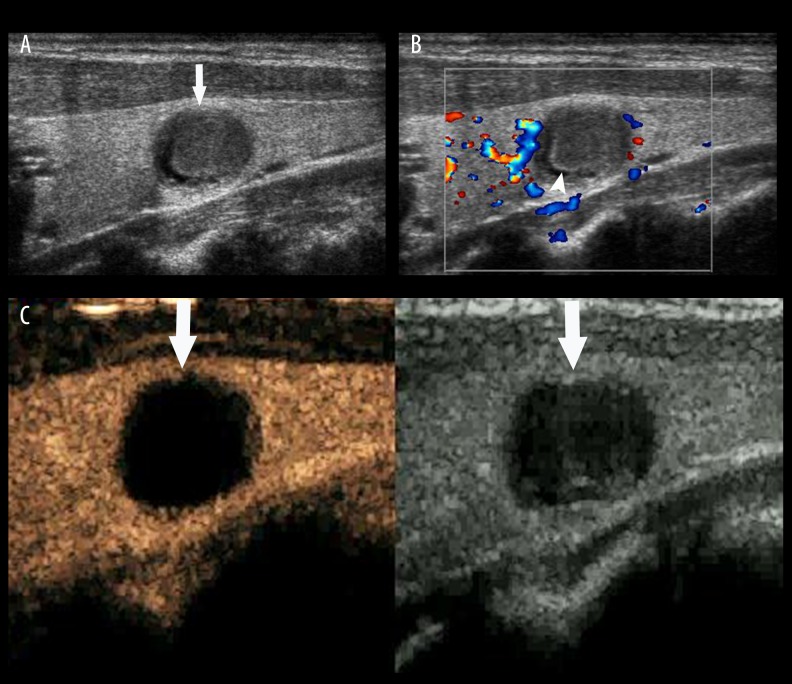
Thyroid adenoma. (A) A hypoechoic lesion (arrow) with clear boundaries and regular shape was found in the left lobe of the thyroid gland, and a regular hypoechoic halo was displayed in its periphery. (B) Iso-enhancement within the lesion and regular non-enhancing ring (arrow head) are shown by CEUS (arrow).
Figure 4.
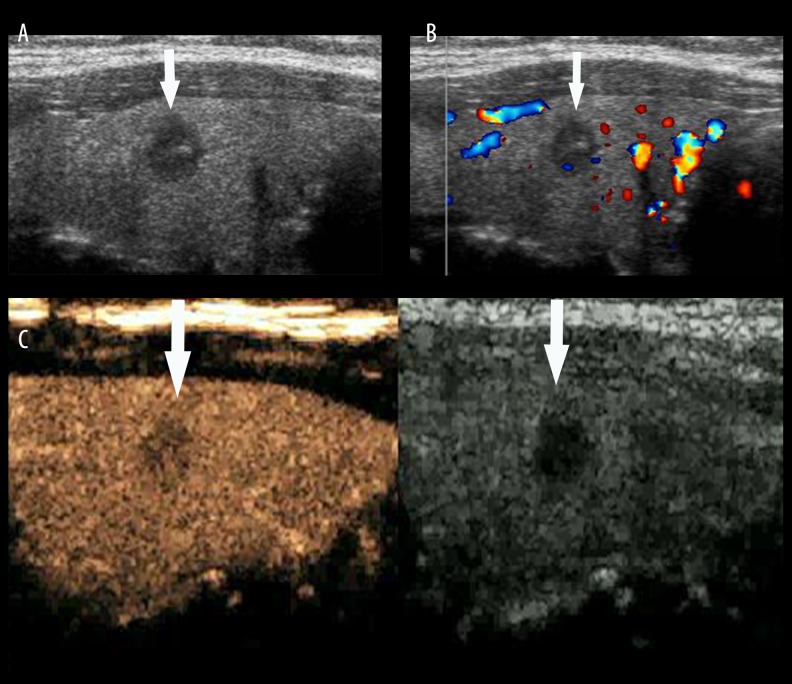
Thyroid papillary carcinoma. (A) A hypoechoic lesion (arrow) with ill-defined boundaries and a lot of microcalcifications was found in the left lobe of the thyroid gland by conventional US. (B) Rich blood signals were detected in lesion by CDFI. (C) Heterogeneous enhancement was seen in the lesion, and an irregular high-enhanced ring (arrow head) was shown in the periphery by CEUS (arrow).
Twenty lesions were cystic-solid mixed (Figure 5). Among them, 15 lesions were showed iso-enhancement coexisting with no-enhancement (14 benign, 1 malignant), and 5 lesion was showed no-enhancement.
Figure 5.
Nodular goiter with accompanying hemorrhage into a cyst. (A) A cystic-solid mixed lesion with clear boundaries and regular shape was found in the left lobe of the thyroid gland by conventional US (arrow). (B) No blood signal was detected in the solid part of lesion by CDFI (arrow head). (C) No-enhancement was seen in the lesion by CEUS (left image, arrow).
In this group, all lesions with homogeneous high-enhancement and no-enhancement patterns were benign. With regards to lesions with low-enhancement appearance as malignant, the diagnostic sensitivity, specificity, and accuracy for TC were 84.15%, 65.33%, and 75.16%, respectively, for CEUS scanning.
Peripheral enhancement patterns of thyroid lesions
No ring enhancement was seen in 117 lesions (74 malignant, 43 benign) that had ill-defined margins. Peripheral ring enhancement was seen in 40 lesions. Regular ring high-enhancement was seen in 28 lesions; all were benign. Irregular ring high-enhancement was seen in 2 lesions (Figure 4C); both were malignant. Regular ring non-enhancement was seen in 6 lesions (Figure 3B); 4 (67%) were benign and the other 2 (33%) were malignant. Irregular ring non-enhancement was seen in 4 lesions; all were malignant. The differences of peripheral ring enhancement between malignant and benign lesions were significant (χ2=22.66, p=0.000). With regards to lesions with irregular rings as malignant, the diagnostic sensitivity, specificity, and accuracy for TC were 100%, 94.12%, and 95%, respectively, for CEUS scanning.
Comparisons between conventional ultrasound scanning and CEUS scanning
With regards to lesions with low-enhancement and/or a peripheral irregular ring as malignant criterion, the diagnostic sensitivity, specificity, and accuracy for TC were 88%, 65.33%, and 88.32%, respectively, for CEUS scanning, whereas they were 98.88%, 42.67% and 71.97%, respectively, for TC by conventional US. The misdiagnosis rate was 57.33% for conventional ultrasound scanning and 34.67% for CEUS scanning (χ2=7.76, p=0.005).
Enhancement patterns and size of thyroid lesions
There was a significant difference in the size of thyroid lesions between low-enhancement, iso-enhancement, high-enhancement, iso-enhancement with no-enhancement area, and no-enhancement (F=11.44, p=0.000) (Table 2). No significant difference was found between lesions with high-enhancement and iso-enhancement with a non-enhancing area (p>0.05).
Table 2.
The enhancement pattern related to the size of thyroid lesion.
| Enhancement degree | Lesions number | Maximum diameter, cm | Mean ±SD, cm |
|---|---|---|---|
| Low-enhancement | 56 | 0.3–2.6 | 0.80±0.47 |
| Iso-enhancement | 33 | 0.4–3.3 | 1.21±0.82 |
| Hyper-enhancement | 16 | 0.6–5.7 | 2.0±1.37 |
| Iso-enhancement with no enhancement area and whole no enhancement | 11 | 0.5–4.1 | 1.86±1.28 |
Discussion
CEUS enhancement patterns were found to be different in benign and malignant thyroid lesions, but its contribution to the diagnosis of thyroid cancer is controversial. In our study, we found a variety of patterns of contrast enhancement in benign and malignant thyroid lesions. Two of these patterns, iso-enhancement with a focal low-enhancement region within the lesion and a non-enhancing ring in the periphery of the lesion, have not been reported previously. We do not clearly understand the pathology that produces these appearances. However, it is possible that they may offer useful diagnostic information.
We found the pattern of iso-enhancement with a focal low-enhancing area to be common with malignant lesions. On biopsy, both the focal low-enhancing area and the surrounding part of the lesion showing iso-enhancement contained malignant cells, and the focal low-enhancing area also showed evidence of interstitial fibrosis. It is possible that the malignant cells induce the interstitial fibrosis [23]. The same pattern was also often encountered with benign lesions, particularly inflammatory ones. Hypo-vascularity is often observed in focal HT [24], which is associated with focal hypothyroidism with severe follicular degeneration [25]. It is also observed in subacute thyroiditis. The pathology is different in the course of subacute thyroiditis. Here, there is a heterogeneous distribution of inflammatory cells with focal fiber hyperplasia. The latter may give rise to focal areas of low-enhancement.
Lesions with low-enhancement were commonly malignant in our study. This is in agreement with previous reports [13,26]. Twenty lesions were benign, including 2 that were HT, 2 that were chronic inflammation, 1 case of subacute thyroiditis, and 1 postoperative suture. These lesions demonstrated heterogeneous low-enhancement within the lesion, without a peripheral ring. Homogeneous iso- and high-enhancement tended to be seen in benign lesions [23].
The enhancement pattern in the periphery of lesions may provide useful diagnostic information, especially for those lesions with internal iso-enhancement appearances. Regular high-enhancing rings were only found in benign lesions, especially in adenoma and nodular goiter. The vascularity of the surface capsule or the compressed tissue around lesion is the explanation for the high-enhancement ring at the periphery of the lesion. The ability of CEUS to detect these features is useful in differentiating cancer from benign lesions [29]. Regular no-enhancement could be found both in benign and malignant nodules. In this group, 6 nodules had peripheral regular no-enhancement rings, in which 4 were benign (2 were adenoma) and 2 malignant (all were PTC). The presence of a non-enhancing ring in the periphery of lesions may reflect changes in tissues adjacent to the lesion [27], which might be a consequence of inflammatory exudate, interstitial edema, or mucoid degeneration because of strong pressure from the nodule.
Both irregular high-enhancement and no-enhancement ring predict malignancy. The pathological cause for an irregular non-enhancing peripheral ring is unclear, but it is possible that it reflects irregular invasion of cancerous tissue into adjacent tissues. Peripheral irregular high-enhancement rings may result from interstitial angiogenesis hyperplasia caused by the cancerous cells invasion [17,28]. Unfortunately, conversional US cannot detect the microvessel signals. Generally, the visible range of lesions was larger on CEUS than on gray-scale US in lesions with irregular high-enhancement ring.
One of 15 cystic-solid mixed lesions in this group was PTC, and it showed iso-enhancement with non-enhancing areas. On conventional US, some tiny ‘spots’ of blood signals were shown in the solid component of the lesion. With CEUS scanning, the solid component showed heterogeneous iso-enhancement without a peripheral ring. The other 14 benign lesions showed homogeneous iso-enhancement in solid component with regular high-enhancing rings or non-enhancing rings.
All lesions that failed to show any enhancement were benign. On conventional US scanning, they had a cystic-solid appearance. Pathological results showed that many of these lesions were nodular goiters with areas of hemorrhage and the CEUS appearances, suggesting that the apparently solid areas were not perfused by contrast agent. This finding can reduce unnecessary biopsies.
We found the degree of enhancement on CEUS was related to the size of lesions, which is in agreement with a previous report [30]. In our study, most malignant lesions showing low-enhancement were papillary thyroid microcarcinomas, perhaps due to the small or immature vascular network in microcarcinomas. The compression of blood vessels by cancer cells can increase resistance to blood flow and impair blood supply [17,31]. The uneven proliferation of tumor cells may cause the heterogeneous enhancement found with CEUS.
Conventional US detected TC with a high sensitivity, so the misdiagnosis rate is our concern. This study showed that CEUS can clearly reduce the misdiagnosis rate compared with conventional US (p<0.01). Our study shows that: (1) with CEUS, the appearances of HT and other inflammatory disorders of the thyroid gland overlap with those of malignant thyroid disease. The patient’s history may help in differentiating them. (2) CEUS appearances of homogeneous hyper-, iso-, and no- enhancement within lesions and/or regular high-enhancement ring in the peripheries of lesions are suggestive of benign thyroid lesions. If there is doubt, conventional US scanning these appearances on CEUS scanning may be helpful pre-operatively. (3) Lesions showing iso-enhancement with a focal low-enhancement region should be considered malignant if inflammatory disease has been excluded. (4) An irregular ring appearance in the periphery of lesions suggests malignancy.
Conclusions
In patients with suspicious US characteristics, CEUS had high specificity and contributed to establishing the diagnosis. Therefore, use of CEUS could avoid unnecessary biopsies.
Footnotes
Conflict of interests
We declare that we have no conflicts of interest in the authorship or publication of this article.
Source of support: Departmental sources
References
- 1.Kobawala TP, Patel GH, Gajjar DR, et al. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. J Thyroid Res. 2011;2011:270149. doi: 10.4061/2011/270149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127:2918–27. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 3.Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–31. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei Y, Ting-ting S, Yan-nan Y, et al. Time trends and pathological characteristics of thyroid cancer in urban Beijing, 1995–2010. Chin J Prev Med. 2013;2:109–12. [PubMed] [Google Scholar]
- 5.Batawil N, Alkordy T. Ultrasonographic features associated with malignancy in cytologically indeterminate thyroid nodules. Eur J Surg Oncol. 2014;40:182–86. doi: 10.1016/j.ejso.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Misra S, Meiyappan S, Heus L, et al. Patients’ experiences following local-regional recurrence of thyroid cancer: A qualitative study. J Surg Oncol. 2013;108:47–51. doi: 10.1002/jso.23345. [DOI] [PubMed] [Google Scholar]
- 7.Marcy PY, Thariat J, Chevenet C, Lacout A. Jugular vein invasion diagnosis and prognosis in thyroid carcinomas. Pol J Radiol. 2016;81:268–69. doi: 10.12659/PJR.896757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen BT, Jain AB, Dagis A, et al. Comparison of the efficacy and safety of ultrasound-guided core-needle biopsy versus fine-needle aspiration for evaluating thyroid nodules. Endocr Pract. 2015;21:128–35. doi: 10.4158/EP14303.OR. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Chen BD, Zhu HF, et al. Comparison of pre-operation diagnosis of thyroid cancer with fine needle aspiration and core needle biopsy: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:7187–93. doi: 10.7314/apjcp.2014.15.17.7187. [DOI] [PubMed] [Google Scholar]
- 10.Han ZY, Li JL, An LC, Li X. The pathological results and clinical features analysis on the thyroid nodules that is difficult for diagnosis by ultrasonography. Chin J Med Ultrasound (Electronic Version) 2008;5:750–56. [Google Scholar]
- 11.Rosario PW, Silva AL, Borges MA, Calsolari MR. Is Doppler ultrasound of additional value to gray-scale ultrasound in differentiating malignant and benign thyroid nodules? Arch Endocrinol Metab. 2015;59:79–83. doi: 10.1590/2359-3997000000014. [DOI] [PubMed] [Google Scholar]
- 12.Puxeddu E, Durante C, Avenia N, et al. Clinical implications of BRAF mutation in thyroid carcinoma. Trends Endocrinol Metab. 2008;19:138–45. doi: 10.1016/j.tem.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Ma JJ, Ding H, Xu BH, et al. Diagnostic performances of various gray-scale, color Doppler, and contrast-enhanced ultrasonography findings in predicting malignant thyroid nodules. Thyroid. 2014;24:355–63. doi: 10.1089/thy.2013.0150. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Luo H. Comparative study of thyroid puncture biopsy guided by contrast-enhanced ultrasonography and conventional ultrasound. Exp Ther Med. 2013;5:1381–84. doi: 10.3892/etm.2013.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giusti M, Orlandi D, Melle G, et al. Is there a real diagnostic impact of elastosonography and contrast-enhanced ultrasonography in the management of thyroid nodules? J Zhejiang Univ Sci B. 2013;14:195–206. doi: 10.1631/jzus.B1200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giusti M, Campomenosi C, Gay S, et al. The use of semi-quantitative ultrasound elastosonography in combination with conventional ultrasonography and contrast-enhanced ultrasonography in the assessment of malignancy risk of thyroid nodules with indeterminate cytology. Thyroid Res. 2014;7:9. doi: 10.1186/s13044-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–89. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 18.NCCN Guidelines, Thyroid carcinoma, Version 2, 2013
- 19.Endocrinology branch of Chinese medical association, Endocrine surgery of Chinese medical association, China anti-cancer association of professional committee of the head and neck cancer, Chinese Society of Nuclear Medicine. [Guide for diagnosis and treatment of thyroid nodules and differentiated thyroid cancer]. Chin J Endocrinol. 2012;28:779–97. [in Chinese] [Google Scholar]
- 20.Zhang B, Jiang YX, Liu JB, et al. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid. 2010;20:51–57. doi: 10.1089/thy.2009.0045. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Kim SY, Yang KR. Ultrasonographic criteria for fine needle aspiration of nonpalpable thyroid nodules 1–2 cm in diameter. Eur J Radiol. 2013;82:321–26. doi: 10.1016/j.ejrad.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology. 2011;260:892–99. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Yang WW, Wen QT, et al. TGF- beta induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM [corrected] Exp Mol Pathol. 2009;87:189–94. doi: 10.1016/j.yexmp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Fu X, Guo L, Zhang H, et al. “Focal thyroid inferno” on color Doppler ultrasonography: A specific feature of focal Hashimoto’s thyroiditis. Eur J Radiol. 2012;81:3319–25. doi: 10.1016/j.ejrad.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Hayshi N, Tamaki N, Konishi J, et al. Sonography of Hashimoto’s thyroiditis. J Clin Ultrasound. 1986;14:123–26. doi: 10.1002/jcu.1870140208. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, Xu YB, Jiang J, et al. [Differential diagnostic value of contrast-enhanced ultrasound in calcified thyroid nodules]. Zhong hua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48:726–29. [in Chinese] [PubMed] [Google Scholar]
- 27.Yoon JH, Kim EK, Youk JH, et al. Better understanding in the differentiation of thyroid follicular adenoma, follicular carcinoma and follicular variant of papillary carcinoma: a tetrospective study. Int J Endocrinol. 2014;2014:321595. doi: 10.1155/2014/321595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahbar G, Sie AC, Hansen GC, et al. Benign versus malignant solid breast masses: US differentiation. Radiology. 1999;213:889–94. doi: 10.1148/radiology.213.3.r99dc20889. [DOI] [PubMed] [Google Scholar]
- 29.Schleder S, Janke M, Agha A, et al. Preoperative differentiation of thyroid adenomas and thyroid carcinomas using high resolution contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2015;61:13–22. doi: 10.3233/CH-141848. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Z, Quan L, Lian C, et al. Relationship between characteristics of thyroid carcinomas in real-time contrast-enhanced ultrasound and tumor sizes. Chin J Med Imaging Technol. 2012;1:82–85. [Google Scholar]
- 31.Averkious M, Powers J, Skyba D, et al. Ultrasound contrast imaging research. Ultrasound Q. 2003;19:27–37. doi: 10.1097/00013644-200303000-00004. [DOI] [PubMed] [Google Scholar]